Efficient Solar Desalination of Seawater Using a Novel Carbon Nanotube-Based Composite Aerogel
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
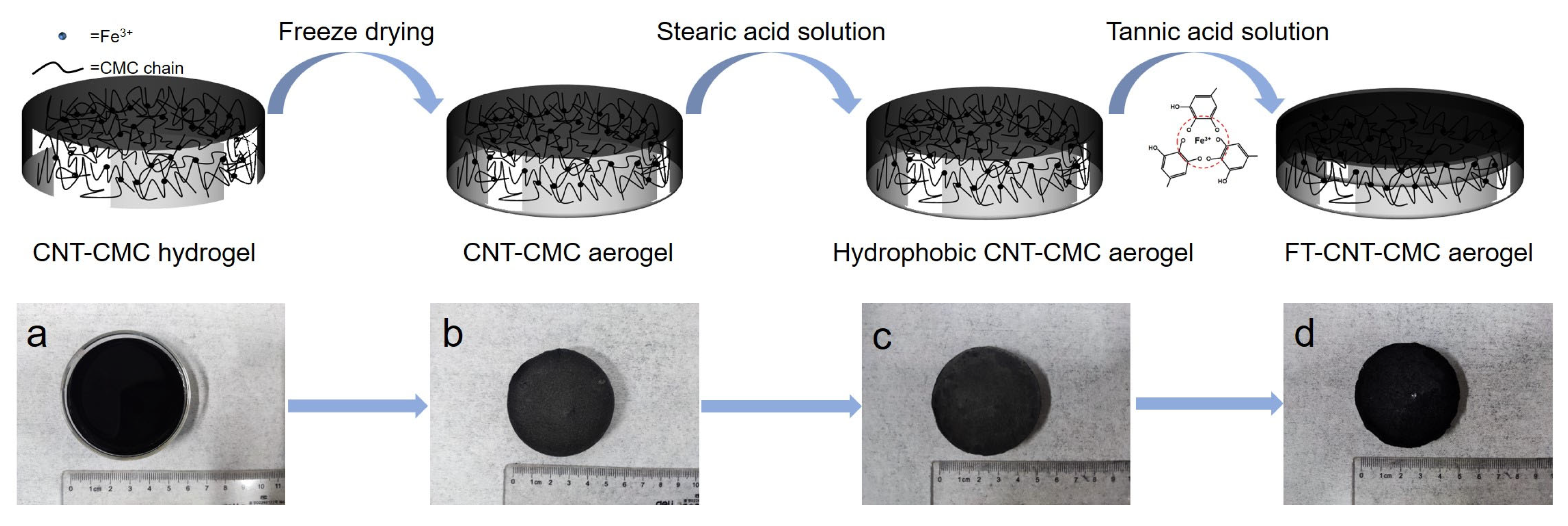
2.2. Synthesis of the CNT-CMC Aerogel
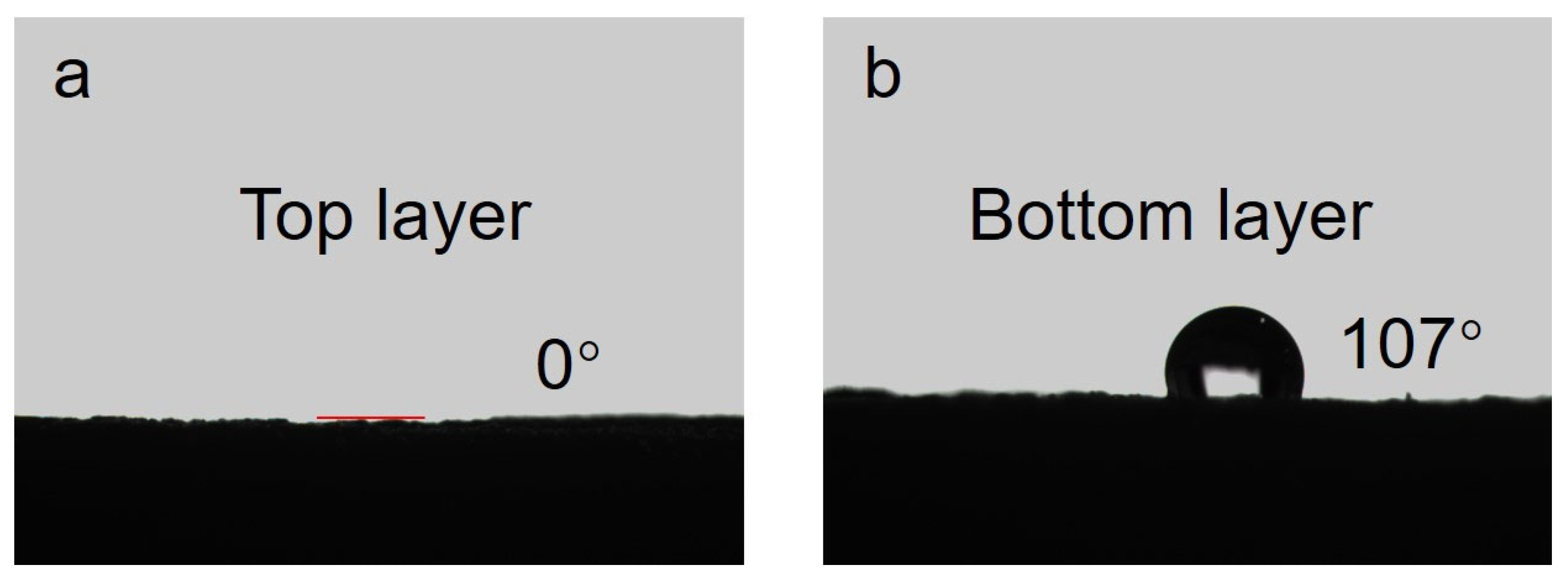
2.3. Synthesis of the FT-CNT-CMC Aerogel
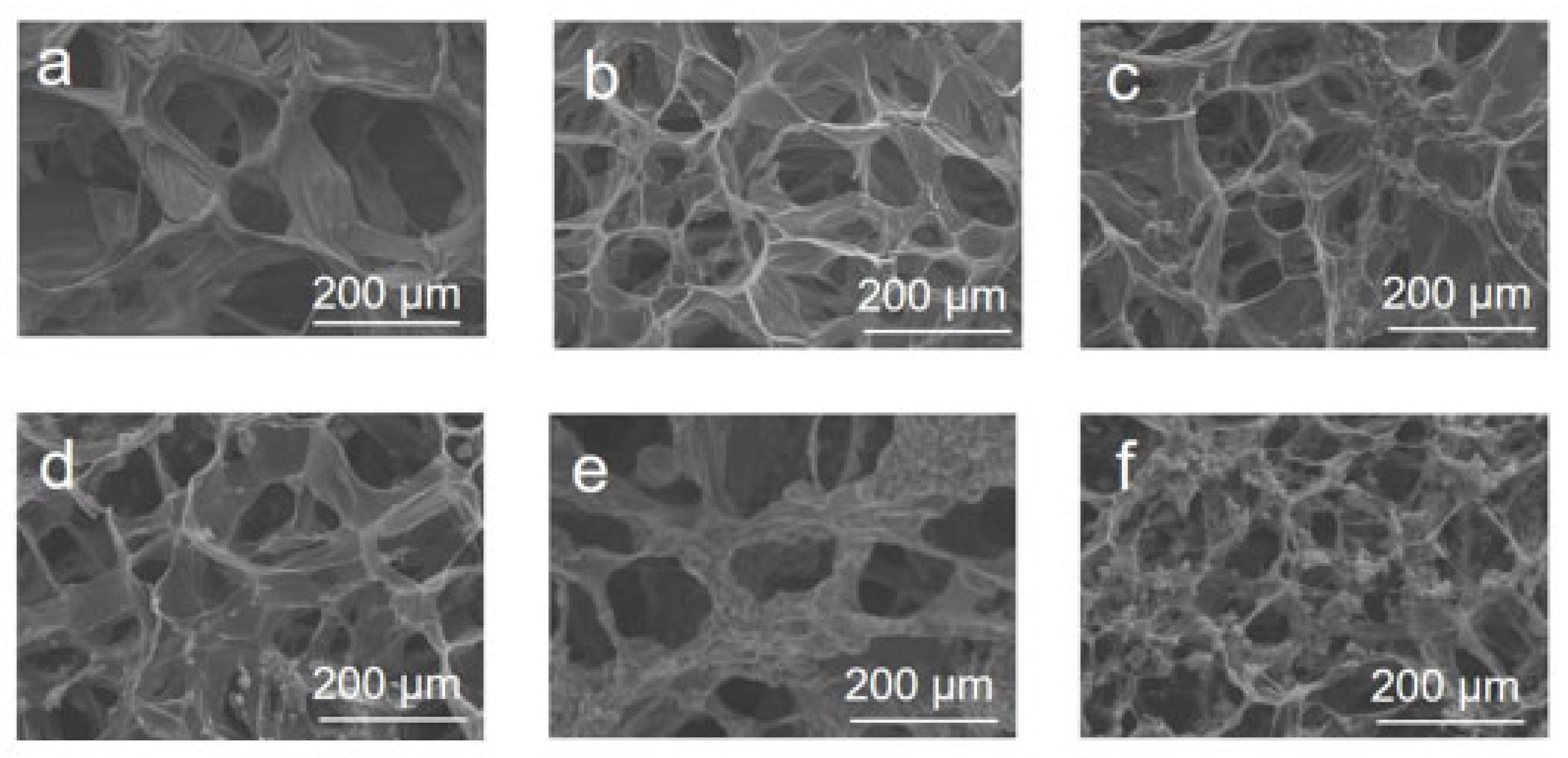
2.4. Characterization
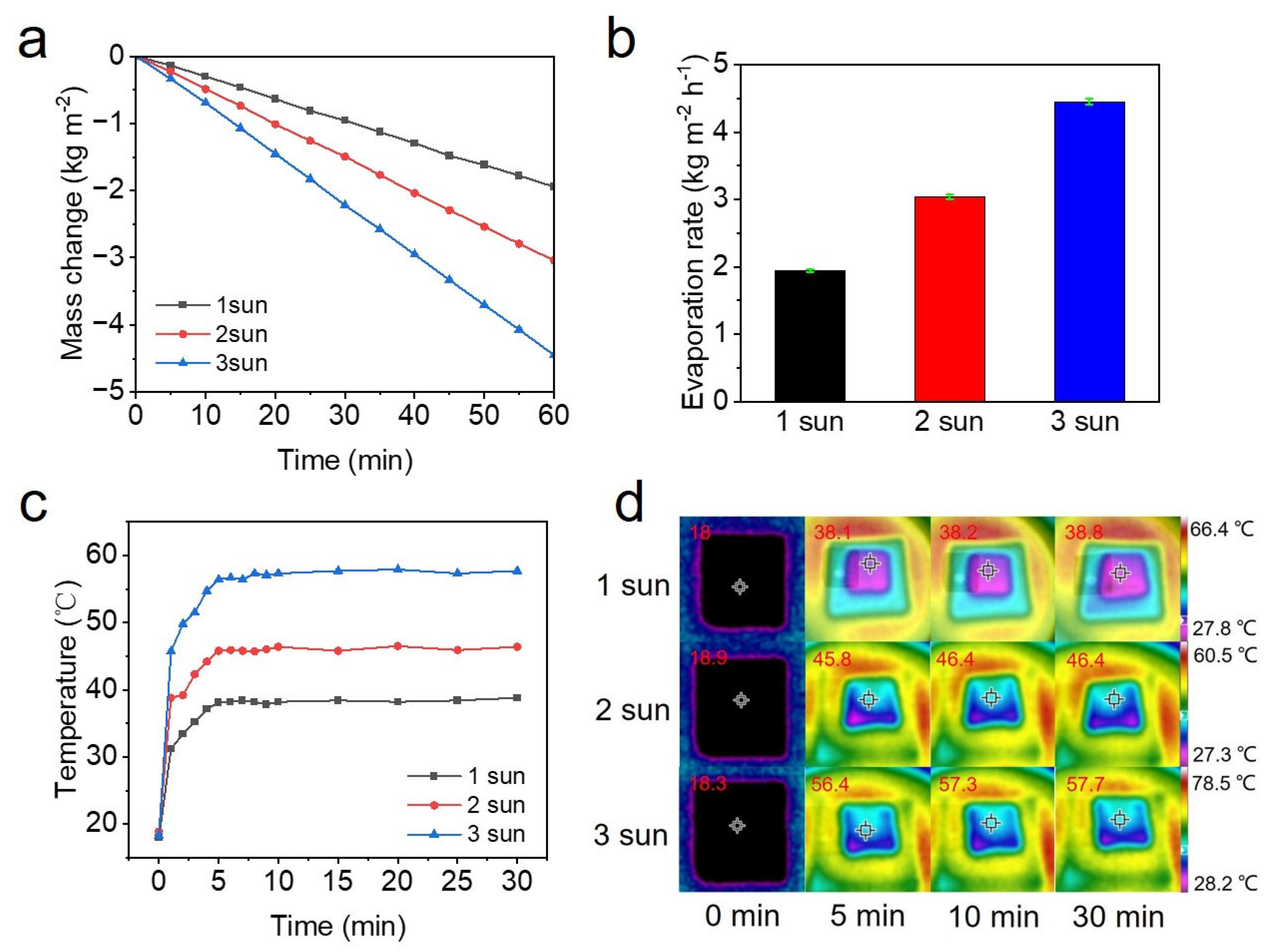
2.5. Solar Evaporation Measurement
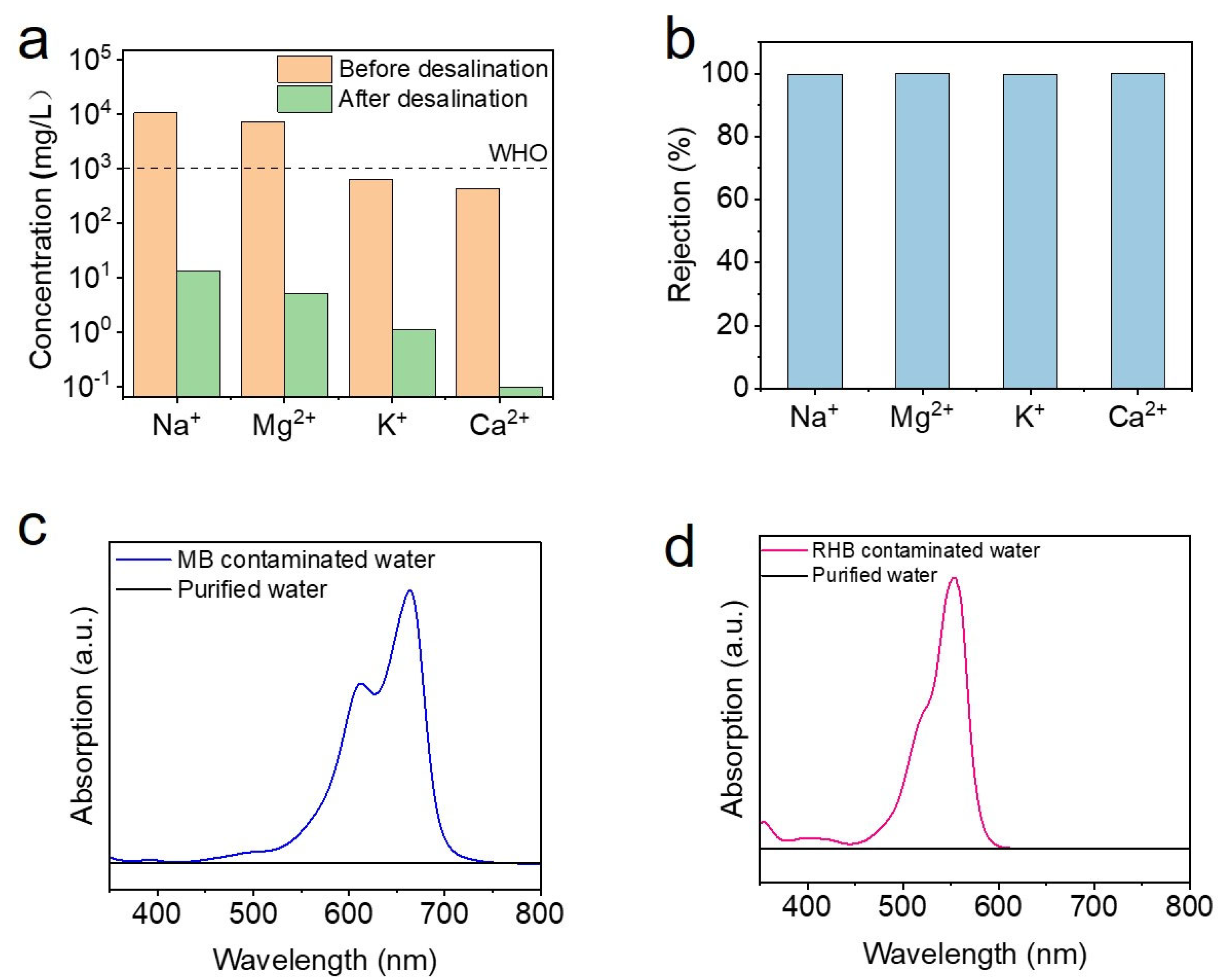
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ito, Y.; Tanabe, Y.; Han, J.H.; Fujita, T.; Tanigaki, K.; Chen, M.W. Multifunctional porous graphene for high-efficiency steam generation by heat localization. Adv. Mater. 2015, 27, 4302. [Google Scholar] [CrossRef]
- Ibrahim, I.; Seo, D.H.; McDonagh, A.M.; Shon, H.K.; Tijing, L. Semiconductor photothermal materials enabling efficient solar steam generation toward desalination and wastewater treatment. Desalination 2021, 500, 114853. [Google Scholar] [CrossRef]
- Storer, D.P.; Phelps, J.L.; Wu, X.; Owens, G.; Khan, N.I.; Xu, H.L. Graphene and rice-straw-fiber-based 3D Photothermal aerogels for highly efficient solar evaporation. ACS Appl. Mater. Interfaces 2020, 12, 15279. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Yan, M.; Zhang, W.; Wang, P.; Guan, W.; Zhang, S.; Yu, L.; Feng, J.; Gan, Z.; et al. Highly efficient self-floating jellyfish-like solar steam generators based on the partially carbonized enteromorpha aerogel. J. Colloid Interf. Sci. 2023, 630, 297. [Google Scholar] [CrossRef]
