Silica-Based Materials Containing Inorganic Red/NIR Emitters and Their Application in Biomedicine
Abstract
1. Introduction
2. Amorphous Silicon Dioxide (Silica, SiO2)
3. Lanthanides (Ln) and Upconversion Nanoparticles (UCNP)
Ln-Containing Silica-Based Materials
4. Quantum Dots (QD)
QD-Containing Silica-Based Materials
5. Ruthenium Complexes
Ruthenium Complexes-Containing Silica-Based Materials
6. Octahedral Metal Cluster Complexes
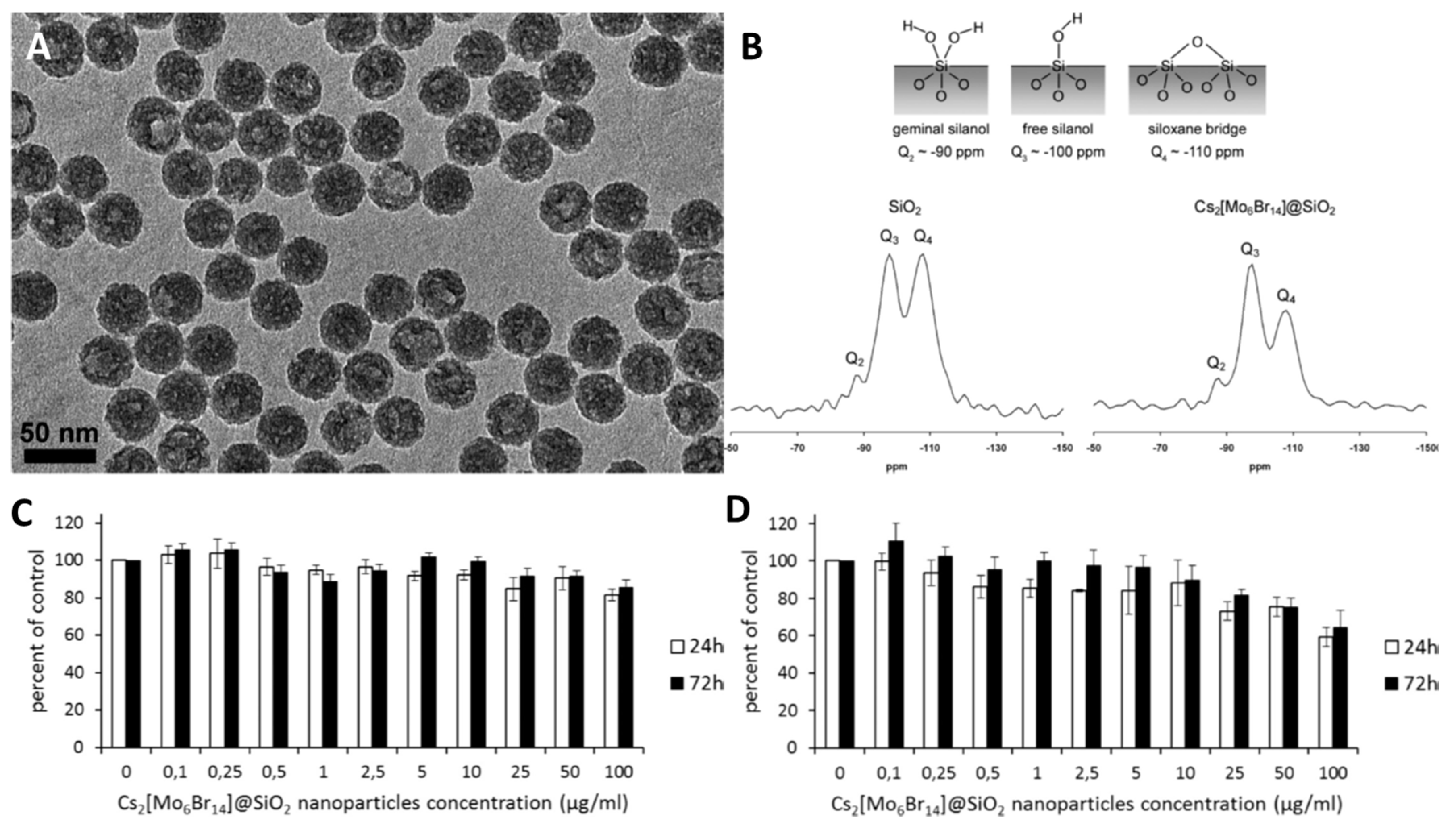
Cluster-Containing Silica-Based Materials
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Wang, S.; Zhang, F. Near-Infrared Luminescence High-Contrast in vivo Biomedical Imaging. Nat. Rev. Bioeng. 2023, 1, 60–78. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Z.; Yuan, Q. Recent Advances in Autofluorescence-Free Biosensing and Bioimaging Based on Persistent Luminescence Nanoparticles. Chin. Chem. Lett. 2019, 30, 1547–1556. [Google Scholar] [CrossRef]
- Li, S.; Wei, J.; Yao, Q.; Song, X.; Xie, J.; Yang, H. Emerging Ultrasmall Luminescent Nanoprobes for in vivo Bioimaging. Chem. Soc. Rev. 2023, 52, 1672–1696. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, C.S.; Yoon, H. Nanoparticulate Photoluminescent Probes for Bioimaging: Small Molecules and Polymers. Int. J. Mol. Sci. 2022, 23, 4949. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.D.; Jolliffe, K.A.; Pfeffer, F.M. Luminescent Probes for the Bioimaging of Small Anionic Species in vitro and in vivo. Chem. Soc. Rev. 2015, 44, 4547–4595. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Tang, T.; Wu, T.; Yu, X.; Zhang, Y.; Wang, M.; Zheng, J.; Ying, Y.; Chen, S.; Zhou, J.; et al. Perfecting and Extending the Near-Infrared Imaging Window. Light Sci. Appl. 2021, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular Luminescent Sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Wolfbeis, O.S.; Meier, R.J. Luminescent Probes and Sensors for Temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef]
- Nawrot, W.; Drzozga, K.; Baluta, S.; Cabaj, J.; Malecha, K. A Fluorescent Biosensors for Detection Vital Body Fluids’ Agents. Sensors 2018, 18, 2357. [Google Scholar]
- Huang, W.-T.; Rajendran, V.; Chan, M.-H.; Hsiao, M.; Chang, H.; Liu, R.-S. Near-Infrared Windows I and II Phosphors for Theranostic Applications: Spectroscopy, Bioimaging, and Light-Emitting Diode Photobiomodulation. Adv. Opt. Mater. 2023, 11, 2202061. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Yaneva, Z.; Ivanova, D.; Nikolova, N.; Toneva, M. Organic Dyes in Contemporary Medicinal Chemistry and Biomedicine. I. From the Chromophore to the Bioimaging/Bioassay Agent. Biotechnol. Biotechnol. Equip. 2022, 36, 1–14. [Google Scholar] [CrossRef]
- Li, L.; Dong, X.; Li, J.; Wei, J. A Short Review on NIR-II Organic Small Molecule Dyes. Dyes Pigm. 2020, 183, 108756. [Google Scholar] [CrossRef]
- Schnermann, M.J. Organic Dyes for Deep Bioimaging. Nature 2017, 551, 176–177. [Google Scholar] [CrossRef]
- Shen, J.; Rees, T.W.; Ji, L.; Chao, H. Recent Advances in Ruthenium(II) and Iridium(III) Complexes Containing Nanosystems for Cancer Treatment and Bioimaging. Coordin. Chem. Rev. 2021, 443, 214016. [Google Scholar] [CrossRef]
- Catalano, A.; Mariconda, A.; Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Longo, P. Biological Activities of Ruthenium NHC Complexes: An Update. Antibiotics 2023, 12, 365. [Google Scholar] [CrossRef]
- Perini, G.; Palmieri, V.; Ciasca, G.; De Spirito, M.; Papi, M. Unravelling the Potential of Graphene Quantum Dots in Biomedicine and Neuroscience. Sciences 2020, 21, 3712. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon Quantum Dots and Their Biomedical and Therapeutic Applications: A Review. Rsc Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Kargozar, S.; Hoseini, S.J.; Milan, P.B.; Hooshmand, S.; Kim, H.-W.; Mozafari, M. Quantum Dots: A Review from Concept to Clinic. Biotechnol. J. 2020, 15, 2000117. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum Dots in Biomedical Applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Mettenbrink, E.M.; Yang, W.; Wilhelm, S. Bioimaging with Upconversion Nanoparticles. Adv. Photonics Res. 2022, 3, 2200098. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, C.; Shi, Z. Current Advances in Lanthanide-Doped Upconversion Nanostructures for Detection and Bioapplication. Adv. Sci. 2016, 3, 1600029. [Google Scholar] [CrossRef]
- Borse, S.; Rafique, R.; Murthy, Z.V.P.; Park, T.J.; Kailasa, S.K. Applications of Upconversion Nanoparticles in Analytical and Biomedical Sciences: A Review. Analyst 2022, 147, 3155–3179. [Google Scholar] [CrossRef]
- Xian, T.; Meng, Q.; Gao, F.; Hu, M.; Wang, X. Functionalization of Luminescent Lanthanide Complexes for Biomedical Applications. Coordin. Chem. Rev. 2023, 474, 214866. [Google Scholar] [CrossRef]
- Li, J.; Khalid, A.; Verma, R.; Abraham, A.; Qazi, F.; Dong, X.; Liang, G.; Tomljenovic-Hanic, S. Silk Fibroin Coated Magnesium Oxide Nanospheres: A Biocompatible and Biodegradable Tool for Noninvasive Bioimaging Applications. Nanomaterials 2021, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Karle, T.J.; Khalid, A.; Abraham, A.N.; Shukla, R.; Gibson, B.C.; Simpson, D.A.; Djurišic, A.B.; Amekura, H.; Tomljenovic-Hanic, S. Room-Temperature Single-Photon Emission from Zinc Oxide Nanoparticle Defects and Their in vitro Photostable Intrinsic Fluorescence. Nanophotonics 2017, 6, 269–278. [Google Scholar] [CrossRef]
- Khalid, A.; Norello, R.; Abraham, A.N.; Tetienne, J.-P.; Karle, T.J.; Lui, E.W.C.; Xia, K.; Tran, P.A.; O’Connor, A.J.; Mann, B.G.; et al. Biocompatible and Biodegradable Magnesium Oxide Nanoparticles with in vitro Photostable Near-Infrared Emission: Short-Term Fluorescent Markers. Nanomaterials 2019, 9, 1360. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, Antimicrobial and Photocatalytic Activities of Green Synthesized Magnesium Oxide Nanoparticles (MgONPs) Using Aqueous Extract of Sargassum Wightii. J. Photochem. Photobiol. B 2019, 190, 86–97. [Google Scholar] [CrossRef]
- Verma, R.; Pathak, S.; Srivastava, A.K.; Prawer, S.; Tomljenovic-Hanic, S. ZnO Nanomaterials: Green Synthesis, Toxicity Evaluation and New Insights in Biomedical Applications. J. Alloys Compd. 2021, 876, 160175. [Google Scholar] [CrossRef]
- Kaur, N.; Aditya, R.N.; Singh, A.; Kuo, T.-R. Biomedical Applications for Gold Nanoclusters: Recent Developments and Future Perspectives. Nanoscale Res. Lett. 2018, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, Y.; Hao, Y.; Li, X.; Zvyagin, A.V.; Whittaker, A.K.; Cui, Y.; Yang, B.; Lin, Q.; Li, Y. Ultrasmall Red Fluorescent Gold Nanoclusters for Highly Biocompatible and Long-Time Nerve Imaging. Part. Part. Syst. Charact. 2021, 38, 2100001. [Google Scholar] [CrossRef]
- Mordini, D.; Mavridi-Printezi, A.; Menichetti, A.; Cantelli, A.; Li, X.; Montalti, M. Luminescent Gold Nanoclusters for Bioimaging: Increasing the Ligand Complexity. Nanomaterials 2023, 13, 648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, X.; Chen, W.; He, M.; Yu, Y.; Gao, G.; Sun, T. Advances of Gold Nanoclusters for Bioimaging. iScience 2022, 25, 105022. [Google Scholar] [CrossRef]
- Kirakci, K.; Shestopalov, M.A.; Lang, K. Recent Developments on Luminescent Octahedral Transition Metal Cluster Complexes Towards Biological Applications. Coordin. Chem. Rev. 2023, 481, 215048. [Google Scholar] [CrossRef]
- Svechkarev, D.; Mohs, M.A. Organic Fluorescent Dye-based Nanomaterials: Advances in the Rational Design for Imaging and Sensing Applications. Curr. Med. Chem. 2019, 26, 4042–4064. [Google Scholar] [CrossRef]
- Li, P.; Li, H. Recent Progress in the Lanthanide-Complexes Based Luminescent Hybrid Materials. Coordin. Chem. Rev. 2021, 441, 213988. [Google Scholar] [CrossRef]
- Cotruvo, J.A., Jr. The Chemistry of Lanthanides in Biology: Recent Discoveries, Emerging Principles, and Technological Applications. ACS Cent. Sci. 2019, 5, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Karges, J.; Kuang, S.; Maschietto, F.; Blacque, O.; Ciofini, I.; Chao, H.; Gasser, G. Rationally Designed Ruthenium Complexes for 1- and 2-Photon Photodynamic Therapy. Nat. Commun. 2020, 11, 3262. [Google Scholar] [CrossRef]
