Features of Helium and Tritium Release from Li2TiO3 Ceramic Pebbles under Neutron Irradiation
Abstract
:1. Introduction
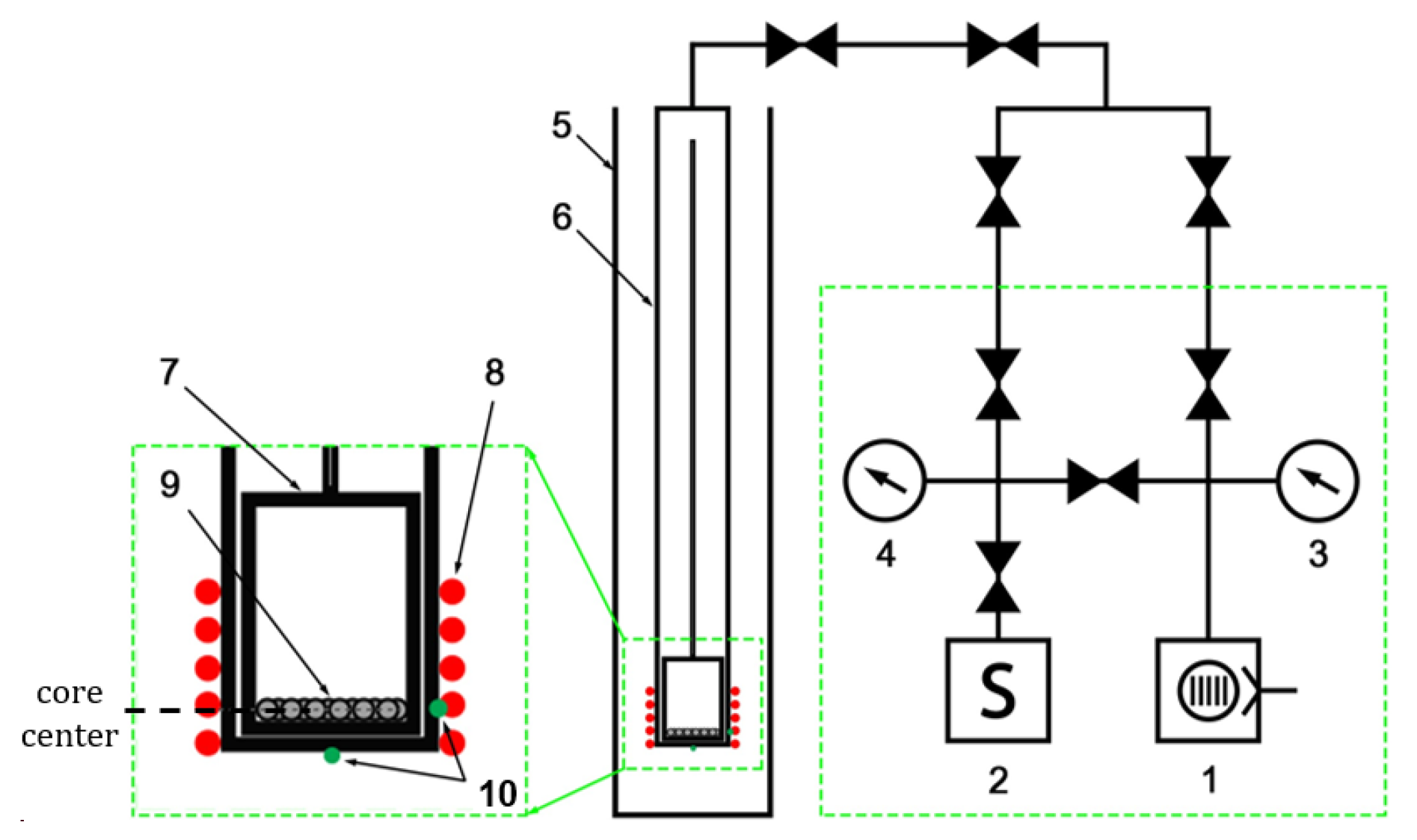
2. Materials and Methods
