Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing
Abstract
:1. Introduction
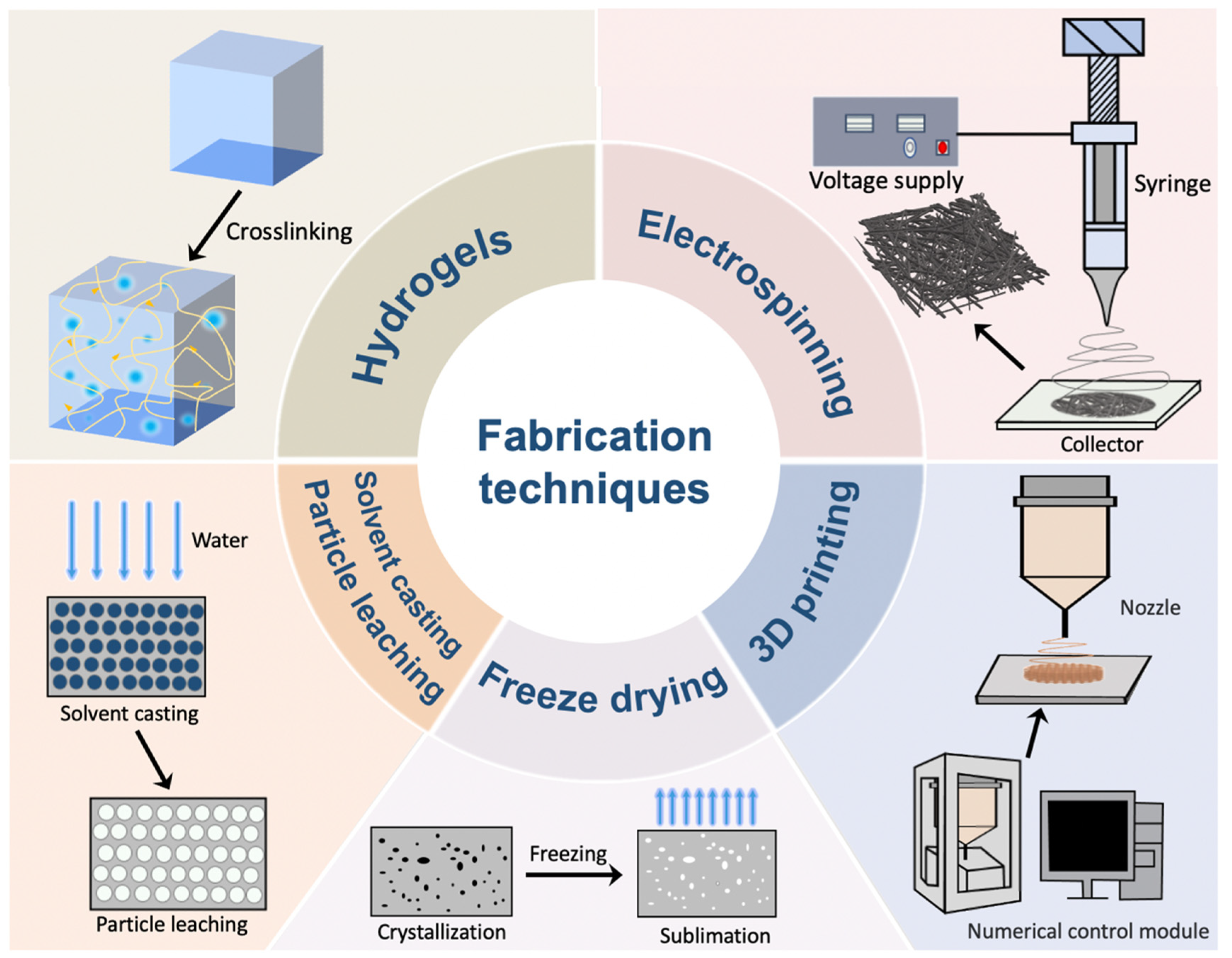
2. Fabrication Techniques Applied in Skin Wound Healing
2.1. Hydrogel Formation
2.2. Electrospinning
2.3. Freeze Drying
2.4. Solvent Casting/Particle Leaching
2.5. Three-Dimensional (3D) Printing
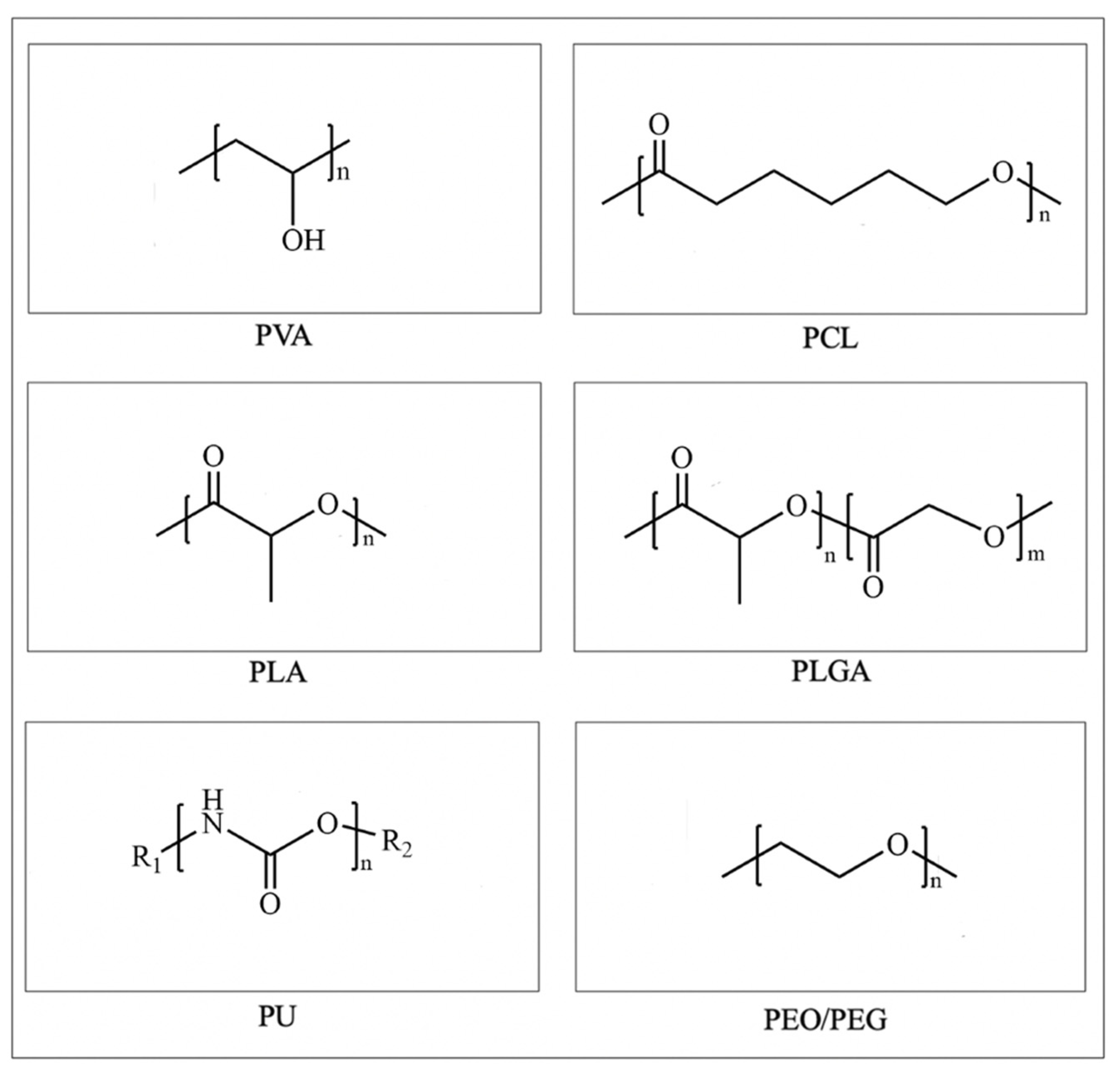
3. Synthetic Polymer-Based Skin Scaffolds
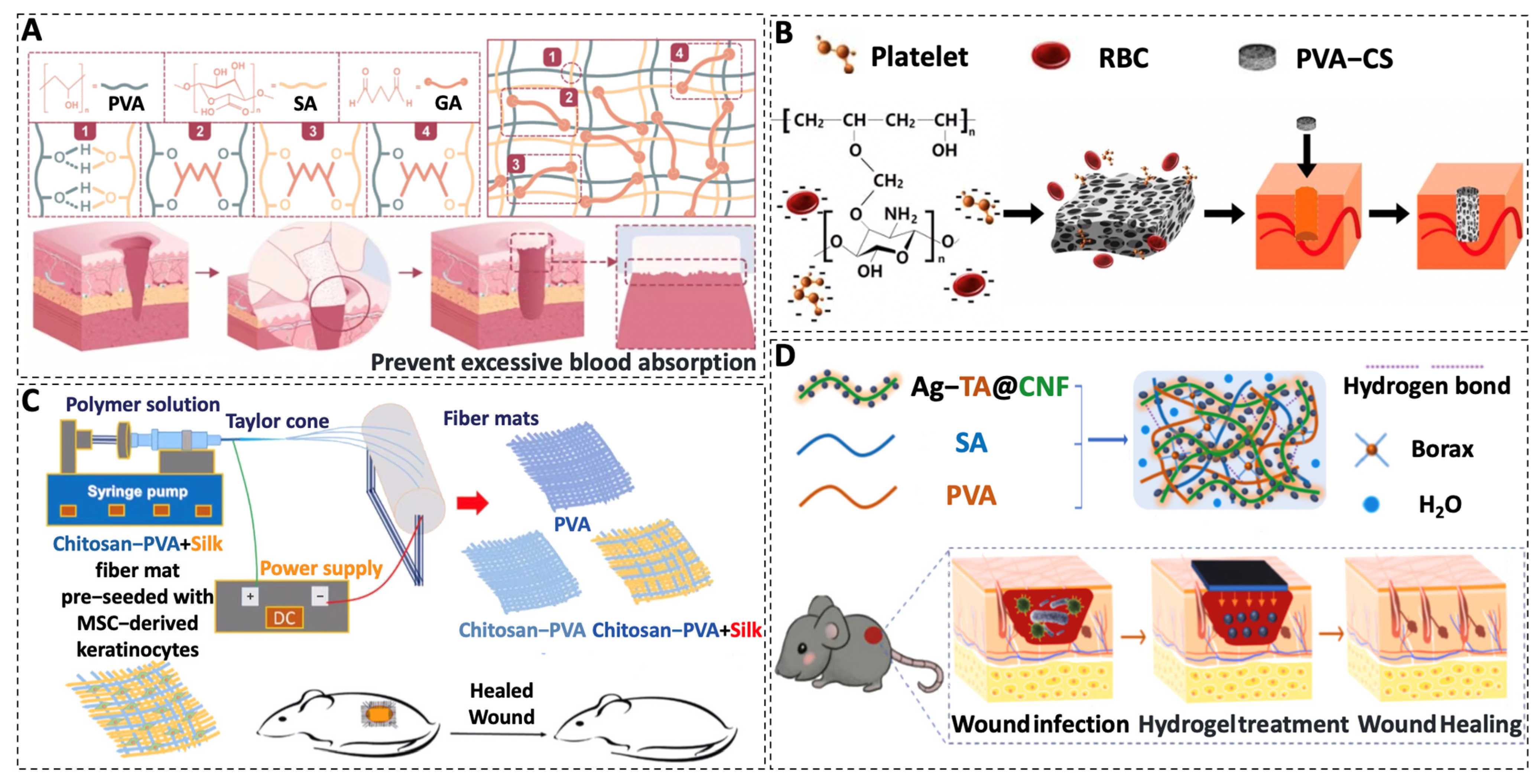
3.1. PVA
3.2. PCL
3.3. PLA
3.4. PLGA
3.5. PU
3.6. PEO/PEG
| Polymers | Form of Scaffold | Composition | Main Effects | Degradation Time | References |
|---|---|---|---|---|---|
| PVA | Sponge | PVA–SA | Improve hemostatic efficiency | - | [73] |
| Sponge | PVA–CS | Procoagulant, high biocompatibility, increased reepithelialization, and reduced granulation tissue | - | [74] | |
| Electrospun fibers | PVA–CS–SF | Beneficial to cell adhesion and proliferation and stimulate wound healing and skin tissue regeneration | At 16 days, the weight loss rate was nearly 70 percent | [75] | |
| Hydrogels | PVA–SA–ATC | Antibacterial to prevent wound infection, excellent biocompatibility, and absorb blood and tissue exudate | - | [77] | |
| Hydrogels | PVA–SA–Microspheres | Antibacterial and promote cell proliferation, epithelialisation, and collagen deposition | Degradation at the wound site occurred within 3 to 4 weeks | [83] | |
| Electrospun fibers | PCL–Collagen–EGF | Promote cell proliferation and differentiation and significantly up-regulate the expression of skin-related genes loricrin | The wound was degraded by 30% in 7 days | [93] | |
| Electrospun fibers | PCL–SF–CU | Strong mechanical strength accelerates wound healing | Close to complete degradation within 14 days | [94] | |
| PCL | Electrospun fibers | PCL–GT | Antibacterial and enhance fibroblast adhesion and proliferation | The nanofiber scaffolds retained their morphology after 30 days | [99] |
| Electrospun fibers | PCL–PVA–GT | Antibacterial and enhance cell proliferation | After 15 days, the hybrid nanofibers were slightly degraded and after 30 days the nanofibers were more expansive | [101] | |
| Electrospun fibers | PCL/Gel–PU/EEP | The outer layer is antibacterial and protects the wound while the inner layer promotes cell adhesion and proliferation | After 28 days, only 1.9% weight loss was observed on the PU/EEP membrane and 76% reduction in the PCL/gel scaffold | [102] | |
| PLA | Electrospun fibers | PLA–PCL–KGF | The rate of peripheral epithelial reformation, keratinocyte proliferation, and granulation reaction were improved | Degradation was observed between day 14 and 28 | [114] |
| Electrospun fibers | PLA–Gelatin | Enhance cell adhesion and proliferation | After ten weeks, there was significant weight damage | [115] | |
| Electrospun fibers | PLA–SF–Gelatin | Promote cell proliferation | Significant degradation occurred one month after subcutaneous implantation | [117] | |
| Electrospun fibers | PLA–Ibuprofen | Promote the growth and reproduction of human epidermal keratinocytes and human dermal fibroblasts and support angiogenesis | The scaffolds were slightly degraded after 14 days on the wound | [62] | |
| PLGA | 3D printed bilayer membrane | PLGA–Alginate | Increase neovascularization and promote collagen deposition | After four weeks, less than 60 percent of its weight remained | [125] |
| 3D printed bilayer membrane | PLGA–dECM | Inhibit collagen fiber deposition and angiogenesis and inhibit hypertrophic scar formation | After four weeks, the degradation rate was close to 70% | [104] | |
| Solvent cast nanocomposite films | PLGA–Ag | Antibacterial | The quality remained unchanged for 25 days and decreased significantly after 1 month | [133] | |
| Electrospun fibers | PLGA–CS | Promote fibroblast attachment and proliferation | At 56 days, mass loss is greater than 20% | [131] | |
| PU | Foams | PU–Lignin–Ag | Antibacterial and absorb wound exudate | In the alkali-methanol solution system, it was completely degraded from the original solid to the clarified solution at 60 °C for 5 h | [147] |
| Foams | PU–PTK | Promote extracellular matrix production and re-epithelialization and relieve inflammation | It was incubated in 20% H2O2/0.1M CoCl2 solution and completely degraded within 20 days | [105] | |
| PEO | Electrospun fibers | PEO–PCL/CS | Antibacterial and cytocompatible | The scaffolds were significantly degraded within 28 days and the earliest was 7 days | [155] |
| Hydrogels | PEO–CS–Cu | Antibacterial and promote cell adhesion | The membrane was degraded in vitro and most of the membrane was degraded after 30 days | [154] | |
| PEG | Hydrogels | PLGA–PEG–PLGA | Reduce inflammation, promote collagen deposition, and accelerate vascularization | - | [106] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K. Human wounds and its burden: An updated compendium of estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.W.; An, S.; Yoon, S.S.; Yarin, A.L. Advances in self-healing materials based on vascular networks with mechanical self-repair characteristics. Adv. Colloid. Interface Sci. 2018, 252, 21–37. [Google Scholar] [CrossRef]
