Extraction of Germanium from Low-Grade Germanium-Bearing Lignite by Reductive Volatilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment
2.3. Characterization and Analysis
3. Results and Discussion
3.1. Thermodynamic Analyses
3.1.1. Chemical Reaction Equations for Ge–C–O
3.1.2. Equilibrium Compositions Diagram of Ge–C–O Systems
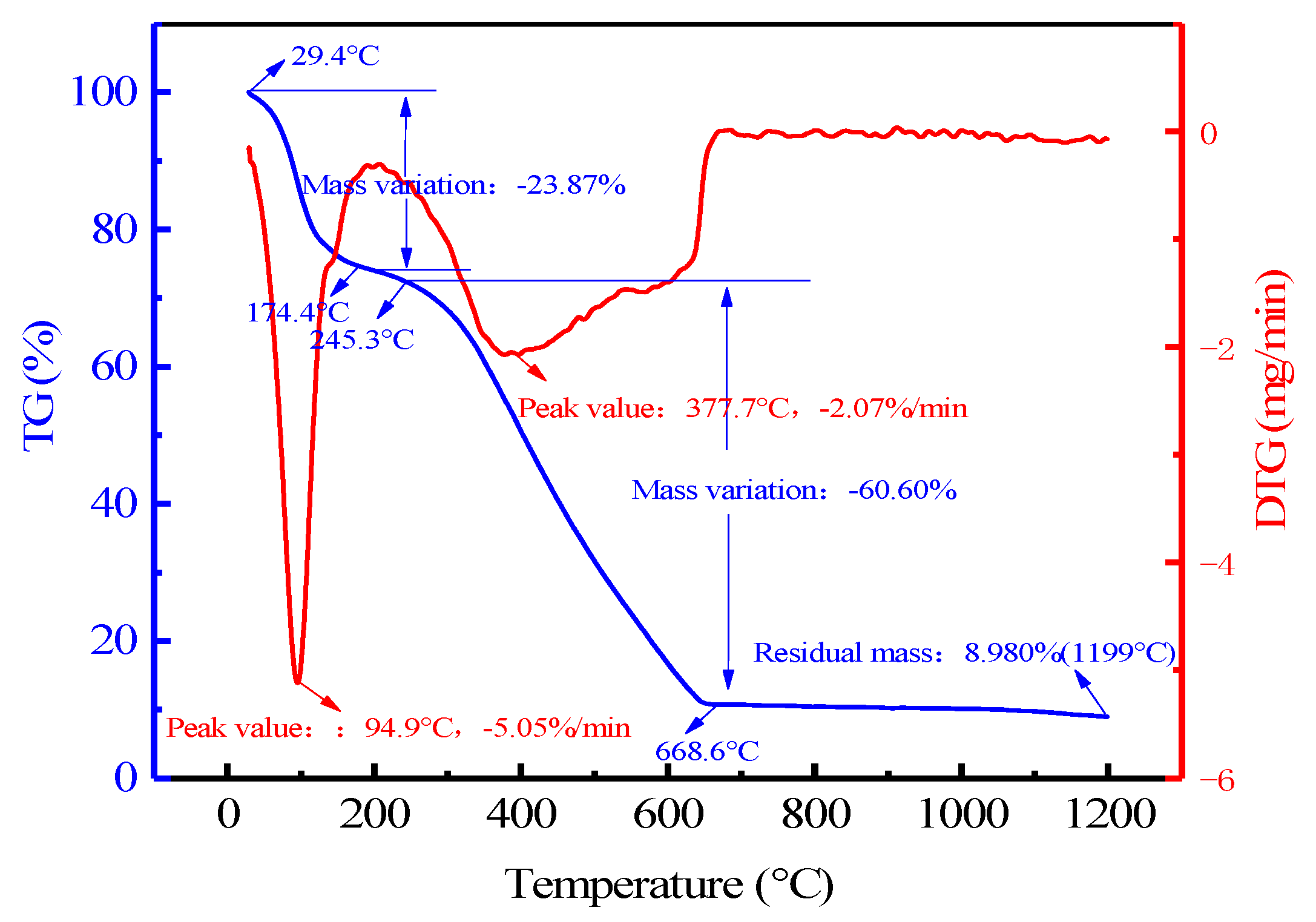
3.1.3. Primary Combustion Thermogravimetric Analysis
3.2. Combustion Experiments
3.2.1. Effect of Roasting System
3.2.2. Effect of Air Flow Rate
3.2.3. Effect of Temperature
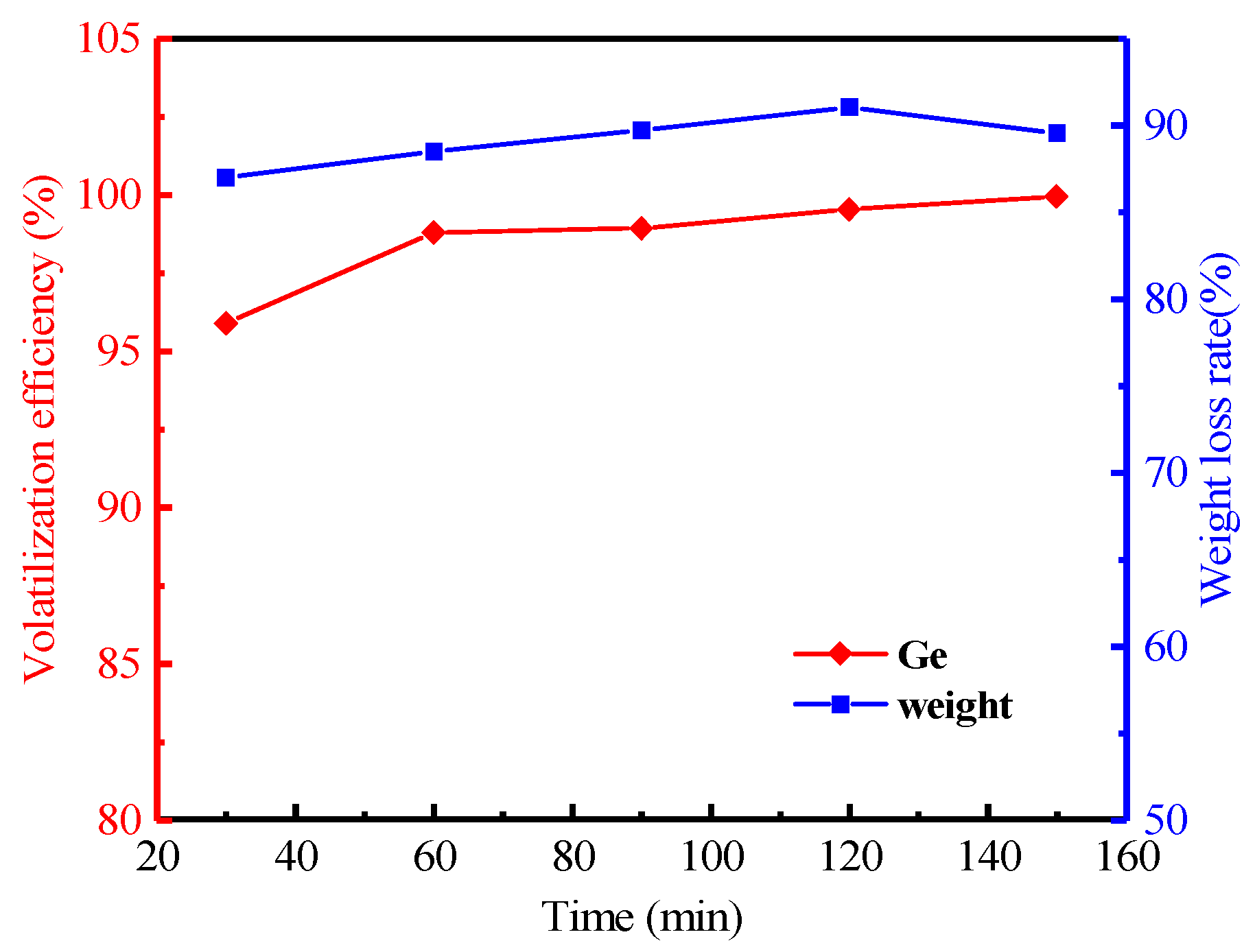
3.2.4. Effect of Heating Time
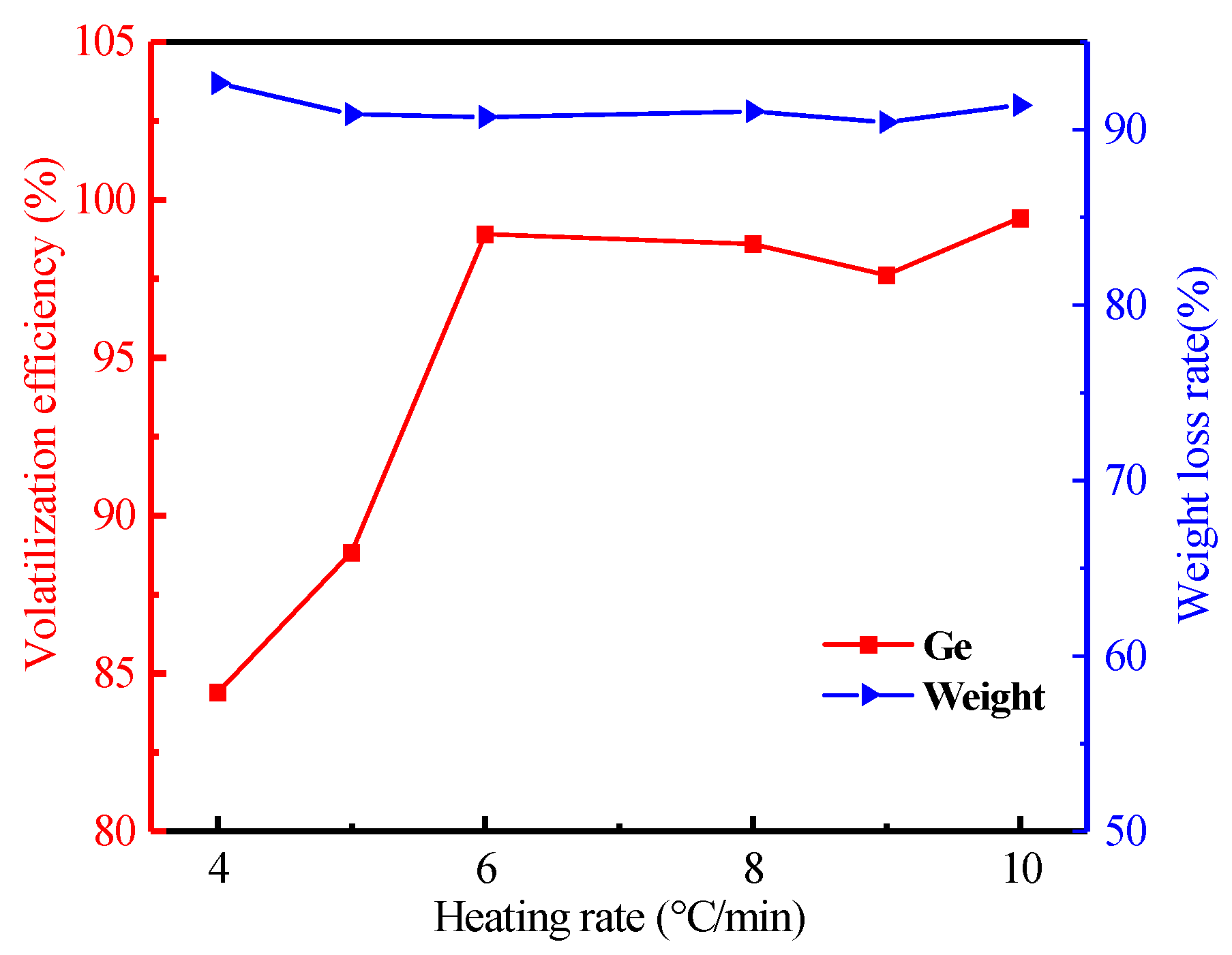
3.2.5. Effect of Heating Rate
3.3. Analysis of Residues and Concentrate
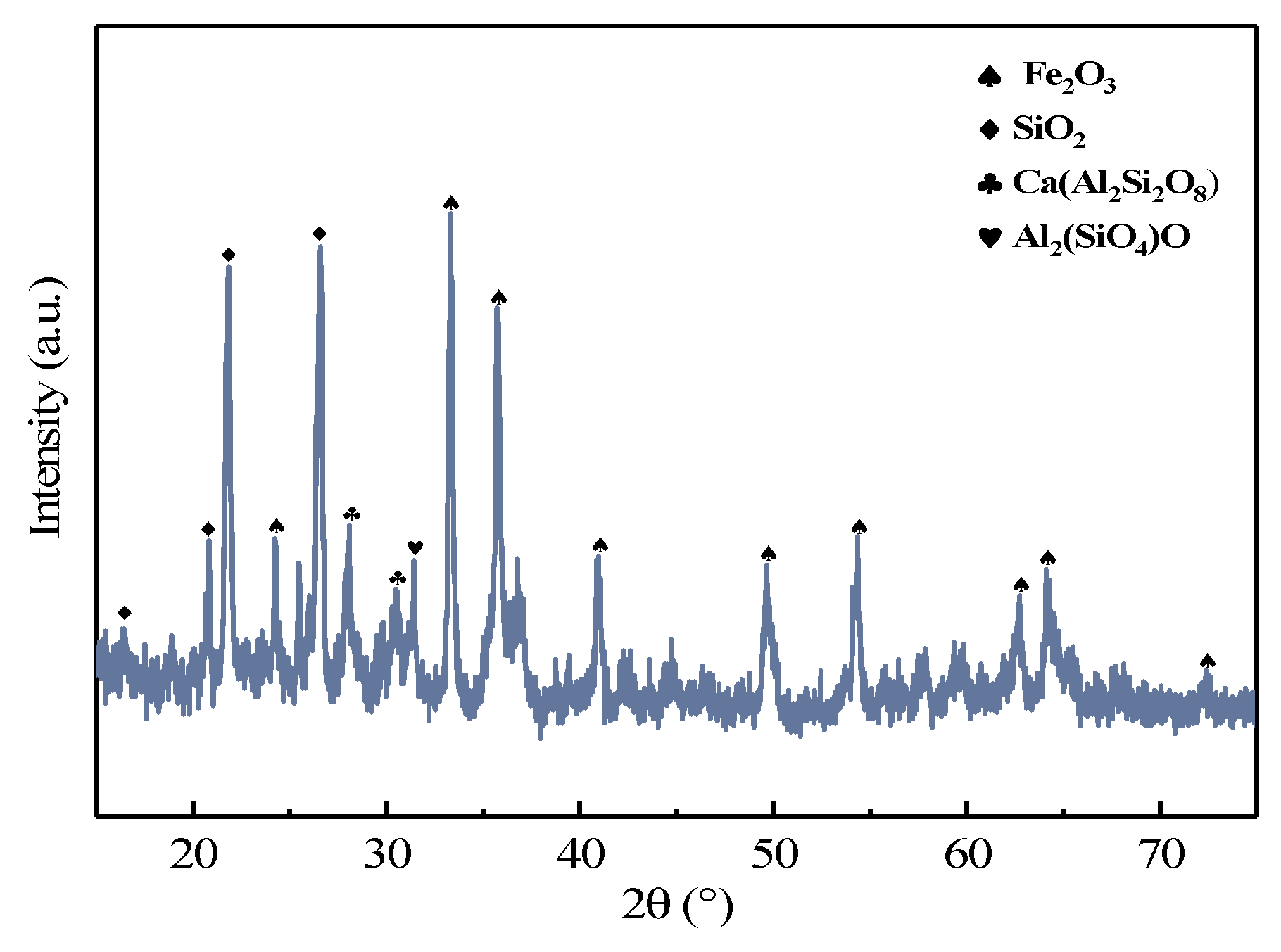
3.3.1. Characterization of the Combustion Residue
3.3.2. Characterization of the Combustion Concentrate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akiyama, S.; Miyaji, A.; Hayashi, Y.; Hiza, M.; Sekiguchi, Y.; Koyama, T.-R.; Shiga, A.; Baba, T. Selective conversion of ethanol to 1,3-butadiene using germanium talc as catalyst. J. Catal. 2018, 359, 184–197. [Google Scholar] [CrossRef]
