1. Introduction
Tailings refer to the remnants left after ore extraction, which are widely concerning due to their harmful substances and potential environmental risks. There are heavy metals within tailings; some can potentially permeate the soil and groundwater via natural weathering or water erosion, posing threats to biological systems and human health [
1]. Additionally, tailings can disrupt the balance of ecosystems. Organic and other toxic substances within tailings can alter soil and water quality, leading to certain risks to aquatic animals and plants and the destruction of entire ecosystems. Furthermore, the storage and treatment of tailings require significant amounts of land and water resources; severe disturbances might be caused [
2]. In order to minimize the detrimental impact of tailings on the environment, there is a great necessity to reduce or remove harmful substances from tailings [
3,
4], meanwhile ensuring by containment that they are safe and stable [
5], reducing their impacts on both the ecosystem and human health. Therefore, it is of utmost importance that we urgently develop new technical approaches that make tailings reinforcement easy and efficient while remaining green and pollution-free, effectively reducing the environmental pollution risk associated with tailings.
In recent years, there has been a surge of interest in microbial-induced calcite precipitation (MICP), considered a highly effective and sustainable reinforcement technology; it has been evidenced by numerous domestic and international studies [
6]. These studies have demonstrated that MICP can induce the formation of crystals with exceptional cementing properties through the metabolic activities of microorganisms. When applied to geotechnical materials, this technology significantly boosted their strength [
7,
8].
MICP technology empowers an ecologically friendly approach to enhance various objects since it can improve the earth and rock stiffness, bearing capacity, permeability, and liquefaction resistance. Song et al. [
9] conducted several cycles of MICP grouting on sandstone. They acquired remarkable results: a 229% increase in uniaxial compressive strength, a 179% increase in elastic modulus, and a 177% increase in brittleness index compared to the pre-grouting conditions. The overall mechanical properties and permeability of sandstone were shown to be primarily impacted by the amount of cemented minerals present, which, in turn, directly governs the microscopic distribution of CaCO
3, amplifying the efficacy of bio-cementation in sandstone. Banik et al. [
10] explored the microstructure and mechanical characteristics of standard sand specimens treated with MICP. The resulting stress–strain data and the gain of strength observed under different pore volume cementing fluids provided insight into quantifying the microbial-reinforced sand’s strength and enhancing the low-strain shear modulus. Zhao et al. [
11] carried out a solution and cyclic triaxial tests while regulating the concentration of NaCl and observed the gradual decrease of stiffness and cyclic resistance of the additive solids as the NaCl concentration increased. However, these values remained higher than those of unreinforced sand, and it was discovered that the decline in liquefaction resistance resulted from the conversion of calcium carbonate crystals from clusters to single crystals induced by the technology. Xiao et al. [
12] leveraged the application of reinforced coral sand with MICP by acclimating microorganisms to various environments to improve their enzymatic activities; a novel three-stage reinforcement method was developed through their study and its ability to increase the strength of MICP-treated objects and the induced calcium carbonate content was confirmed by experimental outcomes. Additionally, suitable cementing solution concentrations were proven to be advantageous in enhancing the reinforcement strength.
MICP technology has shown enormous potential for producing biological bricks [
13] that conform to specific strength standards. This technology offers advantages such as low costs, environmental compatibility, and brief reinforcement periods when applied to tailings reinforcement. Unlike traditional cement and chemical grouting, MICP reinforcement grout does not cause intricate concrete tailings to dissociate. Furthermore, the bio-cement produced is simpler to grind, dissociate, and recycle. The effects of bio-cementation and cement treatment were examined and compared by Yin et al. [
14] for oil-contaminated sand. The results demonstrate that while the effects of both bio-cementation and cement treatment were diminished under oil pollution, sand treated with MICP was four times stronger than sand treated with cement using the same quantity of cementing fluid. The unconfined compressive strength of sand treated with MICP surpassed 1 MPa after only eight reinforcement cycles, reflecting the tremendous potential of MICP in improving soil and rock in oil-contaminated regions. However, due to the bacterial fluid’s influence on the cementation rate of microbial reinforcement technology, the strengthening effect might be diminished in unexpected environments, resulting in a different curing strength than expected. Therefore, it is necessary to investigate new methods which can regulate MICP to enhance its strengthening effect and extend its engineering applications.
