Study of the Structure and Infrared Spectra of LiF, LiCl and LiBr Using Density Functional Theory (DFT)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. DFT Calculations
2.3. Far Infrared Spectroscopy
3. Results
3.1. Model of Crystals of Anhydrous Salts: LiF, LiCl and LiBr
3.1.1. Geometry
3.1.2. Charge Distribution in Model Structure of LiF, LiCl and LiBr
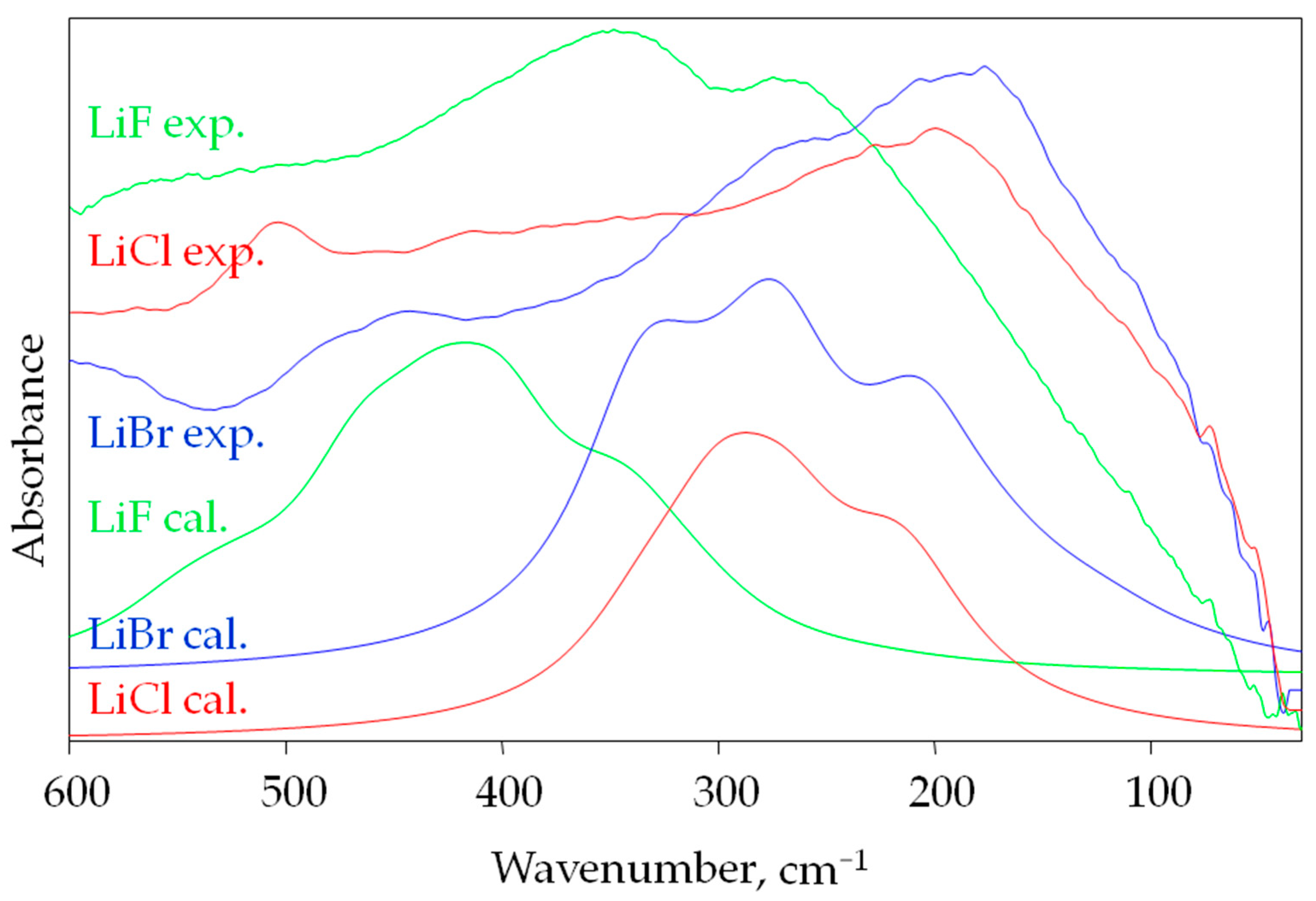
3.2. Infrared Spectra of LiF, LiCl and LiBr
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindagato, P.; Li, Y.; Macháček, J.; Yang, G.; Mungwarakarama, I.; Ndahimana, A.; Ntwali, H.P.K. Lithium metal: The key to green transportation. Appl. Sci. 2022, 13, 405. [Google Scholar] [CrossRef]
- Bandini, G.; Caposciutti, G.; Marracci, M.; Buffi, A.; Tellini, B. Characterization of lithium-batteries for high power applications. J. Energy Storage 2022, 50, 104607. [Google Scholar] [CrossRef]
- Garcia, L.V.; Ho, Y.-C.; Myo Thant, M.M.; Han, D.S.; Lim, J.W. Lithium in a sustainable circular economy: A comprehensive review. Processes 2023, 11, 418. [Google Scholar] [CrossRef]
- Jaskula, B.W. Mineral Commodity Summary: Lithium; U.S. Geological Survey: Washington, DC, USA, 2022; pp. 100–101. [Google Scholar]
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research–global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Linneen, N.; Bhave, R.; Woerner, D. Purification of industrial grade lithium chloride for the recovery of high purity battery grade lithium carbonate. Sep. Purif. Technol. 2019, 214, 168–173. [Google Scholar] [CrossRef]
- Xu, Z.-G.; Sun, S.-Y. Preparation of battery-grade lithium carbonate with lithium-containing desorption solution. Metals 2021, 11, 1490. [Google Scholar] [CrossRef]
- Stober, H.C. Lithium carbonate. In Analytical Profiles of Drug Substances; Academic Press: Cambridge, MA, USA, 1986; Volume 15, pp. 367–391. ISBN 978-0-12-260815-5. [Google Scholar]
- Brooker, M.H.; Bates, J.B. Raman and infrared spectral studies of anhydrous Li2CO3 and Na2CO3. J. Chem.Phys. 2003, 54, 4788–4796. [Google Scholar] [CrossRef]
- Lillley, S.A. Lattice Vibrations of Lithium Hydroxide: Isotopic Effects and Factor Group Analysis. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 1971. [Google Scholar]
- Takeuchi, M.; Kurosawa, R.; Ryu, J.; Matsuoka, M. Hydration of LiOH and LiCl—Near-infrared spectroscopic analysis. ACS Omega 2021, 6, 33075–33084. [Google Scholar] [CrossRef]
- Parker, S.F.; Refson, K.; Bewley, R.I.; Dent, G. Assignment of the vibrational spectra of lithium hydroxide monohydrate, LiOH·H2O. J. Chem. Phys. 2011, 134, 084503. [Google Scholar] [CrossRef]