- Selli, D.; Baburin, I.A.; Martoňák, R.; Leoni, S. Novel metastable metallic and semiconducting germaniums. Sci. Rep. 2013, 3, srep01466. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetty, R. Academic and industry research progress in germanium nanodevices. Nature 2011, 479, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Yellishetty, M.; Huston, D.; Graedel, T.E.; Werner, T.T.; Reck, B.K.; Mudd, G.M. Quantifying the potential for recoverable resources of gallium, germanium and antimony as companion metals in Australia. Ore Geol. Rev. 2017, 82, 148–159. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.E. Dynamic analysis of the global metals flows and stocks in electricity generation technologies. J. Clean. Prod. 2013, 59, 260–273. [Google Scholar] [CrossRef]
- Meng, Y.-M.; Qi, H.-W.; Hu, R.-Z. Determination of germanium isotopic compositions of sulfides by hydride generation MC-ICP-MS and its application to the Pb–Zn deposits in SW China. Ore. Geol. Rev. 2015, 65, 1095–1109. [Google Scholar] [CrossRef]
- Dai, S.F.; Seredin, V.V.; Ward, C.R.; Jiang, J.H.; Hower, J.C.; Song, X.L.; Jiang, Y.F.; Wang, X.B.; Gornostaeva, T.; Li, X.; et al. Composition and modes of occurrence of minerals and elements in coal combustion products derived from high-Ge coals. Int. J. Coal Geol. 2014, 121, 79–97. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. A Review on Germanium Resources and its Extraction by Hydrometallurgical Method. Miner. Process. Extr. Met. Rev. 2020, 42, 406–426. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, X.G.; Querol, X.; Font, O.; Izquierdo, M.; Wang, Z.M. New data on mineralogy and geochemistry of high-Ge coals in the Yimin coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2014, 125, 10–21. [Google Scholar] [CrossRef]