- Kim, S.; Tahir, Z.; Rashid, M.U.; Jang, J.I.; Kim, Y.S. Highly efficient solar vapor generation via a simple morphological alteration of TiO2 films grown on a glassy carbon foam. ACS Appl. Mater. Interfaces 2021, 13, 50911. [Google Scholar] [CrossRef]
- Selvam, A.; Jain, G.; Chaudhuri, R.G.; Mandal, M.K.; Chakrabarti, S. Avant-garde solar-thermal nanostructures: Nascent strategy into effective photothermal desalination. Sol. RRL 2022, 6, 2200321. [Google Scholar] [CrossRef]
- Politano, A.; Argurio, P.; Di Profio, G.; Sanna, V.; Cupolillo, A.; Chakraborty, S.; Arafat, H.A.; Curcio, E. Photothermal membrane distillation for seawater desalination. Adv. Mater. 2017, 29, 1603504. [Google Scholar] [CrossRef] [PubMed]
- Kiriarachchi, H.D.; Awad, F.S.; Hassan, A.A.; Bobb, J.A.; El Shall, M.S. Plasmonic chemically modified cotton nanocomposite fibers for efficient solar water desalination and wastewater treatment. Nanoscale 2018, 10, 18531. [Google Scholar] [CrossRef]
- Wani, T.A.; Garg, P.; Bera, S.; Bhattacharya, S.; Dutta, S.; Kumar, H.; Bera, A. Narrow-bandgap LaMO3 (M=Ni, Co) nanomaterials for efficient interfacial solar steam generation. J. Colloid Interface Sci. 2022, 612, 203. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Hong, Z.; Pu, Z.; Yao, Q.; Shi, J.; Yang, G.; Mi, B.; Yang, B.; Liu, X.; et al. A bioinspired capillary-driven pump for solar vapor generation. Nano Energy 2017, 42, 115. [Google Scholar] [CrossRef]
- Joo, B.S.; Kim, I.S.; Han, I.K.; Ko, H.; Kang, J.G.; Kang, G. Plasmonic silicon nanowires for enhanced heat localization and interfacial solar steam generation. Appl. Surf. Sci. 2022, 583, 152563. [Google Scholar] [CrossRef]
- Chen, R.; Wu, Z.; Zhang, T.; Yu, T.; Ye, M. Magnetically recyclable self-assembled thin films for highly efficient water evaporation by interfacial solar heating. Rsc Adv. 2017, 7, 19849. [Google Scholar] [CrossRef]
- Duang, H.M.; Myint, S.M.; Tran, T.Q.; Le, D.K. Chapter 6—Post-spinning treatments to carbon nanotube fibers. In Carbon Nanotube Fibers and Yarns; Miao, M., Ed.; Woodhead Publishing: Sawston, UK, 2020; pp. 103–134. [Google Scholar]
- Duong, H.M.; Tran, T.Q.; Kopp, R.; Myint, S.M.; Peng, L. Chapter 1—Direct Spinning of Horizontally Aligned Carbon Carbon Nanotube Fibers and Films from the Floating Catalyst Method. In Nanotube Superfiber Materials, 2nd ed.; Schulz, M.J., Shanov, V., Yin, Z., Cahay, M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 3–29. [Google Scholar]
