Effects of Heat Treatment on Microstructure and Mechanical Properties of Weldable Al–Mg–Zn–Sc Alloy with High Strength and Ductility
Abstract
:1. Introduction
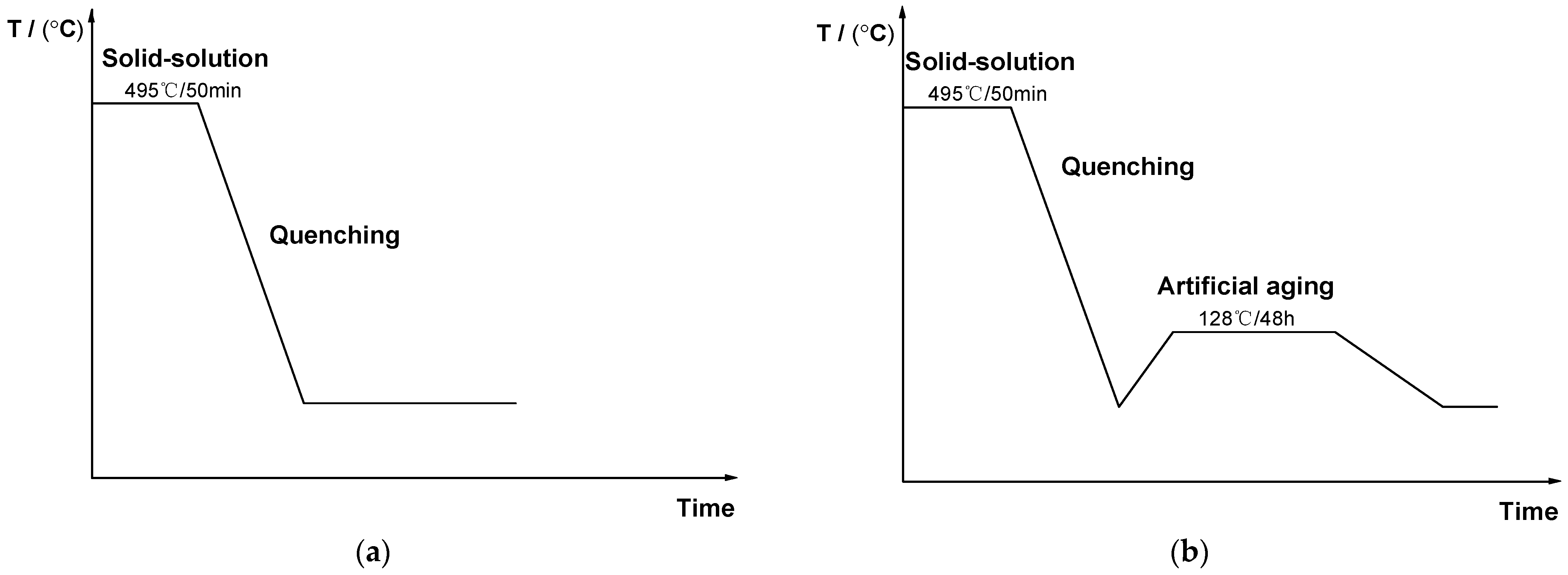
2. Experimental Section
3. Results
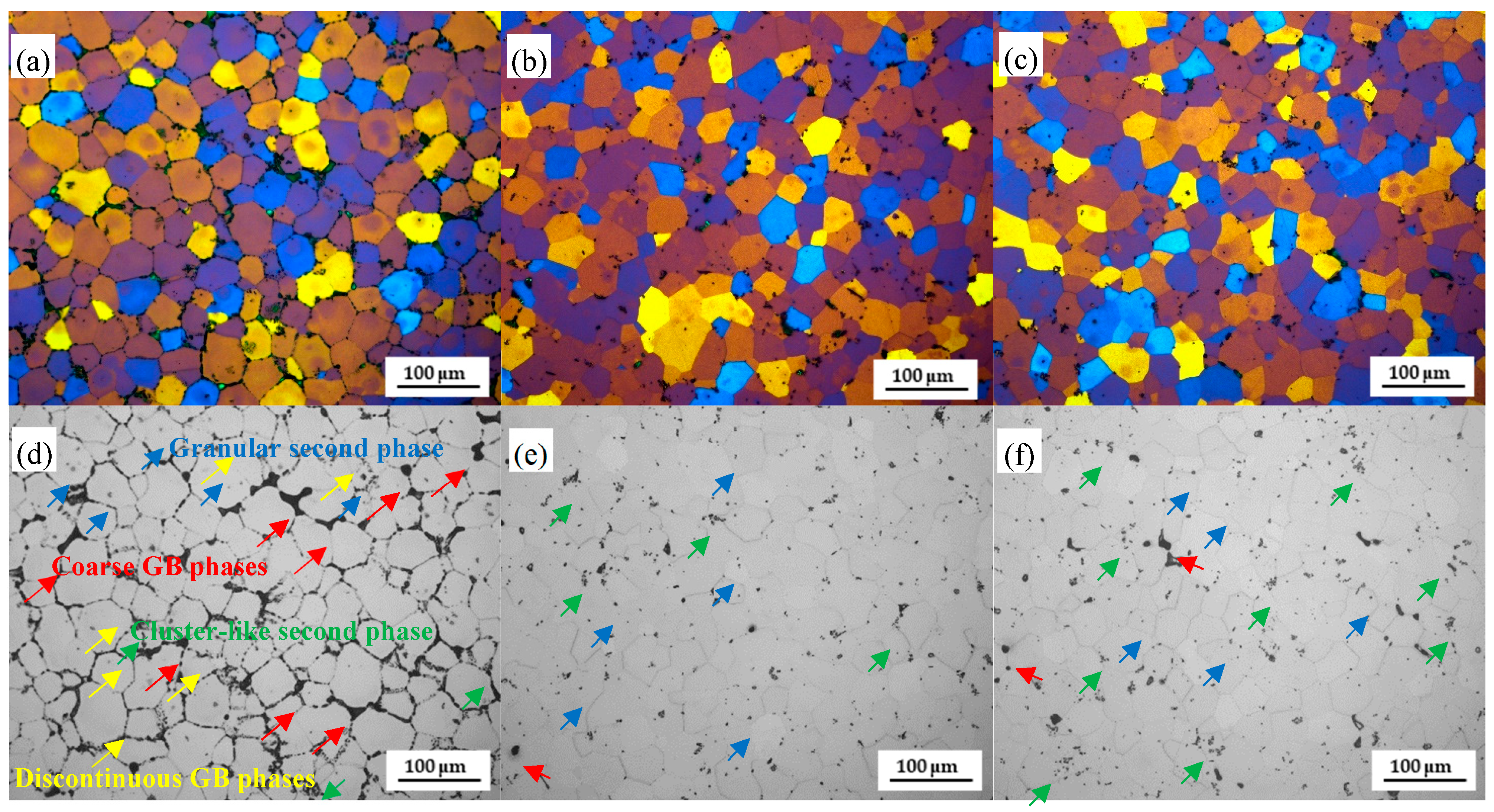
