Enhancement of Hydrogen Adsorption on Spray-Synthesized HKUST-1 via Lithium Doping and Defect Creation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Precursor Solution of Li-Doped HKUST-1 (Li-HKUST-1)
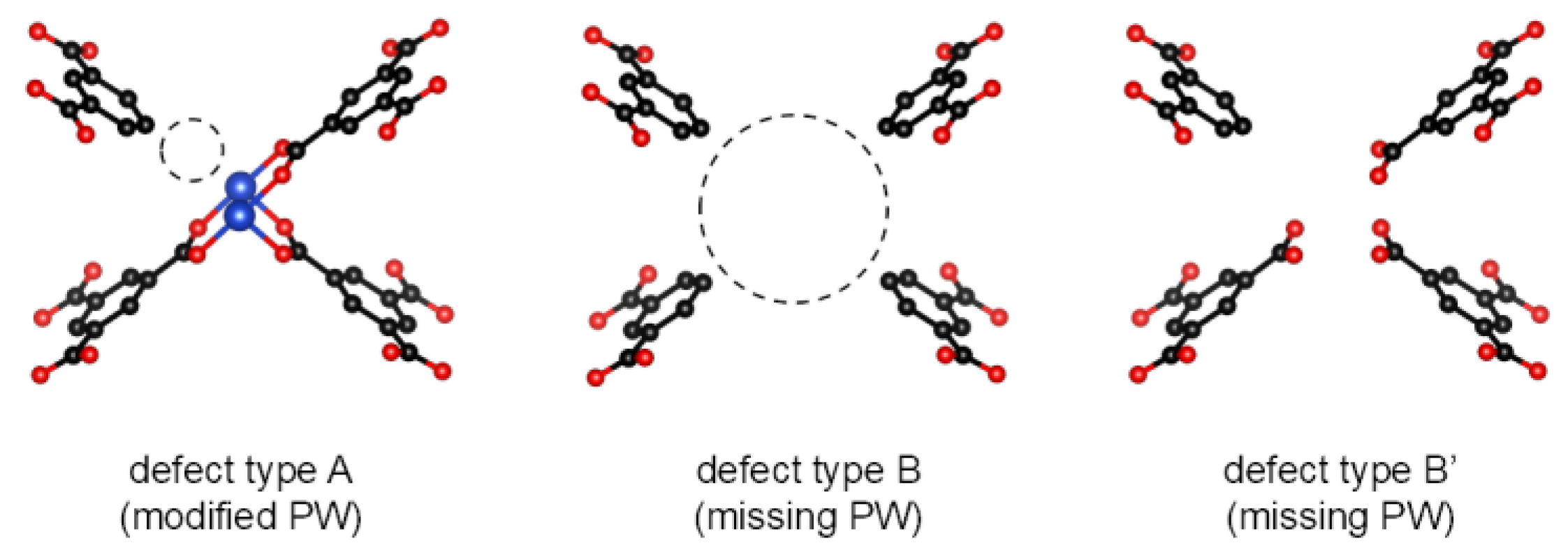
2.3. Preparation of Precursor Solutions of Defect-HKUST-1 (d-HKUST-1) and Li-Doped Defect-HKUST-1 (Li-d-HKUST-1)
2.4. Spray Synthesis of Li-HKUST-1, d-HKUST-1, and Li-d-HKUST-1
2.5. Characterization
3. Results
3.1. Li-HKUST-1
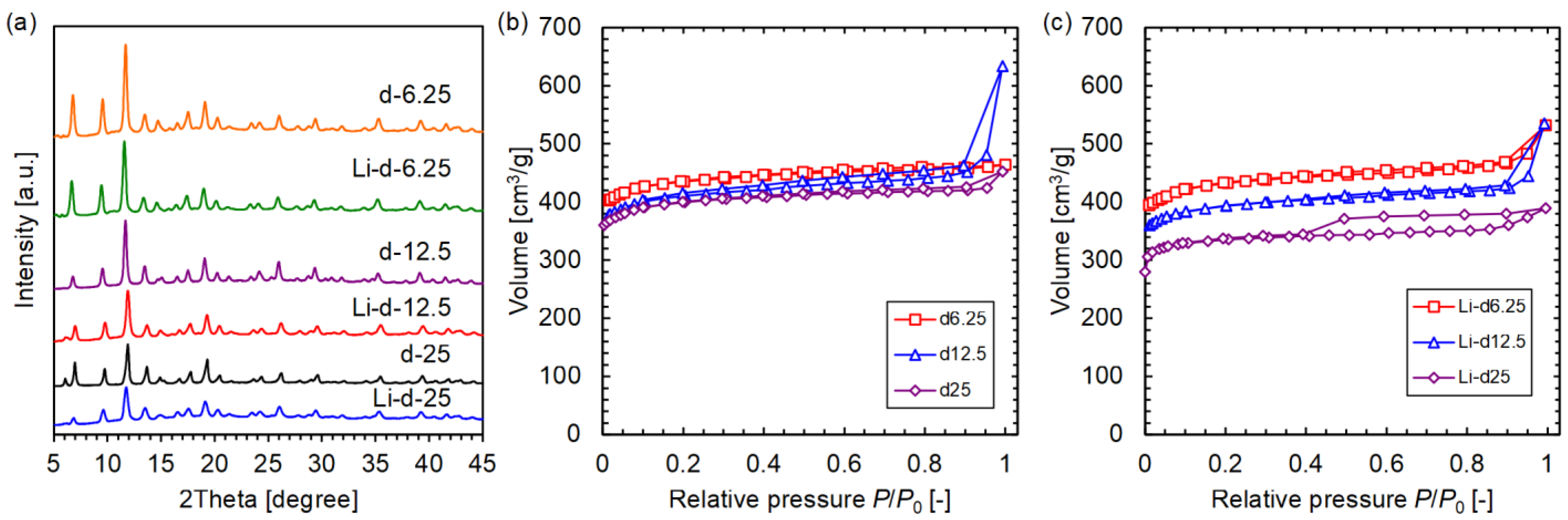
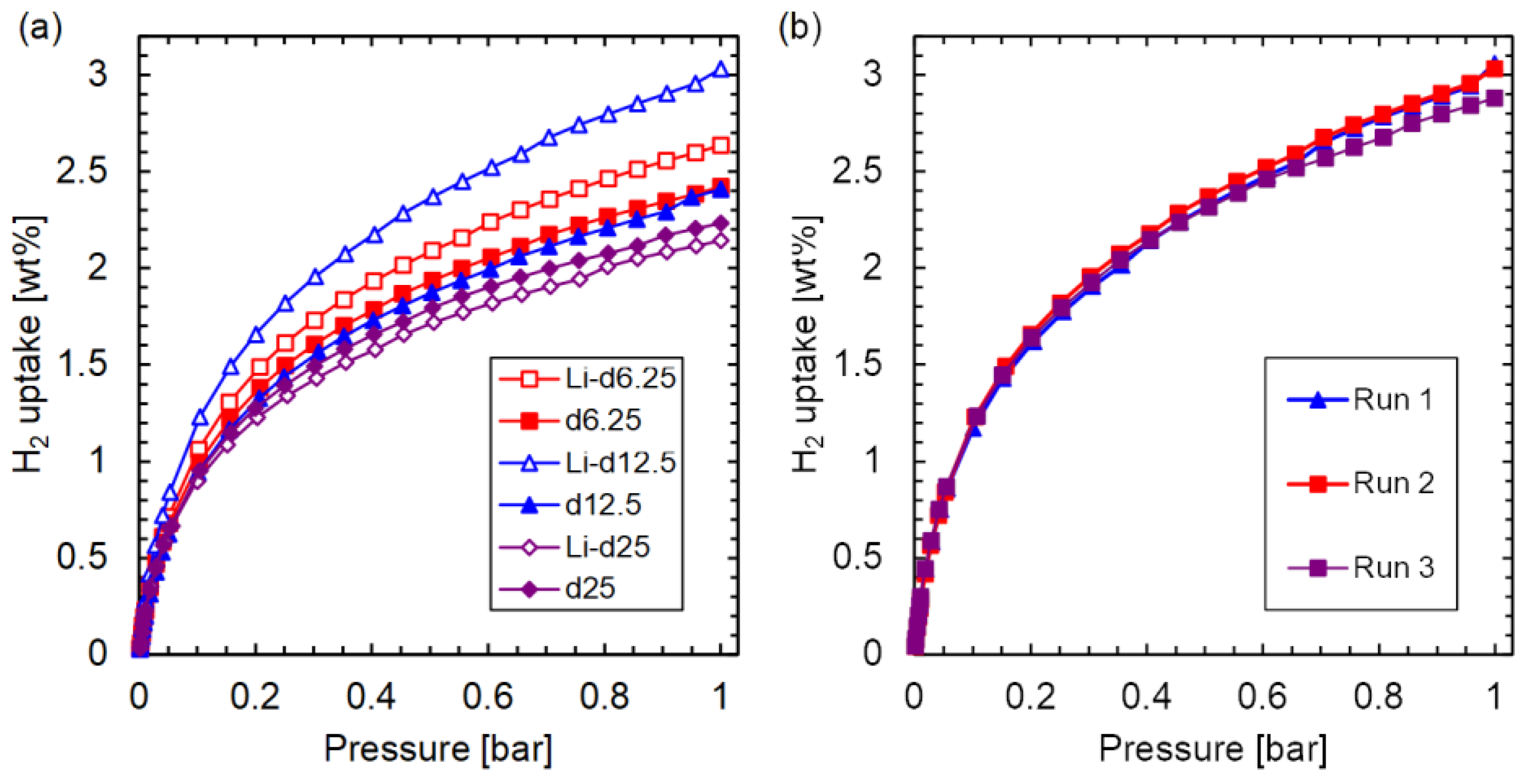
3.2. Li-d-HKUST-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stocker, T.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P. (Eds.) Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 27. [Google Scholar]
- Jena, P. Materials for Hydrogen Storage: Past, Present, and Future. J. Phys. Chem. Lett. 2011, 2, 206–211. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Sule, R.; Mishra, A.K.; Nkambule, T.T. Recent Advancement in Consolidation of MOFs as Absorbents for Hydrogen Storage. Int. J. Energy Res. 2021, 45, 12481–12499. [Google Scholar] [CrossRef]
- DOE. Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 3 October 2022).
- Murray, L.J.; Dincă, M.; Long, J.R. Hydrogen Storage in Metal–Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Langmi, H.W.; North, B.C.; Mathe, M. Review on Processing of Metal-Organic Framework (MOF) Materials towards System Integration for Hydrogen Storage. Int. J. Energy Res. 2015, 39, 607–620. [Google Scholar] [CrossRef]
