Two-Dimensional Graphene-Based Potassium Channels Built at an Oil/Water Interface
Abstract
:1. Introduction
2. Results and Discussion
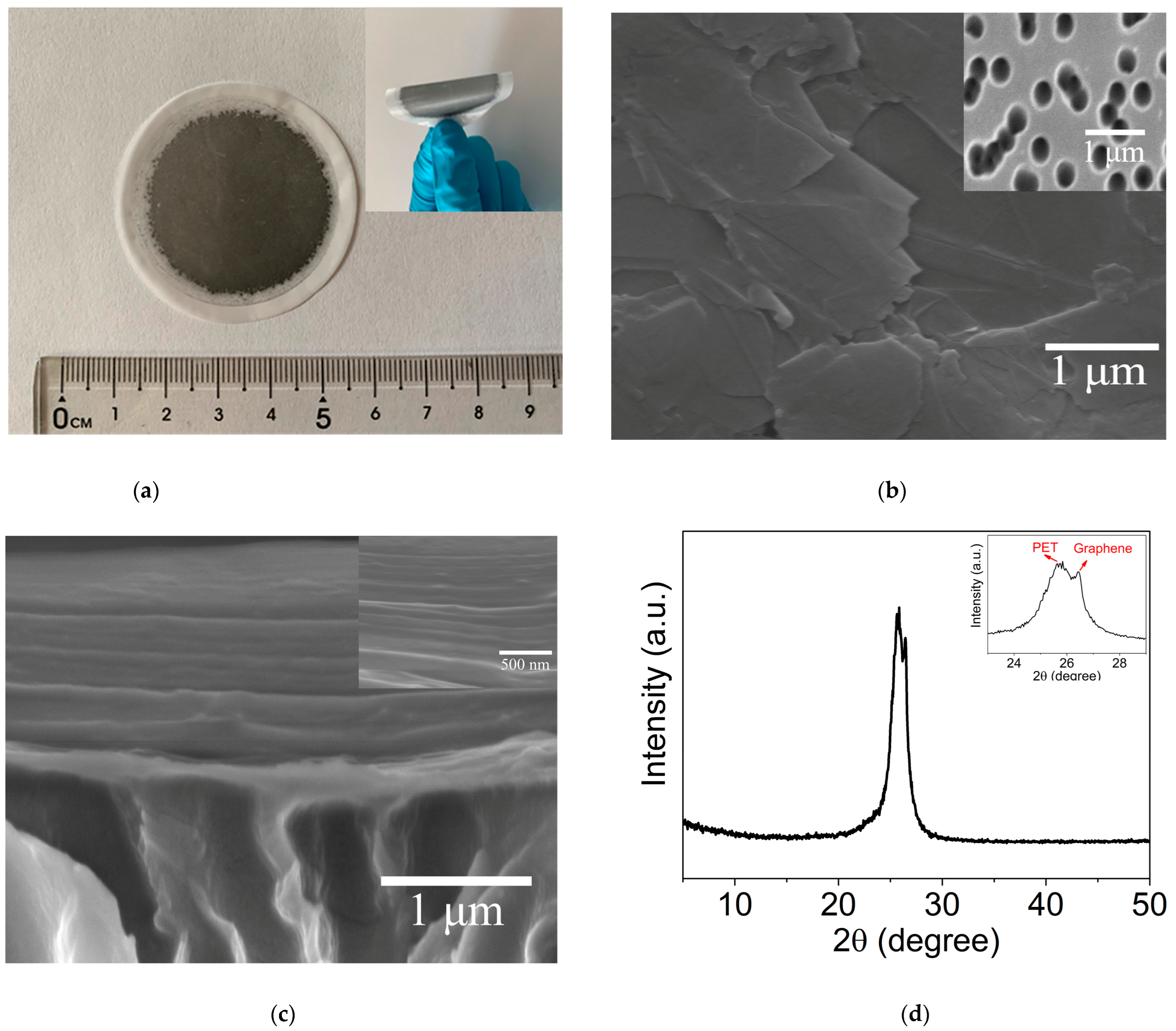
2.1. Microstructures of GLM/PET
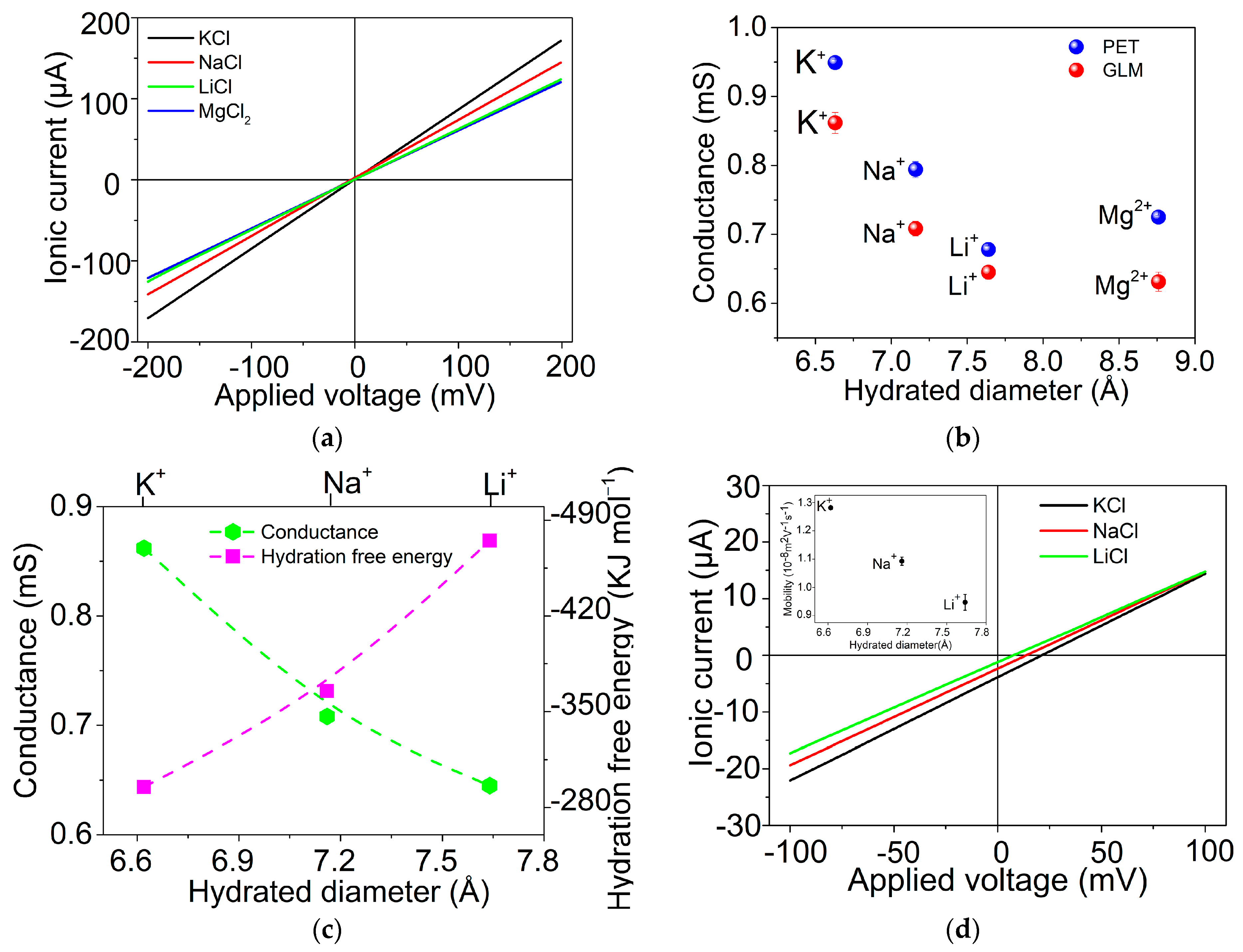
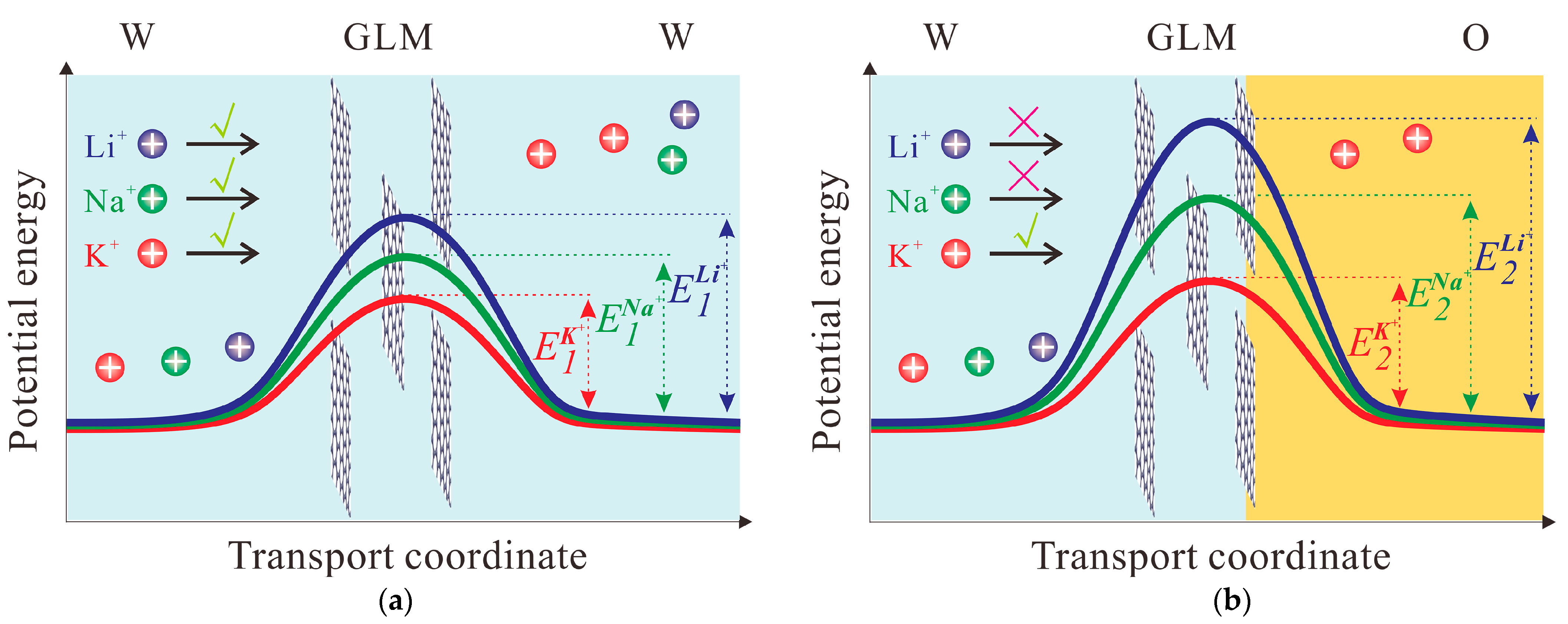
2.2. Ion Transport through GLM in the System of W/GLM/W
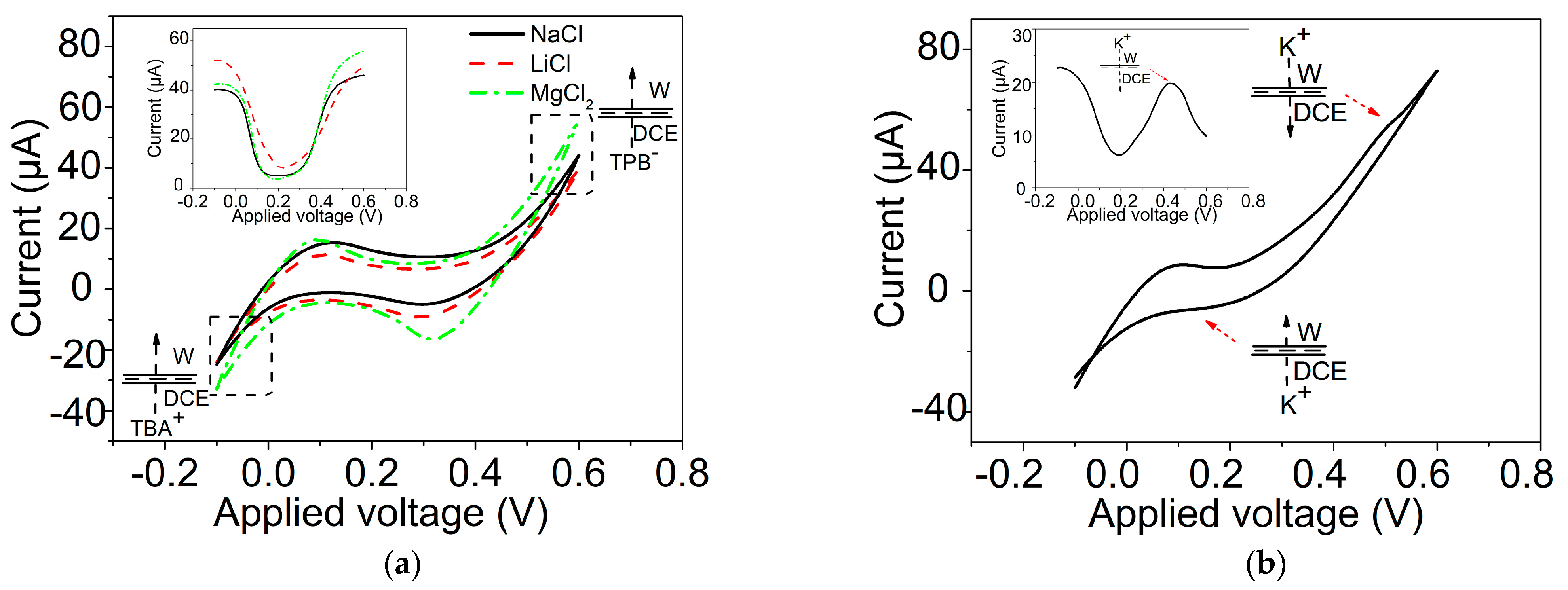
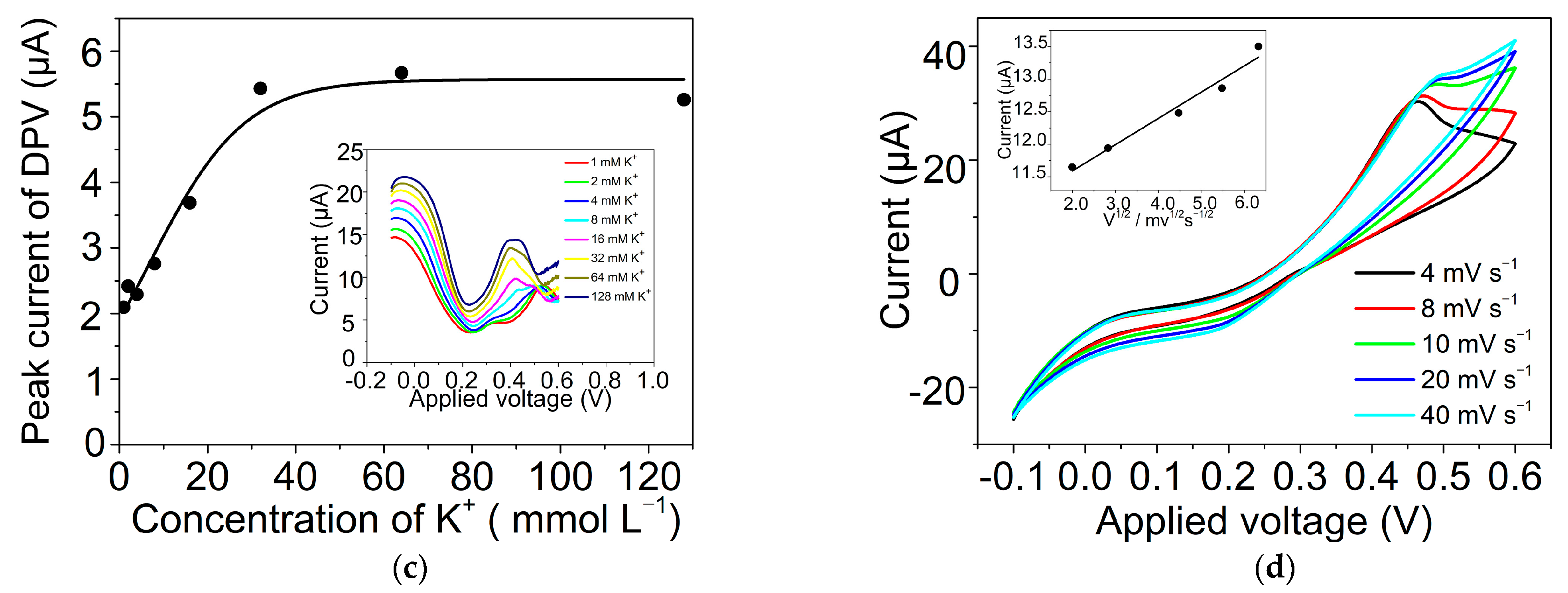
2.3. Ion Transfer across the GLM-Supported W/DCE Interface in the System of W/GLM/O
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gouaux, E.; MacKinnon, R. Principles of selective ion transport in channels and pumps. Science 2005, 310, 1461–1465. [Google Scholar] [CrossRef] [Green Version]
- Doyle, D.A.; Cabral, J.M.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of potassium channels. Cell. Mol. Life Sci. 2015, 72, 3677–3693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epsztein, R.; DuChanois, R.M.; Ritt, C.L.; Noy, A.; Elimelech, M. Towards single-species selectivity of membranes with subnanometre pores. Nat. Nanotechnol. 2020, 15, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, G.; Han, Y.; Jin, W. Artificial channels for confined mass transport at the sub-nanometre scale. Nat. Rev. Mater. 2021, 6, 294–312. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hou, J.; Jiang, L.; Wang, H. Angstrom-scale ion channels towards single-ion selectivity. Chem. Soc. Rev. 2022, 51, 2224–2254. [Google Scholar] [CrossRef]
