Theoretical Study on the Influence of the Anharmonic Effect on the Ionic Conductivity and Thermal Stability of 8 mol% Yttria-Stabilized Zirconia Solid Electrolyte Material
Abstract
1. Introduction
2. Methods
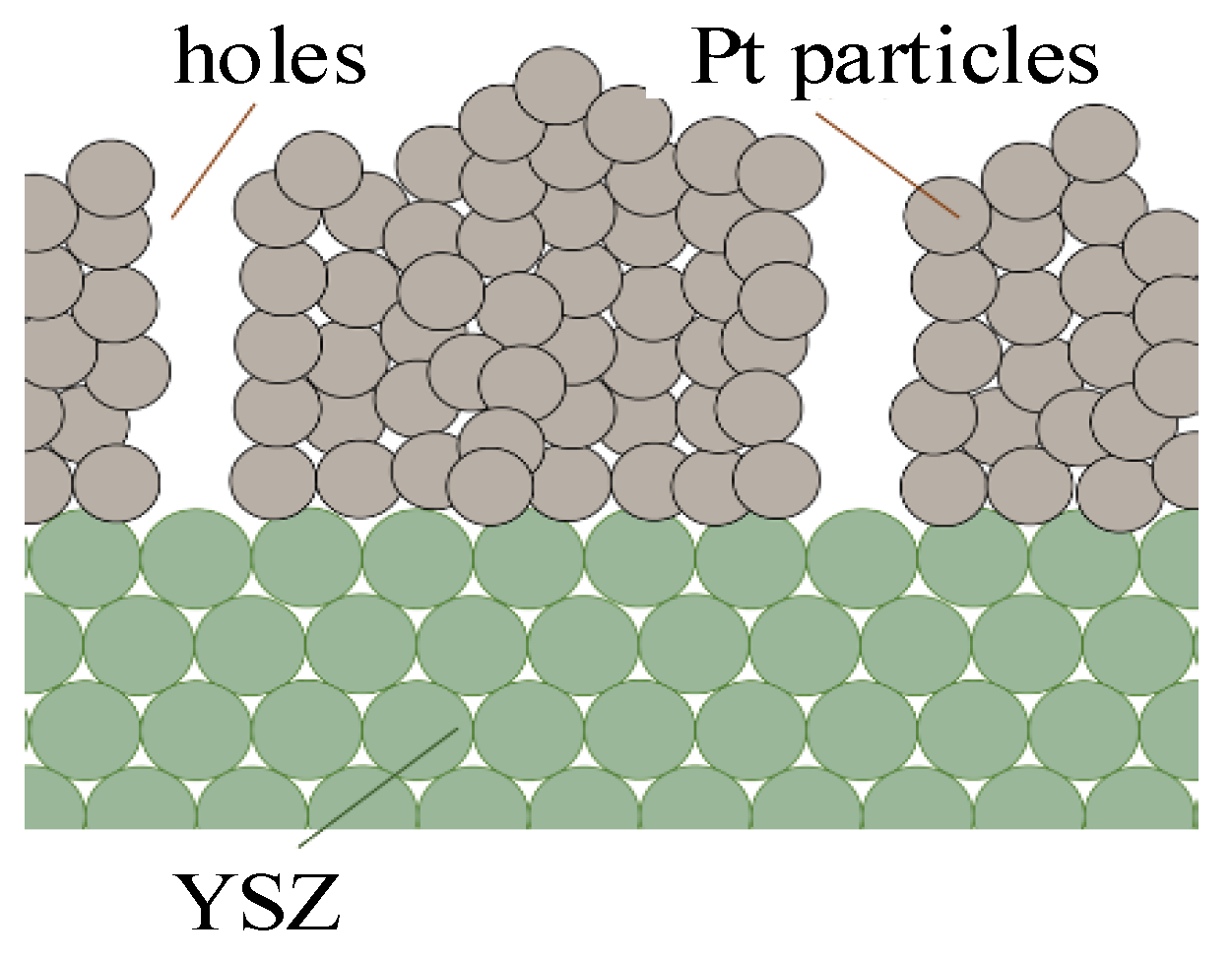
2.1. Physical Model
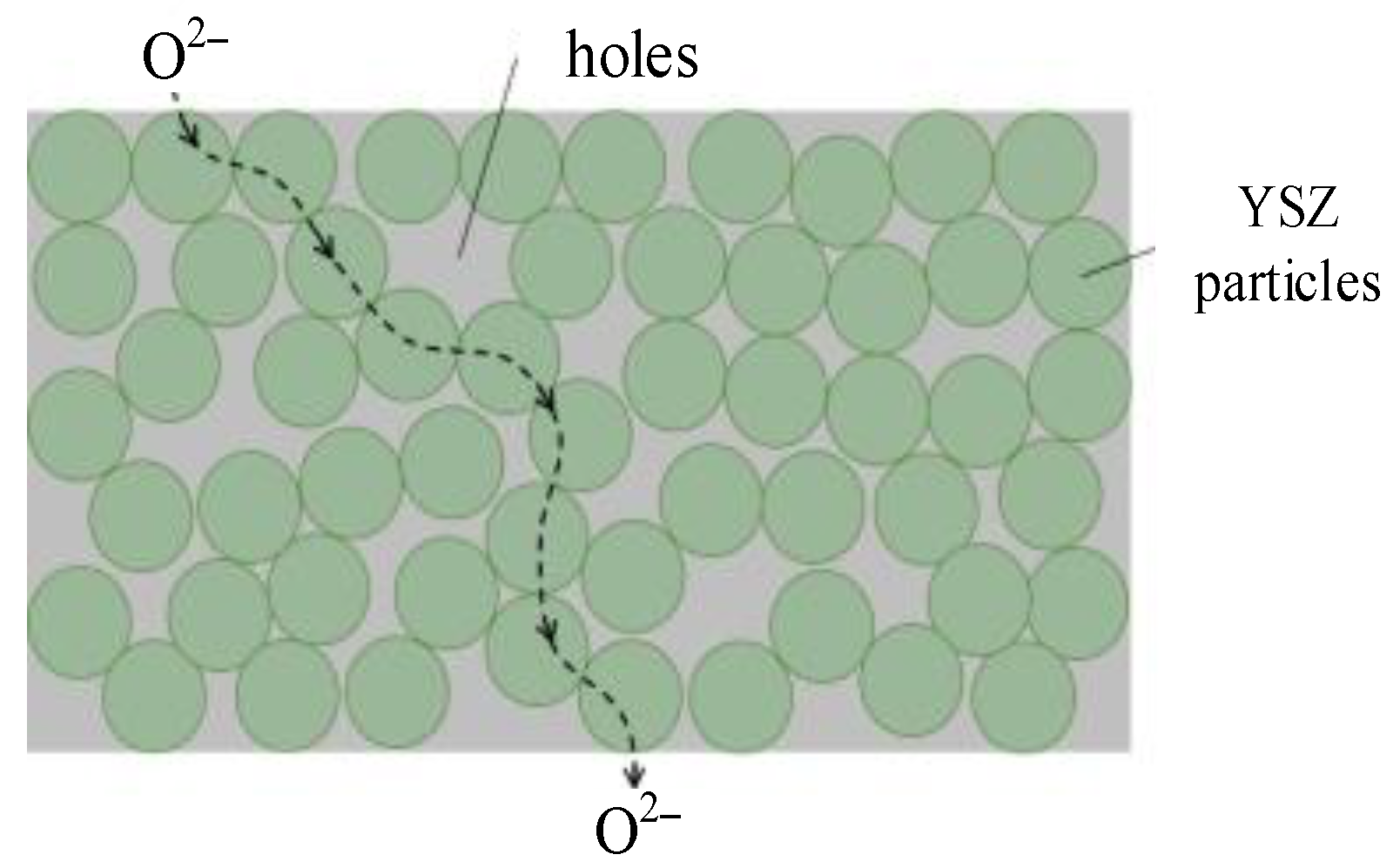
2.2. Conduction Mechanism of YSZ Electrolyte
2.3. Ionic Conductivity in YSZ
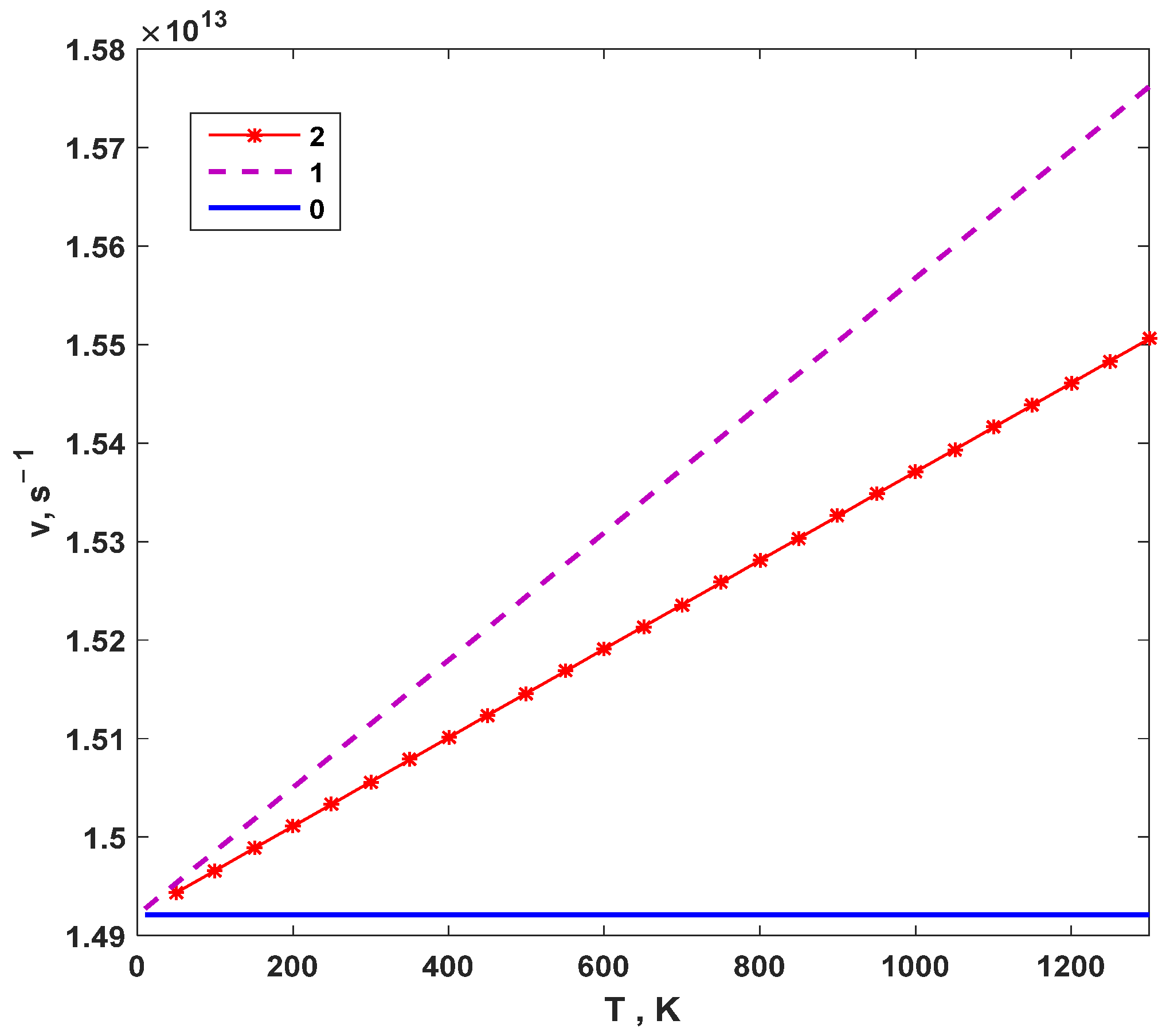
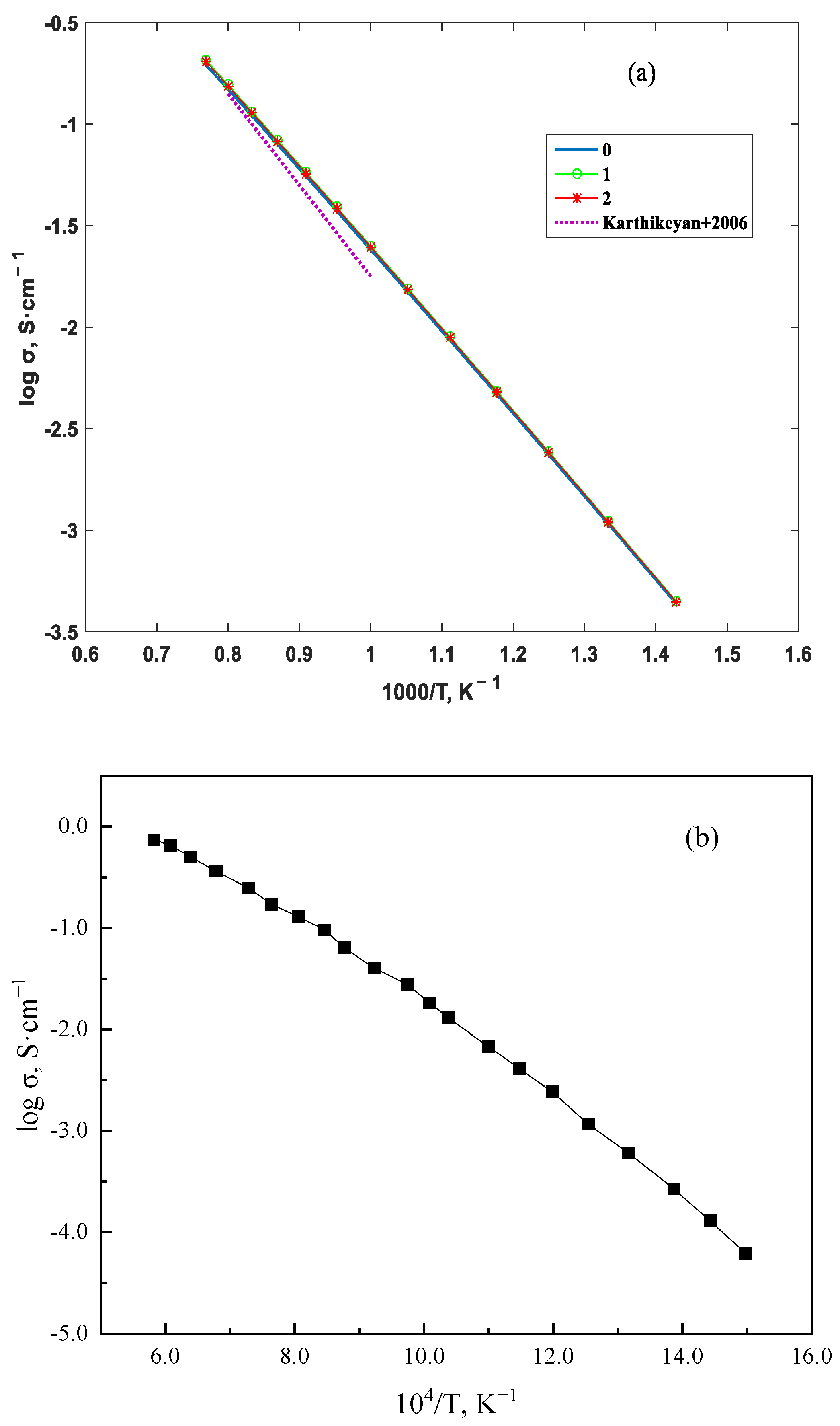
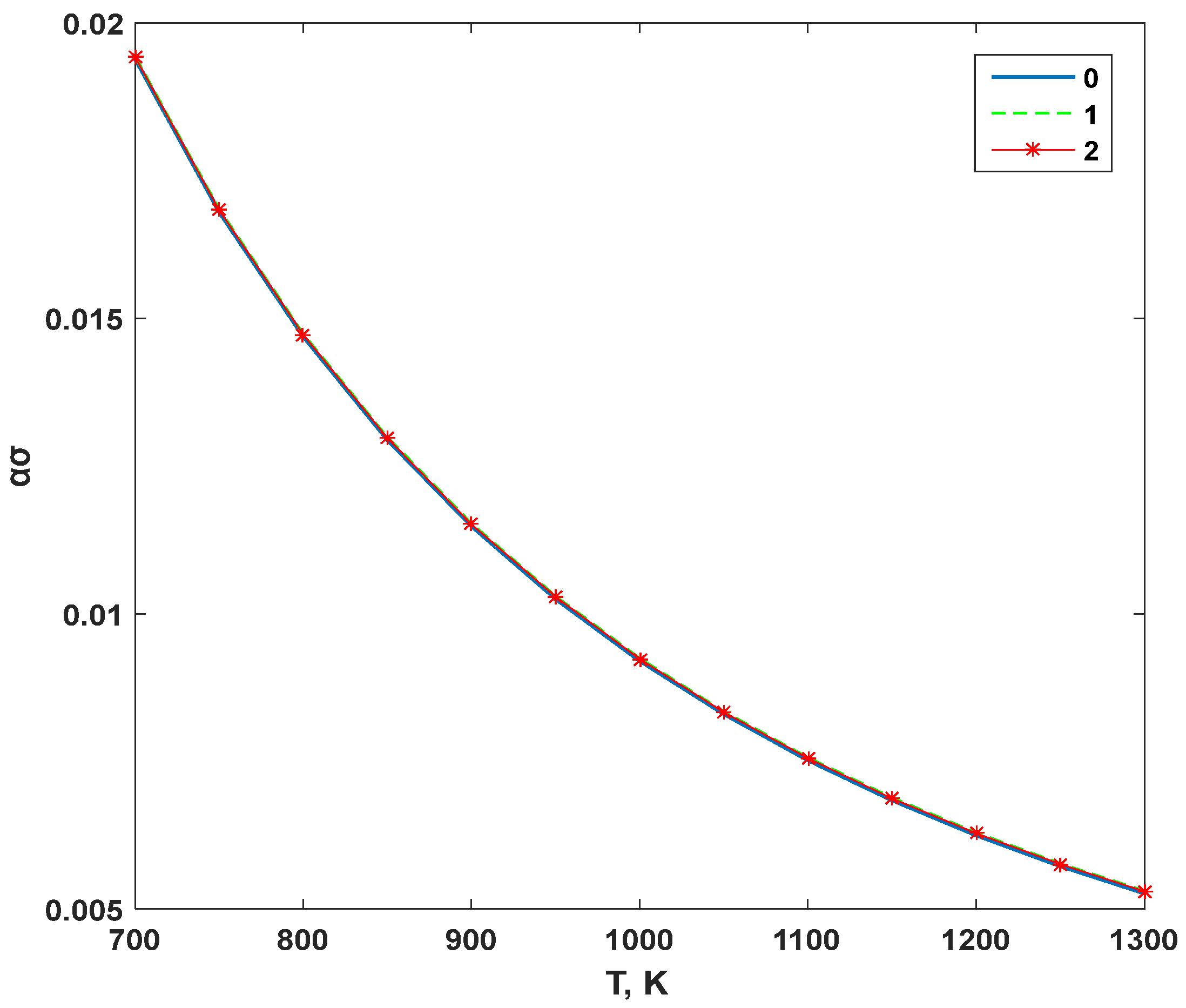
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tikhov, S.; Gorshkov, O.; Antonov, I.; Morozov, A.; Koryazhkina, M.; Filatov, D. Ion Migration Polarization in the Yttria Stabilized Zirconia Based Metal-Oxide-Metal and Metal-Oxide-Semiconductor Stacks for Resistive Memory. Adv. Condens. Matter Phys. 2018, 2018, 2028491. [Google Scholar] [CrossRef]
- Gorshkov, O.N.; Antonov, I.N.; Belov, A.I.; Kasatkin, A.P.; Mikhaylov, A.N. Resistive switching in metal-insulator-metal structures based on germanium oxide and stabilized zirconia. Tech. Phys. Lett. 2014, 40, 101. [Google Scholar] [CrossRef]