- Shet, S.P.; Shanmuga Priya, S.; Sudhakar, K.; Tahir, M. A Review on Current Trends in Potential Use of Metal-Organic Framework for Hydrogen Storage. Int. J. Hydrogen Energy 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Farha, O.K.; Yazaydın, A.Ö.; Eryazici, I.; Malliakas, C.D.; Hauser, B.G.; Kanatzidis, M.G.; Nguyen, S.T.; Snurr, R.Q.; Hupp, J.T. De Novo Synthesis of a Metal-Organic Framework Material Featuring Ultrahigh Surface Area and Gas Storage Capacities. Nat. Chem. 2010, 2, 944–948. [Google Scholar] [CrossRef]
- Lochan, R.C.; Head-Gordon, M. Computational Studies of Molecular Hydrogen Binding Affinities: The Role of Dispersion Forces, Electrostatics, and Orbital Interactions. Phys. Chem. Chem. Phys. 2006, 8, 1357–1370. [Google Scholar] [CrossRef]
- Kubo, M.; Ushiyama, H.; Shimojima, A.; Okubo, T. Investigation on Specific Adsorption of Hydrogen on Lithium-Doped Mesoporous Silica. Adsorption 2011, 17, 211–218. [Google Scholar] [CrossRef]
- Han, S.S.; William, A.G., 3rd. Lithium-Doped Metal-Organic Frameworks for Reversible H2 Storage at Ambient Temperature. J. Am. Chem. Soc. 2007, 129, 8422–8423. [Google Scholar] [CrossRef]
- Xu, G.; Meng, Z.; Liu, Y.; Guo, X.; Deng, K.; Ding, L.; Lu, R. Porous MOF-205 with Multiple Modifications for Efficiently Storing Hydrogen and Methane as Well as Separating Carbon Dioxide from Hydrogen and Methane. Int. J. Energy Res. 2019, 43, 7517–7528. [Google Scholar] [CrossRef]
- Himsl, D.; Wallacher, D.; Hartmann, M. Improving the Hydrogen-Adsorption Properties of a Hydroxy-Modified MIL-53(Al) Structural Analogue by Lithium Doping. Angew. Chem. Int. Ed. Engl. 2009, 48, 4639–4642. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Shimojima, A.; Okubo, T. Effect of Lithium Doping into MIL-53(Al) through Thermal Decomposition of Anion Species on Hydrogen Adsorption. J. Phys. Chem. C 2012, 116, 10260–10265. [Google Scholar] [CrossRef]