- Geim, A.K. Exploring two-dimensional empty space. Nano Lett. 2021, 21, 6356–6358. [Google Scholar] [CrossRef]
- Zhan, H.; Xiong, Z.; Cheng, C.; Liang, Q.; Liu, J.Z.; Li, D. Solvation-involved nanoionics: New opportunities from 2D nanomaterial laminar membranes. Adv. Mater. 2020, 32, 1904562. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, W.; Zhao, J.; Che, H.; Chen, L.; Sui, X. Construction and application of bioinspired nanochannels based on two-dimensional materials. Chin. Chem. Lett. 2022, 33, 2291–2300. [Google Scholar] [CrossRef]
- Gao, T.; Ai, X.; Li, Y.H.; Li, Y.W.; Zhou, K.G. Recent progress on the smart membranes based on two-dimensional materials. Chin. Chem. Lett. 2022, 33, 2832–2844. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, J.; Wu, Q.; Song, Q.; Xie, W.; Liu, B. Environmental applications of graphene oxide composite membranes. Chin. Chem. Lett. 2022, 33, 5001–5012. [Google Scholar] [CrossRef]
- Tu, W.; Liu, Y.; Chen, M.; Ma, L.; Li, L.; Yang, B. A mussel-induced approach to secondary functional cross-linking 3-aminopropytriethoxysilane to modify the graphene oxide membrane for wastewater purification. Chin. Chem. Lett. 2023, 34, 107322. [Google Scholar] [CrossRef]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Hirunpinyopas, W.; Iamprasertkun, P.; Bissett, M.A.; Dryfe, R.A.W. Tunable charge/size selective ion sieving with ultrahigh water permeance through laminar graphene membranes. Carbon 2020, 156, 119–129. [Google Scholar] [CrossRef]