- Kwon, O.H.; Jang, C.; Lee, J.; Jeong, H.Y.; Kwon, Y.; Joo, J.Y.; Kim, H. Investigation of the electrical conductivity of sintered monoclinic zirconia (ZrO2). Ceram. Int. 2017, 43, 8236. [Google Scholar] [CrossRef]
- Kumar, A.; Rajdev, D.; Douglass, D.L. Effect of oxide defect structure on the electrical properties of ZrO2. J. Am. Ceram. Soc. 2010, 93, 439–446. [Google Scholar] [CrossRef]
- Park, M.S.; Jo, K.; Lee, H.; Yoon, B.; Lee, H. Oxygen-ion conductivity and mechanical properties of Lu2O3-doped ZrO2 as a solid electrolyte. Ceram. Int. 2021, 47, 20844. [Google Scholar] [CrossRef]
- Borik, M.A.; Bredikhin, S.I.; Bublik, V.T.; Kulebyakin, A.V.; Kuritsyna, I.E.; Lomonova, E.E.; Milovich, F.O.; Myzina, V.A.; Osiko, V.V.; Seryakov, S.V.; et al. Change in the mechanism of conductivity in ZrO2-based crystals depending on the content of stabilizing Y2O3 additive. Tech. Phys. Lett. 2017, 43, 289. [Google Scholar] [CrossRef]