Current research has focused on regulating bacteria to enhance the efficacy of MICP technology. Researchers have explored the control of pH, culture temperature, bacterial types, and concentration. Wen et al. [
15] used urease activity monitoring through changes in conductivity and pH values before MICP reaction to maintain consistent MICP application. Results indicated that the conductivity and pH value rapidly increased after mixing bacteria with urea solution. The optimum conductivity range was 1.5–1.8 ms/cm within 60 min, and the pH range was 8.82–9.02. Using a 0.25 m calcium source in reinforced sand samples produced consistent unconfined compressive strength, improving rock and soil engineering performance. In his study on the effects of bacterial concentration and activity on the MICP process under different temperatures, Wang et al. [
16] demonstrated that adjusting temperature controlled induced calcium carbonate’s size and crystal form. Urease activity did not decrease at low temperatures, but precipitation amounts were limited. Low temperatures reduced bacterial growth and the precipitation rate of induced calcium carbonate. High temperatures rendered urease inactive, but repeated grouting increased calcium carbonate quantity, optimizing the application field of MICP. Lv, Tang, Zhang, Pan, and Liu [
7] explored different calcium sources and magnesium ions’ effects on the treatment of calcareous sand during MICP treatment. Results indicated that calcium acetate had the highest calcium carbonate content, but calcium nitrate or calcium chloride increased solid strength. Solid strength under different calcium sources increased after adding 0.05 M of magnesium. When the magnesium ion concentration reached 0.5 M, using calcium chloride as the calcium source produced the highest strength and calcium carbonate content, providing novel guiding principles for MICP reinforcement technology.
Researchers are exploring various breakthrough points in the regulation process of bacterial induction, including screening new bacterial strains, adding additives, and externalization techniques. Fazelikia et al. [
17] analyzed the ecosystem and screened various strains with MICP performance, comparing the induced mineralization functions of each strain through wind tunnel tests and scanning electron microscopes. The tests demonstrated that the diversity of Bacillus strains provides significant potential for environmental adaptation and plays a critical role in resisting soil and rock erosion.
Furthermore, Shan et al. [
18] investigated the feasibility of adding activated carbon, thus improving the adsorption rate of bacteria during MICP reinforcement. The results have shown a significant enhancement in the axial strain strength of samples with increased activated carbon content, along with an improvement in bacteria adsorption rate and strength. At 0.75% activated carbon, the liquefaction resistance of sand treated with MICP was significantly improved, which, in turn, helped to enhance the liquefaction resistance of sand strengthened through MICP. Liang et al. [
19] explored the strength mechanism of MICP-treated sand by adding different fibers and varying fiber lengths. The results indicate that segment fibers effectively promote the precipitation of calcium carbonate, limit the movement of sand particles, and enhance the strength of the solidified body. However, overly long fibers can lead to uneven calcium carbonate, thus reducing the strength. Zhang et al. [
20] conducted experiments measuring the effects of different protein concentrations on MICP-reinforced tailings. The results showed that with the increase in protein concentration, the solidification heterogeneity of tailings increased. When the protein concentration was 5%, the solidification strength of tailings was at its best, with calcite as the primary induced calcium carbonate. Deng et al. [
21] improved the effectiveness of MICP-induced precipitation by introducing an electric field, thus increasing the amount of calcium carbonate induced by the microorganisms. The results reveal that the growth and activity of mineralized bacteria were substantially improved under an electric field of 0.5 V/cm, leading to increased calcium carbonate content. Finally, Su et al. [
22] have proposed a new technology that improves reinforcement and reduces byproduct emissions in microbial-induced precipitation. By combining MICP with zeolite, the good adsorption function of zeolite is utilized, leading to significant enhancements in reinforcement strength, reduced permeability, and a reduction in ammonia emissions. This innovation represents a significant improvement in standard MICP technology.
The research on regulating bacterial induction has made remarkable progress, particularly in adding organic matrices to effectively modulate calcium carbonate precipitation induced by bacteria. Organic matrices have been found to directly control calcium carbonate’s nucleation, growth, and aggregation. As early as 1984, Wheeler and Sikes [
23] discovered that organic matrices, as an initial factor of crystal growth, can regulate crystal formation, provide a lattice template, or stabilize the core surface of calcium carbonate. Paul and Das [
24] also observed that the use of organic substrates had a significant effect on biomineralization. Using organic molecules such as curcumin and quercetin as biological templates, researchers successfully synthesized the most unstable vaterite crystal and stable calcite in calcium carbonate, with vaterite being stable in the liquid phase for up to 18 h.
