Study of Electroless-Deposited Zn on the Surface of Mg-Li Alloy
Abstract
:1. Introduction
2. Materials and Methods
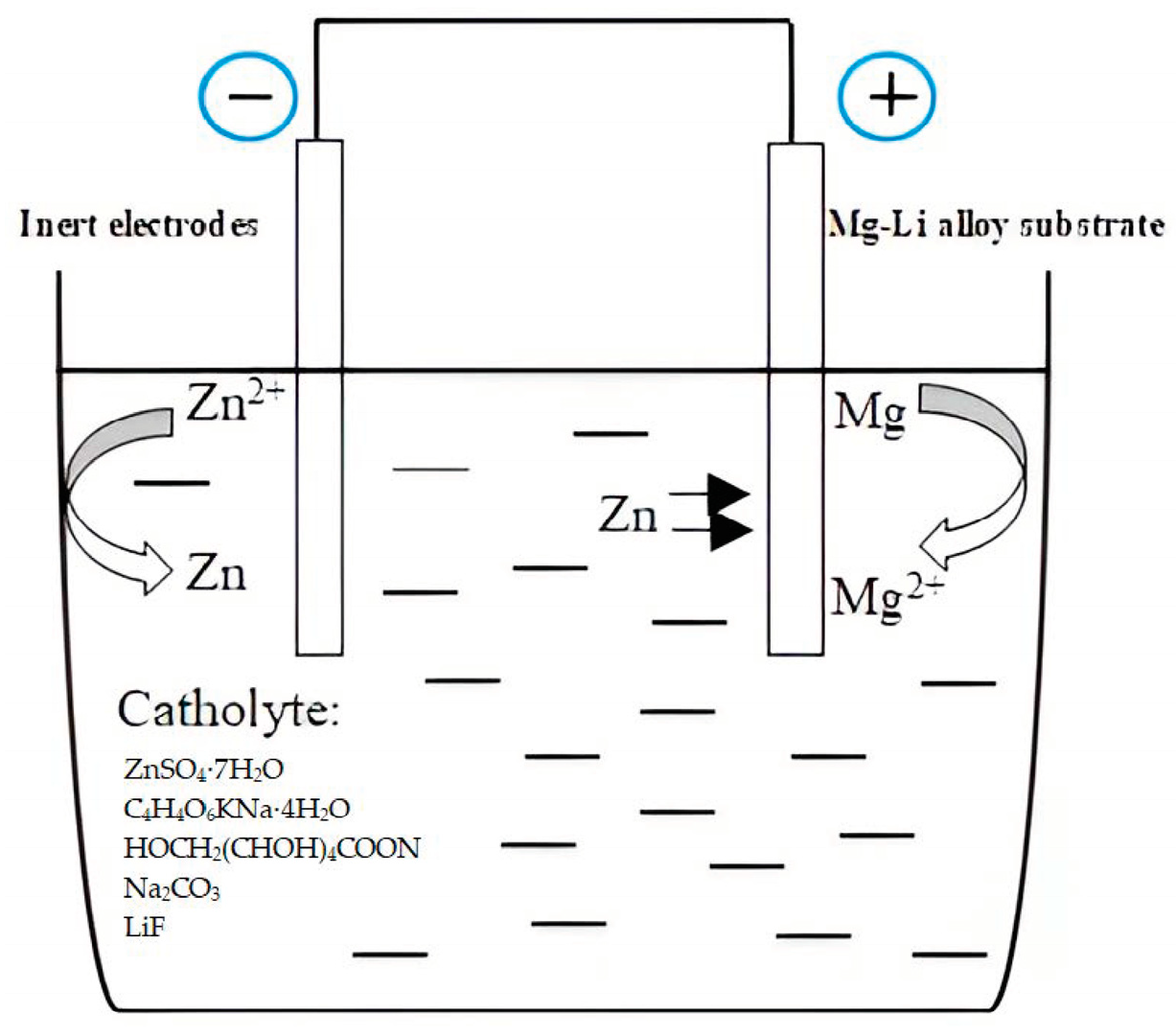
2.1. Electroless Plating of Zn
- Alkaline degreasing: The alkaline wash solution was heated to 70 °C and the sonicated samples were immersed in this solution for 6 min. Afterward, the samples were immediately rinsed with hot distilled water to prevent the generation of insoluble silicic acid during the subsequent steps. Finally, the samples were rinsed with cold distilled water.
- Acid pickling: The acid wash solution was heated to 30 °C, and the alkaline washed samples previously subjected to alkaline degreasing were immersed in the solution for 45 s. Throughout the cleaning process, the sample was continuously shaken to prevent over-etching of the surface. Subsequently, the samples were thoroughly rinsed with an abundant amount of distilled water.
- Activation: Acid activation was used in this experiment at room temperature. The samples were immersed in the activation solution for 45 s, followed by a thorough rinse with distilled water.
- Immersion in the zinc bath: The samples were immersed in the zinc bath for 1–10 min at a temperature ranging from 20–70 °C. After immersion, the samples were extensively rinsed with distilled water and dried using cold air.