- Tanaka, A.; Nagate, T.; Matsuda, H. Acceleration of wound healing by gelatin film dressings with epidermal growth factor. J. Vet. Med. Sci. 2005, 67, 909–913. [Google Scholar] [CrossRef] [Green Version]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Ousey, K.; Cutting, K.F.; Rogers, A.A.; Rippon, M.G. The importance of hydration in wound healing: Reinvigorating the clinical perspective. J. Wound Care 2016, 25, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural polymers-based materials: A contribution to a greener future. Molecules 2021, 27, 94. [Google Scholar] [CrossRef]
- Schink, B.; Janssen, P.H.; Frings, J. Microbial degradation of natural and of new synthetic polymers. FEMS Microbiol. Rev. 1992, 9, 311–316. [Google Scholar] [CrossRef]
- Ou, Y.; Tian, M. Advances in multifunctional chitosan-based self-healing hydrogels for biomedical applications. J. Mater. Chem. B 2021, 9, 7955–7971. [Google Scholar] [CrossRef]
- Naomi, R.; Ratanavaraporn, J.; Fauzi, M.B. Comprehensive review of hybrid collagen and silk fibroin for cutaneous wound healing. Materials 2020, 13, 3097. [Google Scholar] [CrossRef] [PubMed]
- Price, R.D.; Myers, S.; Leigh, I.M.; Navsaria, H.A. The role of hyaluronic acid in wound healing: Assessment of clinical evidence. Am. J. Clin. Dermatol. 2005, 6, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Katas, H.; Hussain, F. Recent advances in polymer-based wound dressings for the treatment of diabetic foot ulcer: An overview of state-of-the-art. Curr. Drug. Targets 2018, 19, 527–550. [Google Scholar] [CrossRef] [PubMed]
- Khew, S.T.; Yang, Q.J.; Tong, Y.W. Enzymatically crosslinked collagen-mimetic dendrimers that promote integrin-targeted cell adhesion. Biomaterials 2008, 29, 3034–3045. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Corrigan, N.; Wong, E.H.H.; Boyer, C. Bioactive synthetic polymers. Adv. Mater. 2022, 34, e2105063. [Google Scholar] [CrossRef]
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-based (bio)degradable polymers as environmentally friendly materials for sustainable development. Int. J. Mol. Sci. 2014, 16, 564–596. [Google Scholar] [CrossRef] [Green Version]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? the impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. Engl. 2019, 58, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Ma, P.X. Conducting polymers for tissue engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y. 3D bioprinting of skin: A state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 8, 032001. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [Green Version]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104 Pt A, 1003–1011. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research status of self-healing hydrogel for wound management: A review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef]
- Acharya, G.; Shin, C.S.; McDermott, M.; Mishra, H.; Park, H.; Kwon, I.C.; Park, K. The hydrogel template method for fabrication of homogeneous nano/microparticles. J. Control Release 2010, 141, 314–319. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, D.H.; Liu, B.H.; Nian, G.D.; Li, X.K.; Yin, J.; Qu, S.X.; Yang, W. 3D printing of multifunctional hydrogels. Adv. Funct. Mater. 2019, 29, 1900971. [Google Scholar] [CrossRef]
- Khaliq, T.; Sohail, M.; Minhas, M.U.; Ahmed Shah, S.; Jabeen, N.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M.; Rashid, H. Self-crosslinked chitosan/κ-carrageenan-based biomimetic membranes to combat diabetic burn wound infections. Int. J. Biol. Macromol. 2022, 197, 157–168. [Google Scholar] [CrossRef]
- Rusu, A.G.; Nita, L.E.; Simionescu, N.; Ghilan, A.; Chiriac, A.P.; Mititelu-Tartau, L. Enzymatically-crosslinked gelatin hydrogels with nanostructured architecture and self-healing performance for potential use as wound dressings. Polymers 2023, 15, 780. [Google Scholar] [CrossRef]
- Op’t Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F. Design Considerations for hydrogel wound dressings: Strategic and molecular advances. Tissue Eng. Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, P.; Yang, Z.; Liu, Y.; Yang, K.; Cheng, Y.; Yao, H.; Zhang, Z. Current progress and outlook of nano-based hydrogel dressings for wound healing. Pharmaceutics 2022, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel trends in hydrogel development for biomedical applications: A review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced hydrogels as wound dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- He, J.H.; Liu, Y.; Xu, L. Apparatus for preparing electrospun nanofibres: A comparative review. Mater. Sci. Technol. 2010, 26, 1275–1287. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Tian, L.; Wu, Z.; Wang, D.; Jiao, T. Preparation and antibacterial properties of a composite fiber membrane material loaded with cationic antibacterial agent by electrospinning. Nanomaterials 2023, 13, 583. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Boroujeni, S.M.; Omidvarkordshouli, N.; Soleimani, M. Advances in skin regeneration: Application of electrospun scaffolds. Adv. Healthcare Mater. 2015, 4, 1114–1133. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liao, S.; Ngiam, M.; Chan, C.K.; Ramakrishna, S. Degradation behaviors of electrospun resorbable polyester nanofibers. Tissue Eng. Part B Rev. 2009, 15, 333–351. [Google Scholar] [CrossRef]
