X-ray Photoelectron Spectroscopy Analysis of Scandia-Ceria-Stabilized Zirconia Composites with Different Transport Properties
Abstract
1. Introduction
2. Materials and Methods
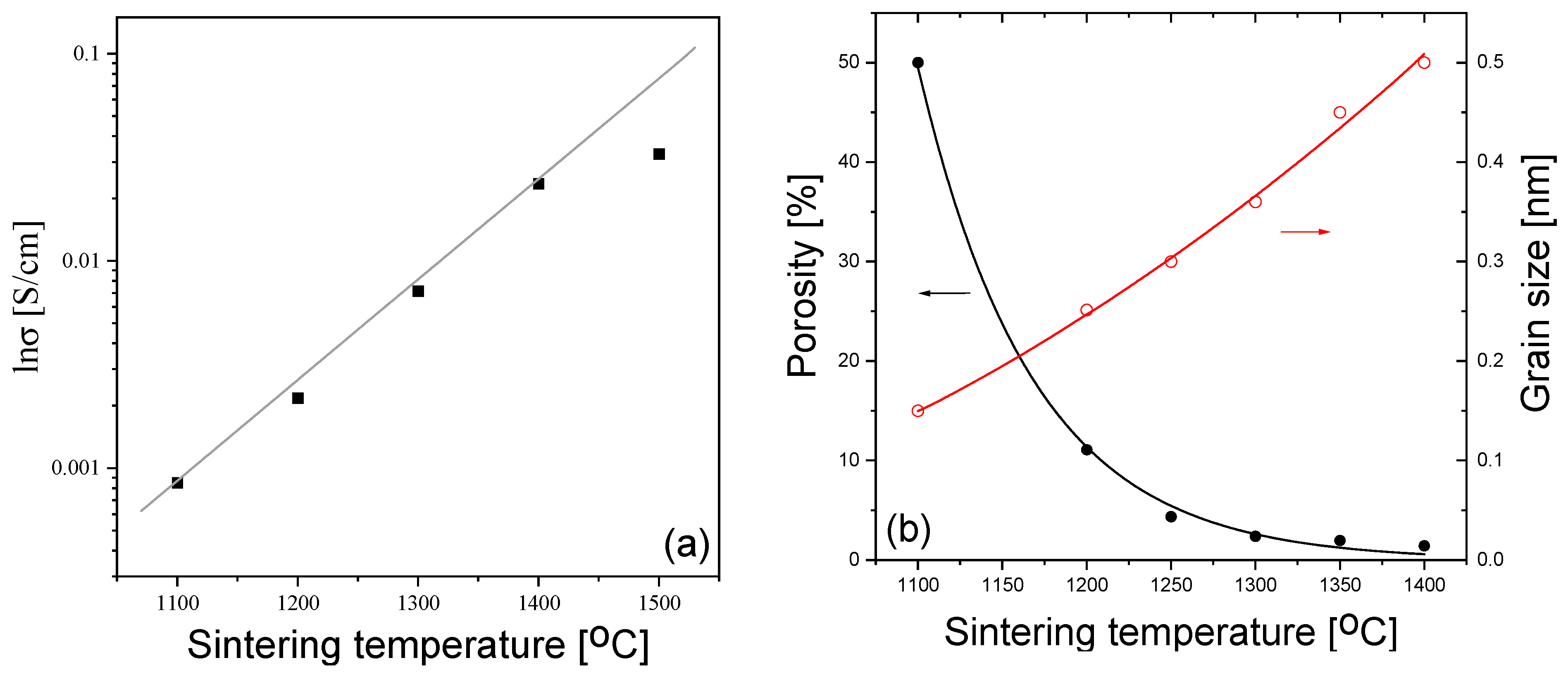
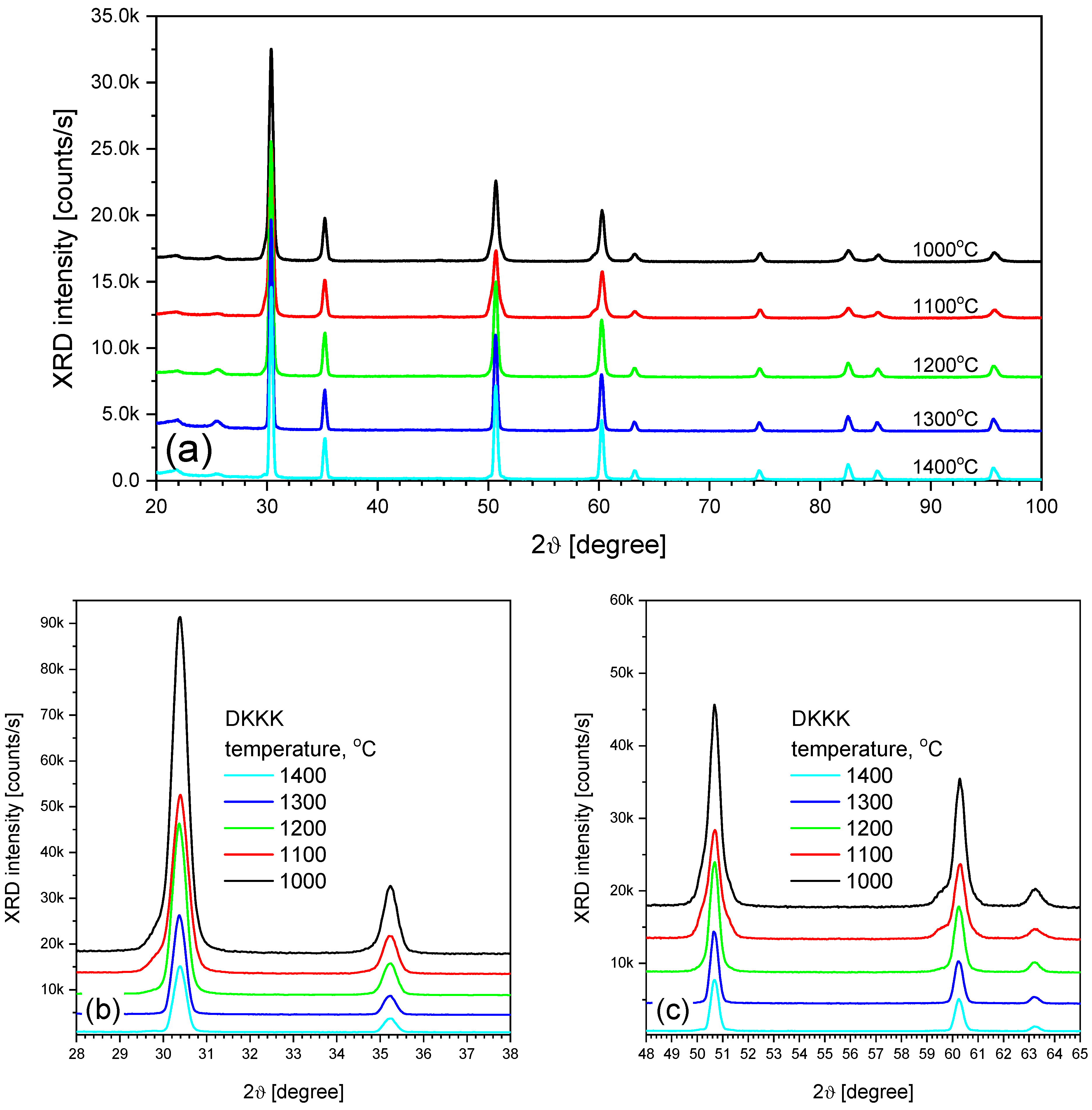
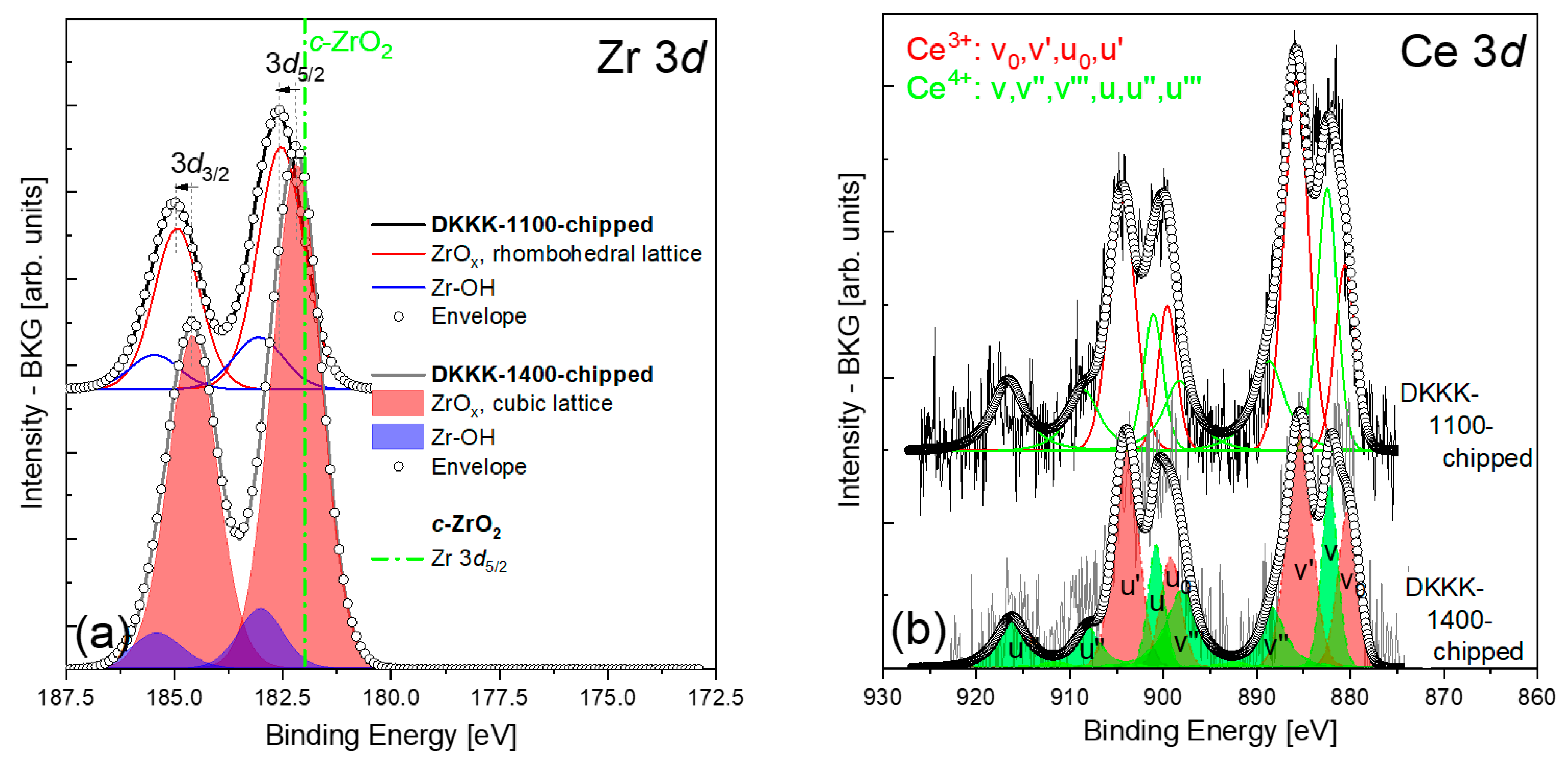
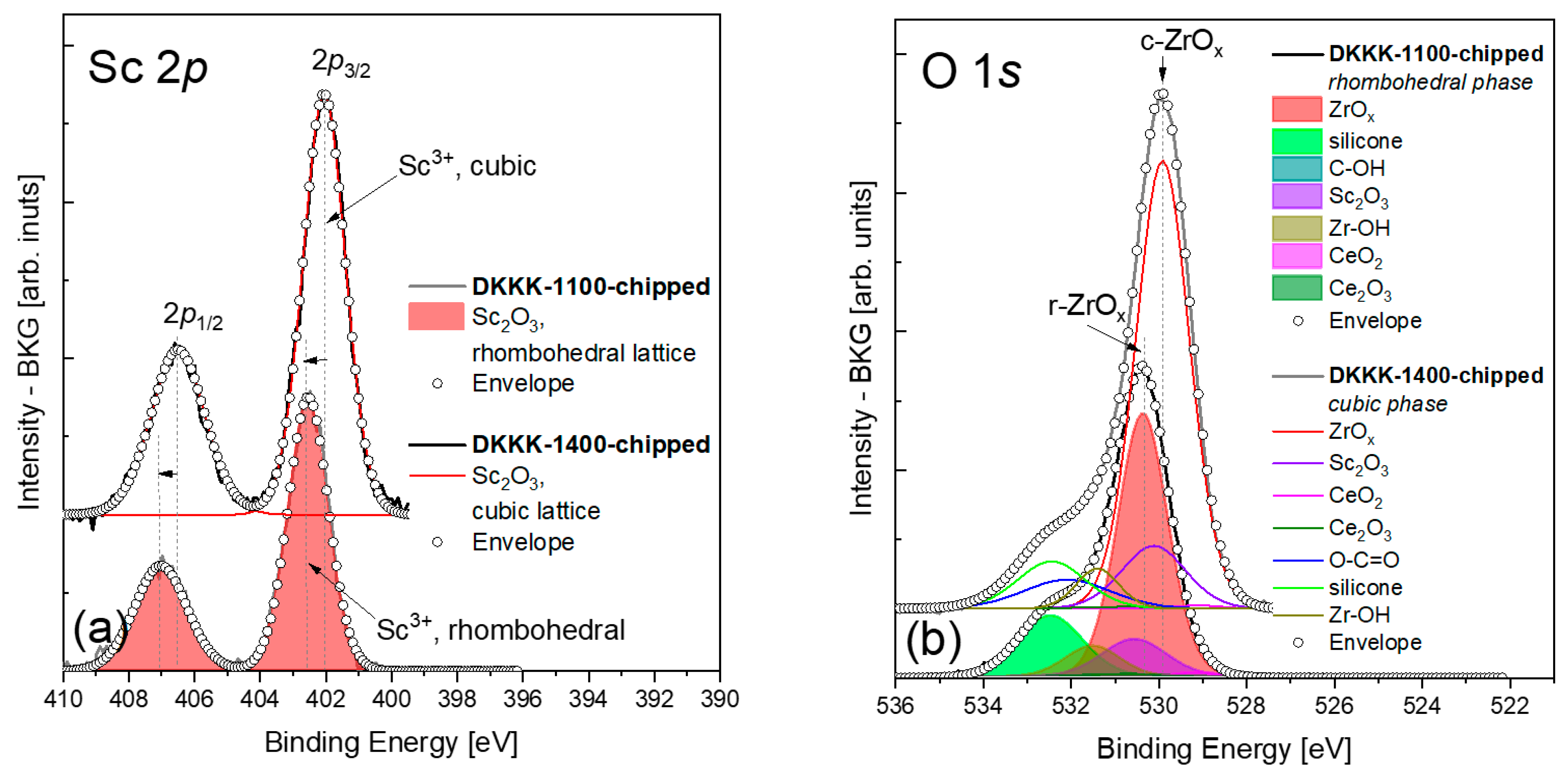
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Dwivedi, S. Solid oxide fuel cell: Materials for anode, cathode and electrolyte. Int. J. Hydrogen Energy 2020, 45, 23988–24013. [Google Scholar] [CrossRef]
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Sajid, A.; Pervaiz, E.; Ali, H.; Noor, T.; Baig, M.M. A perspective on development of fuel cell materials: Electrodes and electrolyte. Int. J. Energy Res. 2022, 46, 6953–6988. [Google Scholar] [CrossRef]
- Terauchi, S.; Takizawa, H.; Endo, T.; Uchida, S.; Terui, T.; Shimada, M. High ionic conductivity and high fracture strength of cubic zirconia, (Y0.16−xScx)Zr0.84O1.92, alumina composites. Mater. Lett. 1995, 23, 273–275. [Google Scholar] [CrossRef]
- Chiba, R.; Yoshimura, F.; Yamaki, J.; Ishii, T.; Yonezawa, T.; Endou, K. Ionic conductivity and morphology in Sc2O3 and Al2O3 doped ZrO2 films prepared by the sol-gel method. Solid State Ion. 1997, 104, 259–266. [Google Scholar] [CrossRef]