Organic substrates play a critical role in regulating the mineralization process of calcium carbonate in natural organisms. The organic matrix provides a template for the nucleation and growth of calcium carbonate and regulates the crystal orientation, form, and morphology of calcium carbonate crystals [
25]. Yang et al. [
26] studied the deposition of calcium carbonate in the presence of various organic substrates, such as beta-cyclodextrin glycogen and soluble starch. Calcite crystals, produced without an organic matrix, can be induced by β-cyclodextrin, while coral aragonite crystals are induced as a regulatory template. Similarly, glycogen and soluble starch induce vaterite. The following research discovered that dextran could effectively induce leaf-like aragonite crystals when used as a template [
27]. Zhao et al. [
28] studied calcium carbonate induced by amylopectin, Mg
2+, and Fe
3+. The results showed that amylopectin was instrumental in forming pumpkin-like calcite, while rod-like and dumbbell-like calcite were formed in the presence of Mg
2+, and calcite with step depression was formed in Fe
3+. Hosoda and Kato [
29] studied the preparation of calcium carbonate under three organic matrix templates: cellulose, chitosan, and chitin.
The results showed that the organic matrix effectively controlled the morphology of calcium carbonate crystals. Zhang et al. [
30] studied the regulation of dumbbell-shaped calcium carbonate on bacterial cell templates and proposed the possible particle formation and transformation mechanism. Azulay et al. [
31] studied the regulation of ECM protein in extracellular polymer by calcium carbonate and found that the organic matrix affected carbonate nucleation. The calcite nucleus was formed stably in advance under the control of ECM, which aggregated into a calcite crystal form. Azulay and Chai [
32] pointed out that biomineralization can significantly change morphology and structure under the guidance of organic molecules and studied the influence of biopolymer additives on the crystal structure of calcium carbonate. Without additives, carbonate crystals mainly exist in the form of calcite, which is the most thermodynamically stable. Kim et al. [
33] studied the formation of calcium carbonate by agar and polyacrylic acid at different concentrations. The results showed that the pH could not be controlled at low concentrations, leading to changes in organic matter components forming an anisotropic template, resulting in elliptical calcite forms. In contrast, a slow pH increase at high concentrations would result in spherical calcite. Erceg et al. [
34] studied the application of lipids in the formation of calcium carbonate and found that calcium carbonate formed a shell structure under the action of lipids, providing a new idea for adjusting the precipitation process of calcium carbonate.
Previously, researchers focused on utilizing one or more organic matrices as templates for the chemical preparation and control of the crystal form in calcium carbonate crystals. Based on the biomineralization of calcium carbonate induced by microorganisms, the mechanism of researching the addition of organic matrices as regulatory templates in the biomineralization process is relatively complex, and further research is needed. Organic matrices are mainly utilized to adjust calcium carbonate’s crystal type, appearance, and particle size, providing a corresponding means for producing calcium carbonate crystals with unique morphology and ordered structure.
Microbial-induced calcium carbonate precipitation is a complex system, and the specific reaction formulae are shown in Formulas (1)–(5). Bacteria and corresponding ions lead to biominerals that differ from chemically prepared carbonates, exhibiting distinct morphology, structure, thermodynamic stability, and kinetic properties. Furthermore, the reproduction and metabolism of bacteria during induced mineralization generate organic matter, which further influences biomineralization. As a result, biomineralization is affected by numerous factors, and different organic substrates have varying effects on the process.
