Effect of Polydopamine and Curcumin on Physicochemical and Mechanical Properties of Polymeric Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Polydopamine
2.2. Preparation of Polymeric Blend of Polyvinyl Alcohol and Sodium Alginate
2.3. Preparation of Polymeric Blend of Polyvinyl Alcohol, Sodium Alginate, and Curcumin
2.4. Preparation of Polymeric Blend of Polyvinyl Alcohol, Sodium Alginate, Curcumin, and Polydopamine
2.5. Microscopic Analysis
2.6. Atomic Force Microscopy (AFM)
2.7. Scanning Electron Microscopy (SEM)
2.8. Fourier Transform Infrared Spectroscopy
2.9. Mechanical Properties
3. Results
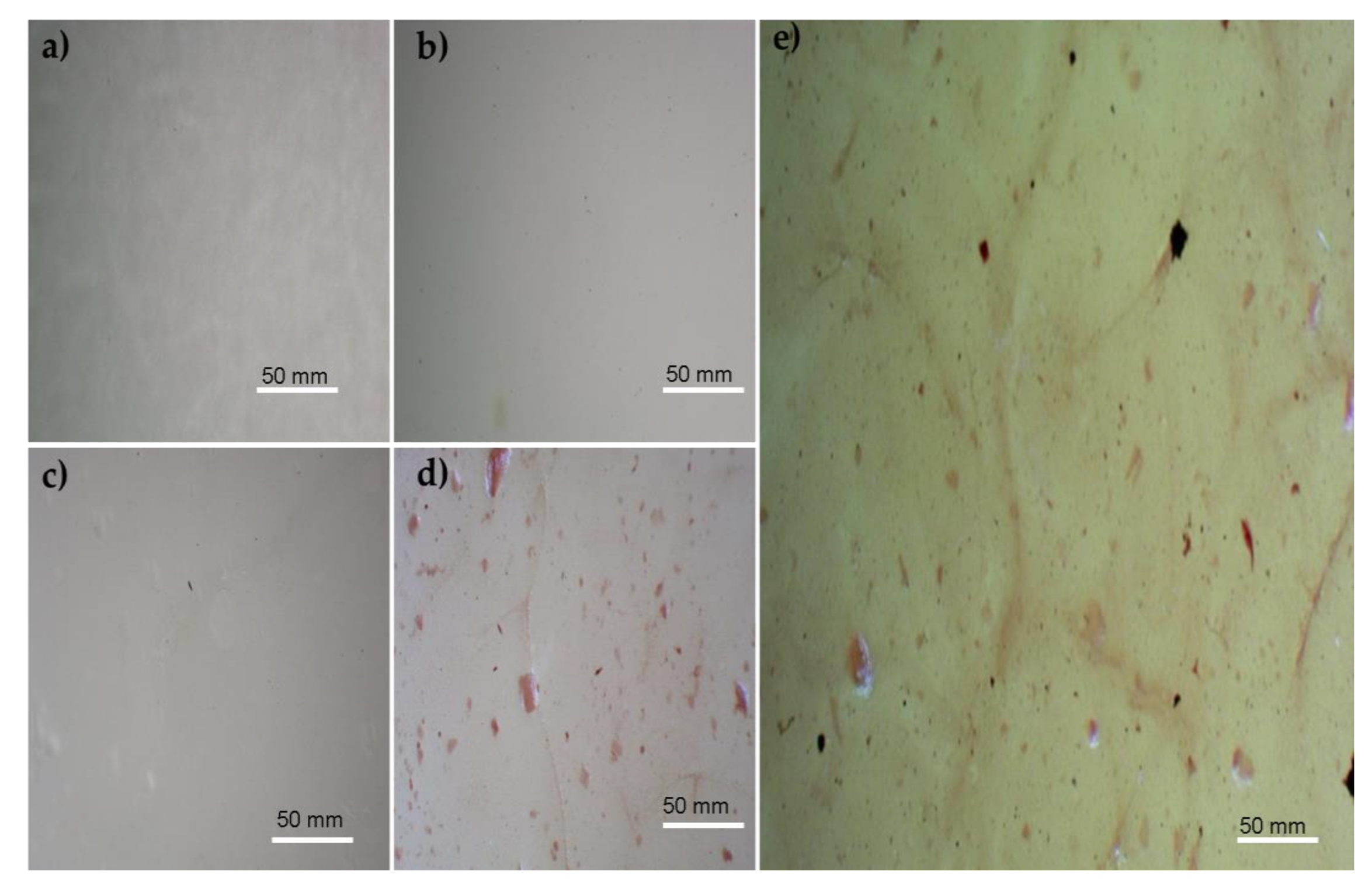
3.1. Microscopic Images
3.2. Atomic Force Microscopy (AFM)
3.3. Scanning Electron Microscopy (SEM)
3.4. FTIR Spectrum
3.5. Mechanical Properties
4. Discussion
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sionkowska, A. Current Research on the Blends of Natural and Synthetic Polymers as New Biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Tabasum, S.; Younas, M.; Zaeem, M.A.; Majeed, I.; Majeed, M.; Noreen, A.; Iqbal, M.N.; Zia, K.M. A Review on Blending of Corn Starch with Natural and Synthetic Polymers, and Inorganic Nanoparticles with Mathematical Modeling. Int. J. Biol. Macromol. 2019, 122, 969–996. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. The Potential of Polymers from Natural Sources as Components of the Blends for Biomedical and Cosmetic Applications. Pure Appl. Chem. 2015, 87, 1075–1084. [Google Scholar] [CrossRef]
- Lin, G.; Yu, F.; Li, D.; Chen, Y.; Zhang, M.; Lu, K.; Wang, N.; Hu, S.; Zhao, Y.; Xu, H. Polydopamine-Cladded Montmorillonite Micro-Sheets as Therapeutic Platform Repair the Gut Mucosal Barrier of Murine Colitis through Inhibiting Oxidative Stress. Mater. Today Bio 2023, 20, 100654. [Google Scholar] [CrossRef] [PubMed]
- Ball, V.; Del Frari, D.; Michel, M.; Buehler, M.J.; Toniazzo, V.; Singh, M.K.; Gracio, J.; Ruch, D. Deposition Mechanism and Properties of Thin Polydopamine Films for High Added Value Applications in Surface Science at the Nanoscale. Bionanoscience 2012, 2, 16–34. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Zhang, H.; Zhang, Y.; Xu, Q.; Lu, J.; Feng, S.; Luo, X.; Wang, S.; Zhao, Q. Smart Polydopamine-Based Nanoplatforms for Biomedical Applications: State-of-Art and Further Perspectives. Coord. Chem. Rev. 2023, 488, 215153. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.R. Polydopamine for Biomedical Application and Drug Delivery System. Med. Chem. 2018, 8, 218–229. [Google Scholar] [CrossRef]
- Král, M.; Dendisová, M.; Matějka, P.; Svoboda, J.; Pop-Georgievski, O. Infrared Imaging of Surface Confluent Polydopamine (PDA) Films at the Nanoscale. Colloids Surf. B Biointerfaces 2023, 221, 112954. [Google Scholar] [CrossRef]