- Yamamura, H.; Utsunomiya, N.; Mori, T.; Atake, T. Electrical conductivity in the system ZrO2-Y2O3-Sc2O3. Solid State Ion. 1998, 107, 185–189. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Ciacchi, F.T.; Milosevic, D. Scandia-zirconia electrolytes for intermediate temperature solid oxide fuel cell operation. Solid State Ion. 2000, 136–137, 91–99. [Google Scholar] [CrossRef]
- Nomura, K.; Mizutani, Y.; Kawai, M.; Nakamura, Y.; Yamamoto, O. Aging and Raman scattering study of scandia and yttria doped zirconia. Solid State Ion. 2000, 132, 235–239. [Google Scholar] [CrossRef]
- Arachi, Y.; Asai, T.; Yamamoto, O.; Takeda, Y.; Imanishi, N.; Kawate, K.; Tamakoshi, C. Electrical Conductivity of ZrO2-Sc2O3 Doped with HfO2, CeO2, and Ga2O3. J. Electrochem. Soc. 2001, 148, A520. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, W.S.; Choi, S.H.; Kim, J.; Lee, H.W.; Lee, J.H. Characterization of ZrO2 co-doped with Sc2O3 and CeO2 electrolyte for the application of intermediate temperature SOFCs. Solid State Ion. 2005, 176, 33–39. [Google Scholar] [CrossRef]
- Du, K.; Kim, C.H.; Heuer, A.H.; Goettler, R.; Liu, Z. Structural Evolution and Electrical Properties of Sc2O3-Stabilized ZrO2 Aged at 850 °C in Air and Wet-Forming Gas Ambients. J. Am. Ceram. Soc. 2008, 91, 1626–1633. [Google Scholar] [CrossRef]