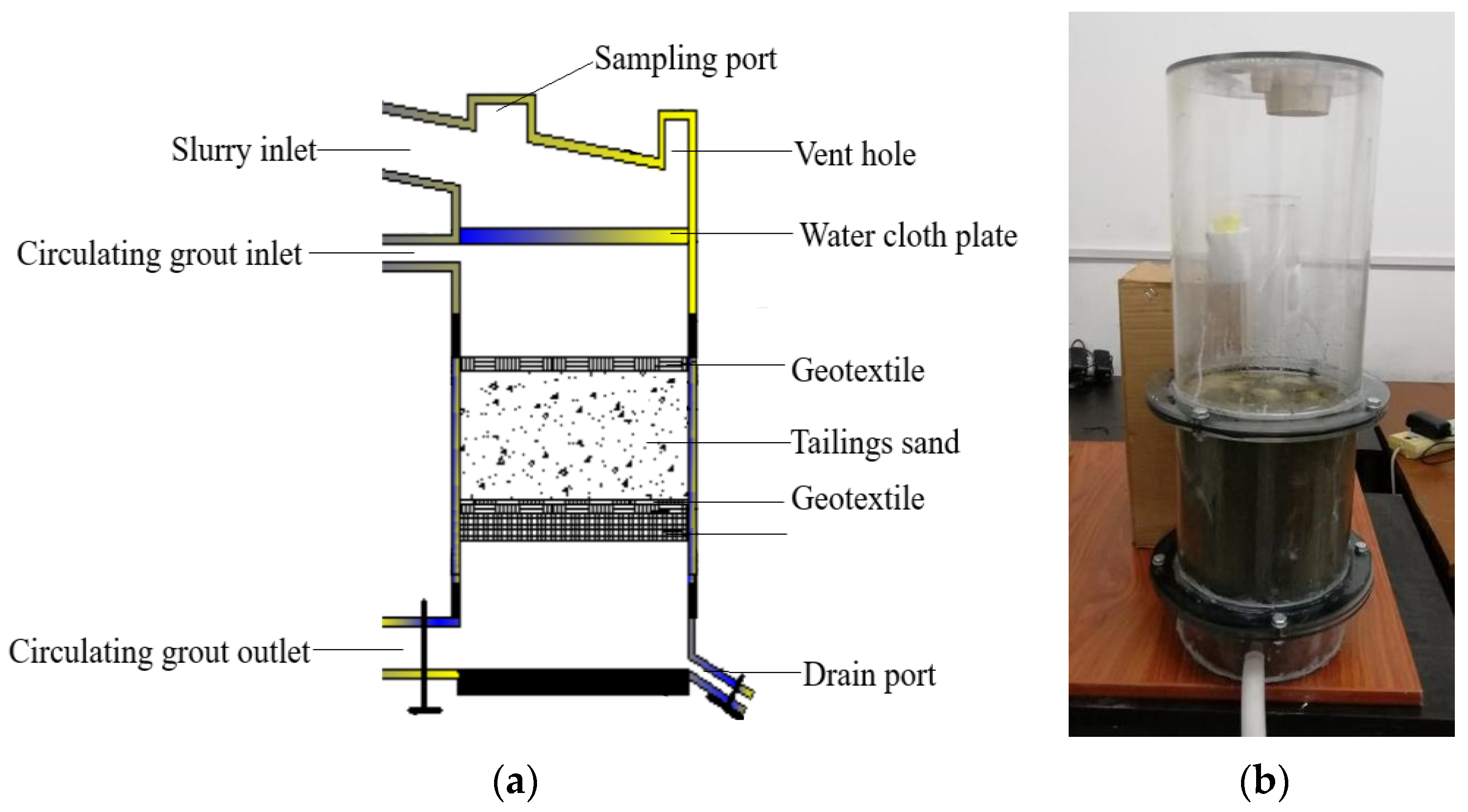
In order to understand the effect of the organic matrix on the mineralization of calcium carbonate prompted by microorganisms and the influence of microorganism-induced calcium carbonate precipitate on the reinforcement of the tailings, this study focused on studying a functional strain, Sporosarcina pasteurii, known for its mineralization capabilities. Initially, the bacteria were cultured using a solution-based experiment. Different concentrations of the organic matrix, sodium citrate (ranging from 0% to 3.5%), were added to the bacterial culture solution to investigate their synergistic effects. The goal was to ascertain the impact of the organic matrix on crystal formation and microstructure during the process of bacterial-induced mineralization, as well as to understand the underlying mechanism by which the organic matrix affects the properties of the sediment throughout this process. The crystal form and microstructure of the induced calcium carbonate samples were characterized using X-ray diffraction, infrared spectrometry, and scanning electron microscopy. After comparing the outcomes of bacteria concentration, calcium carbonate content, and crystal morphology, the optimal dosage of the organic matrix, sodium citrate, was determined. Lastly, employing the bacteria solution with the optimized concentration of organic matrix sodium citrate, tailings reinforcement tests were conducted on tailings and solid samples, incorporating mechanical testing, microscopic detection, and CT scanning. The objective was to explore how the organic matrix reinforces tailings through microbial activity. The results shed light on the role of the organic matrix in regulating the crystal form of calcium carbonate in microbially-induced calcium carbonate precipitation (MICP), facilitating the control of the solidification process in microbial-enhanced tailings and yielding superior reinforcement strength.