- Lynge, M.E.; Van Der Westen, R.; Postma, A.; Städler, B. Polydopamine—A Nature-Inspired Polymer Coating for Biomedical Science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Liu, R.; Zhou, Q.; Lu, H.; Zhang, W.; Zhou, Y. Research Progress of Polydopamine Hydrogel in the Prevention and Treatment of Oral Diseases Research Progress of Polydopamine Hydrogel in the Prevention and Treatment of Oral Diseases. Int. J. Nanomed. 2023, 18, 2623–2645. [Google Scholar] [CrossRef]
- David Dayanidhi, P.; Anithabanu, P.; Vaidyanathan, V.G. Studies on Stabilization of Collagen Using Cr-Doped Polydopamine Complex. Biophys. Chem. 2023, 292, 106917. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.; Wang, K.; Yuan, H.; Fang, L.; Zheng, X.; Chan, C.; Ren, F.; Zhao, C. Pulse Electrochemical Driven Rapid Layer-by-Layer Assembly of Polydopamine and Hydroxyapatite Nanofilms via Alternative Redox in Situ Synthesis for Bone Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 920–928. [Google Scholar] [CrossRef]
- Antunes-ricardo, M.; Martínez-morales, P.; Rivera-hern, G. Polyvinyl Alcohol Based-Drug Delivery Systems for Cancer Treatment. Int. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef]
- Millon, L.E.; Wan, W.K. The Polyvinyl Alcohol-Bacterial Cellulose System as a New Nanocomposite for Biomedical Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 79, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, N.; Bryaskova, R.; Tzoneva, R. New Polyvinyl Alcohol-Based Hybrid Materials for Biomedical Application. Mater. Lett. 2012, 88, 19–22. [Google Scholar] [CrossRef]
- Gao, T.; Jiang, M.; Liu, X.; You, G.; Wang, W.; Sun, Z.; Ma, A.; Chen, J. Patterned Polyvinyl Alcohol Hydrogel Dressings with Stem Cells Seeded for Wound Healing. Polymers 2019, 11, 171. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Feng, W. PVA Hydrogel Properties for Biomedical Application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A Review of Polyvinyl Alcohol and Its Uses in Cartilage and Orthopedic Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100B, 1451–1457. [Google Scholar] [CrossRef]
- Szekalska, M.; Wróblewska, M.; Czajkowska-Kośnik, A.; Sosnowska, K.; Misiak, P.; Wilczewska, A.Z.; Winnicka, K. The Spray-Dried Alginate/Gelatin Microparticles with Luliconazole as Mucoadhesive Drug Delivery System. Materials 2023, 16, 403. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Huang, X.; Arslan, M.; Shi, J.; Zhihua Li, Y.G.; Holmes, M.; Zou, X. Fabrication and Characterization of Polyvinyl Alcohol/Sodium Alginate/Zein/Chitosan Bilayer Film for Dynamic Visualization of Pork Quality. Sci. Total Environ. 2023, 243, 125065. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A Promising Wound Dressing Material with Excellent Cytocompatibility and Proangiogenesis Action for Wound Healing: Strontium Loaded Silk Fibroin/Sodium Alginate (SF/SA) Blend Films. Int. J. Biol. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, J.; Peng, C.; Huang, T.; Zhou, H.; Ou, B.; Chen, J.; Liu, Q.; He, S.; Cao, D.; et al. Fabrication and Physical Properties of Gelatin/Sodium Alginate/Hyaluronic Acid Composite Wound Dressing Hydrogel. J. Macromol. Sci. Part A Pure Appl. Chem. 2014, 51, 318–325. [Google Scholar] [CrossRef]