- Yang, S.; Lin, X.; Blake, A.J.; Walker, G.S.; Hubberstey, P.; Champness, N.R.; Schröder, M. Cation-Induced Kinetic Trapping and Enhanced Hydrogen Adsorption in a Modulated Anionic Metal–Organic Framework. Nat. Chem. 2009, 1, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Hagi, H.; Shimojima, A.; Okubo, T. Facile Synthesis of Hydroxy-Modified MOF-5 for Improving the Adsorption Capacity of Hydrogen by Lithium Doping. Chem. Asian J. 2013, 8, 2801–2806. [Google Scholar] [CrossRef]
- Panchariya, D.K.; Kumar, E.A.; Singh, S.K. Lithium-Doped Silica-Rich MIL-101(Cr) for Enhanced Hydrogen Uptake. Chem. Asian J. 2019, 14, 3728–3735. [Google Scholar] [CrossRef]
- Mulfort, K.L.; Farha, O.K.; Stern, C.L.; Sarjeant, A.A.; Hupp, J.T. Post-Synthesis Alkoxide Formation within Metal-Organic Framework Materials: A Strategy for Incorporating Highly Coordinatively Unsaturated Metal Ions. J. Am. Chem. Soc. 2009, 131, 3866–3868. [Google Scholar] [CrossRef]
- Zhou, L.; Niu, Z.; Jin, X.; Tang, L.; Zhu, L. Effect of Lithium Doping on the Structures and CO2 Adsorption Properties of Metal-organic Frameworks HKUST-1. ChemistrySelect 2018, 3, 12865–12870. [Google Scholar] [CrossRef]
- Chuvikov, S.V.; Berdonosova, E.A.; Krautsou, A.; Kostina, J.V.; Minin, V.V.; Ugolkova, E.A.; Klyamkin, S.N. Peculiarities of High-Pressure Hydrogen Adsorption on Pt Catalyzed Cu-BTC Metal-Organic Framework. Phys. Chem. Chem. Phys. 2021, 23, 4277–4286. [Google Scholar] [CrossRef]