- Xu, Z.; Zou, L.; Xie, F.; Zhang, X.; Ou, X.; Gao, G. Biocompatible carboxymethyl chitosan/go-based sponge to improve the efficiency of hemostasis and wound healing. ACS Appl. Mater. Interfaces 2022, 14, 44799–44808. [Google Scholar] [CrossRef]
- Keirouz, A.; Fortunato, G.; Zhang, M.; Callanan, A.; Radacsi, N. Nozzle-free electrospinning of Polyvinylpyrrolidone/Poly(glycerol sebacate) fibrous scaffolds for skin tissue engineering applications. Med. Eng. Phys. 2019, 71, 56–67. [Google Scholar] [CrossRef]
- SalehHudin, H.S.; Mohamad, E.N.; Mahadi, W.N.L.; Afifi, A.M. Multiple-jet electrospinning methods for nanofiber processing: A review. Mater. Manuf. Process. 2018, 33, 479–498. [Google Scholar] [CrossRef]
- Ward, K.R.; Matejtschuk, P. The principles of freeze-drying and application of analytical technologies. Methods Mol. Biol. 2021, 2180, 99–127. [Google Scholar]
- Abou-Saleh, R.H.; Delaney, A.; Ingram, N.; Batchelor, D.V.B.; Johnson, B.R.G.; Charalambous, A.; Bushby, R.J.; Peyman, S.A.; Coletta, P.L.; Markham, A.F.; et al. Freeze-dried therapeutic microbubbles: Stability and gas exchange. ACS Appl. Bio Mater. 2020, 3, 7840–7848. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Meredith, J.C. Assembly of chitin nanofibers into porous biomimetic structures via freeze drying. ACS Macro Lett. 2014, 3, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Zhou, J.P.; Xu, Y. Effect of freeze-drying on the properties of silk fibroin/polyvinyl alcohol/hydroxyapatite composites. Polym. Compos. 2019, 40, 3131–3139. [Google Scholar] [CrossRef]
- Shyna, S.; Nair, P.D.; Thomas, L.V. A nonadherent chitosan-polyvinyl alcohol absorbent wound dressing prepared via controlled freeze-dry technology. Int. J. Biol. Macromol. 2020, 150, 129–140. [Google Scholar]
- Thongtham, N.; Chai-in, P.; Unger, O.; Boonrungsiman, S.; Suwantong, O. Fabrication of chitosan/collagen/hydroxyapatite scaffolds with encapsulated Cissus quadrangularis extract. Polym. Adv. Technol. 2020, 31, 1496–1507. [Google Scholar] [CrossRef]
- Vaquette, C.; Frochot, C.; Rahouadj, R.; Wang, X. An innovative method to obtain porous PLLA scaffolds with highly spherical and interconnected pores. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 9–17. [Google Scholar] [CrossRef]
- Liao, C.J.; Chen, C.F.; Chen, J.H.; Chiang, S.F.; Lin, Y.J.; Chang, K.Y. Fabrication of porous biodegradable polymer scaffolds using a solvent merging/particulate leaching method. J. Biomed. Mater. Res. 2002, 59, 676–681. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Dogan, F.; Bal, B.S. Freeze-cast hydroxyapatite scaffolds for bone tissue engineering applications. Biomed. Mater. 2008, 3, 025005. [Google Scholar] [CrossRef]
- Wiemann, S.; Keck, C.M. Particle-assisted dermal penetration-a simple formulation strategy to foster the dermal penetration efficacy. Pharmaceutics 2022, 14, 1039. [Google Scholar] [CrossRef]
- Ghosal, K.; Chandra, A.; Roy, S.; Agatemor, C.; Thomas, S.; Provaznik, I. Electrospinning over solvent casting: Tuning of mechanical properties of membranes. Sci. Rep. 2018, 8, 5058. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Cha, R.; Li, J.; Hao, W.; Zhang, Y.; Zhou, F. Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydr. Polym. 2019, 224, 115144. [Google Scholar] [CrossRef] [PubMed]
- Atari, M.; Mohammadalizadeh, Z.; Kharazi, A.Z.; Javanmard, S.H. The effect of different solvent systems on physical properties of electrospun poly(glycerol sebacate)/poly(e-caprolactone) blend. Polym. Plast. Technol. Mater. 2022, 61, 789–802. [Google Scholar] [CrossRef]
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Lu, C.; Jian, Z.; Zhang, T.; Chen, Z.; Zhu, Q.; Tai, Z.; Liu, Y. 3D bioprinting for fabricating artificial skin tissue. Colloids Surf. B Biointerfaces 2021, 208, 112041. [Google Scholar] [CrossRef]
- Singh, D.; Singh, D.; Han, S.S. 3D printing of scaffold for cells delivery: Advances in skin tissue engineering. Polymers 2016, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Gan, S.; Wang, X.; Liu, W.; Li, X. Applications of 3D bioprinting in tissue engineering: Advantages, deficiencies, improvements, and future perspectives. J. Mater. Chem. B 2021, 9, 5385–5413. [Google Scholar] [CrossRef]