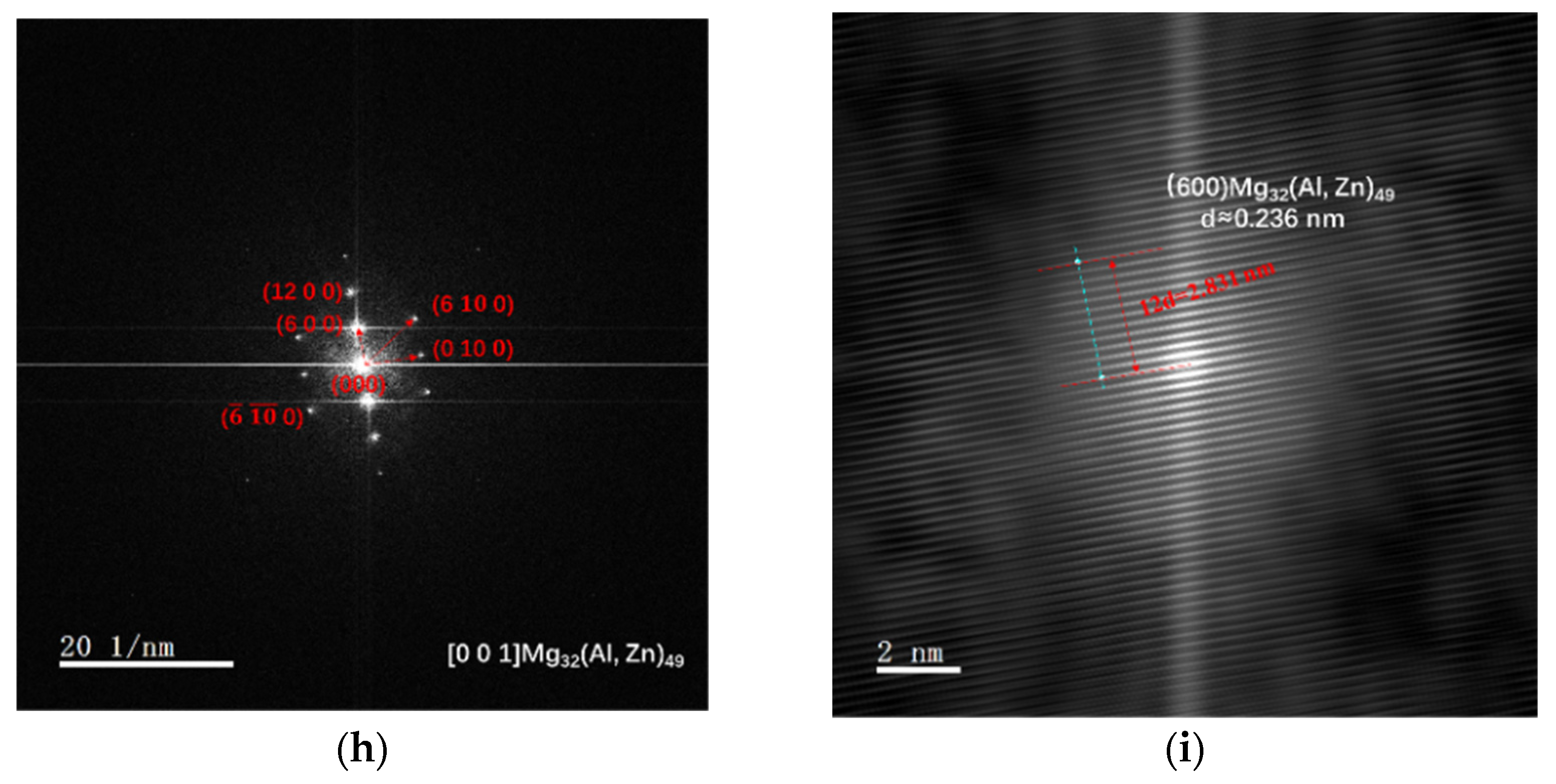
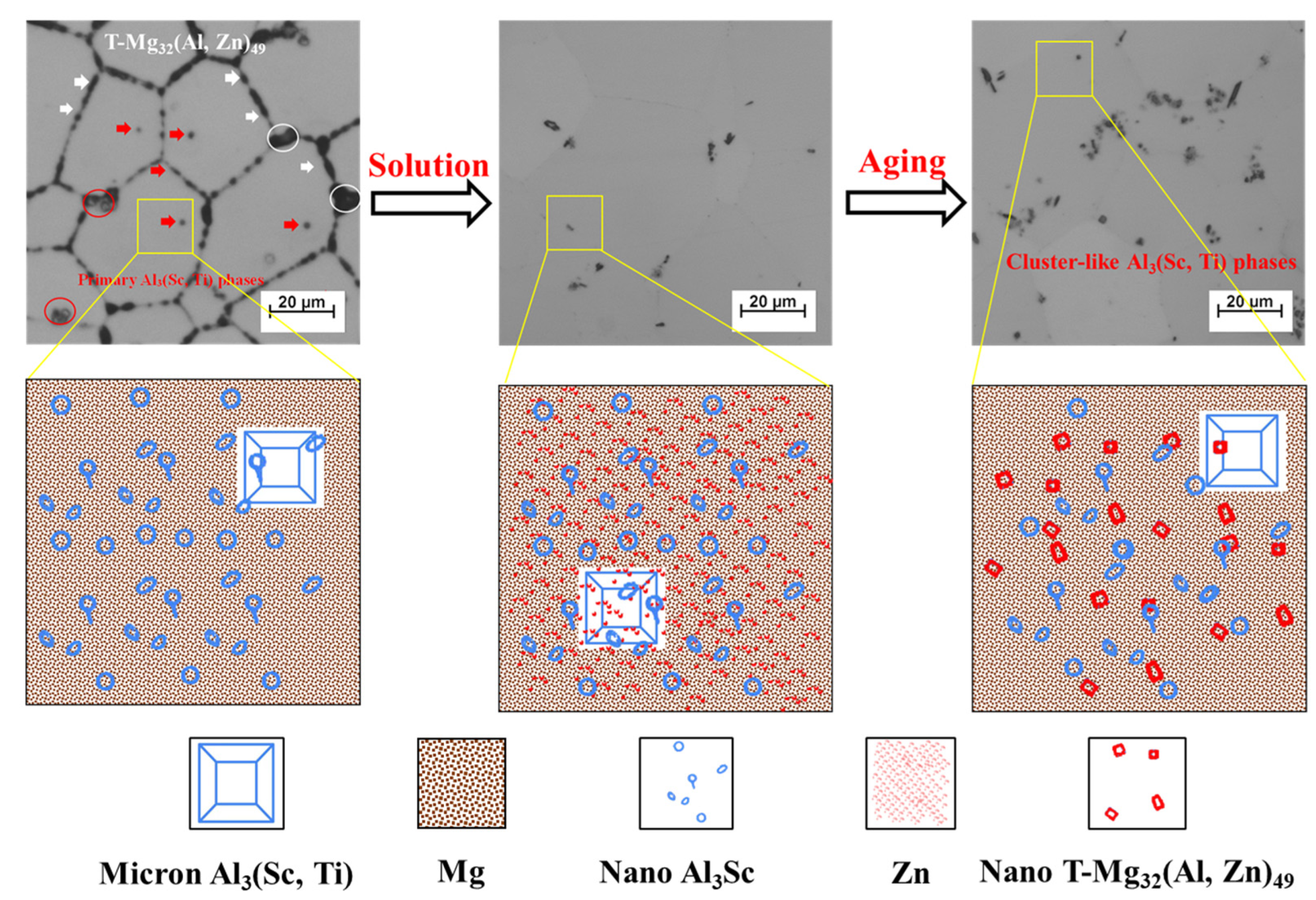
3.1. Microstructure Evolution
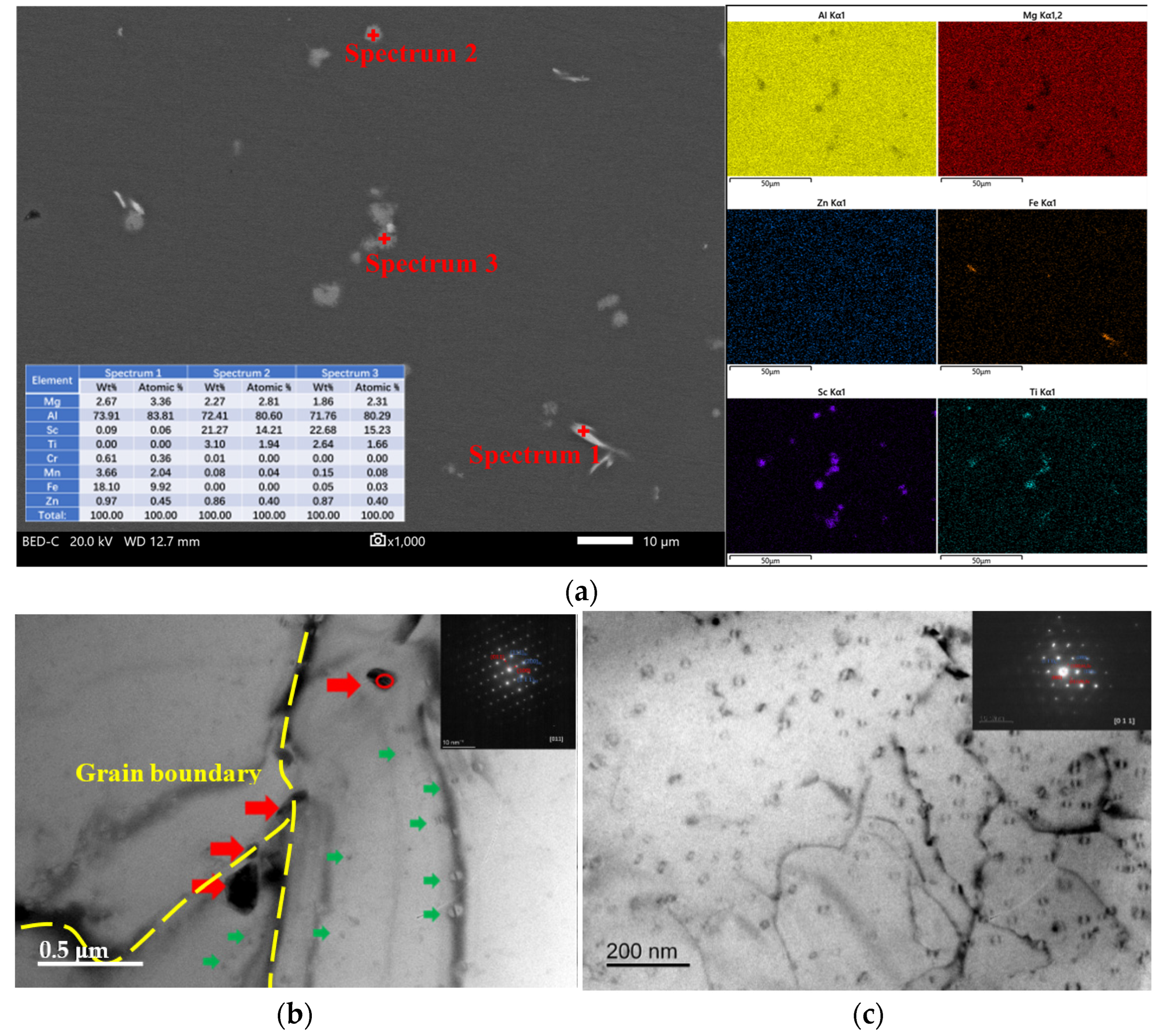
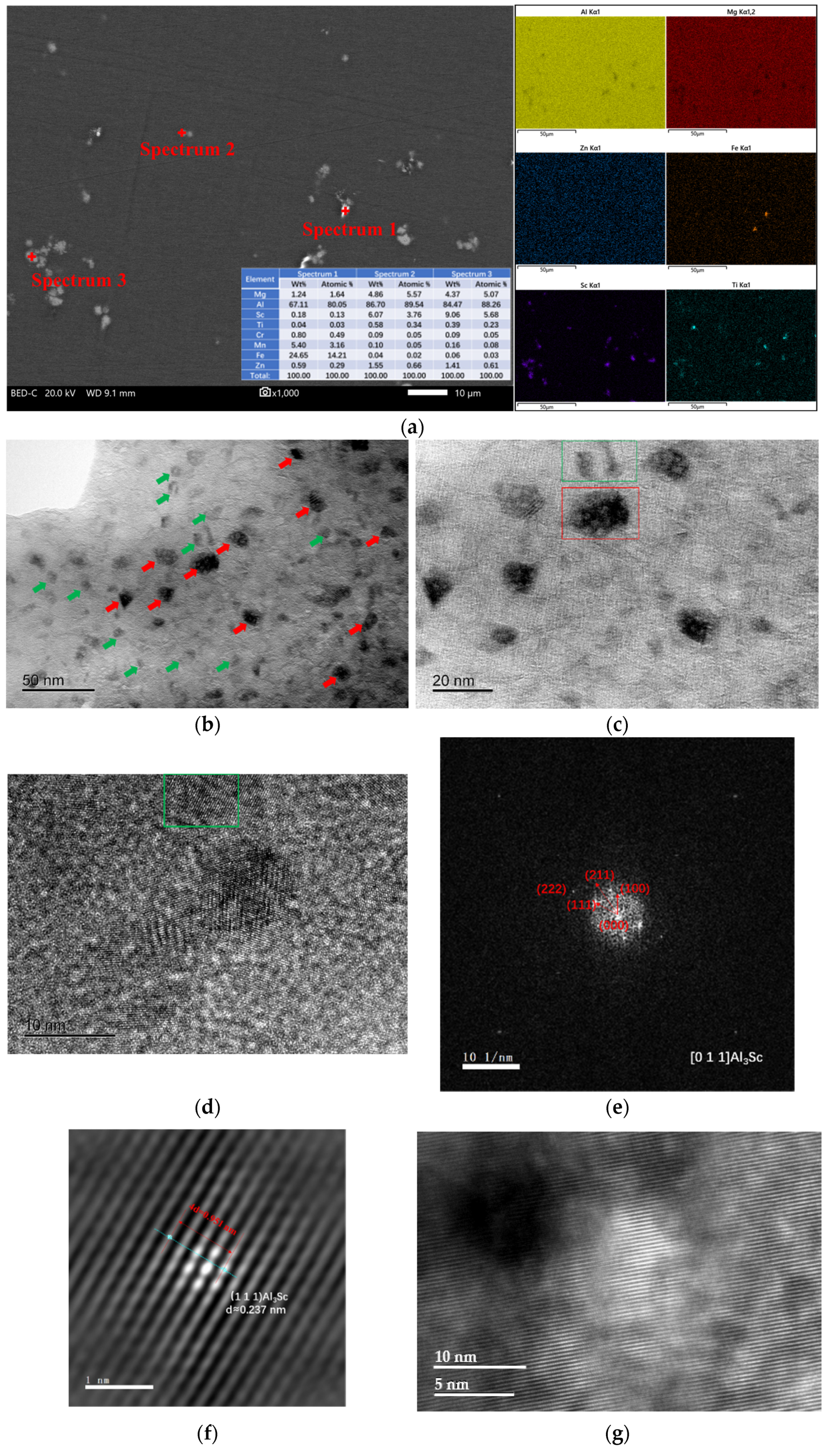
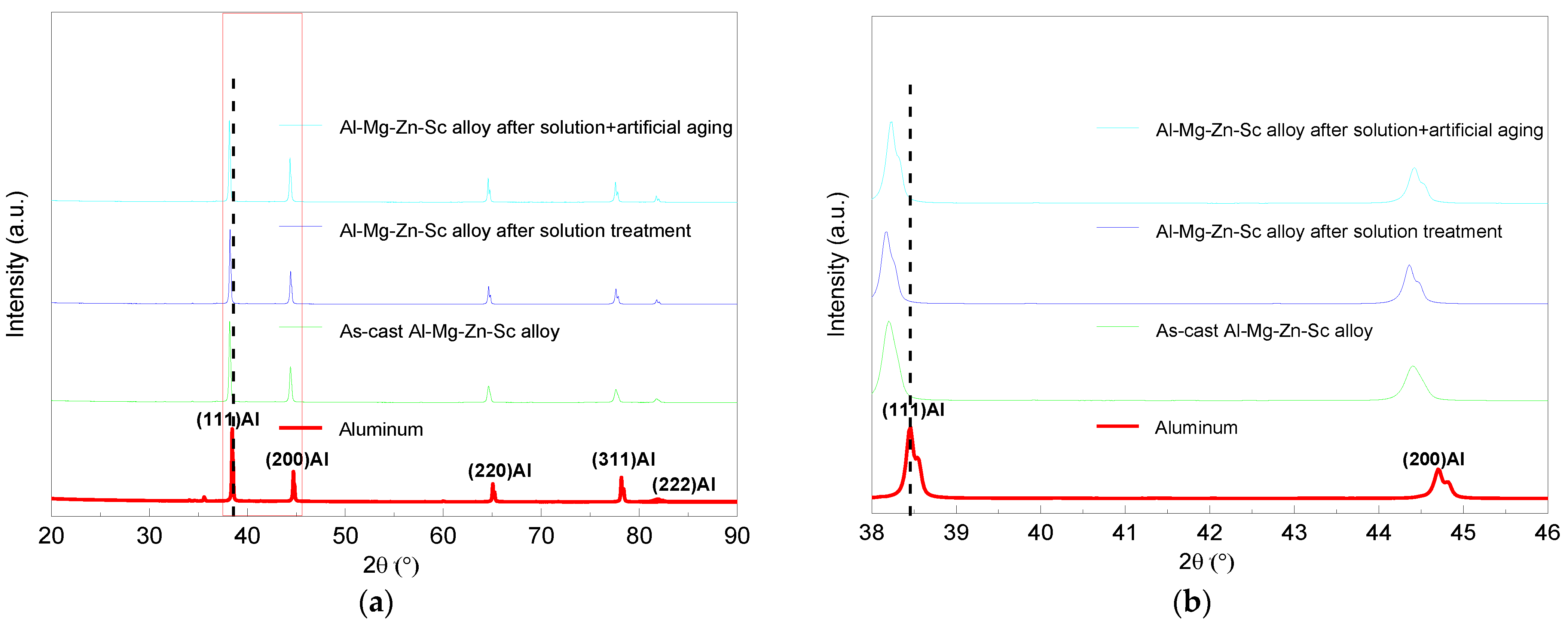
3.2. Characterization of the Solid Solubility