- Zhang, W.; Kauer, M.; Halbherr, O.; Epp, K.; Guo, P.; Gonzalez, M.I.; Xiao, D.J.; Wiktor, C.; LIabrés I Xamena, F.X.; Wöll, C.; et al. Ruthenium Metal-Organic Frameworks with Different Defect Types: Influence on Porosity, Sorption, and Catalytic Properties. Chemistry 2016, 22, 14297–14307. [Google Scholar] [CrossRef]
- Usman, M.; Iqbal, N.; Noor, T.; Zaman, N.; Asghar, A.; Abdelnaby, M.M.; Galadima, A.; Helal, A. Advanced Strategies in Metal-Organic Frameworks for CO2 Capture and Separation. Chem. Rec. 2021, 22, e202100230. [Google Scholar] [CrossRef]
- Iacomi, P.; Formalik, F.; Marreiros, J.; Shang, J.; Rogacka, J.; Mohmeyer, A.; Behrens, P.; Ameloot, R.; Kuchta, B.; Llewellyn, P.L. Role of Structural Defects in the Adsorption and Separation of C3 Hydrocarbons in Zr-Fumarate-MOF (MOF-801). Chem. Mater. 2019, 31, 8413–8423. [Google Scholar] [CrossRef]
- Barin, G.; Krungleviciute, V.; Gutov, O.; Hupp, J.T.; Yildirim, T.; Farha, O.K. Defect Creation by Linker Fragmentation in Metal-Organic Frameworks and Its Effects on Gas Uptake Properties. Inorg. Chem. 2014, 53, 6914–6919. [Google Scholar] [CrossRef] [PubMed]
- Chaemchuen, S.; Luo, Z.; Zhou, K.; Mousavi, B.; Phatanasri, S.; Jaroniec, M.; Verpoort, F. Defect Formation in Metal–Organic Frameworks Initiated by the Crystal Growth-Rate and Effect on Catalytic Performance. J. Catal. 2017, 354, 84–91. [Google Scholar] [CrossRef]
- Kubo, M.; Saito, T.; Shimada, M. Evaluation of the Parameters Utilized for the Aerosol-Assisted Synthesis of HKUST-1. Microporous Mesoporous Mater. 2017, 245, 126–132. [Google Scholar] [CrossRef]