- Esfandiar, A.; Radha, B.; Wang, F.C.; Yang, Q.; Hu, S.; Garaj, S.; Nair, R.R.; Geim, A.K.; Gopinadhan, K. Size effect in ion transport through angstrom-scale slits. Science 2017, 358, 511–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.Z.; Fan, J.C.; Xia, J.; Zhu, Y.B.; Wu, H.A.; Wang, F.C. Dehydration impeding ionic conductance through two-dimensional angstrom-scale slits. Nanoscale 2019, 11, 8449–8457. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Z.; Fan, J.C.; Esfandiar, A.; Zhu, Y.B.; Wu, H.A.; Wang, F.C. Charge asymmetry effect in ion transport through angstrom-scale channels. J. Phys. Chem. C 2019, 123, 1462–1469. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.S.; Shen, J.; Peng, B.Q.; Zhang, B.W.; Wang, Y.Z.; Bian, F.G.; Wang, J.J.; Li, D.Y.; Qian, Z.; et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.Z.; Liu, J.F.; Chen, L.; Liang, S.S.; Fang, H.P. Unexpected ion sieving in graphene oxide membranes. J. Phys. Chem. C 2022, 126, 9572–9579. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, H. Cation-π Interactions in graphene-containing systems for water treatment and beyond. Adv. Mater. 2020, 32, 1905756. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, S.; Wu, H.; Shi, G.; Fang, H. Revisit the hydrated cation-p interaction at the interface: A new view of dynamics and statistics. Langmuir 2022, 38, 2401–2408. [Google Scholar] [CrossRef]