- Hu, L.; Zhong, H.; He, Z. Toxicity Evaluation of Cadmium-Containing Quantum Dots: A Review of Optimizing Physicochemical Properties to Diminish Toxicity. Colloids Surf. B 2021, 200, 111609. [Google Scholar] [CrossRef]
- Singh, D.; Thapa, S.; Singh, K.R.B.; Verma, R.; Singh, R.P.; Singh, J. Cadmium Selenide Quantum Dots and Its Biomedical Applications. Mater. Lett. X 2023, 18, 100200. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, C.; Zhou, J.; Ji, H.; Qin, Y.; Li, G.; Wu, L.; Zhou, X. Conjugated Polymer-Based Luminescent Probes for Ratiometric Detection of Biomolecules. J. Mater. Chem. B 2022, 10, 7309–7327. [Google Scholar] [CrossRef] [PubMed]
- Polícia, R.; Correia, D.M.; Peřinka, N.; Tubio, C.R.; Lanceros-Méndez, S. Influence of Polymer Matrix on the Luminescence of Phosphor Based Printable Electroluminescent Materials and Devices. Polymer 2023, 268, 125700. [Google Scholar] [CrossRef]
- Montalti, M.; Prodi, L.; Rampazzo, E.; Zaccheroni, N. Dye-Doped Silica Nanoparticles as Luminescent Organized Systems for Nanomedicine. Chem. Soc. Rev. 2014, 43, 4243–4268. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, W.; Tang, J.; Wang, Y.; Liu, J.; Huang, L.; Wang, Y.; Guo, F.; Wang, J.; Shen, W.; et al. Classification, Synthesis, and Application of Luminescent Silica Nanoparticles: A Review. Nanoscale Res. Lett. 2019, 14, 190. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Zhang, Y.; Tan, J.-C. Confinement of Luminescent Guests in Metal–Organic Frameworks: Understanding Pathways from Synthesis and Multimodal Characterization to Potential Applications of LG@MOF Systems. Chem. Rev. 2022, 122, 10438–10483. [Google Scholar] [CrossRef]
- Sun, Z.; Khurshid, A.; Sohail, M.; Qiu, W.; Cao, D.; Su, S.-J. Encapsulation of Dyes in Luminescent Metal-Organic Frameworks for White Light Emitting Diodes. Nanomaterials 2021, 11, 2761. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shah, Z.H.; Zhang, S.; Lu, R. Silica-Based Nanocomposites via Reverse Microemulsions: Classifications, Preparations, and Applications. Nanoscale 2014, 6, 4418–4437. [Google Scholar] [CrossRef]
- Shirshahi, V.; Soltani, M. Solid Silica Nanoparticles: Applications in Molecular Imaging. Contrast Media Mol. Imaging 2015, 10, 1–17. [Google Scholar] [CrossRef]
- Atabaev, T.; Hong, N.H. Chapter 4—Silica-Based Nanostructures in Biomedicine. In Nano-Sized Multifunctional Materials; Hong, N.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 73–88. [Google Scholar]
- Ahmadi, F.; Sodagar-Taleghani, A.; Ebrahimnejad, P.; Moghaddam, S.P.H.; Ebrahimnejad, F.; Asare-Addo, K.; Nokhodchi, A. A Review on the Latest Developments of Mesoporous Silica Nanoparticles as a Promising Platform for Diagnosis and Treatment of Cancer. Int. J. Pharm. 2022, 625, 122099. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of Mesoporous Silica Nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, Z.A.; Rubloff, G.W.; Bassous, E. Transmission, Photoconductivity, and the Experimental Band Gap of Thermally Grown SiO2 Films. Phys. Rev. B 1979, 19, 3107–3117. [Google Scholar] [CrossRef]
- Martin-Samos, L.; Bussi, G.; Ruini, A.; Molinari, E.; Caldas, M.J. Unraveling Effects of Disorder on the Electronic Structure of SiO2 from First Principles. Phys. Rev. B 2010, 81, 081202. [Google Scholar] [CrossRef]
- Mallik, A.K.; Moktadir, M.A.; Rahman, M.A.; Shahruzzaman, M.; Rahman, M.M. Progress in Surface-Modified Silicas for Cr(VI) Adsorption: A Review. J. Hazard. Mater. 2022, 423, 127041. [Google Scholar] [CrossRef]
- Mahtabani, A.; Rytöluoto, I.; Anyszka, R.; He, X.; Saarimäki, E.; Lahti, K.; Paajanen, M.; Dierkes, W.; Blume, A. On the Silica Surface Modification and Its Effect on Charge Trapping and Transport in PP-Based Dielectric Nanocomposites. ACS Appl. Polym. Mater. 2020, 2, 3148–3160. [Google Scholar] [CrossRef]
- Choi, M.; Choi, W.-K.; Jung, C.-H.; Kim, S.-B. The Surface Modification and Characterization of SiO2 Nanoparticles for Higher Foam Stability. Sci. Rep. 2020, 10, 19399. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Akhter, F.; Rao, A.A.; Abbasi, M.N.; Wahocho, S.A.; Mallah, M.A. Anees-ur-Rehman and Z. A. Chandio. A Comprehensive Review of Synthesis, Applications and Future Prospects for Silica Nanoparticles (SNPs). Silicon 2022, 14, 8295–8310. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Porrang, S.; Davaran, S.; Rahemi, N.; Allahyari, S.; Mostafavi, E. How Advancing are Mesoporous Silica Nanoparticles? A Comprehensive Review of the Literature. Int. J. Nanomed. 2022, 17, 1803–1827. [Google Scholar] [CrossRef]
- Bunzli, J.-C.G. On the Design of Highly Luminescent Lanthanide Complexes. Coordin. Chem. Rev. 2015, 293, 19–47. [Google Scholar] [CrossRef]
- Quici, S.; Cavazzini, M.; Marzanni, G.; Accorsi, G.; Armaroli, N.; Ventura, B.; Barigelletti, F. Visible and Near-Infrared Intense Luminescence from Water-Soluble Lanthanide [Tb(III), Eu(III), Sm(III), Dy(III), Pr(III), Ho(III), Yb(III), Nd(III), Er(III)] Complexes. Inorg. Chem. 2005, 44, 529–537. [Google Scholar] [CrossRef]
- Duan, C.; Liang, L.; Li, L.; Zhang, R.; Xu, Z.P. Recent Progress in Upconversion Luminescence Nanomaterials for Biomedical Applications. J. Mater. Chem. B 2018, 6, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, F.; Xue, Y.; Gu, R.; Liu, H.; Xia, D.; Liu, Y. The Biological Functions of Europium-Containing Biomaterials: A Systematic Review. Mater. Today Bio 2023, 19, 100595. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; Diamente, P.R.; van Veggel, F.C. Silica-Coated Ln3+-Doped LaF3 Nanoparticles as Robust Down- and Upconverting Biolabels. Chem.-Eur. J. 2006, 12, 5878–5884. [Google Scholar] [CrossRef]
- Shan, J.N.; Ju, Y.G. Controlled Synthesis of Lanthanide-Doped NaYF4 Upconversion Nanocrystals via Ligand Induced Crystal Phase Transition and Silica Coating. Appl. Phys. Lett. 2007, 91, 123103. [Google Scholar] [CrossRef]
- Ehlert, O.; Thomann, R.; Darbandi, M.; Nann, T. A Four-Color Colloidal Multiplexing Nanoparticle System. ACS Nano 2008, 2, 120–124. [Google Scholar] [CrossRef]
- Ocaña, M.; Cantelar, E.; Cussó, F. A Facile Single-Step Procedure for the Synthesis of Luminescent Ln3+:YVO4 (Ln=Eu or Er+Yb)-Silica Nanocomposites. Mater. Chem. Phys. 2011, 125, 224–230. [Google Scholar] [CrossRef]
- Wang, L.L.; Chen, H.A.; Zhang, D.S.; Zhao, D.; Qin, W.P. Dual-Mode Luminescence from Lanthanide Tri-Doped NaYF4 Nanocrystals. Mater. Lett. 2011, 65, 504–506. [Google Scholar] [CrossRef]
- Liu, L.N.; Xiao, H.Y.; An, X.Y.; Zhang, Y.S.; Qin, R.F.; Liu, L.S.; Zhang, D.M.; Sun, R.R.; Chen, L.F. Synthesis and Photoluminescence Properties of Core-Shell Structured YVO4:Eu3+@SiO2 Nanocomposites. Chem. Phys. Lett. 2015, 619, 169–173. [Google Scholar] [CrossRef]
- van Hest, J.J.H.A.; Blab, G.A.; Gerritsen, H.C.; Donega, C.D.; Meijerink, A. Incorporation of Ln-Doped LaPO4 Nanocrystals as Luminescent Markers in Silica Nanoparticles. Nanoscale Res. Lett. 2016, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, J.A.; Xu, J.; Xue, X.J.; Chen, H.Y.; Liu, X.G. Tunable Upconversion Emissions From Lanthanide-Doped Monodisperse-NaYF4 Nanoparticles. Spectrosc. Lett. 2010, 43, 400–405. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Lim, K.M.; Sim, E.K.W.; Ye, L. NIR-to-Visible Upconversion Nanoparticles for Fluorescent Labeling and Targeted Delivery of siRNA. Nanotechnology 2009, 20, 155101. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Chen, M.; Gao, Y.; Wu, D.M.; Feng, W.; Li, F.Y. Gd3+ Complex-Modified NaLuF4-Based Upconversion Nanophosphors for Trimodality Imaging of NIR-to-NIR Upconversion Luminescence, X-ray Computed Tomography and Magnetic Resonance. Biomaterials 2012, 33, 5394–5405. [Google Scholar] [CrossRef]
- Chien, Y.H.; Chou, Y.L.; Wang, S.W.; Hung, S.T.; Liau, M.C.; Chao, Y.J.; Su, C.H.; Yeh, C.S. Near-Infrared Light Photocontrolled Targeting, Bioimaging, and Chemotherapy with Caged Upconversion Nanoparticles In Vitro and In Vivo. ACS Nano 2013, 7, 8516–8528. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.Y.; He, X.X.; Liu, L.; You, H.P.; Zhang, H.M.; Wang, Z.X. Conjugation of NaGdF4 Upconverting Nanoparticles on Silica Nanospheres as Contrast Agents for Multi-Modality Imaging. Biomaterials 2013, 34, 5218–5225. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, W.; Yang, T.S.; Yi, T.; Li, F.Y. Upconversion Luminescence Imaging of Cells and Small Animals. Nat. Protoc. 2013, 8, 2033–2044. [Google Scholar] [CrossRef]