2.2. Coating Characterization
2.3. Electrochemical Testing
3. Results and Discussion
3.1. Zinc Deposition Process
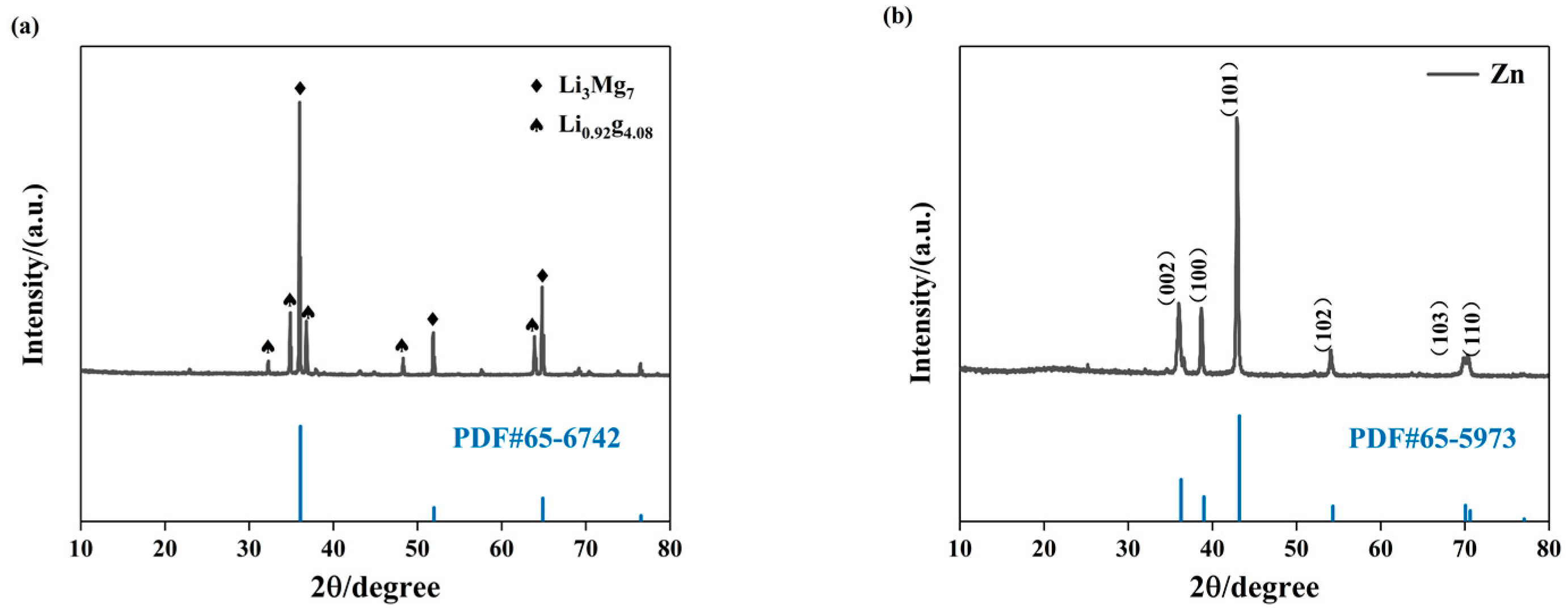
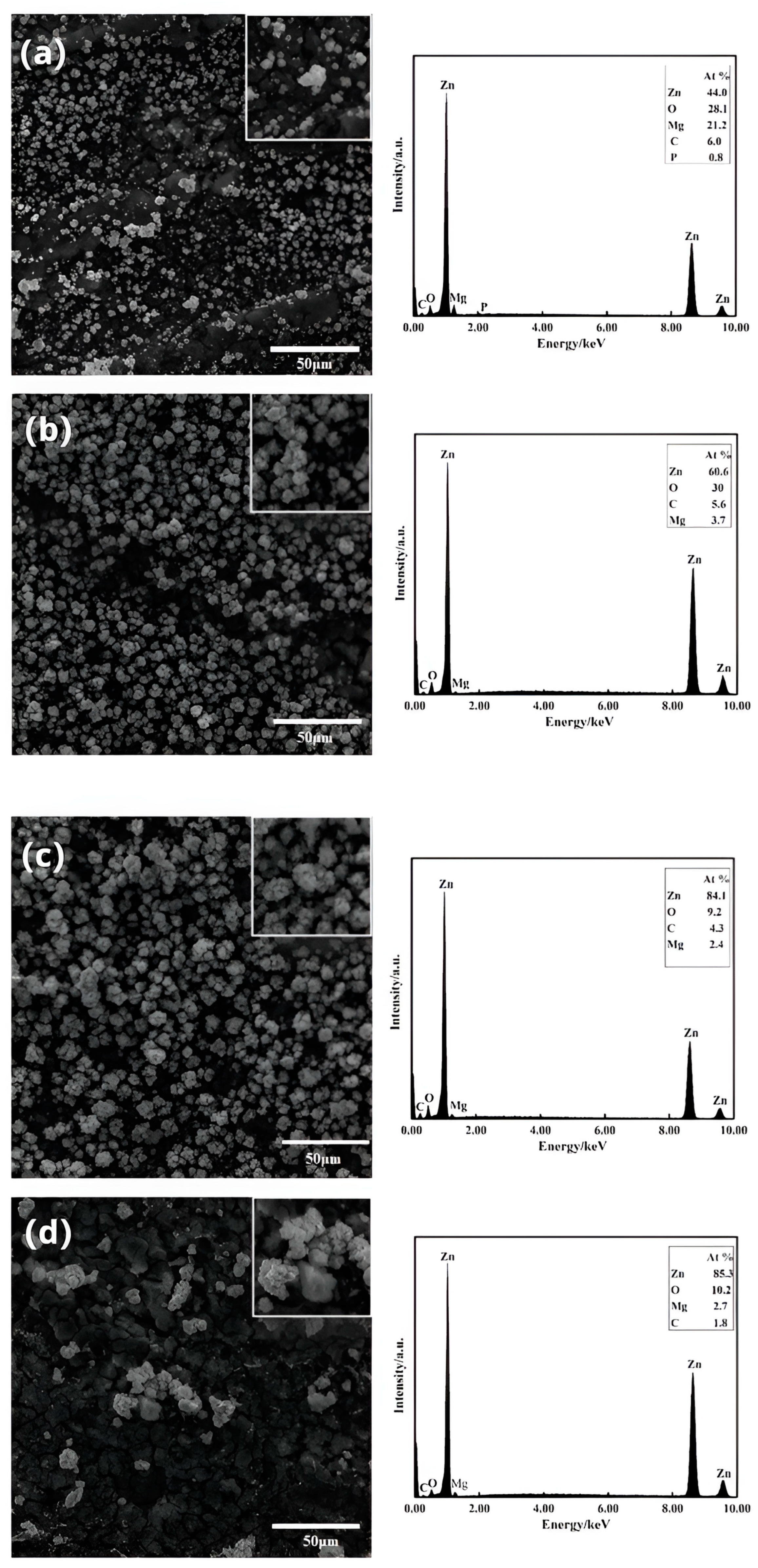