- Díaz Nieto, C.H.; Flexer, V. Is it possible to recover lithium compounds from complex brines employing electromembrane processes exclusively? Curr. Opin. Electrochem. 2022, 35, 101087. [Google Scholar] [CrossRef]
- Stringfellow, W.T.; Dobson, P.F. Technology for the recovery of lithium from geothermal brines. Energies 2021, 14, 6805. [Google Scholar] [CrossRef]
- Knapik, E.; Rotko, G.; Marszałek, M.; Piotrowski, M. Comparative study on lithium recovery with ion-selective adsorbents and extractants: Results of multi-stage screening test with the use of brine simulated solutions with increasing complexity. Energies 2023, 16, 3149. [Google Scholar] [CrossRef]
- Ertas, A.; Anderson, E.E.; Kiris, I. Properties of a new liquid desiccant solution—Lithium chloride and calcium chloride mixture. Sol. Energy 1992, 49, 205–212. [Google Scholar] [CrossRef]
- Peeters, R.; Vanderschaeghe, H.; Rongé, J.; Martens, J.A. Fresh water production from atmospheric air: Technology and innovation outlook. iScience 2021, 24, 103266. [Google Scholar] [CrossRef] [PubMed]
- N’Tsoukpoe, K.E.; Schmidt, T.; Rammelberg, H.U.; Watts, B.A.; Ruck, W.K.L. A systematic multi-step screening of numerous salt hydrates for low temperature thermochemical energy storage. Appl. Energy 2014, 124, 1–16. [Google Scholar] [CrossRef]
- Mastronardo, E.; Piperopoulos, E.; Palamara, D.; Frazzica, A.; Calabrese, L. Morphological observation of LiCl deliquescence in PDMS-based composite foams. Appl. Sci. 2022, 12, 1510. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Tong, L.; Yin, S.; Wang, L.; Ding, Y. Salt hydrate adsorption material-based thermochemical energy storage for space heating application: A review. Energies 2023, 16, 2875. [Google Scholar] [CrossRef]
- Bodrikov, I.V.; Guerasimov, Y.A.; Kurnikov, N.A. Effect of lithium compounds on the functional characteristics of fluxes for brazing aluminium. Weld. Int. 2015, 29, 558–560. [Google Scholar] [CrossRef]
- Eftekhari-Sis, B.; Khalili, B.; Abdollahifar, A.; Hashemi, M.M. Transition metal free oxidation of alcohols to carbonyl ompounds using hydrogen peroxide catalyzed with LiCl on montmorillonite K10. Acta Chim. Slov. 2007, 54, 635–637. [Google Scholar]