- You, S.; Xiang, Y.; Hwang, H.H.; Berry, D.B.; Kiratitanaporn, W.; Guan, J.; Yao, E.; Tang, M.; Zhong, Z.; Ma, X.; et al. High cell density and high-resolution 3D bioprinting for fabricating vascularized tissues. Sci. Adv. 2023, 9, eade7923. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Douroumis, D. Recent advances in 3D printing for wound healing: A systematic review. J. Drug Deliv. Sci. Technol. 2022, 74, 103564. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, H.J.; Chung, J.Y.; Lee, J.H.; Young, S.B.; Kim, Y.H. Natural and synthetic biomaterials for controlled drug delivery. Arch. Pharm. Res. 2014, 37, 60–68. [Google Scholar] [CrossRef]
- Ujjwal, R.R.; Yadav, A.; Tripathi, S.; Krishna, S. Polymer-based nanotherapeutics for burn wounds. Curr. Pharm. Biotechnol. 2022, 23, 1460–1482. [Google Scholar]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef] [PubMed]
- Mohiti-Asli, M.; Saha, S.; Murphy, S.V.; Gracz, H.; Pourdeyhimi, B.; Atala, A.; Loboa, E.G. Ibuprofen loaded PLA nanofibrous scaffolds increase proliferation of human skin cells in vitro and promote healing of full thickness incision wounds in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Wedmore, I.; McManus, J.G.; Pusateri, A.E.; Holcomb, J.B. A special report on the chitosan-based hemostatic dressing: Experience in current combat operations. J. Trauma 2006, 60, 655–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Guo, H. Research progress of polyvinyl alcohol water-resistant film materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Sun, J. Degradable poly(vinyl alcohol)-based supramolecular plastics with high mechanical strength in a watery environment. Adv. Mater. 2021, 33, e2007371. [Google Scholar] [CrossRef]
- Allen, M.J.; Schoonmaker, J.E.; Bauer, T.W.; Williams, P.F.; Higham, P.A.; Yuan, H.A. Preclinical evaluation of a poly (vinyl alcohol) hydrogel implant as a replacement for the nucleus pulposus. Spine 2004, 29, 515–523. [Google Scholar] [CrossRef]
- Wilkinson, A.C.; Ishida, R.; Kikuchi, M.; Sudo, K.; Morita, M.; Crisostomo, R.V.; Yamamoto, R.; Loh, K.M.; Nakamura, Y.; Watanabe, M.; et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature 2019, 571, 117–121. [Google Scholar] [CrossRef]
- Zhai, W.; Wang, Y.; Wang, X.; Wu, Q.; Li, K. Research Progress of Polyvinyl Alcohol Foam in Biomedicine. IOP Conf. Series Earth Environ. Sci. 2019, 295, 032078. [Google Scholar] [CrossRef]
- Jin, S.G. Production and application of biomaterials based on polyvinyl alcohol (pva) as wound dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef]
- Yin, Z.; Cao, J.; Li, Z.; Qiu, D. Optimizing the interaction between poly (vinyl alcohol) and sandy soil for enhanced water retention performance. RSC Adv. 2016, 6, 13377–13383. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cao, L.; Sang, F.; Zhang, B.; Meng, Z.; Pan, L.; Hao, J.; Yang, X.; Ma, Z.; Shi, C. Polyvinyl alcohol/sodium alginate composite sponge with 3D ordered/disordered porous structure for rapidly controlling noncompressible hemorrhage. Biomater. Adv. 2022, 134, 112698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Zhao, J.Y.; Hu, W.Z.; Ma, K.; Chao, Y.; Sun, P.J.; Fu, X.B.; Zhang, H. Synthetic poly(vinyl alcohol)-chitosan as a new type of highly efficient hemostatic sponge with blood-triggered swelling and high biocompatibility. J. Mater. Chem. B 2019, 7, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Khanmohammadi, M.; Goodarzi, A.; Foroutani, L.; Mobarakeh, Z.T.; Saremi, J.; Arabpour, Z.; Ai, J. Fabrication of chitosan-polyvinyl alcohol and silk electrospun fiber seeded with differentiated keratinocyte for skin tissue regeneration in animal wound model. J. Biol. Eng. 2020, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhu, J.; Inverarity, C.; Fang, Y.; Zhang, Z.; Ye, H.; Cui, Z.; Nguyen, L.; Wan, H.; Dye, J.F. Fabrication of fibrin/polyvinyl alcohol scaffolds for skin tissue engineering via emulsion templating. Polymers 2023, 15, 1151. [Google Scholar] [CrossRef]
- Liu, K.; Dai, L.; Li, C. A lignocellulose-based nanocomposite hydrogel with pH-sensitive and potent antibacterial activity for wound healing. Int. J. Biol. Macromol. 2021, 191, 1249–1254. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stanciuc, A.M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef]
- Hermosilla, J.; Pastene-Navarrete, E.; Acevedo, F. Electrospun fibers loaded with natural bioactive compounds as a biomedical system for skin burn treatment. A review. Pharmaceutics 2021, 13, 2054. [Google Scholar] [CrossRef]
- Su, W.Y.; Yang, Z.W.; Wang, H.Y.; Fang, J.; Li, C.L.; Lyu, G.J.; Li, H. Synergistic effect of sodium alginate and lignin on the properties of biodegradable poly(vinyl alcohol) mulch films. ACS Sustain. Chem. Eng. 2022, 10, 11800–11814. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Johnson, M.L.; Bilski, J.J.; Redmer, D.A.; Reynolds, L.P.; Abdullah, A.; Abdullah, K.M. Wound healing: The role of growth factors. Drugs Today 2003, 39, 787–800. [Google Scholar] [CrossRef]