4. Results of Tailings Reinforcement
4.1. Setting of Sodium Citrate Addition Amount in an Organic Matrix
According to the analysis results of the solution test after synergistic culture of organic substrate sodium citrate and bacteria, the most extended growth duration of the bacterial solution and a steady increase in urease activity were observed upon adding 3% organic substrate sodium citrate. Moreover, experimental outcomes also demonstrated that the most significant amount of induced calcium carbonate sediment containing various crystal structures and tight cementation was obtained under the control of 3% organic matrix sodium citrate. Consequently, the appropriate addition mode and amount (3% soluble organic matrix sodium citrate) were determined by the reinforcement test of tailings involving regulation of the calcium carbonate crystal form. A grouting reinforcement test of tailings was subsequently implemented to explore the reinforcement effect of tailings after adding organic matrix sodium citrate. In this test, the mechanical properties of the tailings after grouting, the distribution of calcium carbonate cement types in the tailings, calcium carbonate content, micro-cementation statuses among tailings particles, crystal form and appearance of calcium carbonate crystals, particle size characteristics, and pore distribution of calcium carbonate crystals were analyzed and calculated through shear strength testing and micro-morphology testing (e.g., SEM, XRD, CT scan, etc.) of the reinforced tailings.
By comparing the test results with the joint MICP reinforcement test group, this study explored the mechanism of tailings reinforcement upon the control of organic matrix sodium citrate.
4.2. The Triaxial Test Results
The strain-controlled triaxial apparatus based on the Mohr–Coulomb strength theory (as shown in Formula (7)) was utilized for the undrained shear test. Confining pressures of 200 kPa, 250 kPa, and 300 kPa were set, and the axial force was applied immediately when the specimen became stable, resulting in the tailings specimen’s shear failure under undrained conditions. During the test, critical parameters such as the shear strength index of tailings were monitored and analyzed, and their cohesion and internal friction angle were calculated. The stress–strain curves of tailings samples strengthened by each group of tailings under the consolidated undrained triaxial shear test are depicted in
Figure 8.
τ is shear strength; σ is normal stress; φ is the angle of internal friction; c is the cohesion.
The stress–strain curve reveals the changing trend of the tailings samples’ stress–strain response under axial load, broadly categorized into four stages. In the initial loading stage, the pores within the tailings sample begin to compact under the axial force, leading to small transverse deformation and a decrease in sample volume over time. This is the pore compaction stage. Moving into the second stage, the load increases and the deviator stress of the sample reaches its peak value, followed by entry into the third stage. In this stage, the deviator stress decreases gradually, and the specimen incurs some destruction. Continuous loading leads to shear failure along cracks within the sample. As the fourth stage begins, the axial deformation increases while the deviator stress decreases and stabilizes gradually. Once the set axial strain value is reached, the specimen undergoes destruction but retains some strength due to the mutual embedding and occlusion between particles.
Compared to the unstrengthened group, the tailings’ peak deviator stress value of ordinary MICP increases strengthened. In contrast, the peak deviator stress value of tailings strengthened with organic matrix sodium citrate has an even more significant change. The Mohr–Coulomb strength theory formula processes triaxial shear strength test data, obtaining the characteristic values of mechanical parameters of tailings samples under different confining pressures, as displayed in
Table 2. The cohesive force, internal friction angle, effective stress ratio, and maximum shear stress of the reinforced tailings samples demonstrate a significant increase. Compared to the experimental group without the addition of organic matrix sodium citrate, the cohesive force and shear stress of the tailings experimental group treated with organic matrix sodium citrate show a significant improvement. Therefore, adding organic matrix sodium citrate can effectively enhance the mechanical properties of tailings while improving the effectiveness of MICP reinforcement of tailings.
4.3. Comparative Analysis of Micro-Morphology of Cement
To analyze the microstructure of original tailings and reinforced tailings samples, a scanning electron microscope (SEM) was used. As illustrated in
Figure 9, the unreinforced undisturbed tailings particles exhibit irregular distribution forms comprising flake, irregular grain, diamond, and other structures. Furthermore, the surface layer displays numerous large pores, representing a granular medium with relatively high permeability. Upon comparing
Figure 9b,c, it becomes evident that adding a 3% organic matrix sodium citrate group induces the formation of more tightly cemented calcium carbonate. Compared to the group lacking organic matrix sodium citrate, the calcium carbonate precipitate obtained by adding 3% organic matrix sodium citrate features a diverse range of structures, shapes, and sizes. Furthermore, various calcium carbonate crystals and tailings particles are encapsulated, extruded, and adhered to one another, thus generating irregular reinforced aggregates. This further highlights how organic matrix sodium citrate can effectively enhance tailings’ stability and strength and facilitate tailings’ reinforcement treatment.