- Wei, W.; Guan, Q.; You, C.; Yu, J.; Yuan, Z.; Qiang, P.; Zhou, C.; Ren, Y.; You, Z.; Zhang, F. Highly compact nanochannel thin films with exceptional thermal conductivity and water pumping for efficient solar steam generation. J. Mater. Chem. A 2020, 8, 13927. [Google Scholar] [CrossRef]
- Awad, F.S.; Kiriarachchi, H.D.; AbouZeid, K.M.; Ozgur, U.; El Shall, M.S. Plasmonic graphene polyurethane nanocomposites for efficient solar water deslination. J. Ind. Eng. Chem. 2018, 1, 976. [Google Scholar]
- Loo, S.L.; Vasquez, L.; Zahid, M.; Costantino, F.; Athanassiou, A.; Fragouli, D. 3D photothermal cryogels for solar-driven deslination. ACS Appl. Mater. Interfaces 2021, 13, 30542. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Q.; Wu, S.; Xu, B.; Xu, H. Multilayer polypyrrole nanosheets with self-organized surface structures for flexible and efficient solar-thermal energy conversion. Adv. Mater. 2019, 31, 1807716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xu, J.; Zhao, W.; Huang, F. Constructing Black Titania with Unique Nanocage Structure for Solar Desalination. ACS Appl. Mater. Interfaces 2016, 8, 31716. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Yang, C.; Tian, Y.; Han, Y.; Liu, J.; Yin, X.; Que, W. A hydrophobic surface enabled salt-blocking 2D Ti3C2 MXene membrane for efficient and stable solar desalination. J. Mater. Chem. A 2018, 6, 16196. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, G.; Fu, Z.; Yu, H.; Chen, S.; Quan, X. Vertically aligned janus MXene-based aerogels for solar desalination with high efficiency and salt resistance. ACS Nano 2019, 13, 13196. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Wei, N.; Xu, R.; Li, X.; Gong, L.; Cui, H. Porous Ni/CNTs composite membrane as solar absorber for highly efficient solar steam generation. Sol. Energy Mater. Sol. Cells 2022, 243, 111815. [Google Scholar] [CrossRef]
- Xiao, C.; Chen, L.; Mu, P.; Jia, J.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. Sugarcane-based photothermal materials for efficient solar steam generation. ChemistrySelect 2019, 4, 7891. [Google Scholar] [CrossRef]
- Lan, K.; Deng, Y.; Huang, A.; Li, S.Q.; Liu, G.; Xie, H.L. Highly-performance polyimide as an efficient photothermal material for solar-driven water evaporation. Polymer 2022, 256, 125177. [Google Scholar] [CrossRef]
- Ghasemi, H.; Ni, G.; Marconnet, A.M.; Loomis, J.; Yerci, S.; Miljkovic, N.; Chen, G. Solar steam generation by heat localization. Nat. Commun. 2014, 5, 4449. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, S.; Nguyen, A.T.; Song, S.H.; Gaillard, N.; Severa, G.; Ma, T.W.; Yoon, S.H.; Lee, W.C. Flexible graphite nanoflake/polydimethylsiloxane nanocomposites with promising solar-thermal conversion performance. ACS Appl. Energy Mater. 2023, 6, 2582. [Google Scholar] [CrossRef]