- Wei, Q.; Zhou, J.; An, Y.; Li, M.; Zhang, J.; Yang, S. Modification, 3D Printing Process and Application of Sodium Alginate Based Hydrogels in Soft Tissue Engineering: A Review. Int. J. Biol. Macromol. 2023, 232, 123450. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.A.J.; Alharbi, K.S.; Almalki, W.H.; Imam, S.S.; Albratty, M.; Meraya, A.M.; Alzarea, S.I.; Kazmi, I.; Al-Abbasi, F.A.; Afzal, O.; et al. Sodium Alginate Based Drug Delivery in Management of Breast Cancer. Carbohydr. Polym. 2022, 292, 119689. [Google Scholar] [CrossRef]
- Zahid, M.; Lodhi, M.; Afzal, A.; Zulfiqar Ahmad Rehan, M.M.; Javed, T.; Shabbir, R.; Siuta, D.; Althobaiti, F.; Dessok, E.S. Development of Hydrogels with the Incorporation of Raphanus sativus L. Seed Extract in Sodium Alginate for Wound-Healing Application. Gels 2021, 7, 107. [Google Scholar] [CrossRef]
- Qin, Y. Alginate Fibres: An Overview of the Production Processes and Applications in Wound Management. Polym. Int. 2008, 57, 171–180. [Google Scholar] [CrossRef]
- Zdiri, K.; Cayla, A.; Elamri, A.; Erard, A.; Salaun, F. Alginate-Based Bio-Composites and Their Potential Applications. J. Funct. Biomater. 2022, 13, 117. [Google Scholar] [CrossRef]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a Polyvinyl Alcohol/Sodium Alginate Hydrogel-Based Scaffold Incorporating BFGF-Encapsulated Microspheres for Accelerated Wound Healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef]
- Yang, N.; Ray, S.D.; Krafts, K. Cell Proliferation. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 761–765. [Google Scholar] [CrossRef]
- Harishkumar, M.; Masatoshi, Y.; Hiroshi, S.; Tsuyomu, I.; Masugi, M. Revealing the Mechanism of in Vitro Wound Healing Properties of Citrus Tamurana Extract. Biomed. Res. Int. 2013, 2013, 963457. [Google Scholar] [CrossRef]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell Migration: Integrating Signals from Front to Back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Golias, C.H.; Charalabopoulos, A.; Charalabopoulos, K. Cell Proliferation and Cell Cycle Control: A Mini Review. Int. J. Clin. Pract. 2004, 58, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.M.; Campelo, M.d.S.; Câmara Neto, J.F.; Lima, A.B.N.; Silva, G.d.A.; Dias, A.T.d.F.F.; Ricardo, N.M.P.S.; Kaplan, D.L.; Ribeiro, M.E.N.P. Alginate/Polyvinyl Alcohol Films for Wound Healing: Advantages and Challenges. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 220–233. [Google Scholar] [CrossRef]
- Muangsri, R.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Sukhavattanakul, P.; Ummartyotin, S. Utilization of Freeze Thaw Process for Polyvinyl Alcohol/Sodium Alginate (PVA/SA) Hydrogel Composite. J. Met. Mater. Miner. 2022, 32, 34–41. [Google Scholar] [CrossRef]