- Frenzel, M.; Ketris, M.P.; Gutzmer, J. On the geological availability of germanium. Miner. Deposita 2013, 49, 471–486. [Google Scholar] [CrossRef]
- Rao, S.; Liu, Z.-Q.; Wang, D.-X.; Cao, H.-Y.; Zhu, W.; Zhang, K.-F.; Tao, J.-Z. Hydrometallurgical process for recovery of Zn, Pb, Ga and Ge from Zn refinery residues. Trans. Nonferrous Met. Soc. China 2021, 31, 555–564. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, H.; Zhang, Q.; Cai, W.; Jiang, G.; Zhang, L. Green and efficient recovery of valuable metals from by-products of zinc hydrometallurgy and reducing toxicity. J. Clean. Prod. 2022, 380, 134993. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, H.; Zhang, Q.; Cai, W.; Jiang, G.; Zhang, L. Efficient recovery of valuable metals from lead-zinc smelting by-products by ultrasonic strengthening. Miner. Eng. 2022, 190, 107915. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. Application of vacuum reduction and chlorinated distillation to enrich and prepare pure germanium from coal fly ash. J. Hazard. Mater. 2017, 321, 18–27. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Zhang, L.; Peng, J.; Li, S.; Yin, S.; Yang, K.; Lin, Y. Microwave-assisted and regular leaching of germanium from the germanium-rich lignite ash. Green Process. Synth. 2018, 7, 538–545. [Google Scholar] [CrossRef]