- Suzuki, Y.; Kawazu, T.; Koyama, H. RNA isolation from siliques, dry seeds, and other tissues of Arabidopsis thaliana. BioTechniques 2004, 37, 542–544. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Park, H.H.; Yong, T.-S.; Jeon, S.-H. Lithium chloride inhibits the migration and invasion of osteosarcoma cells by blocking nuclear translocation of phospho-Erk. Biochem. Biophys. Res. Commun. 2021, 581, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bolmont, M.; Cahen, S.; Fauchard, M.; Guillot, R.; Medjahdi, G.; Berger, P.; Lamura, G.; Lagrange, P.; Hérold, C. LiCl-KCl eutectic molten salt as an original and efficient medium to intercalate metals into graphite: Case of europium. Carbon 2018, 133, 379–383. [Google Scholar] [CrossRef] [Green Version]
- Mullen, E.; Harris, R.; Graham, D.; Rhodes, C.; Hodgson, Z. Transfer characteristics of a lithium chloride–potassium chloride molten salt. Nucl. Eng. Technol. 2017, 49, 1727–1732. [Google Scholar] [CrossRef]
- Ran, P.; Zhou, X.; Wang, Y.; Fan, Q.; Xin, D.; Li, Z. Thermodynamic and exergetic analysis of a novel multi-generation system based on SOFC, micro-gas turbine, s-CO2 and lithium bromide absorption refrigerator. Appl. Therm. Eng. 2023, 219, 119585. [Google Scholar] [CrossRef]
- Beausoleil-Morrison, I.; Johnson, G.; Kemery, B.P. The experimental characterization of a lithium bromide–water absorption chiller and the development of a calibrated model. Sol. Energy 2015, 122, 368–381. [Google Scholar] [CrossRef]
- FathiAlmas, Y.; Ghadamian, H.; Aminy, M.; Moghadasi, M.; Amirian, H.; Hoseinzadeh, S.; Astiaso Garcia, D. Thermo-economic analysis, energy modeling and reconstructing of components of a single effect solar–absorption lithium bromide chiller for energy performance enhancement. Energy Build. 2023, 285, 112894. [Google Scholar] [CrossRef]
- Ketfi, O.; Abdi, H.; Lounici, B.; Bourouis, M. Performance analysis of low-capacity water–LiBr absorption–cooling systems using geothermal heat-sinks in hot climates. Energies 2023, 16, 809. [Google Scholar] [CrossRef]
- Weng, L.; Song, W.; Jacobs, D.J.; Elliott, G.D. Molecular insights into water vapor absorption by aqueous lithium bromide and lithium bromide/sodium formate solutions. Appl. Therm. Eng. 2016, 102, 125–133. [Google Scholar] [CrossRef]
- Zhao, B.; Li, X.; Wang, X.; Jiang, L.; Li, Z.; Du, Y. Synthesis of 3-haloindoles via cascade oxidative cyclization/halogenation of 2-alkenylanilines mediated by PIDA and LiBr/KI. J. Org. Chem. 2023, 88, 1493–1503. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.; Shi, K.; Pan, Y. LiBr-catalyzed C3-disulfuration between indole and N-dithiophthalimide. J. Org. Chem. 2022, 88, 2550–2556. [Google Scholar] [CrossRef]
- Zhu, L.; Shao, X.; Pan, X.; Sun, Z.; Li, X.; Duan, X.; Shi, J. One-pot aqueous-phase conversion of biomass into furfural catalyzed by cupported heteropolyacids with the addition of LiBr. Biomass Bioenergy 2023, 171, 106734. [Google Scholar] [CrossRef]