- Montaser, A.S.; Rehan, M.; El-Naggar, M.E. PH-Thermosensitive Hydrogel Based on Polyvinyl Alcohol/Sodium Alginate/N-Isopropyl Acrylamide Composite for Treating Re-Infected Wounds. Int. J. Biol. Macromol. 2019, 124, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Nikkhah, M.; Mohammadi, S.; Bahrami, S.H.; Sadeghizadeh, M. Nano-Curcumin/Graphene Platelets Loaded on Sodium Alginate/Polyvinyl Alcohol Fibers as Potential Wound Dressing. J. Appl. Polym. Sci. 2021, 138, 50884. [Google Scholar] [CrossRef]
- Hong, N.P.; Chen, X.G.; Li, Y.; Hui, Y.Z. Characterization and Ornidazole Release in Vitro of a Novel Composite Film Prepared with Chitosan/Poly(Vinyl Alcohol)/Alginate. J. Biomed. Mater. Res. A 2008, 85A, 566–572. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, R.; Sun, D.; Zhou, J.; Li, M.; Zhang, Y.; Wang, Y. Design and Evaluation of Sodium Alginate/Polyvinyl Alcohol Blend Hydrogel for 3D Bioprinting Cartilage Scaffold: Molecular Dynamics Simulation and Experimental Method. J. Mater. Res. Technol. 2022, 17, 66–78. [Google Scholar] [CrossRef]
- Mahmood, K.; Zia, K.M.; Zuber, M.; Salman, M.; Anjum, M.N. Recent Developments in Curcumin and Curcumin Based Polymeric Materials for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2015, 81, 877–890. [Google Scholar] [CrossRef]
- Witika, B.A.; Makoni, P.A.; Matafwali, S.K.; Mweetwa, L.L.; Shandele, G.C.; Walker, R.B. Enhancement of Biological and Pharmacological Properties of an Encapsulated Polyphenol: Curcumin. Molecules 2021, 26, 4244. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef]
- Kumari, M.; Nanda, D.K. Potential of Curcumin Nanoemulsion as Antimicrobial and Wound Healing Agent in Burn Wound Infection. Burns 2022. In Press. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.E.; Gilbert, R.J. Curcumin Release from Biomaterials for Enhanced Tissue Regeneration Following Injury or Disease. Bioengineering 2023, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, M.; Foshati, S.; Abdi, M.; Askari, G.; Sukhorukov, V.N.; Bagherniya, M.; Sahebkar, A. The Effectiveness of Nano-Curcumin on Patients with COVID-19: A Systematic Review of Clinical Trials. Phytother. Res. 2023, 37, 1663–1677. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; San Lio, R.M.; Evola, G.; Agodi, A.; Basile, G. Nutrition and Wound Healing: An Overview Focusing on the Beneficial Effects of Curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Dev, A.; Das, S.S.; Kim, H.J.; Kumar, A.; Thakur, V.K.; Han, S.S. Curcumin-Loaded Alginate Hydrogels for Cancer Therapy and Wound Healing Applications: A Review. Int. J. Biol. Macromol. 2023, 232, 123283. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Chen, L.; Zhao, Y.; Yan, X.; Wang, X.; Zhou, C.; Shi, Y.; Xue, W. Methods to Prepare Dopamine/Polydopamine Modified Alginate Hydrogels and Their Special Improved Properties for Drug Delivery. Eur. Polym. J. 2019, 110, 192–201. [Google Scholar] [CrossRef]