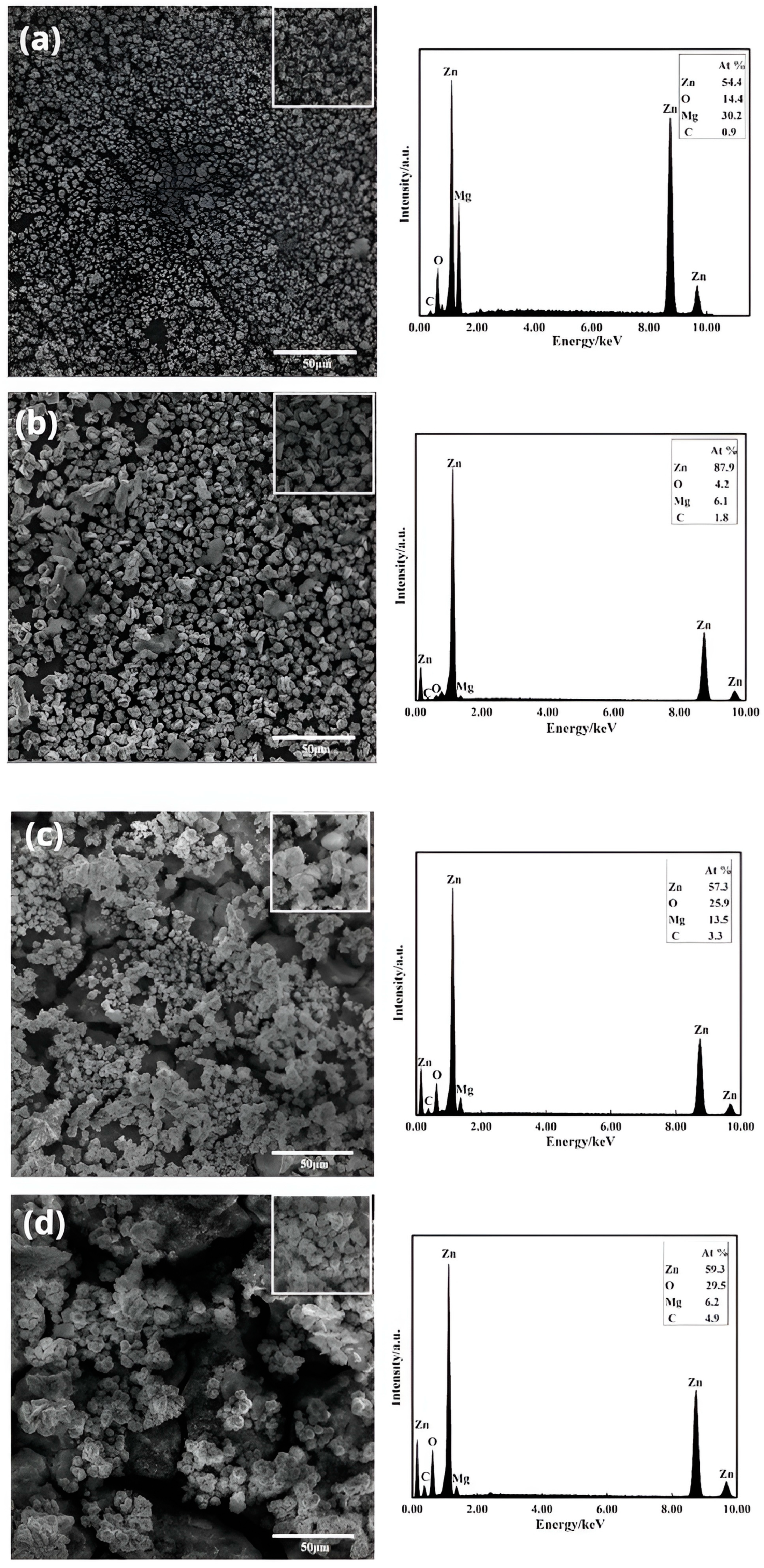
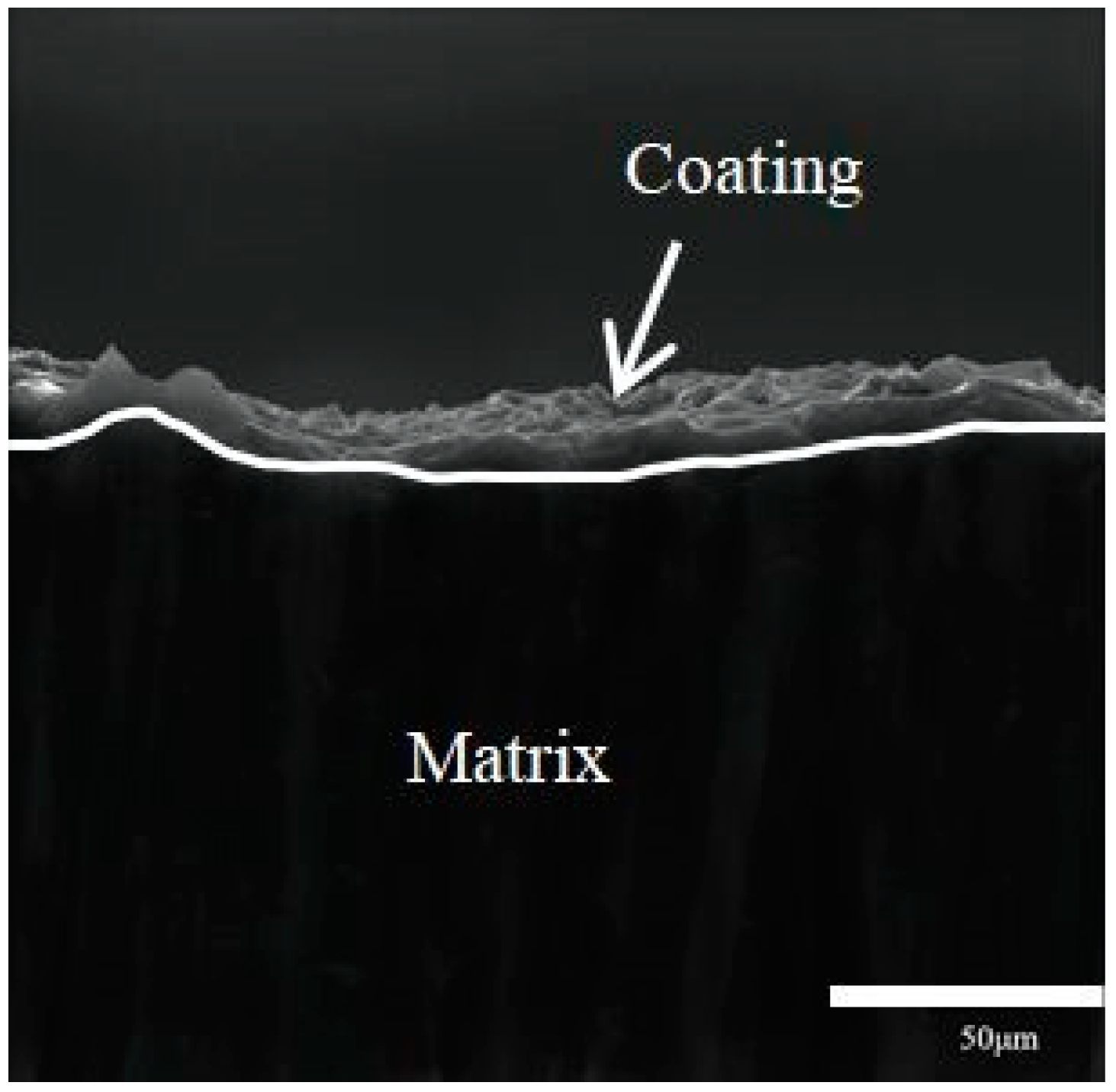
3.2. Microstructural Characteristics
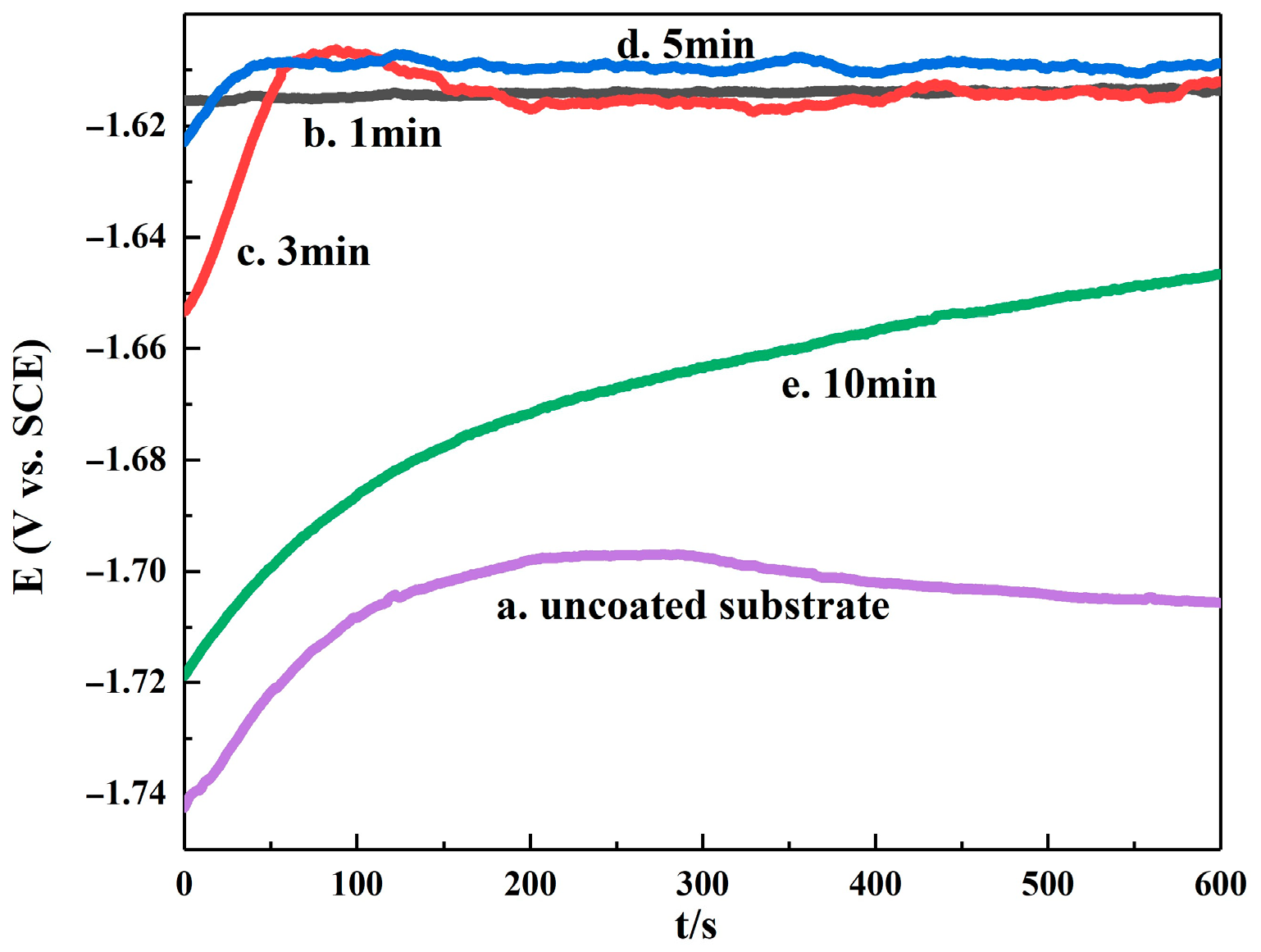
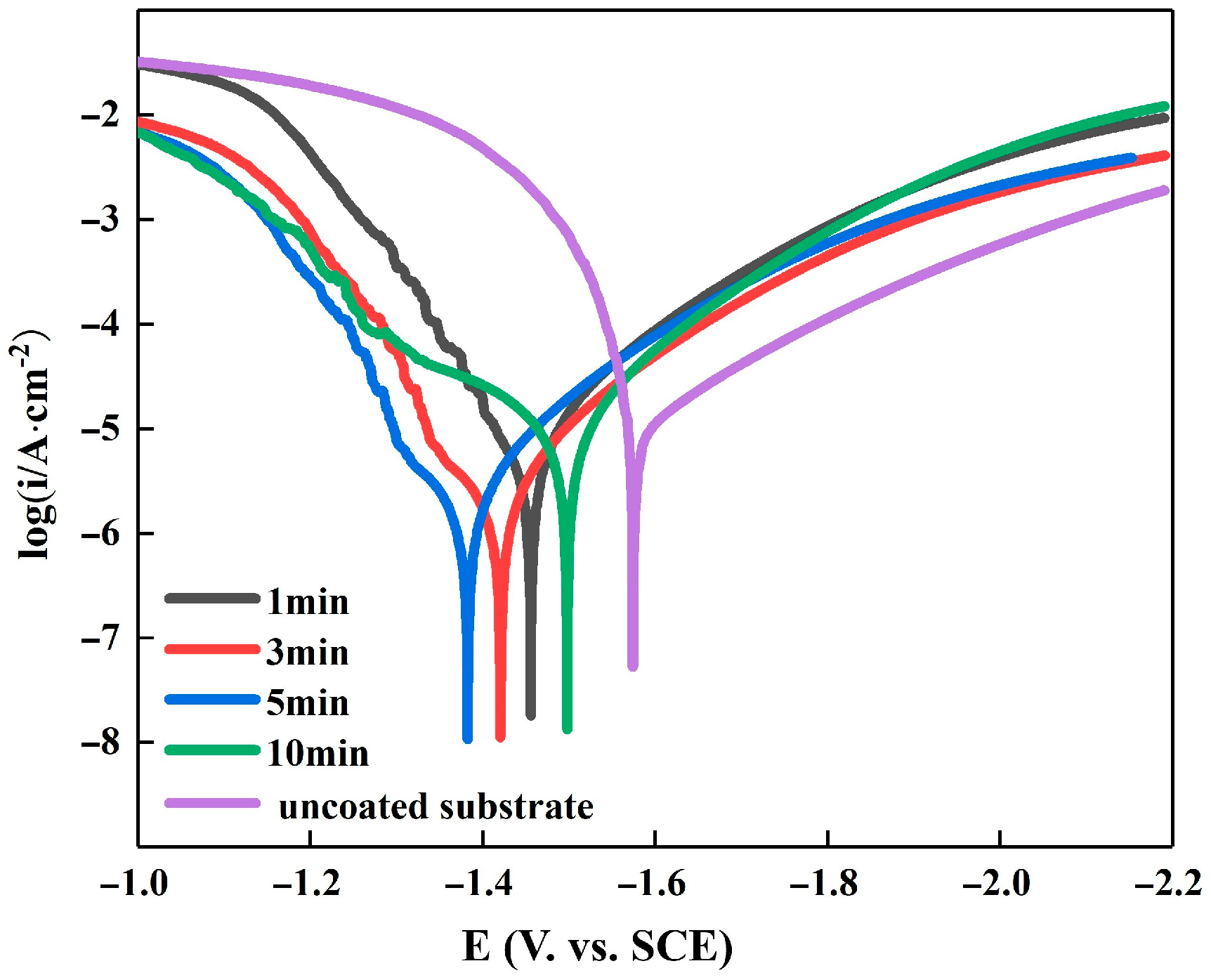