Figure 9c shows calcium carbonate completely covers the tailings particles, effectively binding them together. This coverage and cementation generate a specific stacking morphology. Furthermore, the distribution of calcium carbonate crystals is uniform, with no large-area fixed calcium carbonate crystal form. Irregular calcium carbonate crystals interconnect and stack upon each other to improve their tightness, thus enhancing the reinforcement effect. Owing to the complexation of organic matrix sodium citrate, more calcium ions can be adsorbed and accumulated to generate calcium carbonate crystals. This effect reduces the scouring impact of the solution, thus further strengthening cementation. The various calcium carbonate structures attached to the surface of tailings particles during cementation and solidification also combine calcium carbonate and tailings particles.
Moreover, sodium citrate rapidly dissolves in water, forms complexes with calcium ions, and speeds up the formation and precipitation of calcium carbonate. Numerous tests and practices have proved that this approach can effectively enhance the mechanical properties of tailings, reducing the impact of tailings instability on the environment. The reinforcement strategy based on regulating and inducing the formation of calcium carbonate demonstrates excellent feasibility and economy and is significant in reducing tailings pollution while enhancing the ecological environment.
4.4. Crystal XRD Detection
Calcium carbonate has three crystal forms: calcite calcium carbonate, aragonite calcium carbonate, and vaterite calcium carbonate. To better understand the structure and morphology of calcium carbonate crystals, an X-ray diffraction technique was utilized to detect the structure and morphology of calcium carbonate crystals. The crystal structure and crystal form distribution of samples can be qualitatively analyzed by comparing the detection results with the known standard chart cards. This study derived the XRD results from the common MICP technique and reinforced tailings samples with 3% organic matrix sodium citrate, as shown in
Figure 10. Following analysis, essential discoveries were obtained regarding the crystal structure and crystal form distribution in the samples.
The bacteria solution cultured by common MICP and sodium citrate added with 3% organic matrix was used as a gum in the tailing sand sample. XRD was conducted on the cemented tailings samples, and the data obtained were analyzed. This revealed that when 3% sodium citrate and bacteria solution were added to the tailings samples for reinforcement treatment, the crystal structure of calcium carbonate was mainly calcite calcium carbonate, followed by vaterite calcium carbonate. The dominant crystal planes in the XRD results appeared at (004), (012), (112), (104), (114), (113), (202), (204), and (118), with the (104) crystal plane being the symbolic corresponding crystal plane of calcite calcium carbonate. In contrast, the dominant crystal planes such as (012), (110), (113), (202), and (204) are notable characteristics of calcite calcium carbonate. On the other hand, the corresponding crystal planes of vaterite calcium carbonate are (004), (112), and (114). Therefore, the calcium carbonate cement formed by adding sodium citrate predominantly features calcite calcium carbonate, and its proportion is higher than that of vaterite calcium carbonate mixed crystal. Compared to tailings reinforced by ordinary MICP, adding 3% organic matrix sodium citrate can generate well-formed calcium carbonate precipitated crystals. The proportion of calcite and vaterite is more reasonable, and the mixed crystals can have more effective cementation within tailings.
4.5. Crystal FT-IR Detection
Two techniques were utilized to investigate the reinforcement effect of tailings: typical microbial-induced calcium carbonate precipitation (MICP) reinforcement and reinforcement by adding 3% organic matrix sodium citrate. Samples of reinforced tailings were analyzed using infrared absorption spectroscopy. The corresponding crystal form structure of calcium carbonate was successfully obtained through the detection and analysis of the wave values under the infrared characteristic absorption peaks of each crystal form of calcium carbonate in the sample and by comparing the resulting spectrum with the standard spectrum of wave numbers.