- Kumpf, R.A.; Dougherty, D.A. A mechanism for ion selectivity in potassium channels: Computational studies of cation-π interactions. Science 1993, 261, 1708–1710. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, D.A. The cation−π interaction. Acc. Chem. Res. 2013, 46, 885–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osakai, T. Is the oil | water interface the simplest and best suited model for understanding biomembranes? Anal. Sci. 2019, 35, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, Q.; Shao, Y. Electrochemistry at micro- and nanoscopic liquid/liquid interfaces. Chem. Soc. Rev. 2011, 40, 2236–2253. [Google Scholar] [CrossRef]
- Gschwend, G.C.; Olaya, A.; Peljo, P.; Girault, H.H. Structure and reactivity of the polarised liquid–liquid interface: What we know and what we do not. Curr. Opin. Electrochem. 2020, 19, 137–143. [Google Scholar] [CrossRef]
- Rodgers, A.N.J.; Rabiu, A.K.; Toth, P.S.; Adams, R.W.; Dryfe, R.A.W. Assembly and electrochemistry of carbon nanomaterials at the liquid-liquid interface. Electrochim. Acta 2019, 308, 307–316. [Google Scholar] [CrossRef]
- Qiu, H.Y.; Jiang, T.; Wang, X.Y.; Zhu, L.; Wang, Q.W.; Zhao, Y.; Ge, J.J.; Chen, Y. Electrochemical investigation of adsorption of graphene oxide at an interface between two immiscible electrolyte solutions. RSC Adv. 2020, 10, 25817–25827. [Google Scholar] [CrossRef]
- Lu, X.; Wang, T.; Zhou, X.; Li, Y.; Wu, B.; Liu, X. Investigation of ion transport traversing the “Ion Channels” by scanning electrochemical microscopy (SECM). J. Phys. Chem. C 2011, 115, 4800–4805. [Google Scholar] [CrossRef]