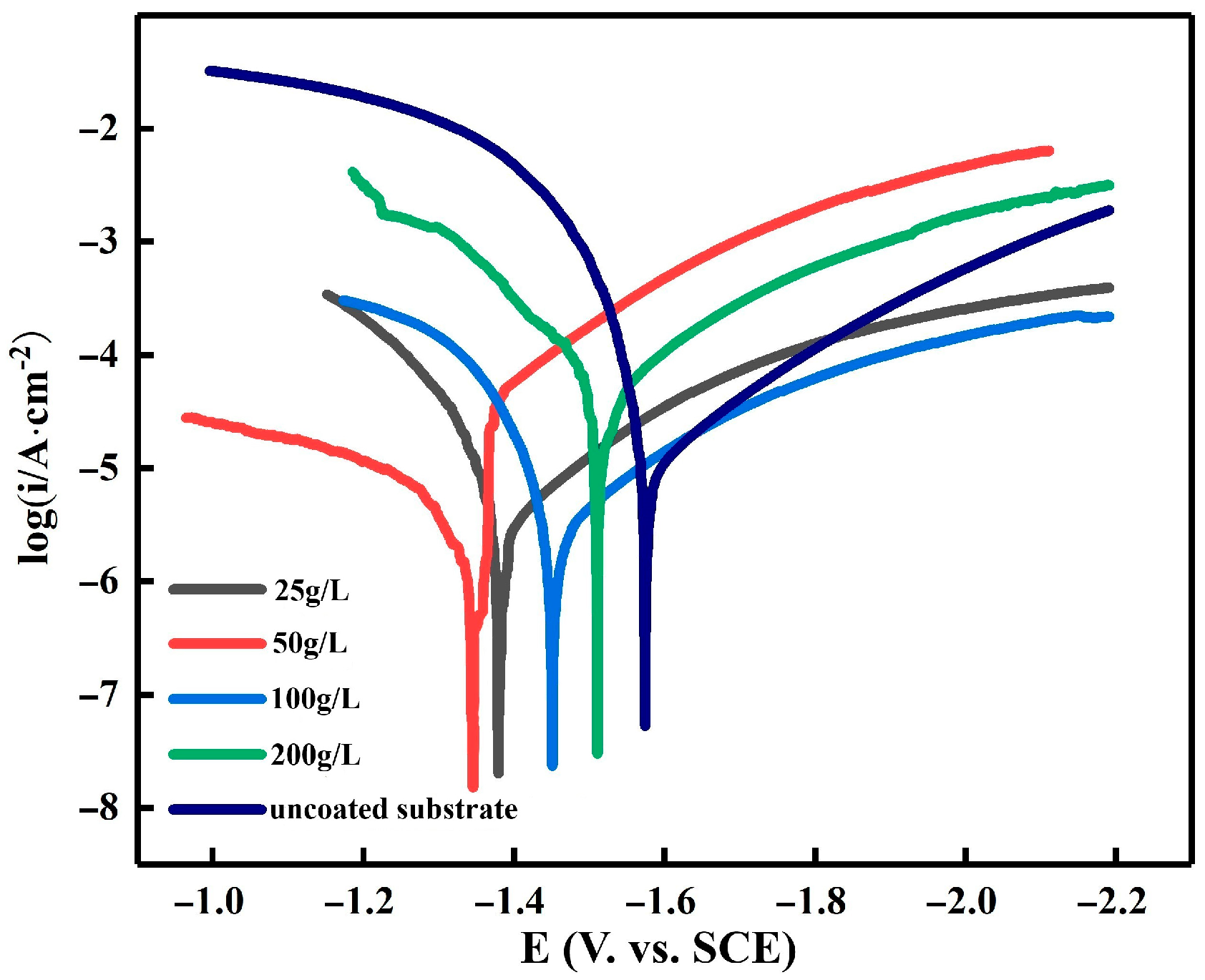
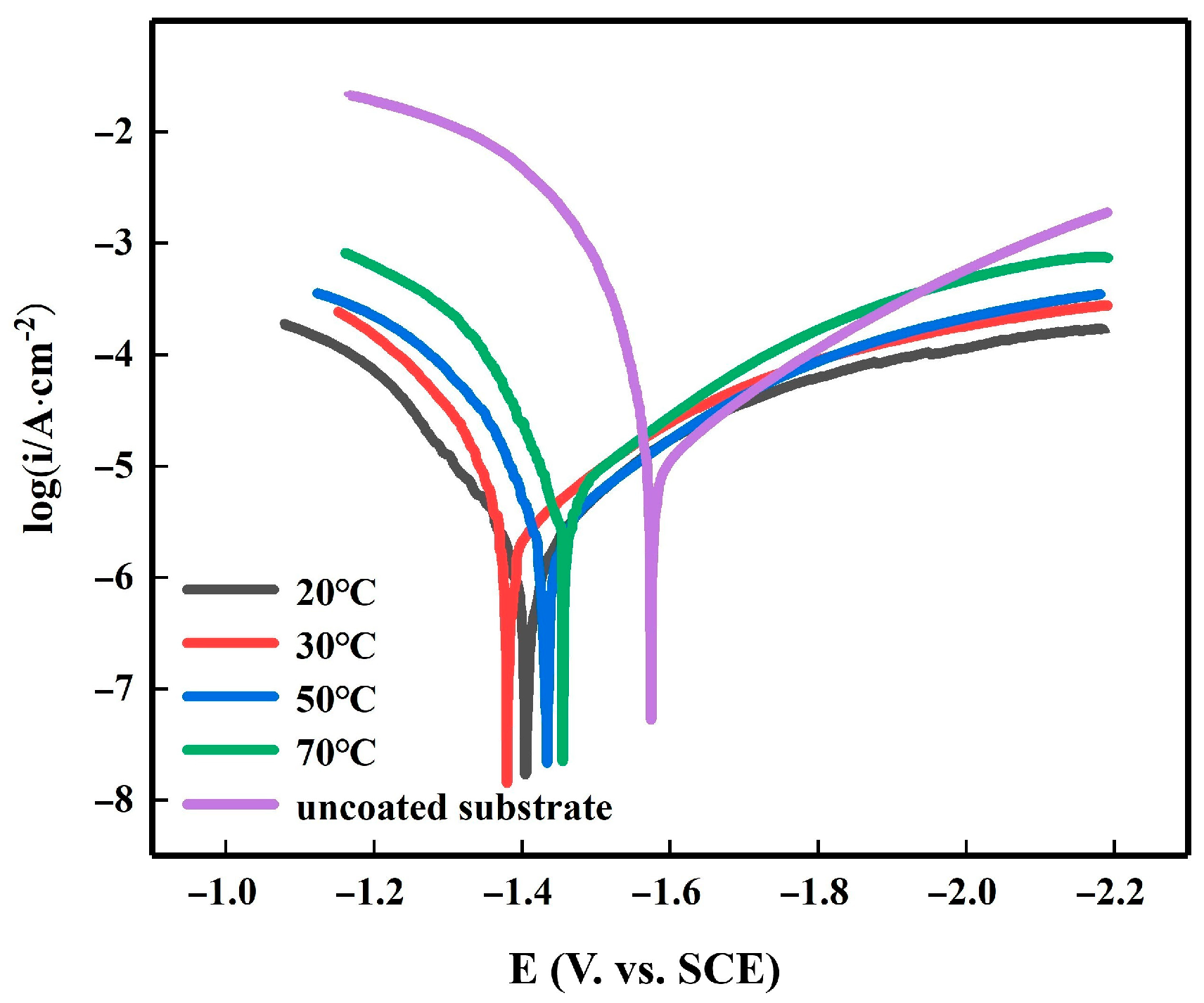
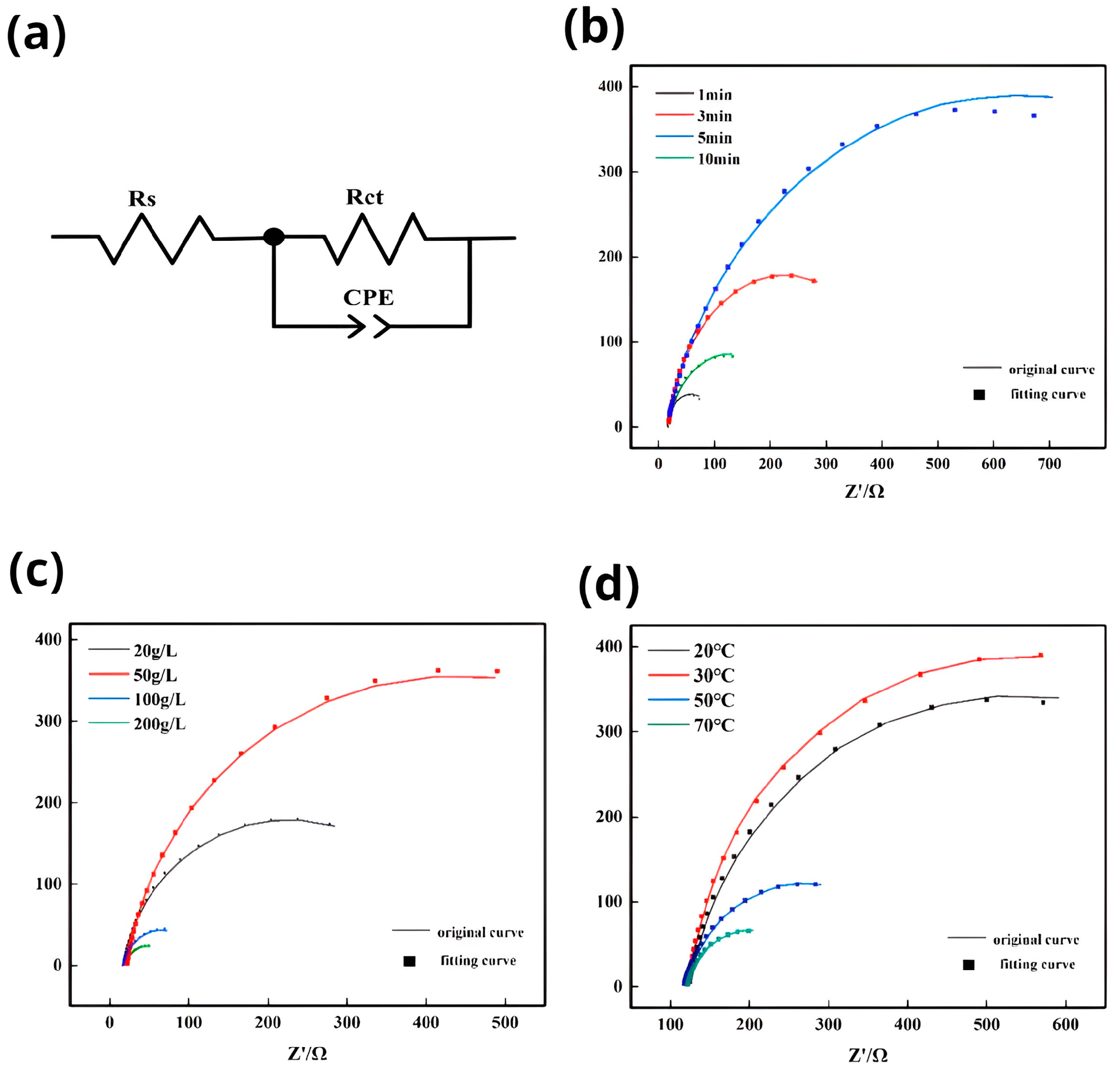
3.3. Electrochemical Characterization of Electroplating
3.4. Test of Binding Force
4. Conclusions
- (1)
- Electroless-deposited zinc follows a process of the nucleation and growth of zinc grains. As the zinc dipping content, time, and temperature increase, the zinc grains gradually cover the surface of the substrate. However, the size of the zinc grains increases while the uniformity of the zinc dipping layer decreases.