The
Figure 11 infrared spectra demonstrate that the blank group exhibits distinctive absorption peaks at 3419.79 cm
−1, 2509.39 cm
−1, 1406.83 cm
−1, 1076.28 cm
−1, 878.68 cm
−1, and 711.73 cm
−1 [
35,
36]. The absorption peaks at 2509.39 cm
−1 and 3419.79 cm
−1 are primarily due to the O-H bond’s symmetrical and asymmetrical stretching vibrations, resulting from the hydroxyl groups and adsorbed water of calcium carbonate particles. Conversely, V
4 and V
2 characteristic absorption peaks of calcite crystals at 711.73 cm
−1 and 878.68 cm
−1 correspond to V
4 of vaterite crystals at 1076.28 cm
−1, respectively. Notably, the IR spectra of calcium carbonate reinforced with 3% sodium citrate register peaks at 1416.03 cm
−1, 1083.03 cm
−1, 873.57 cm
−1, and 711.85 cm
−1. Upon comparison to standard spectra, gravimetric analysis reveals the calcium carbonate reinforced with 3% sodium citrate to be composed of calcite and vaterite. The corresponding wave numbers of calcite are 1416.03 cm
−1, 1083.03 cm
−1, and 711.85 cm
−1; the corresponding peaks of calcite are relatively protracted, signifying that the proportion of calcite calcium carbonate in calcium carbonate exceeds that of vaterite calcium carbonate. This outcome coincides with calcium carbonate crystal’s X-ray Diffraction (XRD) findings. The addition of 3% organic matrix sodium citrate can regulate the calcium carbonate crystal to reinforce the tailings, and consequently, the crystal form of calcium carbonate can be adjusted.
4.6. Quantitative Characterization of Pore Structure Based on CT Scanning
The tailings samples reinforced by typical microbial-induced calcium carbonate precipitation (MICP) and microbial grouting with 3% organic matrix sodium citrate were scanned layer by layer using CT technology. The result data were reconstructed using three-dimensional visualization software ( Dragonfly 2021.1 Build 977), and digital image information was established. By utilizing this microscopic research method, we explored the microscopic changes in the pore structure of tailings following grouting.
To reconstruct the tailings specimen and obtain a quantitative characterization of its pore structure, the data generated by layered scanning were imported into Dragonfly software for further analysis. The Dragonfly software was employed to effectively remove any artifacts and execute multiple iterative reconstruction algorithms to achieve the stereo reconstruction of the data. The resulting three-dimensional reconstruction of the reinforced tailings specimen subjected to ordinary MICP and 3% organic matrix sodium citrate is depicted in
Figure 12a,b. CT technology provided a powerful means to reveal the characteristics of the microstructure change of the tailings following grouting reinforcement. This technique yields significant theoretical and technical support for the subsequent optimization design and facilitates a comprehensive understanding of the changes in pore structure following tailings reinforcement.
The distribution of pores and tailings particles in the tailings samples after grouting reinforcement can be distinguished through the grey values generated by CT scanning in two-dimensional slices of the three-dimensional sectional image. The pore structure of the tailings samples can be obtained by segmented threshold processing of the cut data volume through Dragonfly software and binary conversion, as illustrated in
Figure 12b,c. By analyzing the three-dimensional pore diagram, it can be inferred that the tailings strengthened with 3% organic matrix sodium citrate have significantly fewer pores than those strengthened through ordinary MICP. In contrast, the upper part of the tailings strengthened via ordinary MICP has more pores than the lower part, which may be a result of repeated grouting and slurry erosion-generated deposition and cementation of calcium carbonate particles, leading to a non-uniformity of the tailings sample during ordinary MICP reinforcement. Compared to ordinary MICP, the overall porosity of the tailings reinforced via adding 3% organic matrix sodium citrate is considerably lower, especially in the upper and middle parts of the reinforced samples. The integrity of the samples becomes robust, and the strengthening effect is also significantly improved. This improved behavior may be attributed to the role of organic matrix sodium citrate in enhancing the activity and proliferation of bacterial liquid and providing more nucleation sites for calcium carbonate crystal-induced precipitation during cementation. The pores in the middle and upper parts of the strengthened samples are lower than those in other parts. However, the related mechanism of organic matrix regulation in MICP requires further exploration and improvement.
The Dragonfly software is capable of analyzing the two-dimensional slice diagram of tailings samples in different layers along the Z axis (from the bottom to the top of the tailings samples), layer-by-layer, to obtain the statistical results of porosity of cross-sections, as illustrated in
Figure 13.
Based on the analysis of the results depicted in
Figure 13, it can be observed that the porosity of the tailings column samples exhibits an overall upward trend, with the porosity gradually increasing from the top to the bottom. In the standard MICP reinforcement test without ammonium citrate addition, the porosity of the tailings column fluctuates between 6.85% and 28.21%, with an average value of 12.93%. However, in the reinforcement test using 3% organic matrix sodium citrate, the porosity of the tailings column ranges only from 2.15% to 11.25%, with an average value of 6.54%. Compared to the standard reinforcement test group, the porosity of the tailings reinforced by grouting under organic matrix ammonium sodium citrate control is relatively low, reduced by 49.42%. This indicates that sodium citrate can effectively expedite the precipitation process of calcium carbonate induced by microorganisms.