- Chen, Y.; Bian, S.; Gao, K.; Cao, Y.; Wu, H.; Liu, C.; Jiang, X.; Sun, X. Studies on the meso-sized selectivity of a novel organic/inorganic hybrid mesoporous silica membrane. J. Membr. Sci. 2014, 457, 9–18. [Google Scholar] [CrossRef]
- Jiang, X.; Gao, K.; Hu, D.; Wang, H.; Bian, S.; Chen, Y. Ion-transfer voltammetric determination of folic acid at meso-liquid-liquid interface arrays. Analyst 2015, 140, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Jiang, X.H.; Hu, D.P.; Bian, S.J.; Wang, M.; Chen, Y. Impact of an ionic surfactant on the ion transfer behaviors at meso-liquid/liquid interface arrays. Chin. Chem. Lett. 2015, 26, 285–288. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Gao, K.; Jiang, X.; Wang, M.; Long, Y.; Chen, Y. Anion transfer across “anion channels” at the liquid/liquid interface modified by anion-exchange membrane. RSC Adv. 2014, 4, 57035–57040. [Google Scholar] [CrossRef]
- Zheng, S.; Tu, Q.; Urban, J.J.; Li, S.; Mi, B. Swelling of graphene oxide membranes in aqueous solution: Characterization of interlayer spacing and insight into water transport mechanisms. ACS Nano 2017, 11, 6440–6445. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Thermodynamics of solvation of ions Part 5.-Gibbs free energy of hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Perram, J.W.; Stiles, P.J. On the nature of liquid junction and membrane potentials. Phys. Chem. Chem. Phys. 2006, 8, 4200–4213. [Google Scholar] [CrossRef]