- Sun, L.N.; Ge, X.Q.; Liu, J.L.; Qiu, Y.N.; Wei, Z.W.; Tian, B.; Shi, L.Y. Multifunctional Nanomesoporous Materials with Upconversion (in vivo) and Downconversion (in vitro) Luminescence Imaging Based on Mesoporous Capping UCNPs and Linking Lanthanide Complexes. Nanoscale 2014, 6, 13242–13252. [Google Scholar] [CrossRef]
- Wang, Y.H.; Song, S.Y.; Liu, J.H.; Liu, D.P.; Zhang, H.J. ZnO-Functionalized Upconverting Nanotheranostic Agent: Multi-Modality Imaging-Guided Chemotherapy with On-Demand Drug Release Triggered by pH. Angew. Chem. Int. Ed. 2015, 54, 536–540. [Google Scholar] [CrossRef]
- Kalluru, P.; Vankayala, R.; Chiang, C.S.; Hwang, K.C. Unprecedented “All-in-One” Lanthanide-Doped Mesoporous Silica Frameworks for Fluorescence/MR Imaging and Combination of NIR Light Triggered Chemo-Photodynamic Therapy of Tumors. Adv. Funct. Mater. 2016, 26, 7908–7920. [Google Scholar] [CrossRef]
- Kim, T.; Lee, N.; Park, Y.I.; Kim, J.; Kim, J.; Lee, E.Y.; Yi, M.; Kim, B.G.; Hyeon, T.; Yu, T.; et al. Mesoporous Silica-Coated Luminescent Eu3+ Doped GdVO4 Nanoparticles for Multimodal Imaging and Drug Delivery. Rsc. Adv. 2014, 4, 45687–45695. [Google Scholar] [CrossRef]
- Kostiv, U.; Janoušková, O.; Šlouf, M.; Kotov, N.; Engstová, H.; Smolková, K.; Ježek, P.; Horák, D. Silica-Modified Monodisperse Hexagonal Lanthanide Nanocrystals: Synthesis and Biological Properties. Nanoscale 2015, 7, 18096–18104. [Google Scholar] [CrossRef]
- Lu, S.; Tu, D.T.; Hu, P.; Xu, J.; Li, R.F.; Wang, M.; Chen, Z.; Huang, M.D.; Chen, X.Y. Multifunctional Nano-Bioprobes Based on Rattle-Structured Upconverting Luminescent Nanoparticles. Angew. Chem. Int. Ed. 2015, 54, 7915–7919. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, P.; Elbaum, D.; Mikulski, J.; Fronc, K.; Kamińska, I.; Morais, P.C.; de Souza, P.E.; Nunes, R.B.; Veiga-Souza, F.H.; Gruzeł, G.; et al. Upconversion Fluorescence Imaging of HeLa Cells Using ROS Generating SiO2-Coated Lanthanide-Doped NaYF4 Nanoconstructs. Rsc Adv. 2017, 7, 30262–30273. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Peng, J.J.; Huang, Q.; Li, C.Y.; Chen, M.; Sun, Y.; Lin, Q.N.; Zhu, L.Y.; Li, F.Y. Near-infrared photoregulated drug release in living tumor tissue via yolk-shell upconversion nanocages. Adv. Funct. Mater. 2014, 24, 363–371. [Google Scholar] [CrossRef]
- Saif, M.; Shebl, M.; Nabeel, A.I.; Shokry, R.; Hafez, H.; Mbarek, A.; Damak, K.; Maalej, R.; Abdel-Mottaleb, M.S.A. Novel Non-Toxic and Red Luminescent Sensor Based on Eu3+:Y2Ti2O7/SiO2 Nano-Powder for Latent Fingerprint Detection. Sens. Actuators B Chem. 2015, 220, 162–170. [Google Scholar] [CrossRef]
- Yang, D.M.; Ma, P.A.; Hou, Z.Y.; Cheng, Z.Y.; Li, C.X.; Lin, J. Current Advances in Lanthanide Ion (Ln3+)-Based Upconversion Nanomaterials for Drug Delivery. Chem. Soc. Rev. 2015, 44, 1416–1448. [Google Scholar] [CrossRef]
- Szczeszak, A.; Ekner-Grzyb, A.; Runowski, M.; Szutkowski, K.; Mrówczyńska, L.; Kaźmierczak, Z.; Grzyb, T.; Dąbrowska, K.; Giersig, M.; Lis, S. Spectroscopic, Structural and in vitro Cytotoxicity Evaluation of Luminescent, Lanthanide Doped Core@Shell Nanomaterials GdVO4:Eu3+5%@SiO2@NH2. J. Colloid Interface Sci. 2016, 481, 245–255. [Google Scholar] [CrossRef]
- Runowski, M.; Stopikowska, N.; Szeremeta, D.; Goderski, S.; Skwierczyńska, M.; Lis, S. Upconverting Lanthanide Fluoride Core@Shell Nanorods for Luminescent Thermometry in the First and Second Biological Windows: β-NaYF4:Yb3+– Er3+@SiO2 Temperature Sensor. ACS Appl. Mater. Interfaces 2019, 11, 13389–13396. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Liu, S.; Song, Y.; Xie, S.; Guo, Z.; Xu, J.; Xu, L. A Versatile Luminescent Resonance Energy Transfer (LRET)-Based Ratiometric Upconversion Nanoprobe for Intracellular miRNA Biosensing. J. Mater. Chem. B 2020, 8, 5952–5961. [Google Scholar] [CrossRef]
- Voronovic, E.; Skripka, A.; Jarockyte, G.; Ger, M.; Kuciauskas, D.; Kaupinis, A.; Valius, M.; Rotomskis, R.; Vetrone, F.; Karabanovas, V. Uptake of Upconverting Nanoparticles by Breast Cancer Cells: Surface Coating versus the Protein Corona. ACS Appl. Mater. Interfaces 2021, 13, 39076–39087. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Y.; Jia, M.; Sun, Z.; Ding, B.; Shao, S.; Jiang, F.; Fu, Z.; Ma, P.A.; Lin, J. One-Pot Synthesis of SiO2-Coated Gd2(WO4)3:Yb3+/Ho3+ Nanoparticles for Simultaneous Multi-Imaging, Temperature Sensing and Tumor Inhibition. Dalton T 2019, 48, 10537–10546. [Google Scholar] [CrossRef]
- Sun, X.; Sun, J.; Dong, B.; Huang, G.; Zhang, L.; Zhou, W.; Lv, J.; Zhang, X.; Liu, M.; Xu, L.; et al. Noninvasive Temperature Monitoring for Dual-Modal Tumor Therapy Based on Lanthanide-Doped Up-conversion Nanocomposites. Biomaterials 2019, 201, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zhao, S.; Liu, G.; Chen, H.; Ma, L.; You, H.; Liu, C.; Wang, Z. Construction of Lanthanide-Doped Upconversion Nanoparticle-Uelx Europaeus Agglutinin-I Bioconjugates with Brightness Red Emission for Ultrasensitive in vivo Imaging of Colorectal Tumor. Biomaterials 2019, 212, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, W.; Dong, S.; Zhang, F.; Dong, Y.; Tian, B.; He, F.; Gai, S.; Yang, P. An All-In-One Theranostic Nanoplatform Based on Upconversion Dendritic Mesoporous Silica Nanocomposites for Synergistic Chemodynamic/Photodynamic/Gas Therapy. Nanoscale 2020, 12, 24146–24161. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Ng, M.; Skripka, A.; Cheng, T.; Li, X.; Bhakoo, K.K.; Chang, A.Y.; Rosei, F.; Vetrone, F. One-Pot Synthesis of Theranostic Nanocapsules with Lanthanide Doped Nanoparticles. Chem. Sci. 2020, 11, 6653–6661. [Google Scholar] [CrossRef]
- Bastos, V.; Oskoei, P.; Andresen, E.; Saleh, M.I.; Rühle, B.; Resch-Genger, U.; Oliveira, H. Stability, Dissolution, and Cytotoxicity of NaYF4-Upconversion Nanoparticles with Different coatings. Sci. Rep. 2022, 12, 3770. [Google Scholar] [CrossRef]
- Miletto, I.; Gionco, C.; Paganini, M.C.; Cerrato, E.; Marchese, L.; Gianotti, E. Red Upconverter Nanocrystals Functionalized with Verteporfin for Photodynamic Therapy Triggered by Upconversion. Int. J. Mol. Sci. 2022, 23, 6951. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.C.; Lee, Y.B.; Kim, I.S.; Park, Y.K.; Hur, N.H. Fabrication of Silica-Coated Magnetic Nanoparticles with Highly Photoluminescent Lanthanide Probes. Chem. Commun. 2007, 16, 1644–1646. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Fu, L.S.; Zhou, Y.J.; Su, H.Q. Novel Bifunctional Magnetic-Near-Infrared Luminescent Nanocomposites: Near-Infrared Emission from Nd and Yb. Photoch. Photobio. Sci. 2011, 10, 548–553. [Google Scholar] [CrossRef]
- Yan, B.; Shao, Y.F. Multifunctional Nanocomposites of Lanthanide (Eu3+, Tb3+) Complexes Functionalized Magnetic Mesoporous Silica Nanospheres Covalently Bonded with Polymer Modified ZnO. Dalton T 2013, 42, 9565–9573. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.R.; Zhao, J.; Yu, S.Y.; Song, S.Y. Multifunctional Visible/Near-Infrared Luminescent Core-Shell Magnetic Silica Structured Nanocomposites. Crystengcomm 2014, 16, 6257–6262. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Yang, W.; Li, Q.G. Dual-Lanthanide-Chelated Silica Nanoparticles as Labels for Highly Sensitive Time-Resolved Fluorometry. Chem. Mater. 2007, 19, 5875–5881. [Google Scholar] [CrossRef]
- Ai, K.L.; Zhang, B.H.; Lu, L.H. Europium-Based Fluorescence Nanoparticle Sensor for Rapid and Ultrasensitive Detection of an Anthrax Biomarker. Angew. Chem. Int. Ed. 2009, 48, 304–308. [Google Scholar] [CrossRef]
- Pinho, S.L.C.; Faneca, H.; Geraldes, C.F.G.C.; Delville, M.H.; Carlos, L.D.; Rocha, J. Lanthanide-DTPA Grafted Silica Nanoparticles as Bimodal-Imaging Contrast Agents. Biomaterials 2012, 33, 925–935. [Google Scholar] [CrossRef]