- Lima, R.S.; Piqueira MC, R.; Paskocimas, C.A.; Bueno, P.R. Thermal stability of yttria-stabilized zirconia coatings obtained by plasma spraying. Surf. Coat. Technol. 2011, 206, 1657–1663. [Google Scholar] [CrossRef]
- Glinchuk, M.D.; Bykov, P.I.; Hilczer, B. Specific features of oxygen-ionic conduction in oxide nanoceramics. Phys. Solid State 2006, 48, 2199. [Google Scholar] [CrossRef]
- Zavodinsky, V.G. The mechanism of ionic conductivity in stabilized cubic zirconia. Phys. Solid State 2004, 46, 441. [Google Scholar] [CrossRef]
- Zavodinskij, V.G.; Chibisov, A.N. On stability of cubic zirconium dioxide and stoichiometric nanoparticles of zirconium dioxide. Fiz. Tverd. Tela 2006, 48, 343–347. [Google Scholar]
- Hirschorn, B.; Virkar, A.V. Ionic conductivity of yttria-stabilized zirconia: A comprehensive model. J. Electrochem. Soc. 2002, 149, A1409–A1421. [Google Scholar]
- Song, S.; Zhou, X.; Liu, M. Effects of temperature and oxygen partial pressure on the electrical conductivity of YSZ electrolyte. J. Power Sources 2012, 205, 285–289. [Google Scholar] [CrossRef]
- Etschmann, B.; Gesing, T.M.; Knorr, K. Ionic conductivity of yttria-stabilized zirconia: A molecular dynamics study. J. Am. Ceram. Soc. 2005, 88, 2641–2646. [Google Scholar] [CrossRef]