- Lu, X.; Chen, J.; Lu, J.; Wang, S.; Xia, T. Monosaccharides and carbon nanosphere obtained by acidic concentrated LiBr treatment of raw crop residues via optimizing the synthesis process. Bioresour. Technol. 2020, 310, 123522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-C.; Cai, X.; Li, K.; Ye, Y.-Y.; Zhang, Z.-X.; Liu, Y.-Q.; Wang, D.; Li, S.-R. LiBr hydrate as reaction medium for preparation of carbon spheres from wood powders via hydrothermal carbonization. Diam. Relat. Mater. 2021, 113, 108295. [Google Scholar] [CrossRef]
- Stubblefield, C.B.; Bach, R.O. Solubility of lithium fluoride in water. J. Chem. Eng. Data 1972, 17, 491–492. [Google Scholar] [CrossRef]
- Yılmaz, S.N.; Akbay, İ.K.; Özdemir, T.A. Metal-ceramic-rubber composite for hybrid gamma and neutron radiation shielding. Radiat. Phys. Chem. 2021, 180, 109316. [Google Scholar] [CrossRef]
- Vijayalakshmi, L.; Naveen Kumar, K.; Srinivas, G.; Hwang, P.; Choi, J. Ravishing blue emission from Ce3+ activated lithium borate glasses for photonic applications. Optik 2021, 227, 166025. [Google Scholar] [CrossRef]
- Kaplunov, I.A.; Kropotov, G.I.; Rogalin, V.E.; Shakhmin, A.A. Optical properties of some crystalline fluorides in the terahertz region of the spectrum. Opt. Mater. 2021, 115, 111019. [Google Scholar] [CrossRef]
- Chruszcz-Lipska, K.; Zelek-Pogudz, S.; Solecka, U.; Solecki, M.L.; Szostak, E.; Zborowski, K.K.; Zając, M. Use of the far infrared spectroscopy for NaCl and KCl minerals characterization—A case study of halides from Kłodawa in Poland. Minerals 2022, 12, 1561. [Google Scholar] [CrossRef]
- Śmiechowski, M.; Krakowiak, J.; Bruździak, P.; Stangret, J. Unique agreement of experimental and computational infrared spectroscopy: A case study of lithium bromide solvation in an important electrochemical solvent. Phys. Chem. Chem. Phys. 2017, 19, 9270–9280. [Google Scholar] [CrossRef]
- Roget, S.A.; Heck, T.R.; Carter-Fenk, K.A.; Fayer, M.D. Ion/water network structural dynamics in highly concentrated lithium chloride and lithium bromide solutions probed with ultrafast infrared spectroscopy. J. Phys. Chem. B 2023, 127, 4532–4543. [Google Scholar] [CrossRef]
- Barba, M.I.; Larrechi, M.S.; Coronas, A. Quantitative analysis of the hydration of lithium salts in water using multivariate curve resolution of near-infrared spectra. Anal. Chim. Acta 2016, 919, 20–27. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Borstel, G. Towards a practical rechargeable 5 V Li ion battery. Phys. Stat. Sol. A 2005, 202, R13–R15. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, UK.