- (2)
- This study employed a galvanic solution containing a sodium-gluconate-based complexing agent for the immersion coating of Mg-Li alloys. The most densely packed and homogeneous zinc impregnation layer was obtained with a zinc content of 50 g/L, an immersion plating time of 5 min, and a temperature of 30 °C. This layer effectively covered the Mg-Li alloy substrate. Among the variables tested, the dipping time had the greatest influence on zinc dipping, while the dipping temperature had the least significant impact.
- (3)
- Zinc dipping improves the corrosion resistance of the Mg-Li alloy by increasing the corrosion potential and reducing the corrosion current to some extent. The capacitive arc of the chemically plated layer obtained after zinc dipping increases with zinc content, time, and temperature until reaching a maximum value, after which it starts to decrease. Therefore, the optimal bond between the plated layer and the substrate was achieved with a zinc content of 50 g/L, a zinc dipping time of 5 min, and a temperature in the vicinity of 30 °C. This resulted in improved self-corrosion potential, current, capacitive arc, and surface coverage compared to previous experiments. These findings lay the groundwork for subsequent treatments of Mg-Li alloys, as zinc deposition on the surface of magnesium-lithium alloys exhibits a certain protective effect and facilitates further treatment of such alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Qian, L.; Cui, R.; Liu, J.; Zhang, Q. Machining-induced residual stress analysis and multi-objective optimization for milling process of Mg–Li alloy. Measurement 2022, 204, 112127. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, X.; Li, J.; Tan, J.; Pan, F. Microstructure, texture and mechanical properties of the rolled high modulus Mg-Y-Zn-Al-Li alloy. Mater. Sci. Eng. A 2022, 831, 142242. [Google Scholar] [CrossRef]
- Wu, H.; Zhong, F.; Wu, R.; Wang, Y.; Wang, D.; Ma, X.; Jin, S.; Zhang, S.; Hou, L.; Zhang, J. Microstructure evolution and mechanical behavior of α/β alternative Mg–Li alloy composite sheets with different initial thickness ratios prepared by accumulative roll bonding. Mater. Sci. Eng. A 2022, 859, 144199. [Google Scholar] [CrossRef]
- Hwang, J.H.; Zargaran, A.; Park, G.; Lee, O.; Lee, B.-J.; Kim, N.J. Effect of 1Al addition on deformation behavior of Mg. J. Magnes. Alloys 2021, 9, 489–498. [Google Scholar] [CrossRef]
- Hu, K.; Guan, Y.; Zhai, J.; Li, Y.; Chen, F.; Liu, Y.; Lin, J. Effect on microstructure and properties of LA103Z Mg–Li alloy plate by multi-pass friction stir processing. J. Mater. Res. Technol. 2022, 20, 3985–3994. [Google Scholar] [CrossRef]
- Ouyang, Y.; Kang, H.; Guo, E.; Qiu, R.; Su, K.; Chen, Z.; Wang, T. Thermo-driven oleogel-based self-healing slippery surface behaving superior corrosion inhibition to Mg-Li alloy. J. Magnes. Alloys 2022, in press. [CrossRef]
- Wang, J.; Wu, R.; Feng, J.; Zhang, J.; Hou, L.; Zhang, M. Influence of rolling strain on electromagnetic shielding property and mechanical properties of dual-phase Mg-9Li alloy. Mater. Charact. 2019, 157, 109924. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, X.; Ye, J.; Chen, T.; Dai, Y.; Liu, C.; Luo, Z.; Gao, S.; Zhang, J.; Yao, J.; et al. Simultaneously improving elastic modulus and damping capacity of extruded Mg-Gd-Y-Zn-Mn alloy via alloying with Si. J. Alloys Compd. 2019, 810, 151857. [Google Scholar] [CrossRef]
- Tu, T.; Chen, X.; Chen, T.; Yuan, Y.; Pan, F. New high-modulus and high-strength Mg-Gd-Ag-Mn-Ge alloys. Mater. Sci. Eng. A 2021, 805, 140559. [Google Scholar] [CrossRef]
- Tu, T.; Chen, X.; Zhao, C.; Yuan, Y.; Pan, F. A simultaneous increase of elastic modulus and ductility by Al and Li additions in Mg-Gd-Zn-Zr-Ag alloy. Mater. Sci. Eng. A 2020, 771, 138576. [Google Scholar] [CrossRef]
- Ji, Q.; Ma, Y.; Wu, R.; Zhang, J.; Hou, L.; Zhang, M. Effect of Y and Ce addition on microstructures and mechanical properties of LZ91 alloys. J. Alloys Compd. 2019, 800, 72–80. [Google Scholar] [CrossRef]
- Zhang, X.; Su, K.; Kang, H.; Liu, L.; Guo, E.; Chen, Z.; Wang, T. Improving the mechanical properties of duplex Mg-Li-Zn alloy by mixed rolling processing. Mater. Today Commun. 2022, 31, 103538. [Google Scholar] [CrossRef]
- Karakulak, E. A review: Past, present and future of grain refining of magnesium castings. J. Magnes. Alloys 2019, 7, 355–369. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Pistofidis, N.; Vourlias, G.; Konidaris, S.; Pavlidou, E.; Stergioudis, G. The combined effect of nickel and bismuth on the structure of hot-dip zinc coatings. Mater. Lett. 2007, 61, 2007–2010. [Google Scholar] [CrossRef]