- Higgins, M.W.; Shakeel Rahmaan, A.R.; Devarpalli, R.R.; Shelke, M.V.; Jha, N. Carbon fabric based solar steam generation for wastewater treatment. Sol. Energy 2018, 159, 800. [Google Scholar] [CrossRef]
- Sreedhar, A.; Noh, J. Advancements in solar desalination of seawater by various Ti3C2 MXene based morphologies for freshwater generation: A review. Catalysts 2021, 11, 1435. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, T.H.; Nguyen, T.A.H.; Pham, D.D.; Nguyen, D.C.; Do, D.B.; Nguyen, H.V.; Ha, M.H.; Nguyen, Z.H. Durable, scalable and affordable iron (Ⅲ) based coconut husk photothermal material for highly efficient solar steam generation. Desalination 2021, 518, 115280. [Google Scholar] [CrossRef]
- Lin, S.; Chen, L.; Huang, L.; Cao, S.; Luo, X.; Liu, K. Novel antimicrobial chitosan-cellulose composite films bioconjugated with silver nanoparticles. Ind. Crops Prod. 2015, 70, 395. [Google Scholar] [CrossRef]
- Vasile, C.; Bumbu, G.G.; Dumitriu, R.P.; Staikos, G. Comparative study of the behavior of carboxymethyl cellulose-g-poly(N-isopropylacrylamide) copolymers and their equivalent physical blends. Eur. Polym. J. 2004, 40, 1209. [Google Scholar] [CrossRef]