- Pinho, S.L.C.; Faneca, H.; Geraldes, C.F.G.C.; Rocha, J.; Carlos, L.D.; Delville, M.H. Silica Nanoparticles for Bimodal MRI-Optical Imaging by Grafting Gd3+ and Eu3+/Tb3+ Complexes. Eur. J. Inorg. Chem. 2012, 2012, 2828–2837. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.N.; Liu, J.L.; Peng, Y.X.; Ge, X.Q.; Shi, L.Y.; Huang, W. Multicolor (Vis-NIR) Mesoporous Silica Nanospheres Linked with Lanthanide Complexes Using 2-(5-bromothiophen)imidazo [4,5-f][1,10]phenanthroline for in vitro Bioimaging. Dalton T 2015, 44, 237–246. [Google Scholar] [CrossRef]
- Mutti, A.M.G.; Santos, J.A.O.; Cavalcante, D.G.S.M.; Gomes, A.S.; Job, A.E.; Teixeira, G.R.; Pires, A.M.; Lima, S.A.M. Design of a Red-Emitter Hybrid Material for Bioimaging: Europium Complexes Grafted on Silica Particles. Mater. Today Chem. 2019, 14, 100204. [Google Scholar] [CrossRef]
- Santos, J.A.O.; Brito, L.D.; da Costa, P.I.; Pires, A.M.; Lima, S.A.M. Development of Red-Luminescent Hybrids as Contrast Agents for Cell Imaging: A Correlation Among Surface, Luminescence, and Biological Properties. Opt. Mater. 2023, 139, 113759. [Google Scholar] [CrossRef]
- Mutti, A.M.G.; Canisares, F.S.M.; Santos, J.A.O.; Santos, B.C.; Cavalcante, D.G.S.M.; Job, A.E.; Pires, A.M.; Lima, S.A.M. Silica-Based Nanohybrids Containing Europium Complexes Covalently Grafted: Structural, Luminescent, and Cell Labeling Investigation. J. Sol.-Gel. Sci. Technol. 2023, 107, 754–770. [Google Scholar] [CrossRef]
- Francis, B.; Neuhaus, B.; Reddy, M.L.P.; Epple, M.; Janiak, C. Amine-Functionalized Silica Nanoparticles Incorporating Covalently Linked Visible-Light-Excitable Eu3+ Complexes: Synthesis, Characterization, and Cell-Uptake Studies. Eur. J. Inorg. Chem. 2017, 2017, 3205–3213. [Google Scholar] [CrossRef]
- Song, B.; Li, M.; Ren, J.; Liu, Q.; Wen, X.; Zhang, W.; Yuan, J. A Multifunctional Nanoprobe Based on Europium(III) Complex–Fe3O4 Nanoparticles for Bimodal Time-Gated Luminescence/Magnetic Resonance Imaging of Cancer Cells in vitro and in vivo. New J. Chem. 2022, 46, 9658–9665. [Google Scholar] [CrossRef]
- Wu, J.; Ye, Z.Q.; Wang, G.L.; Jin, D.Y.; Yuan, J.L.; Guan, Y.F.; Piper, J. Visible-Light-Sensitized Highly Luminescent Europium Nanoparticles: Preparation and Application for Time-Gated Luminescence Bioimaging. J. Mater. Chem. 2009, 19, 1258–1264. [Google Scholar] [CrossRef]
- Jiang, H.F.; Wang, G.L.; Zhang, W.Z.; Liu, X.Y.; Ye, Z.Q.; Jin, D.Y.; Yuan, J.L.; Liu, Z.G. Preparation and Time-Resolved Luminescence Bioassay Application of Multicolor Luminescent Lanthanide Nanoparticles. J. Fluoresc. 2010, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Dai, Z.C.; Zhang, L.; Zhang, R.Y.; Ye, Z.Q.; Wu, J.; Jin, D.Y.; Yuan, J.L. Preparation and Time-Gated Luminescence Bioimaging Applications of Long Wavelength-Excited Silica-Encapsulated Europium Nanoparticles. Nanoscale 2012, 4, 3551–3557. [Google Scholar] [CrossRef]
- Guo, X.M.; Canet, J.L.; Boyer, D.; Gautier, A.; Mahiou, R. Sol-Gel Emulsion Synthesis of Biphotonic Core-Shell Nanoparticles Based on Lanthanide Doped Organic-Inorganic Hybrid Materials. J. Mater. Chem. 2012, 22, 6117–6122. [Google Scholar] [CrossRef]
- Lapadula, G.; Bourdolle, A.; Allouche, F.; Conley, M.P.; del Rosal, I.; Maron, L.; Lukens, W.W.; Guyot, Y.; Andraud, C.; Brasselet, S.; et al. Near-IR Two Photon Microscopy Imaging of Silica Nanoparticles Functionalized with Isolated Sensitized Yb(III) Centers. Chem. Mater. 2014, 26, 1062–1073. [Google Scholar] [CrossRef][Green Version]
- Granadeiro, C.M.; Ferreira, R.A.S.; Soares-Santos, P.C.R.; Carlos, L.D.; Trindade, T.; Nogueira, H.I.S. Lanthanopolyoxotungstates in Silica Nanoparticles: Multi-Wavelength Photoluminescent Core/Shell Materials. J. Mater. Chem. 2010, 20, 3313–3318. [Google Scholar] [CrossRef]
- Philippot, C.; Bourdolle, A.; Maury, O.; Dubois, F.; Boury, B.; Brustlein, S.; Brasselet, S.; Andraud, C.; Ibanez, A. Doped Silica Nanoparticles Containing Two-Photon Luminescent Eu(III) Complexes for the Development of Water Stable Bio-Labels. J. Mater. Chem. 2011, 21, 18613–18622. [Google Scholar] [CrossRef]
- Azevedo, C.B.; Batista, T.M.; de Faria, E.H.; Rocha, L.A.; Ciuffi, K.J.; Nassar, E.J. Nanospherical Silica as Luminescent Markers Obtained by Sol-Gel. J. Fluoresc. 2015, 25, 433–440. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Song, B.; Vandevyver, C.D.B.; Chauvin, A.S.; Wacker, J.B.; Bunzli, J.C.G. Increasing the Efficiency of Lanthanide Luminescent Bioprobes: Bioconjugated Silica Nanoparticles as Markers for Cancerous Cells. New J. Chem. 2010, 34, 2915–2921. [Google Scholar] [CrossRef]
- Gaillard, C.; Adumeau, P.; Canet, J.L.; Gautier, A.; Boyer, D.; Beaudoin, C.; Hesling, C.; Morel, L.; Mahiou, R. Monodisperse Silica Nanoparticles Doped with Dipicolinic Acid-Based Luminescent Lanthanide(III) Complexes for Bio-Labelling. J. Mater. Chem. B 2013, 1, 4306–4312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, Y.H.; Cao, Y.F.; Lv, W.; Yu, Q.; Liu, S.J.; Liu, X.M.; Shi, M.; Huang, W. Rational Design of Nanoparticles with Efficient Lanthanide Luminescence Sensitized by Iridium(III) Complex for Time-Gated Luminescence Bioimaging. Adv. Opt. Mater. 2015, 3, 233–240. [Google Scholar] [CrossRef]
- Shi, G.; Li, Z.; Zhang, Z.; Yin, Q.; Li, N.; Wang, S.; Qi, G.; Hao, L. Functionalized Europium-Doped Hollow Mesoporous Silica Nanospheres as a Cell Imaging and Drug Delivery Agents. Biochem. Biophys. Res. Commun. 2023, 674, 1–9. [Google Scholar] [CrossRef]
- Chan, M.H.; Lin, H.M. Preparation and Identification of Multifunctional Mesoporous Silica Nanoparticles for in vitro and in vivo Dual-Mode Imaging, Theranostics, and Targeted Tracking. Biomaterials 2015, 46, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Shi, M.; Zhao, L.Z.; Feng, W.; Li, F.Y.; Huang, C.H. Visible-Light-Excited and Europium-Emissive Nanoparticles for Highly-Luminescent Bioimaging in vivo. Biomaterials 2014, 35, 5830–5839. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ai, K.L.; Liu, J.H.; Sun, G.Y.; Yin, Q.; Lu, L.H. Multifunctional Envelope-Type Mesoporous Silica Nanoparticles for pH-Responsive Drug Delivery and Magnetic Resonance Imaging. Biomaterials 2015, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor Quantum Dots: Technological Progress and Future Challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef]
- Pu, Y.; Cai, F.; Wang, D.; Wang, J.-X.; Chen, J.-F. Colloidal Synthesis of Semiconductor Quantum Dots toward Large-Scale Production: A Review. Ind. Eng. Chem. Res. 2018, 57, 1790–1802. [Google Scholar] [CrossRef]
- Cotta, M.A. Quantum Dots and Their Applications: What Lies Ahead? ACS Appl. Nano Mater. 2020, 3, 4920–4924. [Google Scholar] [CrossRef]
- Farzin, M.A.; Abdoos, H. A Critical Review on Quantum Dots: From Synthesis Toward Applications in Electrochemical Biosensors for Determination of Disease-Related Biomolecules. Talanta 2021, 224, 121828. [Google Scholar] [CrossRef]
- Pandey, S.; Bodas, D. High-Quality Quantum Dots for Multiplexed Bioimaging: A Critical Review. Adv. Colloid Interface Sci. 2020, 278, 102137. [Google Scholar] [CrossRef] [PubMed]
- Gil, H.M.; Price, T.W.; Chelani, K.; Bouillard, J.-S.G.; Calaminus, S.D.J.; Stasiuk, G.J. NIR-Quantum Dots in Biomedical Imaging and Their Future. iScience 2021, 24, 102189. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Kumar, D. Core-Shell Quantum Dots: A Review on Classification, Materials, Application, and Theoretical Modeling. J. Alloys Compd. 2022, 924, 166508. [Google Scholar] [CrossRef]
- Koole, R.; Mulder, W.J.M.; van Schooneveld, M.M.; Strijkers, G.J.; Meijerink, A.; Nicolay, K. Magnetic Quantum Dots for Multimodal Imaging. Nanomed. Nanobiotechnol. 2009, 1, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Selvan, S.T.; Patra, P.K.; Ang, C.Y.; Ying, J.Y. Synthesis of Silica-Coated Semiconductor and Magnetic Quantum Dots and Their Use in the Imaging of Live Cells. Angew. Chem. Int. Ed. 2007, 46, 2448–2452. [Google Scholar] [CrossRef]
- Tan, T.T.; Selvan, S.T.; Zhao, L.; Gao, S.J.; Ying, J.Y. Size Control, Shape Evolution, and Silica Coating of Near-Infrared-Emitting PbSe Quantum Dots. Chem Mater. 2007, 19, 3112–3117. [Google Scholar] [CrossRef]