- Eglitis, R.I.; Purans, J.; Popov, A.I.; Jia, R. Tendencies in ABO3 perovskite and SrF2, BaF2 and CaF2 bulk and surface F-center ab initio computations at high symmetry cubic structure. Symmetry 2021, 13, 1920. [Google Scholar] [CrossRef]
- Finch, G.I.; Fordham, S. The effect of crystal-size on lattice-dimensions. Proc. Phys. Soc. 1936, 48, 85–94. [Google Scholar] [CrossRef]
- Cortona, P. Direct determination of self-consistent total energies and charge densities of solids: A study of the cohesive properties of the alkali halides. Phys. Rev. B 1992, 46, 2008–2014. [Google Scholar] [CrossRef]
- Wang, J.; Deng, M.; Chen, Y.; Liu, X.; Ke, W.; Li, D.; Dai, W.; He, K. Structural, elastic, electronic and optical properties of lithium halides (LiF, LiCl, LiBr, and LiI): First-principle calculations, Mater. Chem. Phys. 2020, 244, 122733. [Google Scholar] [CrossRef]
- Zborowski, K.; Gryboś, R.; Proniewicz, L.M. Vibrational and computational study on maltol (3-hydroxy-2-methyl-4h-pyran-4-one) polymorphism. Vib. Spectrosc. 2005, 37, 233–236. [Google Scholar] [CrossRef]
- Cioslowski, J. A new population analysis based on atomic polar tensors. J. Am. Chem. Soc. 1989, 111, 8333–8336. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 32–751. [Google Scholar] [CrossRef]
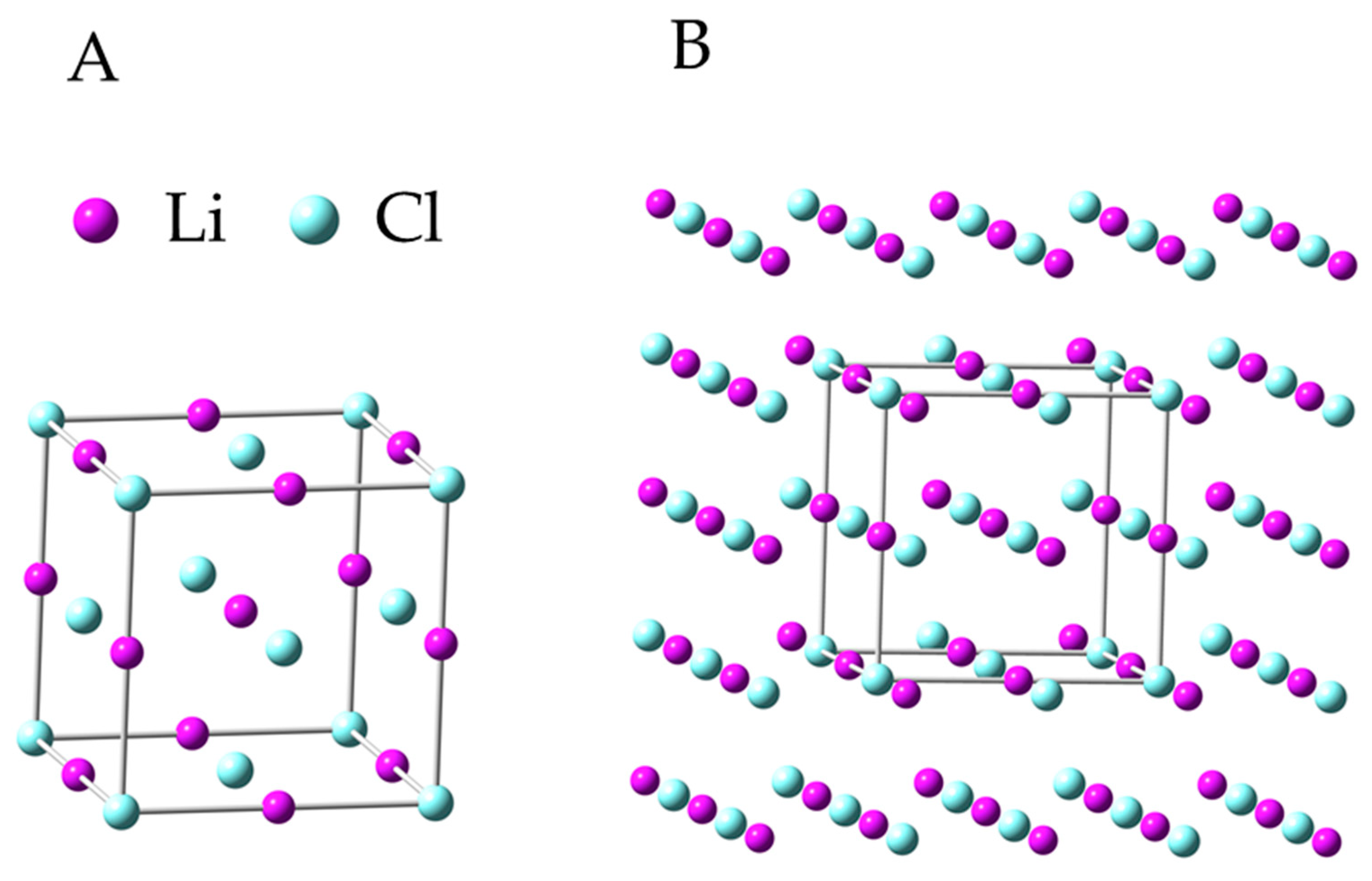
| Chemical Formula | Crystal System | Symmetry Space Group | Unit Cell Parameter | |
|---|---|---|---|---|
| a = b = c (Å) | α = β = γ (°) | |||
| LiF | isometric | Fm-3m | 4.020 ± 0.002 a, 3.906 ± 0.015 b | 90.0 |
| LiCl | isometric | Fm-3m | 5.143 ± 0.006 a, 5.038 ± 0.022 b | 90.0 |
| LiBr | isometric | Fm-3m | 5.489 ± 0.006 a, 5.283 ± 0.003 b | 90.0 |