- Dasari, H.P.; Ahn, J.S.; Ahn, K.; Park, S.-Y.; Hong, J.; Kim, H.; Yoon, K.J.; Son, J.-W.; Lee, H.-W.; Lee, J.-H. Synthesis, sintering and conductivity behavior of ceria-doped Scandia-stabilized zirconia. Solid State Ion. 2014, 263, 103–109. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, K.; Singh, A. A Study on the Present Status of Zirconia based Electrolytes for Solid Oxide Fuel Cell. Indian J. Pure Appl. Phys. 2016, 4, 23–26. [Google Scholar]
- Kumar, A.; Jaiswal, A.; Sanbui, M.; Omar, S. Oxygen-Ion Conduction in Scandia-Stabilized Zirconia-Ceria Solid Electrolyte (xSc2O3–1CeO2–(99 − x)ZrO2, 5 ≤ x ≤ 11). J. Am. Ceram. Soc. 2017, 100, 659–668. [Google Scholar] [CrossRef]
- Xue, Q.N.; Wang, L.G.; Huang, X.W.; Zhang, J.X.; Zhang, H. Influence of codoping of Sc-doped zirconia by first-principles calculations and experiments. Mater. Des. 2018, 160, 131–137. [Google Scholar] [CrossRef]
- Fabris, S.; Paxton, A.T.; Finnis, M.W. A stabilization mechanism of zirconia based on oxygen vacancies only. Acta Mater. 2002, 50, 5171–5178. [Google Scholar] [CrossRef]
- Molenda, J.; Swierczek, K.; Zajac, W. Functional materials for the IT-SOFC. J. Power Sources 2007, 173, 657–670. [Google Scholar] [CrossRef]
- Hearing, C.; Roosen, A.; Schichl, H. Degradation of the electrical conductivity in stabilised zirconia systems: Part I: Yttria-stabilised zirconia. Solid State Ion. 2005, 176, 253–259. [Google Scholar] [CrossRef]