- Wang, Q.; Virkar, A.V. Effect of microstructure on ionic conductivity of yttria-stabilized zirconia. J. Electrochem. Soc. 1996, 143, 1607–1613. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Li, N.; Verma, A.; Singh, P. Electrical conductivity of manganese doped yttria (8 mol%) stabilized zirconia. Solid State Ion. 2014, 253, 223–226. [Google Scholar] [CrossRef]
- Yildiz, B.; Maier, J. Grain-boundary effects on ionic conductivity in yttria-stabilized zirconia. Phys. Rev. Lett. 2004, 93, 265901. [Google Scholar]
- Wan, S.M. Electronic theory of potential energy function of interatomic interaction in solids and elasticity of alkali metals and alkaline earth metals. Chin. Sci. 1987, 02, 170. [Google Scholar]
- Zacate, M.O.; Minervini, L.; Bradfield, D.J.; Grimes, R.W.; Sickafus, K.E. Defect cluster formation in M2O3-doped cubic ZrO2. Solid State Ion. 2000, 128, 243. [Google Scholar] [CrossRef]
- Buckingham, R.A. The classical equation of state of gaseous helium, neon and argon. Proc. R. Soc. Lond. 1938, 168, 264. [Google Scholar] [CrossRef]
- Wang, J.L. Molecular Dynamics Simulation of Solid Electrolyte Material YSZ. Ph.D. Dissertation, Harbin Institute of Technology, Harbin, China, 2019. [Google Scholar]
- Huang, K. Solid State Physics; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Wang, C.Y.; Xu, B.B.; Zhang, X.; Sun, W.P.; Chen, J.; Pan, H.G.; Yan, M.; Jiang, Y.Z. Ion hopping: Design principles for strategies to improve ionic conductivity for inorganic solid electrolytes. Small 2022, 18, e2107064. [Google Scholar] [CrossRef]
- Zheng, R.L.; Hu, X.Q. Solid Theory and Application; Southwest Normal University Press: Chongqing, China, 1996. [Google Scholar]
- Gao, J.H.; Huang, H.; Zeng, C.; Zheng, R.L. Effect of porosity on the electrical conductivity of porous electrode materials for sensors. Mater. Rep. 2021, 35, 18018. [Google Scholar]
- Kittel, C. Introduction to Solid State Physics; Xiang, J.Z.; Wu, X.H., Translators; Chemical Industry Press: Beijing, Germany, 2011. [Google Scholar]