- Legrand, J.M.D.; Martino, M.M. Growth factor and cytokine delivery systems for wound healing. Cold Spring Harb. Perspect. Biol. 2022, 14, a041234. [Google Scholar] [CrossRef]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGF-encapsulated microspheres for accelerated wound healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and recycling of films based on biodegradable polymers: A short review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Dhanaraju, M.D.; Gopinath, D.; Ahmed, M.R.; Jayakumar, R.; Vamsadhara, C. Characterization of polymeric poly(epsilon-caprolactone) injectable implant delivery system for the controlled delivery of contraceptive steroids. J. Biomed. Mater. Res. A 2006, 76, 63–72. [Google Scholar] [CrossRef]
- Ma, G.; Song, C.; Sun, H.; Yang, J.; Leng, X. A biodegradable levonorgestrel-releasing implant made of PCL/F68 compound as tested in rats and dogs. Contraception 2006, 74, 141–147. [Google Scholar] [CrossRef]
- Yao, Z.; Yuan, W.; Xu, J.; Tong, W.; Mi, J.; Ho, P.C.; Chow, D.H.K.; Li, Y.; Yao, H.; Li, X.; et al. Magnesium-encapsulated Injectable Hydrogel and 3D-engineered polycaprolactone conduit facilitate peripheral nerve regeneration. Adv. Sci. 2022, 9, e2202102. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The application of polycaprolactone in Three-dimensional printing scaffolds for bone tissue engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef]
- Morrison, R.J.; Hollister, S.J.; Niedner, M.F.; Mahani, M.G.; Park, A.H.; Mehta, D.K.; Ohye, R.G.; Green, G.E. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci. Transl. Med. 2015, 7, 285ra64. [Google Scholar] [CrossRef] [Green Version]
- Backes, E.H.; Harb, S.V.; Beatrice, C.A.G.; Shimomura, K.M.B.; Passador, F.R.; Costa, L.C.; Pessan, L.A. Polycaprolactone usage in additive manufacturing strategies for tissue engineering applications: A review. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1479–1503. [Google Scholar] [CrossRef] [PubMed]
- Gümüşderelioğlu, M.; Dalkıranoğlu, S.; Aydın, R.S.; Cakmak, S. A novel dermal substitute based on biofunctionalized electrospun PCL nanofibrous matrix. J. Biomed. Mater. Res. A 2011, 98, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, Y.; Rajinikanth, P.S.; Ranjan, S.; Tiwari, U.; Balasubramnaiam, J.; Pandey, P.; Arya, D.K.; Anand, S.; Deepak, P. Curcumin loaded polycaprolactone-/polyvinyl alcohol-silk fibroin based electrospun nanofibrous mat for rapid healing of diabetic wound: An in-vitro and in-vivo studies. Int. J. Biol. Macromol. 2021, 176, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-healing effects of curcumin and its nanoformulations: A comprehensive review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H. Towards the development of polycaprolactone based amphiphilic block copolymers: Molecular design, self-assembly and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 620–634. [Google Scholar] [CrossRef]
- Jafari, A.; Amirsadeghi, A.; Hassanajili, S.; Azarpira, N. Bioactive antibacterial bilayer PCL/gelatin nanofibrous scaffold promotes full-thickness wound healing. Int. J. Pharm. 2020, 583, 119413. [Google Scholar] [CrossRef]
- Moghe, A.; Hufenus, R.; Hudson, S.; Gupta, B. Effect of the addition of a fugitive salt on electrospinnability of poly (ε-caprolactone). Polymer 2009, 50, 3311–3318. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Development of nanofibrous scaffolds containing gum tragacanth/poly (ε-caprolactone) for application as skin scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 71–79. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Kargozar, S.; Bahrami, S.H.; Joghataei, M. Fabrication of curcumin-loaded gum tragacanth/poly (vinyl alcohol) nanofibers with optimized electrospinning parameters. J. Ind. Text. 2017, 46, 1170–1192. [Google Scholar] [CrossRef]
- Zarekhalili, Z.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Fabrication and characterization of PVA/Gum tragacanth/PCL hybrid nanofibrous scaffolds for skin substitutes. Int. J. Biol. Macromol. 2017, 94 Pt A, 679–690. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A novel bilayer wound dressing composed of a dense polyurethane/propolis membrane and a biodegradable polycaprolactone/gelatin nanofibrous scaffold. Sci. Rep. 2020, 10, 3063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Li, W.; Liu, Y.; Yang, Z.; Ma, L.; Zhuang, H.; Wang, E.; Wu, C.; Huan, Z.; Guo, F.; et al. Design of a biofluid-absorbing bioactive sandwich-structured Zn-Si bioceramic composite wound dressing for hair follicle regeneration and skin burn wound healing. Bioact. Mater. 2021, 6, 1910–1920. [Google Scholar] [CrossRef]