- Chen, Y.; Orlovskaya, N.; Payzant, E.A.; Graule, T.; Kuebler, J. A search for temperature induced time-dependent structural transitions in 10 mol% Sc2O3–1 mol% CeO2–ZrO2 and 8 mol% Y2O3–ZrO2 electrolyte ceramics. J. Eur. Ceram. Soc. 2015, 35, 951–958. [Google Scholar] [CrossRef]
- Wang, X.; Kim, J.S.; Atkinson, A. Constrained sintering of 8 mol% Y2O3 stabilised zirconia films. J. Eur. Ceram. Soc. 2012, 32, 4121–4128. [Google Scholar] [CrossRef]
- Mæland, D.; Suciu, C.; Wærnhus, I.; Hoffmann, A.C. Sintering of 4YSZ (ZrO2 + 4 mol% Y2O3) nanoceramics for solid oxide fuel cells (SOFCs), their structure and ionic conductivity. J. Eur. Ceram. Soc. 2009, 29, 2537–2547. [Google Scholar] [CrossRef]
- Hearing, C.; Roosen, A.; Schichl, H.; Schnöller, M. Degradation of the electrical conductivity in stabilized zirconia system: Part II: Scandia-stabilised zirconia. Solid State Ion. 2005, 176, 261–268. [Google Scholar] [CrossRef]
- Yarmolenko, S.; Sankar, J.; Bernier, N.; Klimov, M.; Kapat, J.; Orlovskaya, N. Phase Stability and Sintering Behavior of 10 mol% Sc2O3-1 mol% CeO2-ZrO2 Ceramics. J. Fuel Cell Sci. Technol. 2009, 6, 021007. [Google Scholar] [CrossRef]
- Vasylyev, O.; Smirnova, A.; Brychevskyi, M.; Brodnikovskyi, I.M.; Firstov, S.; Vereshchak, V.; Vereschak, V.; Akimov, G.; Komysa, Y.; Irvine, J.; et al. Structural, mechanical, and electrochemical properties of ceria doped scandia stabilized zirconia. Mater. Sci. Nanostruct. 2011, 1, 70–80. [Google Scholar]