- Karthikeyan, A.; Chang, C.L.; Ramanathan, S. High-temperature conductivity studies on nanoscale yttria-doped zirconia thin films and size effects. Appl. Phys. Lett. 2006, 89, 183116. [Google Scholar] [CrossRef]
- Kharton, V.V.; Naumovich, E.N.; Vecher, A.A. Research on the electrochemistry of oxygen ion conductors in the former Soviet Union. J. Solid State Electrochem. 2001, 5, 160. [Google Scholar] [CrossRef]

| a (Å) | r0 (Å) | λd (Å) | n | g (eV∙Å) | |
|---|---|---|---|---|---|
| Zr | 3.23 | 3.27064 | 22.66438 | 1 | 75 |
| Y | 3.65 | 3.69592 | 25.61145 | 1 | 75 |
| Ion Pairs | A (eV) | ρ (Å) | C (eV ∙ Å6) |
|---|---|---|---|
| O2−—O2− | 9547.96 | 0.2192 | 32.0 |
| Zr4+—O2− | 1502.11 | 0.3477 | 5.1 |
| Y3+—O2− | 1766.40 | 0.3385 | 19.4 |
| ε0 (102 Jm −2) | ε1 (1012 Jm −3) | ε2 (1022 Jm −4) | |
|---|---|---|---|
| O | 2.33525 | −2.30901 | 2.60601 |
| Zr | 9.34374 | −2.44726 | 2.37901 |
| Y | 8.00938 | −0.39169 | 1.72522 |
| YSZ | 5.67260 | −2.12119 | 2.41078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Zhao, X.; Cheng, Z.; Tian, L. Theoretical Study on the Influence of the Anharmonic