- Wei, Q.; Rimmer, S.M. Acid solubility and affinities of trace elements in the high-Ge coals from Wulantuga (Inner Mongolia) and Lincang (Yunnan Province), China. Int. J. Coal Geol. 2017, 178, 39–55. [Google Scholar] [CrossRef]
- Su, H.; Tan, F.; Lin, J. An integrated approach combines hydrothermal chemical and biological treatment to enhance recycle of rare metals from coal fly ash. Chem. Eng. J. 2020, 395, 124640. [Google Scholar] [CrossRef]
- Zhang, L.E.; Xu, Z.M. An environmentally-friendly vacuum reduction metallurgical process to recover germanium from coal fly ash. J. Hazard. Mater. 2016, 312, 28–36. [Google Scholar] [CrossRef]
- Vejahati, F.; Xu, Z.; Gupta, R. Trace elements in coal: Associations with coal and minerals and their behavior during coal utilization—A review. Fuel 2010, 89, 904–911. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, G.; Lei, X.; Xu, H.; Li, L.; Yuan, C.; Wang, Y. Distribution and mode of occurrence of uranium in bottom ash derived from high-germanium coals. J. Environ. Sci. 2016, 43, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y.; Song, Q.; Liu, S.; Yan, J.; Ma, Q.; Ma, L.; Shu, X. Investigation on the thermal behavior characteristics and products composition of four pulverized coals: Its potential applications in coal cleaning. Int. J. Hydrogen Energy 2019, 44, 23620–23638. [Google Scholar] [CrossRef]