- Fang, Y.; Han, Y.; Wang, S.; Chen, J.; Dai, K.; Xiong, Y.; Sun, B. Three-dimensional printing bilayer membranous nanofiber scaffold for inhibiting scar hyperplasia of skin. Biomater. Adv. 2022, 138, 212951. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Russo, K.A.; McCune, J.T.; Pollins, A.C.; Cottam, M.A.; Dollinger, B.R.; DeJulius, C.R.; Gupta, M.K.; D’Arcy, R.; Colazo, J.M.; et al. Reactive oxygen species-degradable polythioketal urethane foam dressings to promote porcine skin wound repair. Sci. Transl. Med. 2022, 14, eabm6586. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-K.; Tang, J.-Y.; Yuan, Z.; Cai, C.-Y.; Chen, X.-B.; Cui, S.-Q.; Liu, P.; Yu, L.; Cai, K.-Y.; Ding, J.-D. Accelerated cutaneous wound healing using an injectable teicoplanin-loaded PLGA-PEG-PLGA thermogel dressing. Chin. J. Polym. Sci. 2019, 37, 548–559. [Google Scholar] [CrossRef]
- Gong, K.; Liu, H.; Huang, C.; Jiang, Q.; Xu, H.; Cao, Z.; Fuenmayor, E.; Major, I. Mass customization of polylactic acid (PLA) parts via a hybrid manufacturing process. Polymers 2022, 14, 5413. [Google Scholar] [CrossRef]
- Hammiche, D.; Boukerrou, A.; Azzeddine, B.; Guermazi, N.; Budtova, T. Characterization of polylactic acid green composites and its biodegradation in a bacterial environment. Int. J. Polym. Anal. Charact. 2019, 24, 236–244. [Google Scholar] [CrossRef]
- Swetha, T.A.; Ananthi, V.; Bora, A.; Sengottuvelan, N.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A review on biodegradable polylactic acid (PLA) production from fermentative food waste—Its applications and degradation. Int. J. Biol. Macromol. 2023, 234, 123703. [Google Scholar] [CrossRef]
- Ying, M.; Guo, C.; Hu, X. The quantitative relationship between isotopic and net contributions of lactate and glucose to the tricarboxylic acid (TCA) cycle. J. Biol. Chem. 2019, 294, 9615–9630. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug. Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhuang, X.; Tang, Z.; Chen, X. Polylactic acid (PLA): Research, development and industrialization. Biotechnol. J. 2010, 5, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Sundar, N.; Keerthana, P.; Kumar, S.A.; Kumar, G.A.; Ghosh, S. Dual purpose, bio-based polylactic acid (PLA)-polycaprolactone (PCL) blends for coated abrasive and packaging industrial coating applications. J. Polym. Res. 2020, 27, 386. [Google Scholar] [CrossRef]
- Kobsa, S.; Kristofik, N.J.; Sawyer, A.J.; Bothwell, A.L.; Kyriakides, T.R.; Saltzman, W.M. An electrospun scaffold integrating nucleic acid delivery for treatment of full-thickness wounds. Biomaterials 2013, 34, 3891–3901. [Google Scholar] [CrossRef] [Green Version]
- Hoveizi, E.; Nabiuni, M.; Parivar, K.; Rajabi-Zeleti, S.; Tavakol, S. Functionalisation and surface modification of electrospun polylactic acid scaffold for tissue engineering. Cell Biol. Int. 2014, 38, 41–49. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A. Electrospun nanofibers as carriers of microorganisms, stem cells, proteins, and nucleic acids in therapeutic and other applications. Front. Bioeng. Biotechnol. 2020, 8, 130. [Google Scholar] [CrossRef]
- Gui-Bo, Y.; You-Zhu, Z.; Shu-Dong, W.; De-Bing, S.; Zhi-Hui, D.; Wei-Guo, F. Study of the electrospun PLA/silk fibroin-gelatin composite nanofibrous scaffold for tissue engineering. J. Biomed. Mater. Res. A 2010, 93, 158–163. [Google Scholar] [CrossRef]
- Koziolová, E.; Venclíková, K.; Etrych, T. Polymer-drug conjugates in inflammation treatment. Physiol. Res. 2018, 67 (Suppl. S2), S281–S292. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug. Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Chegini, Z.; Ghaznavi-Rad, E.; Zare, E.N.; Hosseini, S.M. PLGA-based nanoplatforms in drug delivery for inhibition and destruction of microbial biofilm. Front. Cell. Infect. Microbiol. 2022, 12, 926363. [Google Scholar] [CrossRef]
- Brown, J.; Farquhar, C. An overview of treatments for endometriosis. JAMA 2015, 313, 296–297. [Google Scholar] [CrossRef] [PubMed]
- Tousi, M.S.; Sepehri, H.; Khoee, S.; Farimani, M.M.; Delphi, L.; Mansourizadeh, F. Evaluation of apoptotic effects of mPEG-b-PLGA coated iron oxide nanoparticles as a eupatorin carrier on DU-145 and LNCaP human prostate cancer cell lines. J. Pharm. Anal. 2021, 11, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, J.; Jin, K.; Liu, W.; Qiu, X.; Li, C. Fabrication of functional PLGA-based electrospun scaffolds and their applications in biomedical engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 1181–1194. [Google Scholar] [CrossRef]
- Wang, S.; Xiong, Y.; Chen, J.; Ghanem, A.; Wang, Y.; Yang, J.; Sun, B. Three dimensional printing bilayer membrane scaffold promotes wound healing. Front. Bioeng. Biotechnol. 2019, 7, 348. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Wang, Y.; Zhu, J.; Shen, X. Preparation of biodegradable poly (lactic-co-glycolic acid)/polyethyle-ne glycol copolymer and its application as a carrier for pigment epithelium-derived factor. Chin. J. Org. Chem. 2017, 37, 203. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Ma, Y.; Bao, Y.; Liu, Z.; Chen, L.; Dai, F.; Li, Z. Electrospun PLGA/SF/artemisinin composite nanofibrous membranes for wound dressing. Int. J. Biol. Macromol. 2021, 183, 68–78. [Google Scholar] [CrossRef]