- Shivakumara, L.R.; Demappa, T. Synthesis and Swelling Behavior of Sodium Alginate/Poly(Vinyl Alcohol) Hydrogels. Turk. J. Pharm. Sci. 2019, 16, 252. [Google Scholar] [CrossRef]
- Badita, C.R.; Aranghel, D.; Burducea, C.; Mereuta, P. Characterization of sodium alginate based films. Rom. J. Phys. 2020, 65, 602. [Google Scholar]
- Xie, L.; Jiang, M.; Dong, X.; Bai, X.; Tong, J.; Zhou, J. Controlled Mechanical and Swelling Properties of Poly(Vinyl Alcohol)/Sodium Alginate Blend Hydrogels Prepared by Freeze–Thaw Followed by Ca2+ Crosslinking. J. Appl. Polym. Sci. 2012, 124, 823–831. [Google Scholar] [CrossRef]
- Mirzaie, Z.; Reisi-Vanani, A.; Barati, M. Polyvinyl Alcohol-Sodium Alginate Blend, Composited with 3D-Graphene Oxide as a Controlled Release System for Curcumin. J. Drug Deliv. Sci. Technol. 2019, 50, 380–387. [Google Scholar] [CrossRef]
- Thanyacharoen, T.; Chuysinuan, P.; Techasakul, S.; Sukhavattanakul, P.; Ummartyotin, S. Release Characteristic of Curcumin (Zingiberaceae) from Sodium Alginate and Polyvinyl Alcohol-Based Hydrogel Composite: Antioxidant Properties. Emergent Mater. 2023, 6, 535–542. [Google Scholar] [CrossRef]
- Batul, R.; Tamanna, T.; Khaliq, A.; Yu, A. Recent Progress in the Biomedical Applications of Polydopamine Nanostructures. Biomater. Sci. 2017, 5, 1204–1229. [Google Scholar] [CrossRef]
- Anwar, H.; Ahmad, M.; Minhas, M.U.; Rehmani, S. Alginate-Polyvinyl Alcohol Based Interpenetrating Polymer Network for Prolonged Drug Therapy, Optimization and in-Vitro Characterization. Carbohydr. Polym. 2017, 166, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal–Carboxylate Interactions in Metal–Alginate Complexes Studied with FTIR Spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef]
- Jipa, I.M.; Stoica, A.; Stroescu, M.; Dobre, L.M.; Dobre, T.; Jinga, S.; Tardei, C. Potassium Sorbate Release from Poly(Vinyl Alcohol)-Bacterial Cellulose Films. Chem. Pap. 2012, 66, 138–143. [Google Scholar] [CrossRef]
- Awada, H.; Daneault, C. Chemical Modification of Poly(Vinyl Alcohol) in Water. Appl. Sci. 2015, 5, 840–850. [Google Scholar] [CrossRef]
- Al-Sahaf, Z.; Raimi-Abraham, B.; Licciardi, M.; de Mohac, L.M. Influence of Polyvinyl Alcohol (PVA) on PVA-Poly-N-Hydroxyethyl-Aspartamide (PVA-PHEA) Microcrystalline Solid Dispersion Films. AAPS PharmSciTech 2020, 21, 267. [Google Scholar] [CrossRef]
- Bernal-Ballen, A.; Lopez-Garcia, J.A.; Ozaltin, K. (PVA/Chitosan/Fucoidan)-Ampicillin: A Bioartificial Polymeric Material with Combined Properties in Cell Regeneration and Potential Antibacterial Features. Polymers 2019, 11, 1325. [Google Scholar] [CrossRef] [PubMed]
- Nkhwa, S.; Lauriaga, K.F.; Kemal, E.; Deb, S. Poly(Vinyl Alcohol): Physical Approaches to Designing Biomaterials for Biomedical Applications. Conf. Pap. Sci. 2014, 2014, 403472. [Google Scholar] [CrossRef]