- Zhou, H.; Bhattarai, R.; Li, Y.; Li, S.; Fan, Y. Utilization of coal fly and bottom ash pellet for phosphorus adsorption: Sustainable management and evaluation. Resour. Conserv. Recycl. 2019, 149, 372–380. [Google Scholar] [CrossRef]
- Ge, L.; Liu, X.; Feng, H.; Chu, H.; Xu, C.; Zhang, Y.; Wang, Z. The Influence of Anionic Additives on the Microwave Dehydration Process of Lignite. Energy Fuels 2020, 34, 9401–9410. [Google Scholar] [CrossRef]
- Karasmanaki, E.; Ioannou, K.; Katsaounis, K.; Tsantopoulos, G. The attitude of the local community towards investments in lignite before transitioning to the post-lignite era: The case of Western Macedonia, Greece. Resour. Policy 2020, 68, 101781. [Google Scholar] [CrossRef]
- Rao, S.; Wang, D.; Cao, H.; Zhu, W.; Duan, L.; Liu, Z. Hydrothermal oxidative leaching of Cu and Se from copper anode slime in a diluted H2SO4 solution. Sep. Purif. Technol. 2022, 300, 121696. [Google Scholar] [CrossRef]











| Sample | Lignite | |
|---|---|---|
| Industrial analysis | Mad | 26.32 |
| Vad | 43.11 | |
| Aad | 13.52 | |
| FCad | 43.37 | |
| Chemical analysis | Ge | 0.019 |
| As | 0.053 | |
| C | 34 | |
| S | 2.87 | |
| Si | 3.4 | |
| Pb | <0.001 |
| Spot | C | O | Si | Ge | Ca | Pb | S |