Additionally, because the reinforcement slurry is injected from the top of the sample, the infiltration may remove some fine tailings and calcium carbonate sediments, ultimately increasing surface pores. Generally speaking, adding an organic matrix can effectively decrease the porosity of tailings samples, particularly in the middle and upper parts. The strengthening velocity is significantly faster than that of ordinary MICP, and the cementation uniformity is enhanced.
5. Discussion
The organic matrix plays crucial roles in regulating microbial-induced calcium carbonate precipitation (MICP) and in promoting the reinforcement of soil and rocks with the aid of microorganisms. By providing sufficient nutrients and energy, the organic matrix stimulates the growth and metabolism of microorganisms. It promotes the precipitation of calcium carbonate, ultimately leading to the formation of robust cementation and an increase in the strength and stability of soil and rock. Through its hydrolysis and biodegradability, the organic matrix modifies the physical and chemical properties of soil and rock, improving the living environment of microorganisms and enhancing their adaptability and stress resistance, thus sustaining their performances in diverse environments. In addition, the organic matrix has various sources and types that can be proportioned and modified to suit different rock and soil types and reinforcement requirements, allowing for high flexibility.
Adding an organic matrix, specifically sodium citrate, to the experimental setup had a notable positive impact on the efficacy and quality of the MICP reinforcement. Comparative analysis revealed that introducing an organic matrix in the sample could lead to a reduction in porosity and an enhancement in the strength and stability of the tailings specimens. Nawarathna et al. [
37] also employed an organic matrix, namely chitosan, to augment the amount of induced calcium carbonate precipitation and subsequently solidify sand—the resultant precipitation comprised calcium carbonate and chitosan hydrogel. Implementing the organic matrix, chitosan, altered the morphology of calcium carbonate crystals, yielding a more robust and consolidated structure than traditional methodologies. Nawarathna et al. [
38] investigated the regulation aspects of biomineralization using two different organic substrates. The graphical representation of the relationship between the induced precipitate amount and the poly (L-lysine) concentration exhibited a bell-shaped curve, indicating a concentration-induced regulatory and inhibitory relationship. Conversely, variations in the polyglutamic acid concentration had no impact on the precipitate quantity. Under poly L-lysine’s influence, calcium carbonate crystals’ morphology shifted from well-developed rhombohedral crystals to elliptical aggregates.
In contrast, when polyglutamic acid is introduced, polyhedral and spherical crystals will predominate. Despite a small quantity of organic matrix being added, its presence exerts control over nucleation and effectively manages the size, shape, structure, and growth orientation of calcium carbonate crystals. Additionally, it governs the nucleation site responsible for forming specific calcium carbonate crystal forms [
39]. However, certain concerns are associated with utilizing organic matrix sodium citrate. Firstly, controlling the amount of sodium citrate within a specific range during induced precipitation is crucial. Excessive amounts of sodium citrate can hinder microbial growth and diminish the desired reinforcement effect. Secondly, due to the biological nature of the organic matrix, attention must be given to the potential issue of bacterial contamination during its application to prevent adverse impacts on the environment.
The organic matrix assumes a significant role in the microbial fortification of rock and soil. Still, meticulous attention ought to be directed toward regulating the supplementary quantity and bacterial contamination during its implementation. The successful application of MICP reinforcement necessitates the perpetuation and efficacy of calcium carbonate precipitation to be upheld. The outgassing of ammonia and subsequent biological oxidation of ammonia, serving as the principal byproducts of urea decomposition, will diminish the concentration of ammonium in the surroundings, consequently resulting in a decline in pH value, which may induce a marginal dissolution of the induced calcium carbonate [
40,
41]. In forthcoming investigations, further discernment of the interconnectedness between the organic matrix, microorganisms, and cement strength must be explored to amplify the utilization of MICP technology in engineering practice.
Moreover, novel formulations and production techniques for organic matrices can be delved into, thereby forging more environmentally sustainable, efficacious, and cost-effective microbial reinforcement technologies for rock and soil. Additionally, the bedrock of research pertaining to the microbial bolstering of rock and soil can be fortified, encompassing the exploration of bioactive substances and a persisting in-depth understanding of the consolidation mechanism and regulatory pattern of microorganisms. Enhancing the reinforcement efficacy and widening the scope of application can expedite the extensive implementation of geotechnical engineering.