| Chemical Compound | Li Mean Value (N = 63) | Li Central Atom (N = 1) | Li Surface (N = 13) | X (F,Cl,Br) Mean Value (N = 62) | X (F,Cl,Br) in Direct Surrounding Central Atom (N = 6) | X (F,Cl,Br) Surface (N = 12) |
|---|---|---|---|---|---|---|
| LiF | 0.8227 ±0.0176 | 0.8171 | 0.8342 ±0.0222 | −0.8198 ±0.0057 | −0.8169 ±0.0000 | −0.8212 ±0.0065 |
| LiCl | 0.7831 ±0.0243 | 0.7626 | 0.8001 ±0.0289 | −0.7796 ±0.0070 | −0.7752 ±0.0000 | −0.7822 ±0.0076 |
| LiBr | 0.7449 ±0.0276 | 0.7172 | 0.7645 ±0.0319 | −0.7408 ±0.0046 | −0.7398 ±0.0000 | −0.7412 ±0.0051 |
| No. | LiF | LiCl | LiBr | |||
|---|---|---|---|---|---|---|
| Frequency (cm−1) | IR Int. (KM/Mole) | Frequency (cm−1) | IR Int. (KM/Mole) | Frequency (cm−1) | IR Int. (KM/Mole) | |
| 1 | 111.4 | 1.7 | 63.7 | 2.3 | 42.7 | 0.3 |
| 2 | 160.1 | 0.1 | 89.6 | 0.0 | 67.0 | 1.6 |
| 3 | 184.2 | 0.0 | 101.0 | 0.0 | 73.1 | 1.9 |
| 4 | 213.4 | 0.9 | 118.6 | 0.0 | 81.3 | 20.2 |
| 5 | 217.6 | 0.5 | 121.0 | 0.0 | 84.7 | 0.0 |
| 6 | 235.3 | 3.0 | 130.3 | 1.9 | 95.1 | 0.4 |
| 7 | 250.4 | 25.8 | 143.5 | 15.0 | 107.1 | 0.7 |
| 8 | 253.2 | 0.0 | 146.1 | 0.5 | 110.7 | 9.4 |
| 9 | 255.4 | 0.1 | 158.7 | 1.5 | 121.0 | 38.4 |
| 10 | 265.3 | 2.1 | 159.8 | 7.3 | 124.7 | 35.4 |
| 11 | 280.3 | 0.7 | 172.3 | 0.1 | 137.6 | 2.8 |
| 12 | 292.1 | 4.0 | 174.2 | 4.6 | 142.0 | 111.1 |
| 13 | 314.0 | 5.5 | 183.0 | 1.5 | 159.1 | 37.5 |
| 14 | 315.8 | 8.7 | 195.0 | 13.5 | 173.9 | 0.0 |
| 15 | 335.1 | 4.9 | 201.3 | 14.5 | 175.3 | 119.1 |
| 16 | 341.2 | 161.5 | 205.3 | 0.0 | 194.5 | 201.3 |
| 17 | 346.0 | 142.6 | 210.7 | 28.4 | 215.9 | 10.9 |
| 18 | 357.4 | 56.2 | 212.0 | 2.3 | 229.1 | 1212.2 |
| 19 | 365.0 | 56.8 | 214.0 | 91.0 | 240.1 | 3.7 |