- Jais, A.A.; Ali, S.A.M.; Anwar, M.; Somalu, M.R.; Muchtar, A.; Isahak, W.N.R.W.; Tan, C.Y.; Singh, R.; Brandon, N.P. Enhanced ionic conductivity of scandia-ceria-stabilized-zirconia (10Sc1CeSZ) electrolyte synthesized by the microwave-assisted glycine nitrate process. Ceram. Int. 2017, 43, 8119–8125. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, A.; Yan, Z.; Xu, G.; Liao, C.; Yan, C. (ZrO2)0.85(REO1.5)0.15 (RE=Sc, Y) solid solutions prepared via three Pechini-type gel routes: 2—Sintering and electrical properties. J. Solid State Chem. 2003, 171, 439–443. [Google Scholar] [CrossRef]
- Brodnikovska, I.; Korsunska, N.; Khomenkova, L.; Polishchuk, Y.; Lavoryk, S.; Brychevskyi, M.; Brodnikovskyi, Y.; Vasylyev, O. Grains, grain boundaries and total ionic conductivity of 10Sc1CeSZ and 8YSZ solid electrolytes affected by crystalline structure and dopant content. Mater. Today Proc. 2019, 6, 79–85. [Google Scholar] [CrossRef]
- Brodnikovskyi, Y.; McDonald, N.; Brodnikovskyi, D.; Brodnikovska, I.; Brychevskyi, M.; Kovalenko, L.; Vasylyev, O.; Belous, A.; Steinberger-Wilckens, R. Properties of 10Sc1CeSZ-3.5YSZ (33-, 40-, 50-wt.%) Composite Ceramics for SOFC Application. Mater. Today Proc. 2019, 6, 26–35. [Google Scholar] [CrossRef]
- Fairley, N. Software Package for the Analysis of XPS Results, CasaXPS Version 2.3.17dev6.6o, Casa Software Ltd. 2016. Available online: http://www.casaxps.com (accessed on 2 April 2023).