- Jadbabaei, S.; Kolahdoozan, M.; Naeimi, F.; Ebadi-Dehaghani, H. Preparation and Characterization of Sodium Alginate–PVA Polymeric Scaffolds by Electrospinning Method for Skin Tissue Engineering Applications. RSC Adv. 2021, 11, 30674. [Google Scholar] [CrossRef]
- Sun, X.; Uyama, H. A Poly(Vinyl Alcohol)/Sodium Alginate Blend Monolith with Nanoscale Porous Structure. Nanoscale Res. Lett. 2013, 8, 411. [Google Scholar] [CrossRef]
- Mohan, P.R.K.; Sreelakshmi, G.; Muraleedharan, C.V.; Joseph, R. Water Soluble Complexes of Curcumin with Cyclodextrins: Characterization by FT-Raman Spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and Experimental Studies of the Structure and Vibrational Spectra of Curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef]
- Boscariol, R.; Paulino, T.H.; Oliveira, J.M.; Balcão, V.M.; Vila, M.M.D.C. Characterization of Commercially Available Turmeric for Use in Pharmaceutical Products and Food Supplements. J. Braz. Chem. Soc. 2022, 33, 1392–1401. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Salem, J.K.; Tabasi, N.S. Uptake of Curcumin by Supported Metal Oxides (CaO and MgO) Mesoporous Silica Materials. J. Sol-Gel Sci. Technol. 2018, 87, 647–656. [Google Scholar] [CrossRef]
- Sun, G.; Zu, F.; Koch, N.; Rappich, J.; Hinrichs, K. In Situ Infrared Spectroscopic Monitoring and Characterization of the Growth of Polydopamine (PDA) Films. Phys. Status Solidi (B) 2019, 256, 1800308. [Google Scholar] [CrossRef]
- Ejaz, S.; Ejaz, S.; Shahid, R.; Noor, T.; Shabbir, S.; Imran, M. Chitosan-Curcumin Complexation to Develop Functionalized Nanosystems with Enhanced Antimicrobial Activity against Hetero-Resistant Gastric Pathogen. Int. J. Biol. Macromol. 2022, 204, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Collagen Blended with Natural Polymers: Recent Advances and Trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]







| Polymer Films and Composites | Rq (nm) | Ra (nm) |
|---|---|---|
| SA | 66.6 | 53.4 |
| PVA | 1.6 | 1.26 |
| PVA/SA | 37.4 | 22.5 |
| PVA/SA/Cur | 25.7 | 21.9 |
| PVA/SA/Cur/PD | 25.1 | 21.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, M.; Sionkowska, A. Effect of Polydopamine and Curcumin on Physicochemical and Mechanical Properties of Polymeric Blends. Materials 2023, 16, 5758. https://doi.org/10.3390/ma16175758
Tahir M, Sionkowska A. Effect of Polydopamine and Curcumin on Physicochemical and Mechanical Properties of Polymeric Blends. Materials. 2023; 16(17):5758. https://doi.org/10.3390/ma16175758
Chicago/Turabian StyleTahir, Muhammad, and Alina Sionkowska. 2023. "Effect of Polydopamine and Curcumin on Physicochemical and Mechanical Properties of Polymeric Blends" Materials 16, no. 17: 5758. https://doi.org/10.3390/ma16175758
APA StyleTahir, M., & Sionkowska, A. (2023). Effect of Polydopamine and Curcumin on Physicochemical and Mechanical Properties of Polymeric Blends. Materials, 16(17), 5758. https://doi.org/10.3390/ma16175758