- Kubo, M.; Sugahara, T.; Shimada, M. Facile Fabrication of HKUST-1 Thin Films and Free-Standing MWCNT/HKUST-1 Film Using a Spray-Assisted Method. Microporous Mesoporous Mater. 2021, 312, 110771. [Google Scholar] [CrossRef]
- Kubo, M.; Ishimura, M.; Shimada, M. Improvement of Production Efficiency of Spray-Synthesized HKUST-1. Adv. Powder Technol. 2021, 32, 2370–2378. [Google Scholar] [CrossRef]
- Kubo, M.; Moriyama, R.; Shimada, M. Facile Fabrication of HKUST-1 Nanocomposites Incorporating Fe3O4 and TiO2 Nanoparticles by a Spray-Assisted Synthetic Process and Their Dye Adsorption Performances. Microporous Mesoporous Mater. 2019, 280, 227–235. [Google Scholar] [CrossRef]
- Kubo, M.; Matsumoto, T.; Shimada, M. Spray Synthesis of Pd Nanoparticle Incorporated HKUST-1, and Its Catalytic Activity for 4-Nitrophenol Reduction. Adv. Powder Technol. 2022, 33, 103701. [Google Scholar] [CrossRef]
- Zhang, W.; Kauer, M.; Guo, P.; Kunze, S.; Cwik, S.; Muhler, M.; Wang, Y.; Epp, K.; Kieslich, G.; Fischer, R.A. Impact of Synthesis Parameters on the Formation of Defects in HKUST-1. Eur. J. Inorg. Chem. 2017, 2017, 925–931. [Google Scholar] [CrossRef]
- Yang, S.; Lin, X.; Blake, A.J.; Thomas, K.M.; Hubberstey, P.; Champness, N.R.; Schröder, M. Enhancement of H2 Adsorption in Li+-Exchanged Co-Ordination Framework Materials. Chem. Commun. 2008, 6108–6110. [Google Scholar] [CrossRef] [PubMed]
- Mulfort, K.L.; Wilson, T.M.; Wasielewski, M.R.; Hupp, J.T. Framework Reduction and Alkali-Metal Doping of a Triply Catenating Metal-Organic Framework Enhances and Then Diminishes H2 Uptake. Langmuir 2009, 25, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Hu, Z.; Yang, W.; Cao, D. Lithium Doping on Metal-Organic Frameworks for Enhancing H2 Storage. Int. J. Hydrogen Energy 2012, 37, 946–950. [Google Scholar] [CrossRef]