|---|---|---|---|---|---|---|---|
| 1 | 44.64 | 35.54 | 0.21 | 0.3 | - | - | 4.02 |
| 2 | - | 30.41 | 20.42 | - | - | - | 2.83 |
| 3 | - | 45.57 | - | - | 32.51 | - | 21.69 |
| 4 | - | 44.91 | 0.27 | 4.04 | 0.03 | 15.35 | 24.52 |
| 5 | - | 20.28 | - | 2.53 | 0.07 | 62.95 | 8.63 |
| Reaction Equation | Number |
|---|---|
| C + O2(g) = CO2(g) | 3-1 |
| 2C + O2(g) = 2CO(g) | 3-2 |
| GeO2 (H) + C = Ge + CO2(g) | 3-3 |
| GeO2 (H) + C = GeO(g) + CO(g) | 3-4 |
| GeO2 (H) = GeO(g) + 1/2O2(g) | 3-5 |
| Reaction Stage | Temperature Rage (°C) | Weight Loss (%) | Total Weight Loss (%) | Percent of Weight Loss (%) |
|---|---|---|---|---|
| I | <174.4 | 23.87 | 91.02 | 26.23 |
| II | 174.4–668.6 | 60.60 | 66.58 | |
| III | 668.6–1200 | 6.55 | 7.20 |
| Element | Ge | As | C | S | Si | Pb |
|---|---|---|---|---|---|---|
| Residue | 0.003 | 0.082 | 0.042 | 0.98 | 11.57 | <0.001 |
| Element | Ge | As | C | S | Si | Pb |
|---|---|---|---|---|---|---|
| Concentrate | 7.16 | 6.88 | - | 12.51 | 3.28 | 25.79 |
| Chemical Phase | Content | Percent |
|---|---|---|
| germanium oxide, germanate | 3.2127 | 44.87 |
| germanium sulfide | 2.6712 | 37.31 |
| germanosilicate | 0.0445 | 0.62 |
| tetragonal germanium dioxide | 1.2316 | 17.20 |
| total | 7.16 | - |
| Spot | O | F | Si | S | Ge | As | Pb |
|---|---|---|---|---|---|---|---|
| 1 | 8.26 | 1.84 | 0.45 | 14.06 | 58.93 | 7.67 | - |