- Sabela, A.; Mareček, V.; Samec, Z.; Fuoco, R. Standard Gibbs energies of transfer of univalent ions from water to 1,2-dichloroethane. Electrochim. Acta 1992, 37, 231–235. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Z.; Li, F.; Ge, L.; Zhang, M.; Zhan, D.; Shao, Y. Studies of electron-transfer and charge-transfer coupling processes at a liquid/liquid interface by double-barrel micropipet technique. Anal. Chem. 2003, 75, 6593–6601. [Google Scholar] [CrossRef]
- Wandlowski, T.; Mareček, V.; Samec, Z. Galvani potential scales for water-nitrobenzene and water-1,2-dichloroethane interfaces. Electrochim. Acta 1990, 35, 1173–1175. [Google Scholar] [CrossRef]
- Cabarcos, O.M.; Weinheimer, C.J.; Lisy, J.M. Size selectivity by cation–π interactions: Solvation of K+ and Na+ by benzene and water. J. Chem. Phys. 1999, 110, 8429–8435. [Google Scholar] [CrossRef]
- Rao, J.S. Explicit solvent effect on cation-π interactions: A first principle investigation. J. Phys. Chem. B 2009, 113, 7225–7236. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Liu, J.; Wang, C.; Song, B.; Tu, Y.; Hu, J.; Fang, H. Ion enrichment on the hydrophobic carbon-based surface in aqueous salt solutions due to cation-π interactions. Sci. Rep. 2013, 3, 3436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Mu, L.; Chen, L.; Shi, G.; Fang, H. Precise control of the interlayer spacing between graphene sheets by hydrated cations. Phys. Chem. Chem. Phys. 2019, 21, 7623–7629. [Google Scholar] [CrossRef] [PubMed]