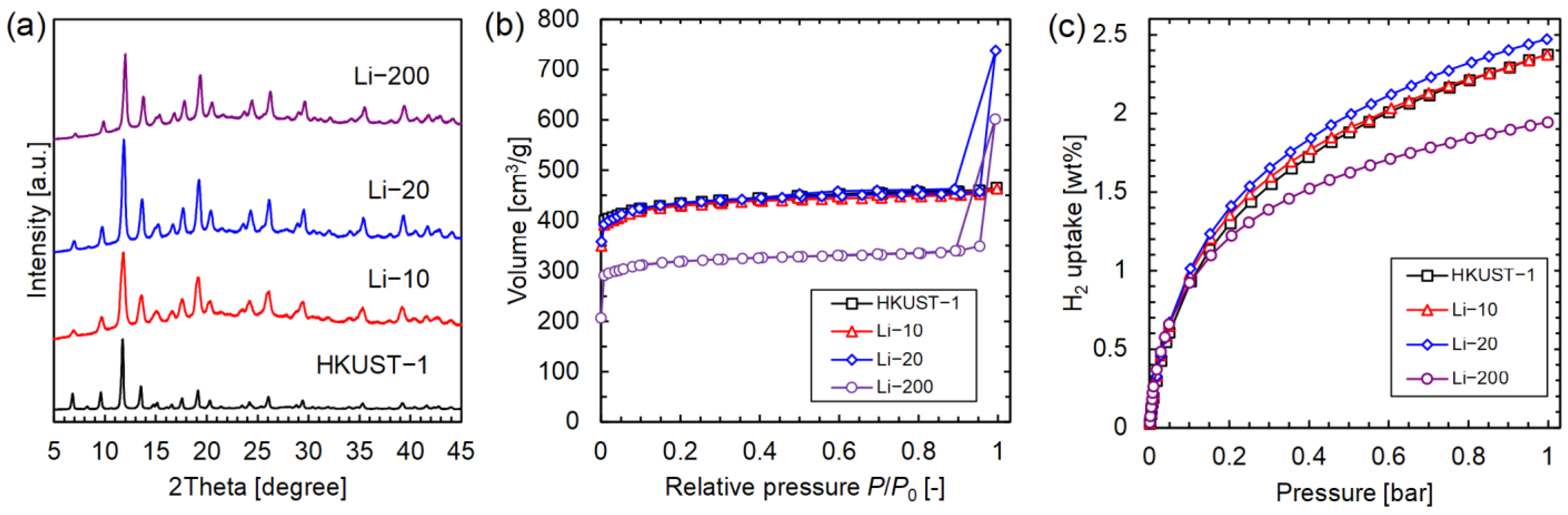

| Sample | Li/Cu (mol%) | BET Surface Area (m2/g) | H2 Uptake at 77 K and 1 Bar (wt%) |
|---|---|---|---|
| HKUST-1 | 0 | 1705 | 2.37 |
| Li-10 | 0.29 | 1548 | 2.37 |
| Li-20 | 0.24 | 1706 | 2.47 |
| Li-200 | 0.93 | 1251 | 1.94 |
| Sample | Li/Cu (mol%) | IP/(BTC+IP) (%) | BET Surface Area (m2/g) | H2 Uptake at 77 K and 1 Bar (wt%) |
|---|---|---|---|---|
| d6.25 | 0 | 4.8 | 1714 | 2.42 |
| d12.5 | 0 | 10.3 | 1609 | 2.41 |
| d25 | 0 | 22.2 | 1569 | 2.23 |
| Li-d6.25 | 0.64 | 4.2 | 1687 | 2.63 |
| Li-d12.5 | 0.85 | 10.9 | 1544 | 3.03 |
| Li-d25 | 0.51 | 19.9 | 1330 | 2.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubo, M.; Matsumoto, T.; Shimada, M. Enhancement of Hydrogen Adsorption on Spray-Synthesized HKUST-1 via Lithium Doping and Defect Creation. Materials 2023, 16, 5416. https://doi.org/10.3390/ma16155416
Kubo M, Matsumoto T, Shimada M. Enhancement of Hydrogen Adsorption on Spray-Synthesized HKUST-1 via Lithium Doping and Defect Creation. Materials. 2023; 16(15):5416. https://doi.org/10.3390/ma16155416
Chicago/Turabian StyleKubo, Masaru, Tomoki Matsumoto, and Manabu Shimada. 2023. "Enhancement of Hydrogen Adsorption on Spray-Synthesized HKUST-1 via Lithium Doping and Defect Creation" Materials 16, no. 15: 5416. https://doi.org/10.3390/ma16155416
APA StyleKubo, M., Matsumoto, T., & Shimada, M. (2023). Enhancement of Hydrogen Adsorption on Spray-Synthesized HKUST-1 via Lithium Doping and Defect Creation. Materials, 16(15), 5416. https://doi.org/10.3390/ma16155416



_CHAINOK.jpg)