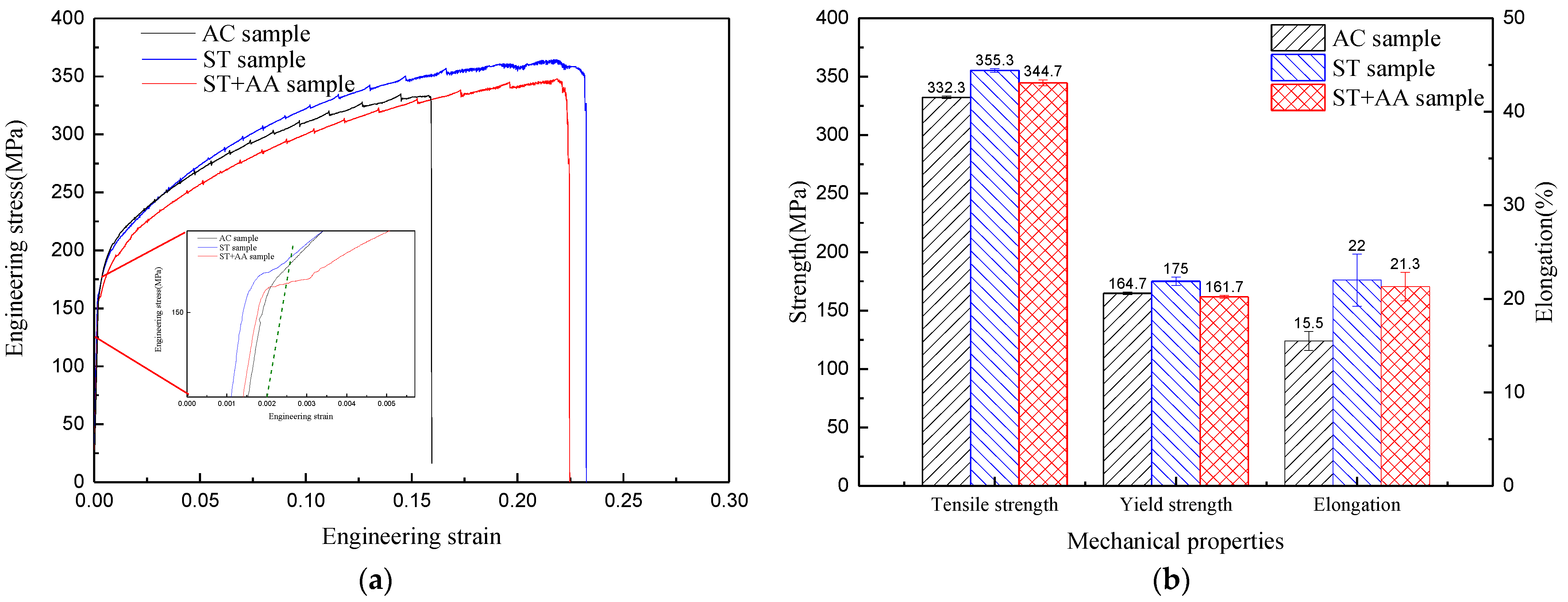
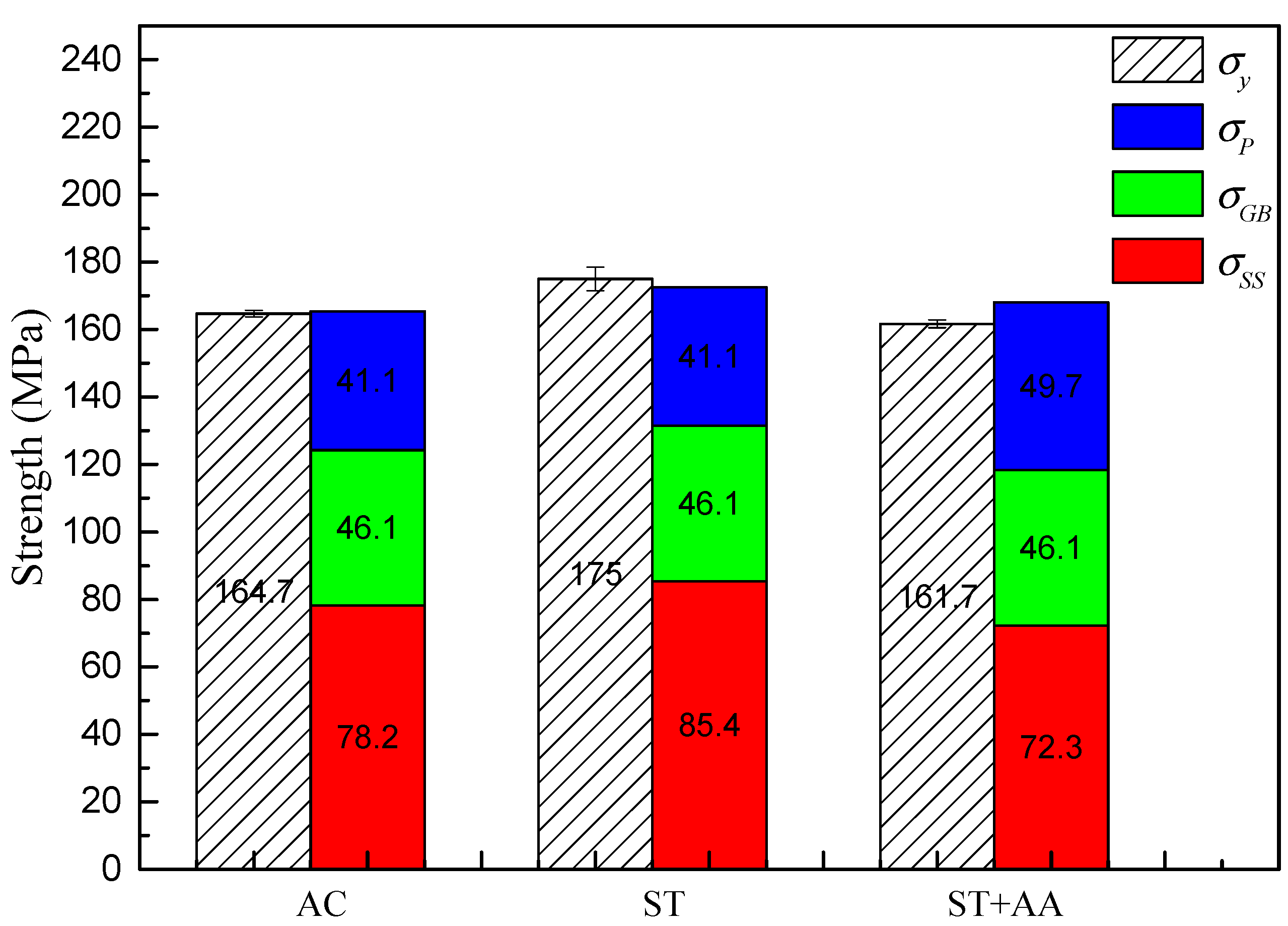
3.3. Mechanical Properties
4. Discussion
5. Conclusions
- (1)
- As-cast Al–Mg–Zn–Sc alloy has fine equiaxed grains 40 μm in size with a high Mg solid solubility of 4.67% and large volume fraction of 0.15% Al3Sc nanoprecipitations. The solid solubility of Mg is increased to 5.33% after solid-solution treatment and dramatically decreased to 4.15% after post-aging treatment, but a dual nanoprecipitation including T-Mg32(Al, Zn)49 with diameters of 10 to 25 nm and Al3Sc with diameters of 5 to 20 nm is observed.
- (2)
- The solid-solution strengthening contributions to the as-cast, post-solid-solution and post-aging Al–Mg–Zn–Sc alloys are 78.2 MPa, 85.4 MPa and 72.3 MPa, respectively. The precipitation strengthening of the post-aging alloy is 49.7 MPa, which is an increase of 21% in comparison to that of both as-cast and post-solid-solution alloy. The tensile strength of 355.3 MPa, yield strength of 175 MPa and elongation of 22% for the alloy are optimally obtained after solid-solution treatment.