- Mu, X.; Gu, Y.; Wang, P.; Wei, A.; Tian, Y.; Zhou, J.; Chen, Y.; Zhang, J.; Sun, Z.; Liu, J.; et al. Strategies for breaking theoretical evaporation limitation in direct solar steam generation. Sol. Energy Mater. Sol. Cells 2021, 220, 110842. [Google Scholar] [CrossRef]
- Matsuda, N.; Santos, J.H.; Takatsu, A.; Kato, K. In situ observation of absorption spectra and adsorbed species of methylene blue on indium-tin-oxide electrode by slab optical waveguide spectroscopy. Thin Solid Films 2003, 445, 313. [Google Scholar] [CrossRef]
- Chakraborty, G.; Bondarde, M.P.; Ray, A.K.; Some, S. Photophysical modulation of Rhodamine-B via π-π stacking with GQD and its further tuning by cucurbit uril. ChemistrySelect 2023, 8, e202203689. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wang, S.; Shuai, S.; Weng, Y.; Zheng, F. Efficient Solar Desalination of Seawater Using a Novel Carbon Nanotube-Based Composite Aerogel. Materials 2023, 16, 5815. https://doi.org/10.3390/ma16175815
Liu S, Wang S, Shuai S, Weng Y, Zheng F. Efficient Solar Desalination of Seawater Using a Novel Carbon Nanotube-Based Composite Aerogel. Materials. 2023; 16(17):5815. https://doi.org/10.3390/ma16175815
Chicago/Turabian StyleLiu, Shuai, Shun Wang, Shunxu Shuai, Yuyan Weng, and Fengang Zheng. 2023. "Efficient Solar Desalination of Seawater Using a Novel Carbon Nanotube-Based Composite Aerogel" Materials 16, no. 17: 5815. https://doi.org/10.3390/ma16175815