| 20 | 377.6 | 896.1 | 219.9 | 4.7 | 247.5 | 0.3 |
| 21 | 384.4 | 106.3 | 231.5 | 176.6 | 266.2 | 51.1 |
| 22 | 394.8 | 403.0 | 236.5 | 326.1 | 272.4 | 9.7 |
| 23 | 439.3 | 497.7 | 241.0 | 1168.7 | 279.6 | 14.3 |
| 24 | 446.3 | 1561.7 | 277.1 | 155.2 | 303.0 | 1647.0 |
| 25 | 478.2 | 1570.5772 | 299.2012 | 1661.4200 | 319.8562 | 40.7289 |
| 26 | 516.3 | 1549.7978 | 320.8487 | 37.8190 | 332.3225 | 247.1439 |
| 27 | 529.9 | 164.5545 | 332.7207 | 2017.2156 | 345.7773 | 60.3065 |
| 28 | 579.3 | 476.5014 | 369.7691 | 766.1095 | 371.0345 | 1523.0904 |
| 29 | 609.4 | 438.1410 | 382.6068 | 75.2121 | 386.6431 | 59.1295 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chruszcz-Lipska, K.; Szostak, E.; Zborowski, K.K.; Knapik, E. Study of the Structure and Infrared Spectra of LiF, LiCl and LiBr Using Density Functional Theory (DFT). Materials 2023, 16, 5353. https://doi.org/10.3390/ma16155353
Chruszcz-Lipska K, Szostak E, Zborowski KK, Knapik E. Study of the Structure and Infrared Spectra of LiF, LiCl and LiBr Using Density Functional Theory (DFT). Materials. 2023; 16(15):5353. https://doi.org/10.3390/ma16155353
Chicago/Turabian StyleChruszcz-Lipska, Katarzyna, Elżbieta Szostak, Krzysztof Kazimierz Zborowski, and Ewa Knapik. 2023. "Study of the Structure and Infrared Spectra of LiF, LiCl and LiBr Using Density Functional Theory (DFT)" Materials 16, no. 15: 5353. https://doi.org/10.3390/ma16155353
APA StyleChruszcz-Lipska, K., Szostak, E., Zborowski, K. K., & Knapik, E. (2023). Study of the Structure and Infrared Spectra of LiF, LiCl and LiBr Using Density Functional Theory (DFT). Materials, 16(15), 5353. https://doi.org/10.3390/ma16155353