- Bardi, G.; Malvindi, M.A.; Gherardini, L.; Costa, M.; Pompa, P.P.; Cingolani, R.; Pizzorusso, T. The Biocompatibility of Amino Functionalized CdSe/ZnS Quantum-Dot-Doped SiO2 Nanoparticles with Primary Neural Cells and Their Gene Carrying Performance. Biomaterials 2010, 31, 6555–6566. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Babaei, M.; Abnous, K.; Taghdisi, S.M.; Peivandi, M.T.; Ramezani, M.; Alibolandi, M. Hybrid Silica-Coated Gd-Zn-Cu-In-S/ZnS Bimodal Quantum Dots as an Epithelial Cell Adhesion Molecule Targeted Drug Delivery and Imaging System. Int. J. Pharm. 2019, 570, 118645. [Google Scholar] [CrossRef]
- Muthunayagam, V.; Abraham, A. Comparative Studies of High Contrast Fluorescence Imaging Efficiency of Silica-coated CdSe Quantum Dots with Green and Red Emission. Nanomed. Res. J. 2020, 5, 182–191. [Google Scholar] [CrossRef]
- Darbandi, M.; Thomann, R.; Nann, T. Single Quantum Dots in Silica Spheres by Microemulsion Synthesis. Chem. Mater. 2005, 17, 5720–5725. [Google Scholar] [CrossRef]
- Yang, H.S.; Santra, S.; Walter, G.A.; Holloway, P.H. GdIII-Functionalized Fluorescent Quantum Dots as Multimodal Imaging Probes. Adv. Mater. 2006, 18, 2890–2894. [Google Scholar] [CrossRef]
- Darbandi, M.; Thomann, R.; Nann, T. Hollow Silica Nanospheres: In situ, semi-in situ, and Two-Step Synthesis. Chem. Mater. 2007, 19, 1700–1703. [Google Scholar] [CrossRef]
- Koole, R.; van Schooneveld, M.M.; Hilhorst, J.; Donegá, C.D.; ‘t Hart, D.C.; van Blaaderen, A.; Vanmaekelbergh, D.; Meijerink, A. On the Incorporation Mechanism of Hydrophobic Quantum Dots in Silica Spheres by a Reverse Microemulsion Method. Chem. Mater. 2008, 20, 2503–2512. [Google Scholar] [CrossRef]
- Yang, Y.H.; Gao, M.Y. Preparation of Fluorescent SiO2 Particles with Single CdTe Nanocrystal Cores by the Reverse Microemulsion Method. Adv. Mater. 2005, 17, 2354–2357. [Google Scholar] [CrossRef]
- Yang, Y.H.; Jing, L.H.; Yu, X.L.; Yan, D.D.; Gao, M.Y. Coating Aqueous Quantum Dots with Silica via Reverse Microemulsion Method: Toward Size-Controllable and Robust Fluorescent Nanoparticles. Chem. Mater. 2007, 19, 4123–4128. [Google Scholar] [CrossRef]
- Zrazhevskiy, P.; Sena, M.; Gao, X.H. Designing Multifunctional Quantum Dots for Bioimaging, Detection, and Drug Delivery. Chem. Soc. Rev. 2010, 39, 4326–4354. [Google Scholar] [CrossRef]
- Jin, H.; Jin, Q.; Liang, Z.; Liu, Y.; Qu, X.; Sun, Q. Quantum Dot Based Fluorescent Traffic Light Nanoprobe for Specific Imaging of Avidin-Type Biotin Receptor and Differentiation of Cancer Cells. Anal. Chem. 2019, 91, 8958–8965. [Google Scholar] [CrossRef]
- Yang, D.; Yao, X.; Dong, J.; Wang, N.; Du, Y.; Sun, S.; Gao, L.; Zhong, Y.; Qian, C.; Hong, H. Design and Investigation of Core/Shell GQDs/hMSN Nanoparticles as an Enhanced Drug Delivery Platform in Triple-Negative Breast Cancer. Bioconjug. Chem. 2018, 29, 2776–2785. [Google Scholar] [CrossRef]
- Dong, J.; Yao, X.; Sun, S.; Zhong, Y.; Qian, C.; Yang, D. In Vivo Targeting of Breast Cancer with a Vasculature-Specific GQDs/hMSN Nanoplatform. Rsc Adv. 2019, 9, 11576–11584. [Google Scholar] [CrossRef]
- Kumar, R.; Ding, H.; Hu, R.; Yong, K.T.; Roy, I.; Bergey, E.J.; Prasad, P.N. In Vitro and in vivo Optical Imaging Using Water-Dispersible, Noncytotoxic, Luminescent, Silica-Coated Quantum Rods. Chem. Mater. 2010, 22, 2261–2267. [Google Scholar] [CrossRef]
- Yao, X.; Qian, C.; Zhong, Y.; Sun, S.; Xu, H.; Yang, D. In Vivo Targeting of Breast Cancer with Peptide Functionalized GQDs/hMSN Nanoplatform. J. Nanoparticle Res. 2019, 21, 263. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Guo, P.; Xu, H.; Wang, Y.; Yang, D. GQDs/hMSN Nanoplatform: Singlet Oxygen Generation for Photodynamic Therapy. J. Drug Deliv. Sci. Technol. 2021, 61, 102127. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Ma, X.-T.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Carbon Dots-Embedded Epitope Imprinted Polymer for Targeted Fluorescence Imaging of Cervical Cancer via Recognition of Epidermal Growth Factor Receptor. Microchim. Acta 2020, 187, 228. [Google Scholar] [CrossRef]
- Zhelev, Z.; Ohba, H.; Bakalova, R. Single Quantum Dot-Micelles Coated with Silica Shell as Potentially Non-Cytotoxic Fluorescent Cell Tracers. J. Am. Chem. Soc. 2006, 128, 6324–6325. [Google Scholar] [CrossRef]
- Bakalova, R.; Zhelev, Z.; Kokuryo, D.; Spasov, L.; Aoki, I.; Saga, T. Chemical Nature and Structure of Organic Coating of Quantum Dots is Crucial for Their Application in Imaging Diagnostics. Int. J. Nanomed. 2011, 6, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Parak, W.J.; Gerion, D.; Zanchet, D.; Woerz, A.S.; Pellegrino, T.; Micheel, C.; Williams, S.C.; Seitz, M.; Bruehl, R.E.; Bryant, Z.; et al. Conjugation of DNA to Silanized Colloidal Semiconductor Nanocrystalline Quantum Dots. Chem. Mater. 2002, 14, 2113–2119. [Google Scholar] [CrossRef]
- Zhang, T.T.; Stilwell, J.L.; Gerion, D.; Ding, L.H.; Elboudwarej, O.; Cooke, P.A.; Gray, J.W.; Alivisatos, A.P.; Chen, F.F. Cellular Effect of High Doses of Silica-Coated Quantum Dot Profiled with High Throughput Gene Expression Analysis and High Content Cellomics Measurements. Nano Lett. 2006, 6, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lv, Y.Y.; Asiri, A.M.; Al-Youbi, A.O.; Tu, B.; Zhao, D.Y. Novel Preparation and Near-Infrared Photoluminescence of Uniform Core-Shell Silver Sulfide Nanoparticle@Mesoporous Silica Nanospheres. J. Mater. Chem. 2012, 22, 7274–7279. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Zhao, Y.; Zhang, X.; Sun, L. Preparation of Triple-Responsive Porous Silica Carriers and Carbon Quantum Dots for Photodynamic-/Chemotherapy and Multicolor Cell Imaging. ChemNanoMat 2020, 6, 648–656. [Google Scholar] [CrossRef]
- Veeranarayanan, S.; Poulose, A.C.; Mohamed, M.S.; Nagaoka, Y.; Iwai, S.; Nakagame, Y.; Kashiwada, S.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Synthesis and Application of Luminescent Single CdS Quantum Dot Encapsulated Silica Nanoparticles Directed for Precision Optical Bioimaging. Int. J. Nanomed. 2012, 7, 3769–3786. [Google Scholar] [CrossRef]
- Radenkovic, D.; Kobayashi, H.; Remsey-Semmelweis, E.; Seifalian, A.M. Quantum Dot Nanoparticle for Optimization of Breast Cancer Diagnostics and Therapy in a Clinical Setting. Nanomed.-Nanotechnol. 2016, 12, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Matsudo, H.; Nakagawa, T.; Kubota, Y.; Gonda, K.; Ohuchi, N. In-vivo Fluorescence Imaging Technique Using Colloid Solution of Multiple Quantum Dots/Silica/Poly(ethylene glycol) Nanoparticles. J. Sol.-Gel. Sci. Technol. 2013, 66, 31–37. [Google Scholar] [CrossRef]
- Bashir, M.; Mantoo, I.A.; Arjmand, F.; Tabassum, S.; Yousuf, I. An Overview of Advancement of Organoruthenium(II) Complexes as Prospective Anticancer Agents. Coordin. Chem. Rev. 2023, 487, 215169. [Google Scholar] [CrossRef]
- Lin, K.; Zhao, Z.-Z.; Bo, H.-B.; Hao, X.-J.; Wang, J.-Q. Applications of Ruthenium Complex in Tumor Diagnosis and Therapy. Front. Pharmacol. 2018, 9, 1323. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, A.-C.; Uivarosi, V. Ruthenium Complexes in the Fight against Pathogenic Microorganisms. An Extensive Review. Pharmaceutics 2021, 13, 874. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, R.; Wiese-Szadkowska, M.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Helmin-Basa, A. Latest Developments in Metal Complexes as Anticancer Agents. Coordin. Chem. Rev 2022, 452, 214307. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton T 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Heinemann, F.; Karges, J.; Gasser, G. Critical Overview of the Use of Ru(II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy. Acc. Chem. Res. 2017, 50, 2727–2736. [Google Scholar] [CrossRef] [PubMed]
- Ankathatti Munegowda, M.; Manalac, A.; Weersink, M.; McFarland, S.A.; Lilge, L. Ru(II) Containing Photosensitizers for Photodynamic Therapy: A Critique on Reporting and an Attempt to Compare Efficacy. Coordin. Chem. Rev. 2022, 470, 214712. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, L.; Zhang, P.; Zhao, H.; Zhou, Q. The Development of Ru(II)-Based Photoactivated Chemotherapy Agents. Molecules 2021, 26, 5679. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Jakupec, M.A.; Zorbas-Seifried, S.; Groessl, M.; Egger, A.; Berger, W.; Zorbas, H.; Dyson, P.J.; Keppler, B.K. KP1019, A New Redox-Active Anticancer Agent—Preclinical Development and Results of a Clinical Phase I Study in Tumor Patients. Chem. Biodivers. 2008, 5, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Trondl, R.; Heffeter, P.; Kowol, C.R.; Jakupec, M.A.; Berger, W.; Keppler, B.K. NKP-1339, The First Ruthenium-Based Anticancer Drug on the Edge to Clinical Application. Chem. Sci. 2014, 5, 2925–2932. [Google Scholar] [CrossRef]