Effect on the Ionic Conductivity and Thermal Stability of 8 mol% Yttria-Stabilized Zirconia Solid Electrolyte Material. Materials 2023, 16, 5345. https://doi.org/10.3390/ma16155345
Gao J, Zhao X, Cheng Z, Tian L. Theoretical Study on the Influence of the Anharmonic Effect on the Ionic Conductivity and Thermal Stability of 8 mol% Yttria-Stabilized Zirconia Solid Electrolyte Material. Materials. 2023; 16(15):5345. https://doi.org/10.3390/ma16155345
Chicago/Turabian StyleGao, Junhua, Xiaofeng Zhao, Zhengfu Cheng, and Liangliang Tian. 2023. "Theoretical Study on the Influence of the Anharmonic Effect on the Ionic Conductivity and Thermal Stability of 8 mol% Yttria-Stabilized Zirconia Solid Electrolyte Material" Materials 16, no. 15: 5345. https://doi.org/10.3390/ma16155345
APA StyleGao, J., Zhao, X., Cheng, Z., & Tian, L. (2023). Theoretical Study on the Influence of the Anharmonic Effect on the Ionic Conductivity and Thermal Stability of 8 mol% Yttria-Stabilized Zirconia Solid Electrolyte Material. Materials, 16(15), 5345. https://doi.org/10.3390/ma16155345