- Yin, T.; Wu, R.; Leng, Z.; Du, G.; Guo, X.; Zhang, M.; Zhang, J. The process of electroplating with Cu on the surface of Mg–Li alloy. Surf. Coat. Technol. 2013, 225, 119–125. [Google Scholar] [CrossRef]
- Luo, H.-J.; Song, B.-N.; Liu, Y.-H.; Yao, G.-C. Electroless Ni-P plating on Mg-Li alloy by two-step method. Trans. Nonferrous Met. Soc. China 2011, 21, 2225–2230. [Google Scholar] [CrossRef]
- Kowase, Y.; Shrestha, N.K.; Saji, T. Preparation of coloured composite films of Ni/organic pigments by immersion plating over zinc surface. Surf. Coat. Technol. 2006, 200, 5526–5531. [Google Scholar] [CrossRef]
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Bastos, A.C.; Quevedo, M.C.; Ferreira, M.G.S. Investigating the separation of anodic and cathodic defects in organic coatings applied on metal substrates. An experimental contribution. Prog. Org. Coat. 2016, 96, 26–31. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Abdullah, A.M.; Hassan, M.K.; Mohamed, A.M.; Wang, C.; Jarjoura, G.; Farhat, Z. Synthesis, Characterization, and Application of Novel Ni-P-Carbon Nitride Nanocomposites. Coatings 2018, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Fayyad, E.M.; Hassan, M.K.; Rasool, K.; Mahmoud, K.A.; Mohamed, A.M.A.; Jarjoura, G.; Farhat, Z.; Abdullah, A.M. Novel electroless deposited corrosion—Resistant and anti-bacterial NiP–TiNi nanocomposite coatings. Surf. Coat. Technol. 2019, 369, 323–333. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Han, E.H. High corrosion resistance of electroless composite plating coatings on AZ91D magnesium alloys. Electrochim. Acta 2008, 53, 2135–2143. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Liu, L.F.; Zhai, C.Q.; Zhu, Y.P.; Ding, W.J. Corrosion behavior of pulse-plated Zn–Ni alloy coatings on AZ91 magnesium alloy in alkaline solutions. Thin Solid Films 2005, 484, 232–237. [Google Scholar] [CrossRef]
- Nan, S.; Zhang, C.H.; Hong, X.M.; Gao, L. Zine Once Dipping and Its Solution for Mg-Li Alloy at Normal Temperature. Corros. Prot. 2008, 28, 752–755. [Google Scholar]
- Lian, J.S.; Li, G.Y.; Niu, L.Y.; Gu, C.D.; Jiang, Z.H.; Jiang, Q. Electroless Ni–P deposition plus zinc phosphate coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2006, 200, 5956–5962. [Google Scholar] [CrossRef]


| Alkaline Degreasing (g/L) | NaOH (AR, 95%) | 18 |
| Na3PO4 (AR, 98%) | 25 | |
| Na2SiO3 (SiO2, 44~47%) | 7 | |
| Acid pickle (g/L) | CrO3 (AR, 99.99%) | 150 |
| Fe(NO3)3 (AR, 99%) | 35 | |
| NaF (99.99% metals basis) | 3.5 | |
| Activation | H3PO4(AR, ≥85 wt.% in H2O) | 50 mL/L |
| NH4HF (AR, 98.5%) | 90 g/L | |
| Zinc leaching (g/L) | ZnSO4·7H2O(AR, ≥99.5%) | 50 |
| HOCH2(CHOH)4COONa (AR, 99%) | 120 | |
| C4H4O6KNa·4H2O (AR,99%) | 50 | |
| Na2CO3 (AR, ≥99.5%) | 5 | |
| LiF (AR, 99%) | 3 |
| Immersion Time Min | E∞rr, V | I∞rr, ×10−6 A/cm2 | Ba, mV | Bc, mV |
|---|---|---|---|---|
| uncoated substrate | −1.63 | 18.64 | 149.44 | 256.12 |
| 1 | −1.44 | 10.19 | 389.6 | 274.93 |
| 3 | −1.44 | 5.53 | 407.04 | 260.73 |
| 5 | −1.39 | 4.07 | 491.25 | 337.32 |
| 10 | −1.63 | 5.75 | 229.846 | 259.21 |
| Zn Content, g/L | E∞rr,V | I∞rr, ×10−6 A/cm2 | Ba, mV | Bc, mV |
|---|---|---|---|---|
| Uncoated substrate | −1.63 | 18.64 | 149.44 | 265.12 |
| 20 | −1.43 | 3.96 | 200.45 | 348.4 |
| 50 | −1.38 | 2.78 | 474.92 | 268.26 |
| 100 | −1.45 | 5.03 | 326.85 | 261.6 |
| 200 | −1.53 | 8.92 | 145.71 | 266.1 |
| Immersion Temperature, °C | E∞rr, V | I∞rr, ×10−6 A/cm2 | Ba, mV | Bc, mV |
|---|---|---|---|---|
| Uncoated substrate | −1.63 | 18.64 | 149.44 | 265.12 |
| 20 | −1.42 | 3.52 | 237.19 | 300.07 |
| 30 | −1.40 | 2.82 | 360.41 | 430.06 |
| 50 | −1.44 | 5.01 | 302.1 | 248.14 |
| 70 | −1.48 | 5.32 | 213.42 | 235.01 |
| Zinc Dipping Time, min | File Test | Scratch Test |
|---|---|---|
| 1 | Completely detached | Peeling, not falling off |
| 3 | Peeling, not falling off | Neither blistering nor peeling |
| 5 | Neither blistering nor peeling | Neither blistering nor peeling |
| 10 | Peeling, not falling off | Peeling, not falling off |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, A.; Cao, Y.; Zhang, Y.; Zhou, S. Study of Electroless-Deposited Zn on the Surface of Mg-Li Alloy. Materials 2023, 16, 5511. https://doi.org/10.3390/ma16165511
Yue A, Cao Y, Zhang Y, Zhou S. Study of Electroless-Deposited Zn on the Surface of Mg-Li Alloy. Materials. 2023; 16(16):5511. https://doi.org/10.3390/ma16165511
Chicago/Turabian StyleYue, Anyu, Yong Cao, Yi Zhang, and Shenggang Zhou. 2023. "Study of Electroless-Deposited Zn on the Surface of Mg-Li Alloy" Materials 16, no. 16: 5511. https://doi.org/10.3390/ma16165511