- Rossi, L.M.; Shi, L.F.; Quina, F.H.; Rosenzweig, Z. Stöber Synthesis of Monodispersed Luminescent Silica Nanoparticles for Bioanalytical Assays. Langmuir 2005, 21, 4277–4280. [Google Scholar] [CrossRef]
- Rossi, L.M.; Shi, L.F.; Rosenzweig, N.; Rosenzweig, Z. Fluorescent Silica Nanospheres for Digital Counting Bioassay of the Breast Cancer Marker HER2/nue. Biosens. Bioelectron. 2006, 21, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Hun, X.; Zhang, Z.J. Fluoroimmunoassay for Tumor Necrosis Factor-Alpha in Human Serum Using Ru(bpy)3Cl2-Doped Fluorescent Silica Nanoparticles as Labels. Talanta 2007, 73, 366–371. [Google Scholar] [CrossRef]
- Babu, E.; Mareeswaran, P.M.; Rajagopal, S. Highly Sensitive Optical Biosensor for Thrombin Based on Structure Switching Aptamer-Luminescent Silica Nanoparticles. J. Fluoresc. 2013, 23, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Long, C.; Wu, Z.; Xiong, H.; Chen, M.; Zhang, X.; Wen, W.; Wang, S. Functional Silica Nanospheres for Sensitive Detection of H9N2 Avian Influenza Virus Based on Immunomagnetic Separation. Sens. Actuators B Chem. 2020, 310, 127831. [Google Scholar] [CrossRef]
- Fent, K.; Weisbrod, C.J.; Wirth-Heller, A.; Pieles, U. Assessment of Uptake and Toxicity of Fluorescent Silica Nanoparticles in Zebrafish (Danio rerio) Early Life Stages. Aquat. Toxicol. 2010, 100, 218–228. [Google Scholar] [CrossRef]
- He, X.X.; Wang, K.M.; Tan, W.H.; Xiao, D.; Li, J.; Yang, X.H. A Novel Fluorescent Label Based on Biological Fluorescent Nanoparticles and Its Application in Cell Recognition. Chin. Sci. Bull. 2001, 46, 1962–1965. [Google Scholar] [CrossRef]
- Kim, J.S.; Rieter, W.J.; Taylor, K.M.L.; An, H.; Lin, W.L.; Lin, W.B. Self-Assembled Hybrid Nanoparticles for Cancer-Specific Multimodal Imaging. J. Am. Chem. Soc. 2007, 129, 8962–8963. [Google Scholar] [CrossRef] [PubMed]
- Bottini, M.; Cerignoli, F.; Mills, D.M.; D’Annibale, F.; Leone, M.; Rosato, N.; Magrini, A.; Pellecchia, M.; Bergamaschi, A.; Mustelin, T. Luminescent Silica Nanobeads: Characterization and Evaluation as Efficient Cytoplasmatic Transporters for T-Lymphocytes. J. Am. Chem. Soc. 2007, 129, 7814–7823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rieter, W.J.; Kim, J.S.; Taylor, K.M.L.; An, H.Y.; Lin, W.L.; Tarrant, T.; Lin, W.B. Hybrid Silica Nanoparticles for Multimodal Imaging. Angew. Chem. Int. Ed. 2007, 46, 3680–3682. [Google Scholar] [CrossRef] [PubMed]
- Mirenda, M.; Levi, V.; Bossi, M.L.; Bruno, L.; Bordoni, A.V.; Regazzoni, A.E.; Wolosiuk, A. Temperature Response of Luminescent Tris(bipyridine)ruthenium(II)-Doped Silica Nanoparticles. J. Colloid Interface Sci. 2013, 392, 96–101. [Google Scholar] [CrossRef]
- Yang, L.; Peng, H.S.; Ding, H.; You, F.T.; Hou, L.L.; Teng, F. Luminescent Ru(bpy)32+-Doped Silica Nanoparticles for Imaging of Intracellular Temperature. Microchim. Acta 2014, 181, 743–749. [Google Scholar] [CrossRef]
- Santoro, S.; Sebastian, V.; Moro, A.J.; Portugal, C.A.M.; Lima, J.C.; Coelhoso, I.M.; Crespo, J.G.; Mallada, R. Development of Fluorescent Thermoresponsive Nanoparticles for Temperature Monitoring on Membrane Surfaces. J. Colloid. Interface Sci. 2017, 486, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.N.; Jiang, H.; Li, Y.X.; Sang, L.J.; Zhou, H.P.; Shahzad, S.A.; Ibupoto, Z.H.; Yu, C. Synthesis of Silica Nanoparticles Doped with [Ru(bpy)3]2+ and Decorated with Silver Nanoclusters for the Ratiometric Photoluminescent Determination and Intracellular Imaging of Cu(II) Ions. Microchim. Acta 2017, 184, 2325–2331. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, J.H.; Wang, Y.; Pang, W.Q. Incorporation of Luminescent Tris(bipyridine)ruthenium(II) Complex in Mesoporous Silica Spheres and Their Spectroscopic and Oxygen-Sensing Properties. Mater. Lett. 2002, 53, 400–405. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, B.; Zhang, L.M.; Liu, L.; Wang, Y.H. Photoluminescent and Oxygen Sensing Properties of Core-Shell Nanospheres Based on a Covalently Grafted Ruthenium(II) Complex. Appl. Organomet. Chem. 2011, 25, 21–26. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, W.; Zhang, Y.; Lin, Z.; Wang, X.-D. Luminescent Silica Nanosensors for Lifetime Based Imaging of Intracellular Oxygen with Millisecond Time Resolution. Anal. Chem. 2019, 91, 15625–15633. [Google Scholar] [CrossRef]
- Harun, S.N.; Ahmad, H.; Lim, H.N.; Chia, S.L.; Gill, M.R. Synthesis and Optimization of Mesoporous Silica Nanoparticles for Ruthenium Polypyridyl Drug Delivery. Pharmaceutics 2021, 13, 150. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Ho, Q.P.; Morand, J.; García, A.; Ortega, E.; Erthal, L.C.S.; Ruiz-Hernandez, E.; Santana, M.D.; Ruiz, J.; Vallet-Regí, M.; et al. Amino-Functionalized Mesoporous Silica Nanoparticle-Encapsulated Octahedral Organoruthenium Complex as an Efficient Platform for Combatting Cancer. Inorg. Chem. 2020, 59, 10275–10284. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Zhu, H.; Pang, G.; Zheng, W.; Wong, Y.-S.; Chen, T. Cancer-Targeted Monodisperse Mesoporous Silica Nanoparticles as Carrier of Ruthenium Polypyridyl Complexes to Enhance Theranostic Effects. Adv. Funct. Mater. 2014, 24, 2754–2763. [Google Scholar] [CrossRef]
- Santra, S.; Bagwe, R.P.; Dutta, D.; Stanley, J.T.; Walter, G.A.; Tan, W.; Moudgil, B.M.; Mericle, R.A. Synthesis and Characterization of Fluorescent, Radio-Opaque, and Paramagnetic Silica Nanoparticles for Multimodal Bioimaging Applications. Adv. Mater. 2005, 17, 2165–2169. [Google Scholar] [CrossRef]
- Lipani, E.; Laurent, S.; Surin, M.; Elst, L.V.; Leclere, P.; Muller, R.N. High-Relaxivity and Luminescent Silica Nanoparticles as Multimodal Agents for Molecular Imaging. Langmuir 2013, 29, 3419–3427. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Chen, Z.F.; Yam, V.W.W.; Zu, Y.B. Multifunctional Ruthenium(II) Polypyridine Complex-Based Core-Shell Magnetic Silica Nanocomposites: Magnetism, Luminescence, and Electrochemiluminescence. ACS Nano 2008, 2, 905–912. [Google Scholar] [CrossRef]
- Sábio, R.M.; Gressier, M.; Caiut, J.M.A.; Menu, M.J.; Ribeiro, S.J.L. Luminescent Multifunctional Hybrids Obtained by Grafting of Ruthenium Complexes on Mesoporous Silica. Mater. Lett. 2016, 174, 1–5. [Google Scholar] [CrossRef]
- Song, C.H.; Ye, Z.Q.; Wang, G.L.; Jin, D.Y.; Yuan, J.L.; Guan, Y.F.; Piper, J. Preparation and Time-Gated Luminescence Bioimaging Application of Ruthenium Complex Covalently Bound Silica Nanoparticles. Talanta 2009, 79, 103–108. [Google Scholar] [CrossRef]
- Liang, W.B.; Zhuo, Y.; Xiong, C.Y.; Zheng, Y.N.; Chai, Y.Q.; Yuan, R. Ultrasensitive Cytosensor Based on Self-Enhanced Electrochemiluminescent Ruthenium-Silica Composite Nanoparticles for Efficient Drug Screening with Cell Apoptosis Monitoring. Anal. Chem. 2015, 87, 12363–12371. [Google Scholar] [CrossRef]
- Deng, X.; Wu, S.; Li, Z.; Zhao, Y.; Duan, C. Ratiometric Detection of DNA and Protein in Serum by a Universal Tripyridinyl RuII Complex–Encapsulated SiO2@Polydopamine Fluorescence Nanoplatform. Anal. Chem. 2020, 92, 15908–15915. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Li, B.; Zhang, L.M.; Song, H. Multifunctional Mesoporous Nanocomposites with Magnetic, Optical, and Sensing Features: Synthesis, Characterization, and Their Oxygen-Sensing Performance. Langmuir 2013, 29, 1273–1279. [Google Scholar] [CrossRef]
- Lechevallier, S.; Mauricot, R.; Gros-Dagnac, H.; Chevreux, S.; Lemercier, G.; Phonesouk, E.; Golzio, M.; Verelst, M. Silica-Based Nanoparticles as Bifunctional and Bimodal Imaging Contrast Agents. Chempluschem 2017, 82, 770–777. [Google Scholar] [CrossRef]
- Umehara, Y.; Kimura, Y.; Kleitz, F.; Nishihara, T.; Kondo, T.; Tanabe, K. Phosphonated Mesoporous Silica Nanoparticles Bearing Ruthenium Complexes Used as Molecular Probes for Tracking Oxygen Levels in Cells and Tissues. Rsc Adv. 2021, 11, 5865–5873. [Google Scholar] [CrossRef] [PubMed]