- (3)
- Solid-solution strengthening is the main strengthening mechanism controlling the ultimate strength of the Al–Mg–Zn–Sc alloys. Contradictory strengthening effects exists in the post-aging alloy with increased precipitation strengthening and reduced solid-solution strengthening. It is an effective way to improve the strengthening effect by increasing the solid solution of Mg in the Al–Mg–Zn–Sc alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Xin, Y.C.; Zhang, B.; Li, X.Y. Stress corrosion cracking resistant nanostructured Al-Mg alloy with low angle grain boundaries. Acta Mater. 2022, 225, 117607. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, B.; Du, K.; Li, X.Y.; Lu, K. Thermally stable nanostructured Al-Mg alloy with relaxed grain boundaries. Acta Mater. 2022, 226, 117640. [Google Scholar] [CrossRef]
- Król, M.; Tański, T.; Snopiński, P.; Tomiczek, B. Structure and properties of aluminium–magnesium casting alloys after heat treatment. J. Therm. Anal. Calorim. 2017, 127, 299–308. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, M.; Yang, B.; Zhao, E.; Liu, F.; Guan, R. Microstructural evolution and mechanical property of Al–Mg–Mn alloys with various solidification cooling rates—ScienceDirect. Mater. Charact. 2021, 184, 111709. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Li, F. Effect of Zr addition and heat treatment on microstructure and mechanical properties of Al-Zn-Mg-Cu alloy. Heat Treat. Met 2018, 43, 27–30. [Google Scholar]
- Lee, B.H.; Kim, S.H.; Park, J.H.; Kim, H.W.; Lee, J.C. Role of Mg in simultaneously improving the strength and ductility of Al–Mg alloys. Mater. Sci. Eng. A 2016, 657, 115–122. [Google Scholar] [CrossRef]
- He, T.; Chen, W.; Wang, W.; Du, S.; Deng, S. Microstructure and hydrogen production of the rapidly solidified Al–Mg-Ga-In-Sn alloy—ScienceDirect. J. Alloys Compd. 2020, 827, 154290. [Google Scholar] [CrossRef]
- Gancarz, T.; Dobosz, A.; Bogno, A.A.; Cempura, G.; Schell, N.; Chulist, R.; Henein, H. Characterization of rapidly solidified Al-Mg-Sc alloys with Li addition. Mater. Charact. 2021, 178, 111290. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Pan, D.; Su, H.; Du, X.; Li, P.; Wu, Y. Effect of Sc on microstructure and mechanical properties of as-cast Al–Mg alloys. Mater. Des. 2015, 90, 1077–1084. [Google Scholar]
- Ratchev, P.; Verlinden, B.; De Smet, P.; Van Houtte, P. Precipitation hardening of anAl–4.2 wt% Mg–0.6 wt% Cu alloy. Acta Mater. 1998, 46, 3523–3533. [Google Scholar] [CrossRef]
- Engler, O.; Marioara, C.D.; Hentschel, T.; Brinkman, H.J. Influence of copper additions on materials properties and corrosion behaviour of Al-Mg alloy sheet. J. Alloys Compd. 2017, 710, 650–662. [Google Scholar] [CrossRef]
- Meng, C.; Zhang, D.; Zhuang, L.; Zhang, J. Correlations between stress corrosion cracking, grain boundary precipitates and Zn content of Al-Mg-Zn alloys. J. Alloys Compd. 2016, 655, 178–187. [Google Scholar] [CrossRef]
- Hou, S.; Liu, P.; Zhang, D.; Zhang, J.; Zhuang, L. Precipitation hardening behavior and microstructure evolution of Al–5.1 Mg–0.15Cu alloy with 3.0Zn (wt%) addition. J. Mater. Sci. 2017, 53, 3846–3861. [Google Scholar] [CrossRef]
- Tändl, J.; Orthacker, A.; Amenitsch, H.; Kothleitner, G.; Poletti, C. Influence of the degree of scandium supersaturation on the precipitation kinetics of rapidly solidified Al-Mg-Sc-Zr alloys. Acta Mater. 2016, 117, 43–50. [Google Scholar] [CrossRef]
- Algendy, A.Y.; Liu, K.; Chen, X.G. Evolution of dispersoids during multistep heat treatments and their effect on rolling performance in an Al-5% Mg-0.8% Mn alloy. Mater. Charact. 2021, 181, 111487. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.A.; Dumitraschkewitz, P.; Mendez-Martin, F.; Tosone, R.; Marchand, D.; Curtin, W.A.; Uggowitzer, P.J.; Pogatscher, S. Giant hardening response in AlMgZn(Cu) alloys. Acta Mater. 2021, 206, 116617. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.A.; Oberhauser, P.; Uggowitzer, P.J.; Pogatscher, S. Age-hardening response of AlMgZn alloys with Cu and Ag additions. Acta Mater. 2020, 195, 541–554. [Google Scholar] [CrossRef]
- Tang, H.-P.; Wang, Q.-D.; Luo, C.; Lei, C.; Liu, T.-W.; Li, Z.-Y.; Jiang, H.-Y.; Ding, W.-J.; Fang, J.; Zhang, J.-W. Effects of aging treatment on the precipitation behaviors and mechanical properties of Al-5.0Mg-3.0Zn-1.0Cu cast alloys. J. Alloys Compd. 2020, 842, 155707. [Google Scholar] [CrossRef]
- Daizen, W.; Chihiro, W.; Ryoichi, M.; Kazue, T. Ostwald ripening of Al3Sc particles in an Al-Mg-Sc alloy. Keikinzoku 2005, 55, 169–174. [Google Scholar]
- Zhu, X.; Ji, S. The formation of Al6 (Fe, Mn) phase in die-cast Al-Mg alloys. IOP Conf. Ser. Mater. Sci. Eng. 2019, 529, 012011. [Google Scholar] [CrossRef] [Green Version]
- Van Dalen, M.E.; Dunand, D.C.; Seidman, D.N. Effects of Ti additions on the nanostructure and creep properties of precipitation-strengthened Al–Sc alloys. Acta Mater. 2005, 53, 4225–4235. [Google Scholar] [CrossRef]