- Demchenko, I.N.; Lisowski, W.; Syryanyy, Y.; Melikhov, Y.; Zaytseva, I.; Konstantynov, P.; Chernyshova, M.; Cieplak, M.Z. Use of XPS to clarify the Hall coefficient sign variation in thin niobium layers buried in silicon. Appl. Surf. Sci. 2017, 399, 32–40. [Google Scholar] [CrossRef]
- Licata, O.G.; Zhu, M.; Hwang, J.; Mazumder, B. Nanoscale chemistry and ion segregation in zirconia-based ceramics at grain boundaries by atom probe tomography. Scr. Mater. 2022, 213, 114603. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Guo, X. Low Temperature Stability of Cubic Zirconia. Phys. Stat. Sol. (A) 2000, 177, 191–201. [Google Scholar] [CrossRef]
- Ingo, G.M.; Paparazzo, E.; Bagnarelli, O.; Zacchetti, N. XPS studies on cerium, zirconium and yttrium valence states in plasma-sprayed coatings. Surf. Interface Anal. 1990, 16, 515–519. [Google Scholar] [CrossRef]
- Pfau, A.; Schierbaum, K.D. The electronic structure of stoichiometric and reduced CeO2 surfaces: An XPS, UPS and HREELS study. Surf. Sci. 1994, 321, 71–80. [Google Scholar] [CrossRef]
- Ram, S.; Mondal, A. X-ray photoelectron spectroscopic studies of Al3+ stabilized t-ZrO2 of nanoparticles. Appl. Sur. Sci. 2004, 221, 237–247. [Google Scholar] [CrossRef]
- Powell, C.J.; Jablonski, A. NIST Electron Effective-Attenuation-Length Database; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011.
- NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000. [CrossRef]
- The International XPS Database of XPS Reference Spectra. Available online: https://xps-database.com/ (accessed on 2 April 2023).
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Guo, X. On the degradation of zirconia ceramics during low-temperature annealing in water or water vapor. J. Phys. Chem. Solids 1999, 60, 539–546. [Google Scholar] [CrossRef]
- Merle-Méjean, T.; Barberis, P.; Othmane, S.B.; Nardou, F.; Quintard, P.E. Chemical forms of hydroxyls on/in Zirconia: An FT-IR study. J. Eur. Ceram. Soc. 1998, 18, 1579–1586. [Google Scholar] [CrossRef]
- Hüfner, S. Photoelectron Spectroscopy: Principles and Applications; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Kotani, A.; Jo, T.; Parlebas, J.C. Many-body effects in core-level spectroscopy of rare-earth compounds. Adv. Phys. 1988, 37, 37–85. [Google Scholar] [CrossRef]
- Paparazzo, E. Use and mis-use of X-ray photoemission spectroscopy Ce3d spectra of Ce2O3 and CeO2. J. Phys. Condens. Matter 2018, 30, 343003, Corrected in J. Phys. Condens. Matter 2020, 32, 099501. [Google Scholar] [CrossRef]
- Thermo Scientific Avantage Data System for XPS, Materials Science Learning System. Available online: https://www.thermofisher.com/pl/en/home/materials-science/learning-center/periodic-table.html (accessed on 2 April 2023).
- X-ray Photoelectron Spectroscopy (XPS) Reference Pages. Available online: http://www.xpsfitting.com (accessed on 2 April 2023).
- XPS (X-ray Photoelectron Spectroscopy) Database. Available online: http://techdb.podzone.net/eindex.html (accessed on 2 April 2023).
| ZrOx, at% | Zr-OH, at% | Ce2O3, at% | CeO2, at% | Sc2O3, at% | C-C, at% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Considered Line | Zr 3d | O 1s | Zr 3d | O 1s | Ce 3d | O 1s | Ce 3d | O 1s | Sc 2p | O 1s | C 1s |
| DKKK-1100 (R) | 21.91 | 40.06 | 5.20 | 5.19 | 0.44 | 0.65 | 0.31 | 0.62 | 5.09 | 7.66 | 12.77 |
| DKKK-1400 (C) | 26.01 | 39.05 | 2.80 | 2.79 | 0.17 | 0.27 | 0.11 | 0.22 | 4.42 | 6.58 | 17.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demchenko, I.N.; Nikiforow, K.; Chernyshova, M.; Melikhov, Y.; Syryanyy, Y.; Korsunska, N.; Khomenkova, L.; Brodnikovskyi, Y.; Brodnikovskyi, D. X-ray Photoelectron Spectroscopy Analysis of Scandia-Ceria-Stabilized Zirconia Composites with Different Transport Properties. Materials 2023, 16, 5504. https://doi.org/10.3390/ma16165504
Demchenko IN, Nikiforow K, Chernyshova M, Melikhov Y, Syryanyy Y, Korsunska N, Khomenkova L, Brodnikovskyi Y, Brodnikovskyi D. X-ray Photoelectron Spectroscopy Analysis of Scandia-Ceria-Stabilized Zirconia Composites with Different Transport Properties. Materials. 2023; 16(16):5504. https://doi.org/10.3390/ma16165504
Chicago/Turabian StyleDemchenko, Iraida N., Kostiantyn Nikiforow, Maryna Chernyshova, Yevgen Melikhov, Yevgen Syryanyy, Nadiia Korsunska, Larysa Khomenkova, Yehor Brodnikovskyi, and Dmytro Brodnikovskyi. 2023. "X-ray Photoelectron Spectroscopy Analysis of Scandia-Ceria-Stabilized Zirconia Composites with Different Transport Properties" Materials 16, no. 16: 5504. https://doi.org/10.3390/ma16165504
APA StyleDemchenko, I. N., Nikiforow, K., Chernyshova, M., Melikhov, Y., Syryanyy, Y., Korsunska, N., Khomenkova, L., Brodnikovskyi, Y., & Brodnikovskyi, D. (2023). X-ray Photoelectron Spectroscopy Analysis of Scandia-Ceria-Stabilized Zirconia Composites with Different Transport Properties. Materials, 16(16), 5504. https://doi.org/10.3390/ma16165504