- Frasconi, M.; Liu, Z.C.; Lei, J.Y.; Wu, Y.L.; Strekalova, E.; Malin, D.; Ambrogio, M.W.; Chen, X.Q.; Botros, Y.Y.; Cryns, V.L.; et al. Photoexpulsion of Surface-Grafted Ruthenium Complexes and Subsequent Release of Cytotoxic Cargos to Cancer Cells from Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2013, 135, 11603–11613. [Google Scholar] [CrossRef] [PubMed]
- Knežević, N.Ž. Visible Light Responsive Anticancer Treatment with an Amsacrine-Loaded Mesoporous Silica-Based Nanodevice. Rsc Adv. 2013, 3, 19388–19392. [Google Scholar] [CrossRef]
- Ellahioui, Y.; Patra, M.; Mari, C.; Kaabi, R.; Karges, J.; Gasser, G.; Gómez-Ruiz, S. Mesoporous Silica Nanoparticles Functionalised with a Photoactive Ruthenium(II) Complex: Exploring the Formulation of a Metal-Based Photodynamic Therapy Photosensitiser. Dalton T 2019, 48, 5940–5951. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, G.; Culita, D.C.; Mocanu, T.; Mitran, R.-A.; Petrescu, S.; Stan, M.S.; Chifiriuc, M.C.; Popa, M. New Nanostructured Materials Based on Mesoporous Silica Loaded with Ru(II)/Ru(III) Complexes with Anticancer and Antimicrobial Properties. Pharmaceutics 2023, 15, 1458. [Google Scholar] [CrossRef] [PubMed]
- Karges, J.; Díaz-García, D.; Prashar, S.; Gómez-Ruiz, S.; Gasser, G. Ru(II) Polypyridine Complex-Functionalized Mesoporous Silica Nanoparticles as Photosensitizers for Cancer Targeted Photodynamic Therapy. ACS Appl. Bio Mater. 2021, 4, 4394–4405. [Google Scholar] [CrossRef]
- Mladenović, M.; Morgan, I.; Ilić, N.; Saoud, M.; Pergal, M.V.; Kaluđerović, G.N.; Knežević, N.Ž. pH-Responsive Release of Ruthenium Metallotherapeutics from Mesoporous Silica-Based Nanocarriers. Pharmaceutics 2021, 13, 460. [Google Scholar] [CrossRef]
- Marinescu, G.; Culita, D.C.; Romanitan, C.; Somacescu, S.; Ene, C.D.; Marinescu, V.; Negreanu, D.G.; Maxim, C.; Popa, M.; Marutescu, L.; et al. Novel Hybrid Materials Based on Heteroleptic Ru(III) Complexes Immobilized on SBA-15 Mesoporous Silica as Highly Potent Antimicrobial and Cytotoxic Agents. Appl. Surf. Sci. 2020, 520, 146379. [Google Scholar] [CrossRef]
- Rudlowski, C.; Friedrichs, N.; Faridi, A.; Füzesi, L.; Moll, R.; Bastert, G.; Rath, W.; Büttner, R. Her-2/neu Gene Amplification and Protein Expression in Primary Male Breast Cancer. Breast Cancer Res. Treat. 2004, 84, 215–223. [Google Scholar] [CrossRef]
- Swain, S.M. HER2-Neu in Breast Cancer: History and Future Directions. Ann. Oncol. 2012, 23, XI10–XI11. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wing, R.M.; Zimmerman, R.A. Far-Infrared Spectra of Metal Atom Cluster Compounds. I. Mo6X84+ Derivatives. Inorg. Chem. 1967, 6, 11–15. [Google Scholar] [CrossRef]
- Efremova, O.A.; Vorotnikov, Y.A.; Brylev, K.A.; Vorotnikova, N.A.; Novozhilov, I.N.; Kuratieva, N.V.; Edeleva, M.V.; Benoit, D.M.; Kitamura, N.; Mironov, Y.V.; et al. Octahedral Molybdenum Cluster Complexes with Aromatic Sulfonate Ligands. Dalton T 2016, 45, 15427–15435. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, M.A.; Brylev, K.A.; Abramov, P.A.; Sakuda, E.; Akagi, S.; Ito, A.; Kitamura, N.; Sokolov, M.N. Synthetic Tuning of Redox, Spectroscopic, and Photophysical Properties of {Mo6I8}4+ Core Cluster Complexes by Terminal Carboxylate Ligands. Inorg. Chem. 2016, 55, 8437–8445. [Google Scholar] [CrossRef]
- Mikhaylov, M.A.; Sokolov, M.N. Molybdenum Iodides—From Obscurity to Bright Luminescence. Eur. J. Inorg. Chem. 2019, 2019, 4181–4197. [Google Scholar] [CrossRef]
- Marchuk, M.V.; Vorotnikov, Y.A.; Ivanov, A.A.; Eltsov, I.V.; Kuratieva, N.V.; Shestopalov, M.A. A Neutral Heteroleptic Molybdenum Cluster trans-[{Mo6I8}(py)2I4]. Symmetry 2022, 14, 2117. [Google Scholar] [CrossRef]
- Vorotnikova, N.A.; Alekseev, A.Y.; Vorotnikov, Y.A.; Evtushok, D.V.; Molard, Y.; Amela-Cortes, M.; Cordier, S.; Smolentsev, A.I.; Burton, C.G.; Kozhin, P.M.; et al. Octahedral Molybdenum Cluster as a Photoactive Antimicrobial Additive to a Fluoroplastic. Mat. Sci. Eng. C-Mater. 2019, 105, 110150. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Mihailov, M.A.; Peresypkina, E.V.; Brylev, K.A.; Kitamura, N.; Fedin, V.P. Highly luminescent complexes [Mo6X8(n-C3F7COO)6]2– (X = Br, I). Dalton T 2011, 40, 6375–6377. [Google Scholar] [CrossRef]
- Akagi, S.; Fujii, S.; Kitamura, N. A study on the redox, spectroscopic, and photophysical characteristics of a series of octahedral hexamolybdenum(II) clusters: [{Mo6X8}Y-6](2-) (X, Y = Cl, Br, or I). Dalton T 2018, 47, 1131–1139. [Google Scholar] [CrossRef]
- Kirakci, K.; Demel, J.; Hynek, J.; Zelenka, J.; Rumlova, M.; Ruml, T.; Lang, K. Phosphinate Apical Ligands: A Route to a Water-Stable Octahedral Molybdenum Cluster Complex. Inorg. Chem. 2019, 58, 16546–16552. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Zelenka, J.; Krizova, I.; Ruml, T.; Lang, K. Octahedral Molybdenum Cluster Complexes with Optimized Properties for Photodynamic Applications. Inorg. Chem. 2020, 59, 9287–9293. [Google Scholar] [CrossRef] [PubMed]
- Molard, Y.; Taupier, G.; Paofai, S.; Cordier, S. Evidencing ((n-C4H9)4N)2[W6I14] Red–NIR Emission and Singlet Oxygen Generation by Two Photon Absorption. Chem. Commun. 2021, 57, 4003–4006. [Google Scholar] [CrossRef]
- Khlifi, S.; Taupier, G.; Amela-Cortes, M.; Dumait, N.; Freslon, S.; Cordier, S.; Molard, Y. Expanding the Toolbox of Octahedral Molybdenum Clusters and Nanocomposites Made Thereof: Evidence of Two-Photon Absorption Induced NIR Emission and Singlet Oxygen Production. Inorg. Chem. 2021, 60, 5446–5451. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Kuba, P.; Fejfarova, K.; Martinc, J.; Nikl, M.; Lang, K. X-ray Inducible Luminescence and Singlet Oxygen Sensitization by an Octahedral Molybdenum Cluster Compound: A New Class of Nanoscintillators. Inorg. Chem. 2016, 55, 803–809. [Google Scholar] [CrossRef]
- Evtushok, D.V.; Melnikov, A.R.; Vorotnikova, N.A.; Vorotnikov, Y.A.; Ryadun, A.A.; Kuratieva, N.V.; Kozyr, K.V.; Obedinskaya, N.R.; Kretov, E.I.; Novozhilov, I.N.; et al. A Comparative Study of Optical Properties and X-ray Induced Luminescence of Octahedral Molybdenum and Tungsten Cluster Complexes. Dalton T 2017, 46, 11738–11747. [Google Scholar] [CrossRef]
- Pozmogova, T.N.; Sitnikova, N.A.; Pronina, E.V.; Miroshnichenko, S.M.; Kushnarenko, A.O.; Solovieva, A.O.; Bogachev, S.S.; Vavilov, G.D.; Efremova, O.A.; Vorotnikov, Y.A.; et al. Hybrid System {W6I8}-Cluster/dsDNA as an Agent for Targeted X-ray Induced Photodynamic Therapy of Cancer Stem Cells. Mater. Chem. Front. 2021, 5, 7499–7507. [Google Scholar] [CrossRef]
- Bardin, V.A.; Vorotnikov, Y.A.; Stass, D.V.; Vorotnikova, N.A.; Shestopalov, M.A. Oxygen-Sensitive Photo- and Radioluminescent Polyurethane Nanoparticles Modified with Octahedral Iodide Tungsten Clusters. Nanomaterials 2022, 12, 3580. [Google Scholar] [CrossRef]
- Kirakci, K.; Pozmogova, T.N.; Protasevich, A.Y.; Vavilov, G.D.; Stass, D.V.; Shestopalov, M.A.; Lang, K. A Water-Soluble Octahedral Molybdenum Cluster Complex as a Potential Agent for X-ray Induced Photodynamic Therapy. Biomater. Sci. 2021, 9, 2893–2902. [Google Scholar] [CrossRef]
- Dierre, B.; Costuas, K.; Dumait, N.; Paofai, S.; Amela-Cortes, M.; Molard, Y.; Grasset, F.; Cho, Y.; Takahashi, K.; Ohashi, N.; et al. Mo6 Cluster-Based Compounds for Energy Conversion Applications: Comparative Study of Photoluminescence and Cathodoluminescence. Sci. Technol. Adv. Mater. 2017, 18, 458–466. [Google Scholar] [CrossRef]
- Jackson, J.A.; Turro, C.; Newsham, M.D.; Nocera, D.G. Oxygen Quenching of Electronically Excited Hexanuclear Molybdenum and Tungsten Halide Clusters. J. Phys. Chem. 1990, 94, 4500–4507. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubát, P.; Kučeráková, M.; Šícha, V.; Gbelcová, H.; Lovecká, P.; Grznárová, P.; Ruml, T.; Lang, K. Water-Soluble Octahedral Molybdenum Cluster Compounds Na2[Mo6I8(N3)6] and Na2[Mo6I8(NCS)6]: Syntheses, Luminescence, and in vitro studies. Inorg. Chim. Acta 2016, 441, 42–49. [Google Scholar] [CrossRef]
- Efremova, O.A.; Brylev, K.A.; Vorotnikov, Y.A.; Vejsadová, L.; Shestopalov, M.A.; Chimonides, G.F.; Mikes, P.; Topham, P.D.; Kim, S.-J.; Kitamura, N.; et al. Photoluminescent Materials Based on PMMA and a Highly-Emissive Octahedral Molybdenum Metal Cluster Complex. J. Mater. Chem. C 2016, 4, 497–503. [Google Scholar] [CrossRef]