| Ion | DH (nm) | ΔGhyd (KJ/mol) | GGLM (mS) | GPET (mS) |
|---|---|---|---|---|
| K+ | 0.662 [35] | −295 [35] | 0.862 ± 0.011 | 0.949 ± 0.003 |
| Na+ | 0.716 [35] | −365 [35] | 0.708 ± 0.008 | 0.794 ± 0.009 |
| Li+ | 0.764 [35] | −475 [35] | 0.645 ± 0.007 | 0.678 ± 0.004 |
| Mg2+ | 0.876 [35] | −1830 [35] | 0.631 ± 0.010 | 0.725 ± 0.002 |
| NH4+ | 0.662 [35] | −285 [35] | 0.848 ± 0.007 | − |
| Ion | Ecation-π (M+⋅⋅⋅C6H6) | Ecation-π (C6H6⋅⋅⋅M+⋅⋅⋅C6H6) | Ecation-π (Gr⋅⋅⋅Hydrated M+⋅⋅⋅Gr) | |
|---|---|---|---|---|
| K+ | 48 [38] | 19.2 [23] | 35.4 [22] | −39.6 [43] |
| Na+ | 54 [38] | 28.0 [23] | 38.6 [22] | −29.5 [43] |
| Li+ | 54 [38] | 38.3 [23] | 47.7 [22] | −25.9 [43] |
| NH4+ | - | 19.3 [23] | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yang, H.; Yu, Z.; Zhang, Z.; Chen, Y. Two-Dimensional Graphene-Based Potassium Channels Built at an Oil/Water Interface. Materials 2023, 16, 5393. https://doi.org/10.3390/ma16155393
Wang X, Yang H, Yu Z, Zhang Z, Chen Y. Two-Dimensional Graphene-Based Potassium Channels Built at an Oil/Water Interface. Materials. 2023; 16(15):5393. https://doi.org/10.3390/ma16155393
Chicago/Turabian StyleWang, Xiaoyuan, Hanhan Yang, Zhenmei Yu, Zengtao Zhang, and Yong Chen. 2023. "Two-Dimensional Graphene-Based Potassium Channels Built at an Oil/Water Interface" Materials 16, no. 15: 5393. https://doi.org/10.3390/ma16155393