| 2 | 0.24 | 1.44 | - | - | - | - | 98.32 |
| 3 | 1.51 | 0.46 | - | 20 | 3.78 | 21.74 | 45.42 |
| 4 | - | - | - | 10.63 | 0.72 | - | 85.47 |
| 5 | 2.89 | 14.59 | 35.54 | 17.62 | 11.75 | 5.18 | - |
| 6 | 10.95 | 0.78 | - | 25.52 | 18.38 | 19.05 | 15.77 |
| 7 | 2.34 | - | - | 26.05 | 4.43 | 23.56 | 32.45 |
| 8 | 7.67 | 3.05 | 0.94 | 17.94 | 13.02 | 11.81 | 35.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Song, W.; Rao, S.; Tao, J.; Wang, D.; Cao, H.; Liu, Z. Extraction of Germanium from Low-Grade Germanium-Bearing Lignite by Reductive Volatilization. Materials 2023, 16, 5374. https://doi.org/10.3390/ma16155374
Yang R, Song W, Rao S, Tao J, Wang D, Cao H, Liu Z. Extraction of Germanium from Low-Grade Germanium-Bearing Lignite by Reductive Volatilization. Materials. 2023; 16(15):5374. https://doi.org/10.3390/ma16155374
Chicago/Turabian StyleYang, Rengao, Weifeng Song, Shuai Rao, Jinzhang Tao, Dongxing Wang, Hongyang Cao, and Zhiqiang Liu. 2023. "Extraction of Germanium from Low-Grade Germanium-Bearing Lignite by Reductive Volatilization" Materials 16, no. 15: 5374. https://doi.org/10.3390/ma16155374
APA StyleYang, R., Song, W., Rao, S., Tao, J., Wang, D., Cao, H., & Liu, Z. (2023). Extraction of Germanium from Low-Grade Germanium-Bearing Lignite by Reductive Volatilization. Materials, 16(15), 5374. https://doi.org/10.3390/ma16155374