- Khlifi, S.; Bigeon, J.; Amela-Cortes, M.; Dumait, N.; Loas, G.H.; Cordier, S.; Molard, Y. Switchable Two-Dimensional Waveguiding Abilities of Luminescent Hybrid Nanocomposites for Active Solar Concentrators. ACS Appl. Mater. Interfaces 2020, 12, 14400–14407. [Google Scholar] [CrossRef] [PubMed]
- Renaud, A.; Nguyen, T.K.N.; Grasset, F.; Raissi, M.; Guillon, V.; Delabrouille, F.; Dumait, N.; Jouan, P.Y.; Cario, L.; Jobic, S.; et al. Preparation by Electrophoretic Deposition of Molybdenum Iodide Cluster-Based Functional Nanostructured Photoelectrodes for Solar Cells. Electrochim. Acta 2019, 317, 737–745. [Google Scholar] [CrossRef]
- Renaud, A.; Jouan, P.-Y.; Dumait, N.; Ababou-Girard, S.; Barreau, N.; Uchikoshi, T.; Grasset, F.; Jobic, S.; Cordier, S. Evidence of the Ambipolar Behavior of Mo6 Cluster Iodides in All-Inorganic Solar Cells: A New Example of Nanoarchitectonic Concept. ACS Appl. Mater. Interfaces 2022, 14, 1347–1354. [Google Scholar] [CrossRef]
- Svezhentseva, E.V.; Vorotnikov, Y.A.; Solovieva, A.O.; Pozmogova, T.N.; Eltsov, I.V.; Ivanov, A.A.; Evtushok, D.V.; Miroshnichenko, S.M.; Yanshole, V.V.; Eling, C.J.; et al. From Photoinduced to Dark Cytotoxicity through an Octahedral Cluster Hydrolysis. Chem.-Eur. J. 2018, 24, 17915–17920. [Google Scholar] [CrossRef]
- Pronina, E.V.; Pozmogova, T.N.; Vorotnikov, Y.A.; Ivanov, A.A.; Shestopalov, M.A. The Role of Hydrolysis in Biological Effects of Molybdenum Cluster with DMSO Ligands. J. Biol. Inorg. Chem. 2022, 27, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Grasset, F.; Dorson, F.; Molard, Y.; Cordier, S.; Demange, V.; Perrin, C.; Marchi-Artzner, V.; Haneda, H. One-Pot Synthesis and Characterizations of Bi-Functional Phosphor-Magnetic @SiO2 Nanoparticles: Controlled and Structured Association of Mo6 Cluster Units and Gamma-Fe2O3 Nanocrystals. Chem. Commun. 2008, 39, 4729–4731. [Google Scholar] [CrossRef]
- Grasset, F.; Dorson, F.; Cordier, S.; Molard, Y.; Perrin, C.; Marie, A.M.; Sasaki, T.; Haneda, H.; Bando, Y.; Mortier, M. Water-In-Oil Microemulsion Preparation and Characterization of Cs2[Mo6X14]@SiO2 Phosphor Nanoparticles Based on Transition Metal Clusters (X = Cl, Br, and I). Adv. Mater. 2008, 20, 143–148. [Google Scholar] [CrossRef]
- Aubert, T.; Cabello-Hurtado, F.; Esnault, M.A.; Neaime, C.; Lebret-Chauvel, D.; Jeanne, S.; Pellen, P.; Roiland, C.; Le Polles, L.; Saito, N.; et al. Extended Investigations on Luminescent Cs2[Mo6Br14]@SiO2 Nanoparticles: Physico-Structural Characterizations and Toxicity Studies. J. Phys. Chem. C 2013, 117, 20154–20163. [Google Scholar] [CrossRef]
- Vorotnikov, Y.A.; Efremova, O.A.; Vorotnikova, N.A.; Brylev, K.A.; Edeleva, M.V.; Tsygankova, A.R.; Smolentsev, A.I.; Kitamura, N.; Mironov, Y.V.; Shestopalov, M.A. On the Synthesis and Characterisation of Luminescent Hybrid Particles: Mo6 Metal Cluster Complex/SiO2. Rsc Adv. 2016, 6, 43367–43375. [Google Scholar] [CrossRef]
- Dechézelles, J.F.; Aubert, T.; Grasset, F.; Cordier, S.; Barthou, C.; Schwob, C.; Maître, A.; Valleé, R.A.L.; Cramail, H.; Ravaine, S. Fine Tuning of Emission Through the Engineering of Colloidal Crystals. Phys. Chem. Chem. Phys. 2010, 12, 11993–11999. [Google Scholar] [CrossRef]
- Vorotnikov, Y.A.; Pozmogova, T.N.; Solovieva, A.O.; Miroshnichenko, S.M.; Vorontsova, E.V.; Shestopalova, L.V.; Mironov, Y.V.; Shestopalov, M.A.; Efremova, O.A. Luminescent Silica Mesoparticles for Protein Transduction. Mat. Sci. Eng. C-Mater. 2019, 96, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Neaime, C.; Amela-Cortes, M.; Grasset, F.; Molard, Y.; Cordier, S.; Dierre, B.; Mortier, M.; Takei, T.; Takahashi, K.; Haneda, H.; et al. Time-Gated Luminescence Bioimaging with New Luminescent Nanocolloids Based on [Mo6I8(C2F5COO)6]2− Metal Atom Clusters. Phys. Chem. Chem. Phys. 2016, 18, 30166–30173. [Google Scholar] [CrossRef]
- Pellen-Mussi, P.; Tricot-Doleux, S.; Neaime, C.; Nerambourg, N.; Cabello-Hurtado, F.; Cordier, S.; Grasset, F.; Jeanne, S. Evaluation of Functional SiO2 Nanoparticles Toxicity by a 3D Culture Model. J. Nanosci. Nanotechnol. 2018, 18, 3148–3157. [Google Scholar] [CrossRef]
- Solovieva, A.O.; Vorotnikov, Y.A.; Trifonova, K.E.; Efremova, O.A.; Krasilnikova, A.A.; Brylev, K.A.; Vorontsova, E.V.; Avrorov, P.A.; Shestopalova, L.V.; Poveshchenko, A.F.; et al. Cellular Internalisation, Bioimaging and Dark and Photodynamic Cytotoxicity of Silica Nanoparticles Doped by {Mo6I8}4+ Metal Clusters. J. Mater. Chem. B 2016, 4, 4839–4846. [Google Scholar] [CrossRef]
- Vorotnikov, Y.A.; Novikova, E.D.; Solovieva, A.O.; Shanshin, D.V.; Tsygankova, A.R.; Shcherbakov, D.N.; Efremova, O.A.; Shestopalov, M.A. Single-Domain Antibody C7b for Address Delivery of Nanoparticles to HER2-Positive Cancers. Nanoscale 2020, 12, 21885–21894. [Google Scholar] [CrossRef]
- Nerambourg, N.; Aubert, T.; Neaime, C.; Cordier, S.; Mortier, M.; Patriarche, G.; Grasset, F. Multifunctional Hybrid Silica Nanoparticles Based on [Mo6Br14]2− Phosphorescent Nanosized Clusters, Magnetic γ-Fe2O3 and Plasmonic Gold Nanoparticles. J. Colloid. Interface Sci. 2014, 424, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Novikova, E.D.; Vorotnikov, Y.A.; Nikolaev, N.A.; Tsygankova, A.R.; Shestopalov, M.A.; Efremova, O.A. The Role of Gold Nanoparticles’ Aspect Ratio in Plasmon-Enhanced Luminescence and the Singlet Oxygen Generation Rate of Mo6 Clusters. Chem. Commun. 2021, 57, 7770–7773. [Google Scholar] [CrossRef]
- Novikova, E.D.; Vorotnikov, Y.A.; Nikolaev, N.A.; Tsygankova, A.R.; Shestopalov, M.A.; Efremova, O.A. Synergetic Effect of Mo6 Clusters and Gold Nanoparticles on the Photophysical Properties of Both Components. Chem.-Eur. J. 2021, 27, 2818–2825. [Google Scholar] [CrossRef]
- Elistratova, J.; Mukhametshina, A.; Kholin, K.; Nizameev, I.; Mikhailov, M.; Sokolov, M.; Khairullin, R.; Miftakhova, R.; Shammas, G.; Kadirov, M.; et al. Interfacial Uploading of Luminescent Hexamolybdenum Cluster Units Onto Amino-Decorated Silica Nanoparticles as New Design of Nanomaterial for Cellular Imaging and Photodynamic Therapy. J. Colloid. Interface Sci. 2019, 538, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, S.; Elistratova, J.; Stepanov, A.; Khazieva, A.; Mikhailov, M.; Sokolov, M.; Kholin, K.; Nizameev, I.; Mendes, R.; Rümmeli, M.; et al. ROS-Generation and Cellular Uptake Behavior of Amino-Silica Nanoparticles Arisen from Their Uploading by Both Iron-Oxides and Hexamolybdenum Clusters. Mater. Sci. Eng. C-Mater. 2020, 117, 111305. [Google Scholar] [CrossRef] [PubMed]
- Elistratova, J.G.; Mikhaylov, M.A.; Sukhikh, T.S.; Kholin, K.V.; Nizameev, I.R.; Khazieva, A.R.; Gubaidullin, A.T.; Voloshina, A.D.; Sibgatullina, G.V.; Samigullin, D.V.; et al. Anticancer Potential of Hexamolybdenum Clusters [{Mo6I8}(L)6]2− (L = CF3COO− and C6F5COO−) Incorporated into Different Nanoparticulate Forms. J. Mol. Liq. 2021, 343, 117601. [Google Scholar] [CrossRef]
- Nguyen, T.K.N.; Grasset, F.; Cordier, S.; Amela-Cortes, M.; Matsui, Y.; Ohashi, N.; Shirahata, N.; Uchikoshi, T. Preparation and Characterization of Hollow Silica Nanocomposite Functionalized with UV Absorbable Molybdenum Cluster. Adv. Powder Technol. 2020, 31, 895–903. [Google Scholar] [CrossRef]





















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorotnikov, Y.A.; Vorotnikova, N.A.; Shestopalov, M.A. Silica-Based Materials Containing Inorganic Red/NIR Emitters and Their Application in Biomedicine. Materials 2023, 16, 5869. https://doi.org/10.3390/ma16175869
Vorotnikov YA, Vorotnikova NA, Shestopalov MA. Silica-Based Materials Containing Inorganic Red/NIR Emitters and Their Application in Biomedicine. Materials. 2023; 16(17):5869. https://doi.org/10.3390/ma16175869
Chicago/Turabian StyleVorotnikov, Yuri A., Natalya A. Vorotnikova, and Michael A. Shestopalov. 2023. "Silica-Based Materials Containing Inorganic Red/NIR Emitters and Their Application in Biomedicine" Materials 16, no. 17: 5869. https://doi.org/10.3390/ma16175869
APA StyleVorotnikov, Y. A., Vorotnikova, N. A., & Shestopalov, M. A. (2023). Silica-Based Materials Containing Inorganic Red/NIR Emitters and Their Application in Biomedicine. Materials, 16(17), 5869. https://doi.org/10.3390/ma16175869