- Aslan, S.; Loebick, C.Z.; Kang, S.; Elimelech, M.; Pfefferle, L.D.; Van Tassel, P.R. Antimicrobial biomaterials based on carbon nanotubes dispersed in poly(lactic-co-glycolic acid). Nanoscale 2010, 2, 1789–1794. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Taghavi Shahraki, B.; Rabiee, N.; Fatahi, Y.; Dinarvand, R.; Tavakolizadeh, M.; Ahmadi, S.; Rabiee, M.; Bagherzadeh, M.; Pourjavadi, A.; et al. Burgeoning polymer nano blends for improved controlled drug release: A review. Int. J. Nanomed. 2020, 15, 4363–4392. [Google Scholar] [CrossRef]
- Azzazy, H.M.E.; Fahmy, S.A.; Mahdy, N.K.; Meselhy, M.R.; Bakowsky, U. Chitosan-coated plga nanoparticles loaded with peganum harmala alkaloids with promising antibacterial and wound healing Activities. Nanomaterials 2021, 11, 2438. [Google Scholar] [CrossRef]
- Ajalloueian, F.; Tavanai, H.; Hilborn, J.; Donzel-Gargand, O.; Leifer, K.; Wickham, A.; Arpanaei, A. Emulsion electrospinning as an approach to fabricate PLGA/chitosan nanofibers for biomedical applications. Biomed. Res. Int. 2014, 2014, 475280. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Lee, J.; Hua, J.; Li, S.; Panchamukhi, A.; Yue, J.; Gou, X.; Xia, Z.; Zhu, L.; et al. A pulsatile release platform based on photo-induced imine-crosslinking hydrogel promotes scarless wound healing. Nat. Commun. 2021, 12, 1670. [Google Scholar] [CrossRef]
- Fortunati, E.; Latterini, L.; Rinaldi, S.; Kenny, J.M.; Armentano, I. PLGA/Ag nanocomposites: In vitro degradation study and silver ion release. J. Mater. Sci. Mater. Med. 2011, 22, 2735–2744. [Google Scholar] [CrossRef]
- Hu, H.; Tang, Y.; Pang, L.; Lin, C.; Huang, W.; Wang, D.; Jia, W. Angiogenesis and full-thickness wound healing efficiency of a copper-doped borate bioactive Glass/Poly(lactic-co-glycolic acid) dressing loaded with vitamin e in vivo and in vitro. ACS Appl. Mater. Interfaces 2018, 10, 22939–22950. [Google Scholar] [CrossRef]
- Wang, C.; Mu, C.; Lin, W.; Xiao, H. Functional-modified polyurethanes for rendering surfaces antimicrobial: An overview. Adv. Colloid. Interface Sci. 2020, 283, 102235. [Google Scholar] [CrossRef] [PubMed]
- Navas-Gómez, K.; Valero, M.F. Why polyurethanes have been used in the manufacture and design of cardiovascular devices: A systematic review. Materials 2020, 13, 3250. [Google Scholar] [CrossRef] [PubMed]
- Kütting, M.; Roggenkamp, J.; Urban, U.; Schmitz-Rode, T.; Steinseifer, U. Polyurethane heart valves: Past, present and future. Expert. Rev. Med. Devices 2011, 8, 227–233. [Google Scholar] [CrossRef]
- Marzec, M.; Kucińska-Lipka, J.; Kalaszczyńska, I.; Janik, H. Development of polyurethanes for bone repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 736–747. [Google Scholar] [CrossRef]
- Morales-González, M.; Díaz, L.E.; Dominguez-Paz, C.; Valero, M.F. Insights into the design of polyurethane dressings suitable for the stages of skin wound-healing: A systematic review. Polymers 2022, 14, 2990. [Google Scholar] [CrossRef] [PubMed]
- Schlottmann, F.; Obed, D.; Bingöl, A.S.; März, V.; Vogt, P.M.; Krezdorn, N. Treatment of complex wounds with novosorb(®) biodegradable temporising matrix (BTM)-a retrospective analysis of clinical outcomes. J. Pers. Med. 2022, 12, 2002. [Google Scholar] [CrossRef]
- Xie, J.-Q.; Yao, Y.-J.; Gao, C.-Y. Synthesis and properties of ROS-responsive biodegradable polyurethanes with unsaturated double bonds. Acta Polym. Sin. 2021, 52, 987–995. [Google Scholar]
- Lu, H.; Dun, C.; Jariwala, H.; Wang, R.; Cui, P.; Zhang, H.; Dai, Q.; Yang, S.; Zhang, H. Improvement of bio-based polyurethane and its optimal application in controlled release fertilizer. J. Control. Release 2022, 350, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Choi, S.M. Advances in waterborne polyurethane-based biomaterials for biomedical applications. Adv. Exp. Med. Biol. 2018, 1077, 251–283. [Google Scholar]
- Li, H.; Liang, Y.; Li, P.; He, C. Conversion of biomass lignin to high-value polyurethane: A review. J. Bioresour. Bioprod. 2020, 5, 163–179. [Google Scholar] [CrossRef]
- Usman, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Rehman, S.; Zia, F. Chitin and chitosan based polyurethanes: A review of recent advances and prospective biomedical applications. Int. J. Biol. Macromol. 2016, 86, 630–645. [Google Scholar] [CrossRef]
- Gondaliya, A.; Nejad, M. Lignin as a partial polyol replacement in polyurethane flexible foam. Molecules 2021, 26, 2302. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ma, X.; Qiu, S.; Chen, J.; Lu, G.; Jia, Z.; Zhu, J.; Yang, Q.; Chen, J.; et al. Antimicrobial lignin-based polyurethane/ag composite foams for improving wound healing. Biomacromolecules 2022, 23, 1622–1632. [Google Scholar] [CrossRef]