- Li, Y.J.; Arnberg, L. Quantitative study on the precipitation behavior of dispersoids in DC-cast AA3003 alloy during heating and homogenization. Acta Mater. 2003, 51, 3415–3428. [Google Scholar] [CrossRef]
- Zha, M.; Zhang, H.-M.; Meng, X.-T.; Jia, H.-L.; Jin, S.-B.; Sha, G.; Wang, H.-Y.; Li, Y.-J.; Roven, H.J. Stabilizing a severely deformed Al–7Mg alloy with a multimodal grain structure via Mg solute segregation. J. Mater. Sci. Technol. 2021, 89, 141–149. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Enikeev, N.A.; Murashkin, M.Y.; Kazykhanov, V.U.; Sauvage, X. On the origin of extremely high strength of ultrafine-grained Al alloys produced by severe plastic deformation. Scr. Mater. 2010, 63, 949–952. [Google Scholar] [CrossRef] [Green Version]
- Meng, C.; Zhang, D.; Cui, H.; Zhuang, L.; Zhang, J. Mechanical properties, intergranular corrosion behavior and microstructure of Zn modified Al–Mg alloys. J. Alloys Compd. 2014, 617, 925–932. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, D.; He, Z.; Zhuang, L.; Zhang, J. Enhanced and accelerated age hardening response of Al-5.2Mg-0.45Cu (wt%) alloy with Zn addition. Mater. Sci. Eng. A 2016, 666, 34–42. [Google Scholar] [CrossRef]
- Mondol, S.; Kumar, S.; Chattopadhyay, K. Effect of thermo-mechanical treatment on microstructure and tensile properties of 2219ScMg alloy. Mater. Sci. Eng. A 2019, 759, 583–593. [Google Scholar] [CrossRef]
- Ryen, Ø.; Holmedal, B.; Nijs, O.; Nes, E.; Sjölander, E.; Ekström, H.E. Strengthening mechanisms in solid solution aluminum alloys. Metall. Mater. Trans. A 2006, 37, 1999–2006. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, D.; Liu, H.; Zhuang, L.; Zhang, J. Precipitation hardening and intergranular corrosion behavior of novel Al-Mg-Zn(-Cu) alloys. J. Alloys Compd. 2021, 853, 157199. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, B.; Gao, M.; Zhao, E.; Guan, R. Microstructure evolution, mechanical property response and strengthening mechanism induced by compositional effects in Al–6Mg alloys. Mater. Des. 2022, 220, 110849. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, X.; Wang, H.; Li, Y.; Sun, B.; Zhang, D. Influence of Mg on tensile deformation behavior of high Mg-content Al-Mg alloys. Int. J. Plast. 2022, 157, 103405. [Google Scholar] [CrossRef]
- Shen, J.; Chen, B.; Wan, J.; Shen, J.; Li, J. Effect of annealing on microstructure and mechanical properties of an Al–Mg-Sc-Zr alloy. Mater. Sci. Eng. A 2022, 838, 142821. [Google Scholar] [CrossRef]
- Dong, B.; Xia, Y.; Cai, X.; Lin, S.; Fan, C. Addition of Sc in wire-based directed energy deposition of Al–Mg–Zn–Cu alloy: Microalloying to refine grains and improve mechanical properties. Addit. Manuf. 2023, 67, 103494. [Google Scholar] [CrossRef]
- Baek, M.-S.; Shah, A.W.; Kim, Y.-K.; Kim, S.-K.; Kim, B.-H.; Lee, K.-A. Microstructures, tensile properties, and strengthening mechanisms of novel Al-Mg alloys with high Mg content. J. Alloys Compd. 2023, 950, 169866. [Google Scholar] [CrossRef]
- Ma, K.; Wen, H.; Hu, T.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J.; Schoenung, J.M. Mechanical behavior and strengthening mechanisms in ultrafine grain precipitation-strengthened aluminum alloy. Acta Mater. 2014, 62, 141–155. [Google Scholar] [CrossRef]

| Elements | Mg | Zn | Sc | Mn | Cr | Ti | Fe | Si | Al |
|---|---|---|---|---|---|---|---|---|---|
| Al–Mg–Zn–Sc | 5.45 | 1.49 | 0.40 | 0.152 | 0.062 | 0.103 | 0.022 | 0.008 | Bal. |
| Sample | Lattice Parameter/nm | Crystal Indices | Solid Solubility/% | |
|---|---|---|---|---|
| (111) | (200) | |||
| Pure aluminum | d | 0.23379 | 0.20247 | / |
| a | 0.40494 | 0.40494 | ||
| Average value of a | 0.40494 | |||
| AC | d | 0.23513 | 0.20343 | 4.67 |
| a | 0.40726 | 0.40692 | ||
| Average value of a | 0.40709 | |||
| ST | d | 0.23529 | 0.20362 | 5.33 |
| a | 0.40753 | 0.40724 | ||
| Average value of a | 0.40739 | |||
| ST + AA | d | 0.23495 | 0.20337 | 4.15 |
| a | 0.40694 | 0.40675 | ||
| Average value of a | 0.40685 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Zhang, Z.; Bai, Y.; Mao, W. Effects of Heat Treatment on Microstructure and Mechanical Properties of Weldable Al–Mg–Zn–Sc Alloy with High Strength and Ductility. Materials 2023, 16, 5435. https://doi.org/10.3390/ma16155435
Jiang L, Zhang Z, Bai Y, Mao W. Effects of Heat Treatment on Microstructure and Mechanical Properties of Weldable Al–Mg–Zn–Sc Alloy with High Strength and Ductility. Materials. 2023; 16(15):5435. https://doi.org/10.3390/ma16155435
Chicago/Turabian StyleJiang, Long, Zhifeng Zhang, Yuelong Bai, and Weimin Mao. 2023. "Effects of Heat Treatment on Microstructure and Mechanical Properties of Weldable Al–Mg–Zn–Sc Alloy with High Strength and Ductility" Materials 16, no. 15: 5435. https://doi.org/10.3390/ma16155435