- Ceonzo, K.; Gaynor, A.; Shaffer, L.; Kojima, K.; Vacanti, C.A.; Stahl, G.L. Polyglycolic acid-induced inflammation: Role of hydrolysis and resulting complement activation. Tissue Eng. 2006, 12, 301–308. [Google Scholar] [CrossRef]
- Nakajima-Kambe, T.; Shigeno-Akutsu, Y.; Nomura, N.; Onuma, F.; Nakahara, T. Microbial degradation of polyurethane, polyester polyurethanes and polyether polyurethanes. Appl. Microbiol. Biotechnol. 1999, 51, 134–140. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic acid: A key ingredient in the therapy of inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, J.; Ou, Y.; He, X.; Wang, Y.; Zou, C.; Jiang, Y.; Luo, F.; Lu, D.; Li, Z.; et al. Shape-recoverable hyaluronic acid-waterborne polyurethane hybrid cryogel accelerates hemostasis and wound healing. ACS Appl. Mater. Interfaces 2022, 14, 17093–17108. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Zhu, J.; Yan, N. Lignin-based polyurethane: Recent advances and future perspectives. Macromol. Rapid Commun. 2021, 42, e2000492. [Google Scholar] [CrossRef]
- Qian, Q.; Zhu, L.; Zhu, X.; Sun, M.; Yan, D. Drug-polymer hybrid macromolecular engineering: Degradable PEG integrated by platinum (IV) for cancer therapy. Matter 2019, 1, 1618–1630. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.K.; Mary, D.S.; Kannan, S. Copper incorporated microporous chitosan-polyethylene glycol hydrogels loaded with naproxen for effective drug release and anti-infection wound dressing. Int. J. Biol. Macromol. 2017, 95, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Asghari, F.; Rabiei Faradonbeh, D.; Malekshahi, Z.V.; Nekounam, H.; Ghaemi, B.; Yousefpoor, Y.; Ghanbari, H.; Faridi-Majidi, R. Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydr. Polym. 2022, 278, 118926. [Google Scholar] [CrossRef] [PubMed]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug. Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Judge, A.; McClintock, K.; Phelps, J.R.; Maclachlan, I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol. Ther. 2006, 13, 328–337. [Google Scholar] [CrossRef]
- Hu, Z.; Cao, X.; Zhang, X.; Wu, B.; Luo, W.; Huang, H.; Li, L.; Chen, Y. Catalytically controlled ring-opening polymerization of 2-oxo-15-crown-5 for degradable and recyclable peg-like polyesters. ACS Macro Lett. 2022, 11, 792–798. [Google Scholar] [CrossRef]
- Golba, B.; Soete, M.; Zhong, Z.; Sanders, N.; Du Prez, F.E.; Houck, H.A.; De Geest, B.G. Visible light conjugation with triazolinediones as a route to degradable poly(ethylene glycol)-lipids for mrna lipid nanoparticle formulation. Angew. Chem. Int. Ed. Engl. 2023, 62, e202301102. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, A.; Yuan, C.; Chen, X.; Liu, Y. Recent trends on burn wound care: Hydrogel dressings and scaffolds. Biomater. Sci. 2021, 9, 4523–4540. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Feng, Z.; Han, B.; Yu, D.G.; Wang, K. Advances in the preparation of nanofiber dressings by electrospinning for promoting diabetic wound healing. Biomolecules 2022, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.J.; Tse, J.W.; Zhou, Y.; Mao, W.; Yim, E.K.F.; Yoo, H.S. Biomaterials and controlled release strategy for epithelial wound healing. Biomater. Sci. 2019, 7, 4444–4471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Long, L.; Hu, C.; Kong, Q.; Wang, Y. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Control. Release 2022, 341, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ou, X.; Guan, L.; Li, X.; Liu, A.; Li, L.; Zvyagin, A.V.; Qu, W.; Yang, B.; Lin, Q. Pomegranate-inspired multifunctional nanocomposite wound dressing for intelligent self-monitoring and promoting diabetic wound healing. Biosens. Bioelectron. 2023, 235, 115386. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, M.; Xu, T.; Zhang, X. Multifunctional hydrogel as wound dressing for intelligent wound monitoring. Chem. Eng. J. 2022, 433, 134625. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H.; Guo, M.; Chu, J.; Gao, B.; He, B. Personalized and programmable microneedle dressing for promoting wound healing. Adv. Healthcare Mater. 2022, 11, e2101659. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Fang, Y.; Zhang, Z.; Cao, Y.; Yan, Y.; Gan, L.; Xu, J.; Zhou, G. Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing. Materials 2023, 16, 5459. https://doi.org/10.3390/ma16155459
Xu R, Fang Y, Zhang Z, Cao Y, Yan Y, Gan L, Xu J, Zhou G. Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing. Materials. 2023; 16(15):5459. https://doi.org/10.3390/ma16155459
Chicago/Turabian StyleXu, Ruojiao, Yifeng Fang, Zhao Zhang, Yajie Cao, Yujia Yan, Li Gan, Jinbao Xu, and Guoying Zhou. 2023. "Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing" Materials 16, no. 15: 5459. https://doi.org/10.3390/ma16155459
APA StyleXu, R., Fang, Y., Zhang, Z., Cao, Y., Yan, Y., Gan, L., Xu, J., & Zhou, G. (2023). Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing. Materials, 16(15), 5459. https://doi.org/10.3390/ma16155459