3. Results and Discussion
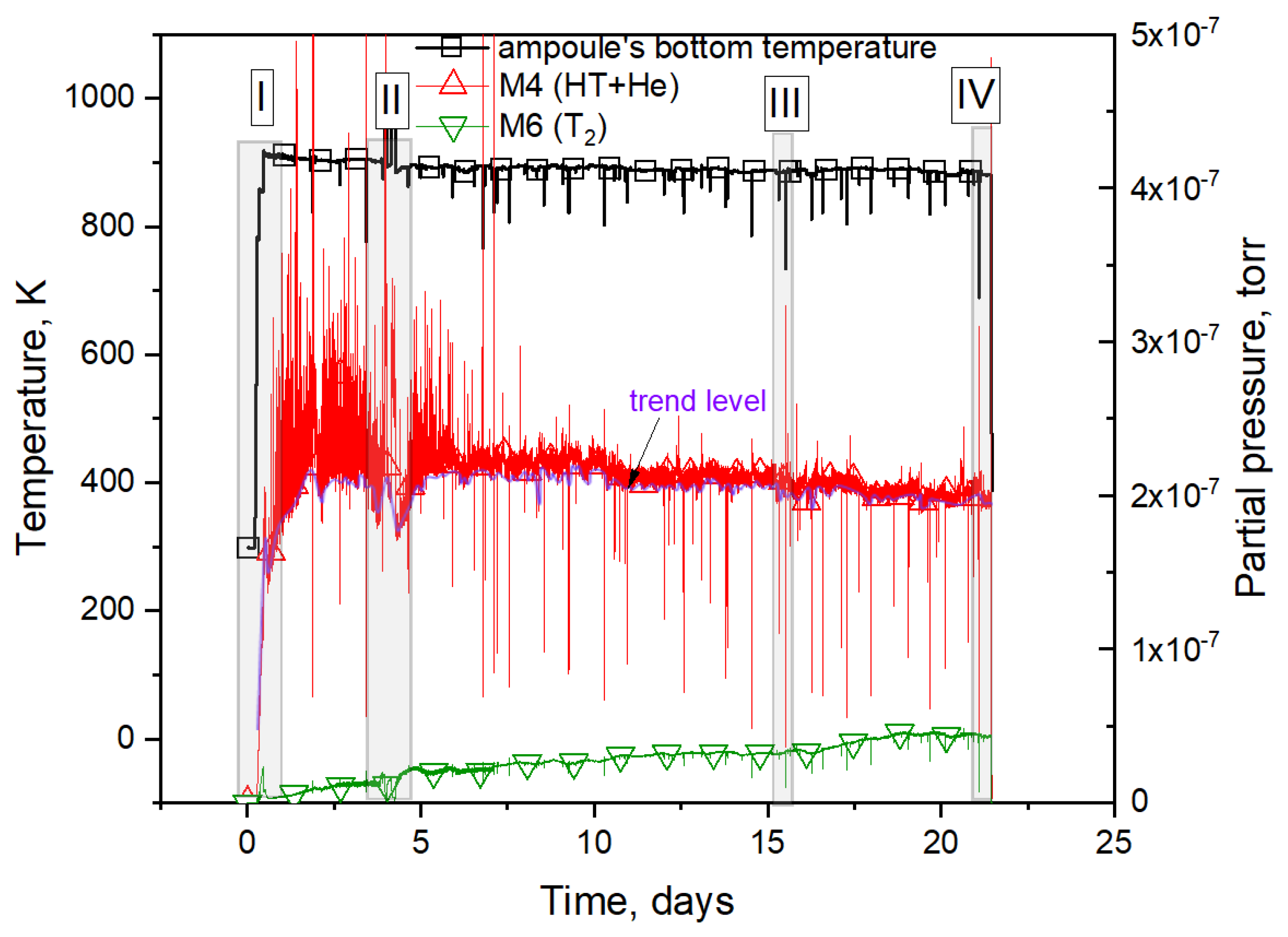
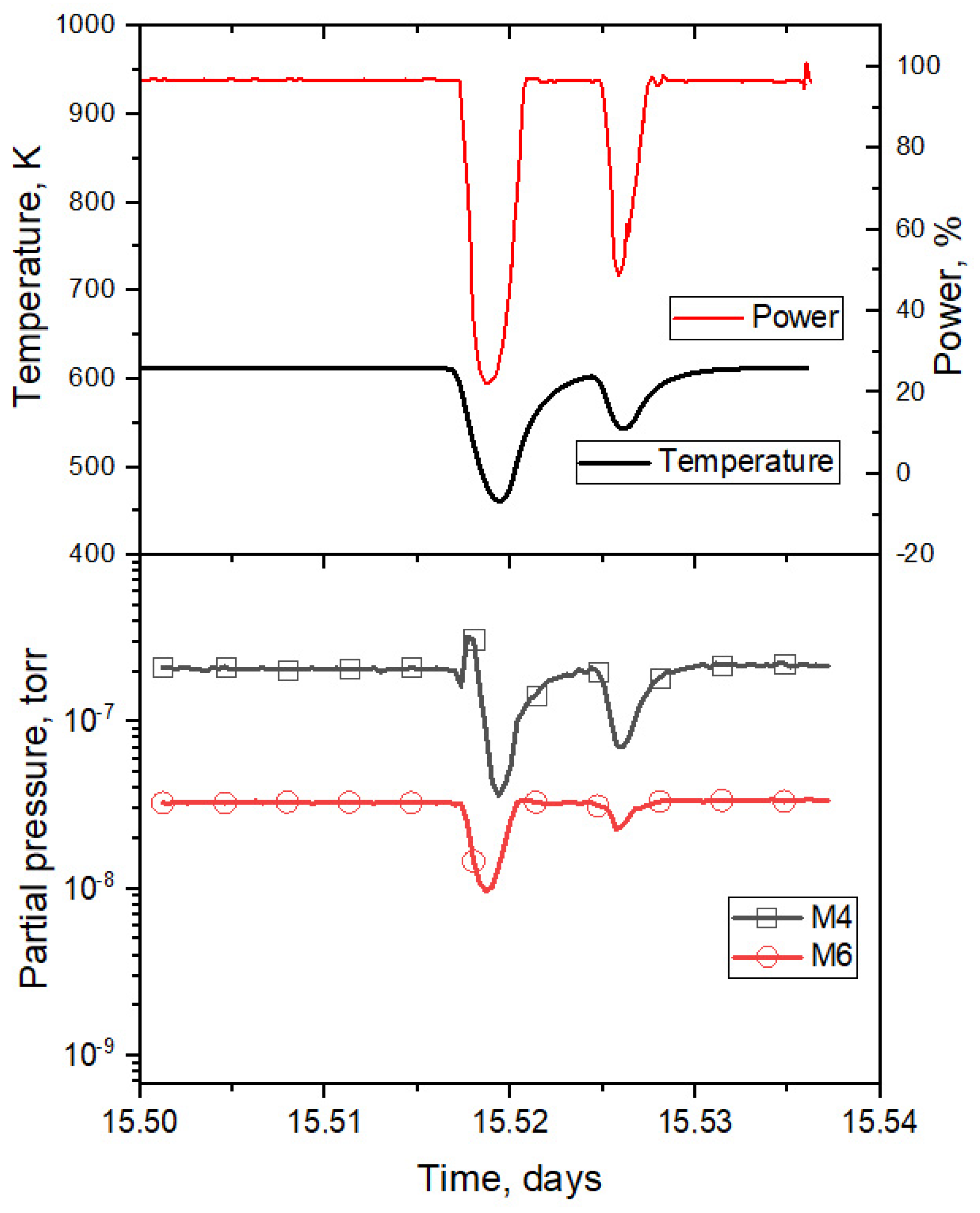
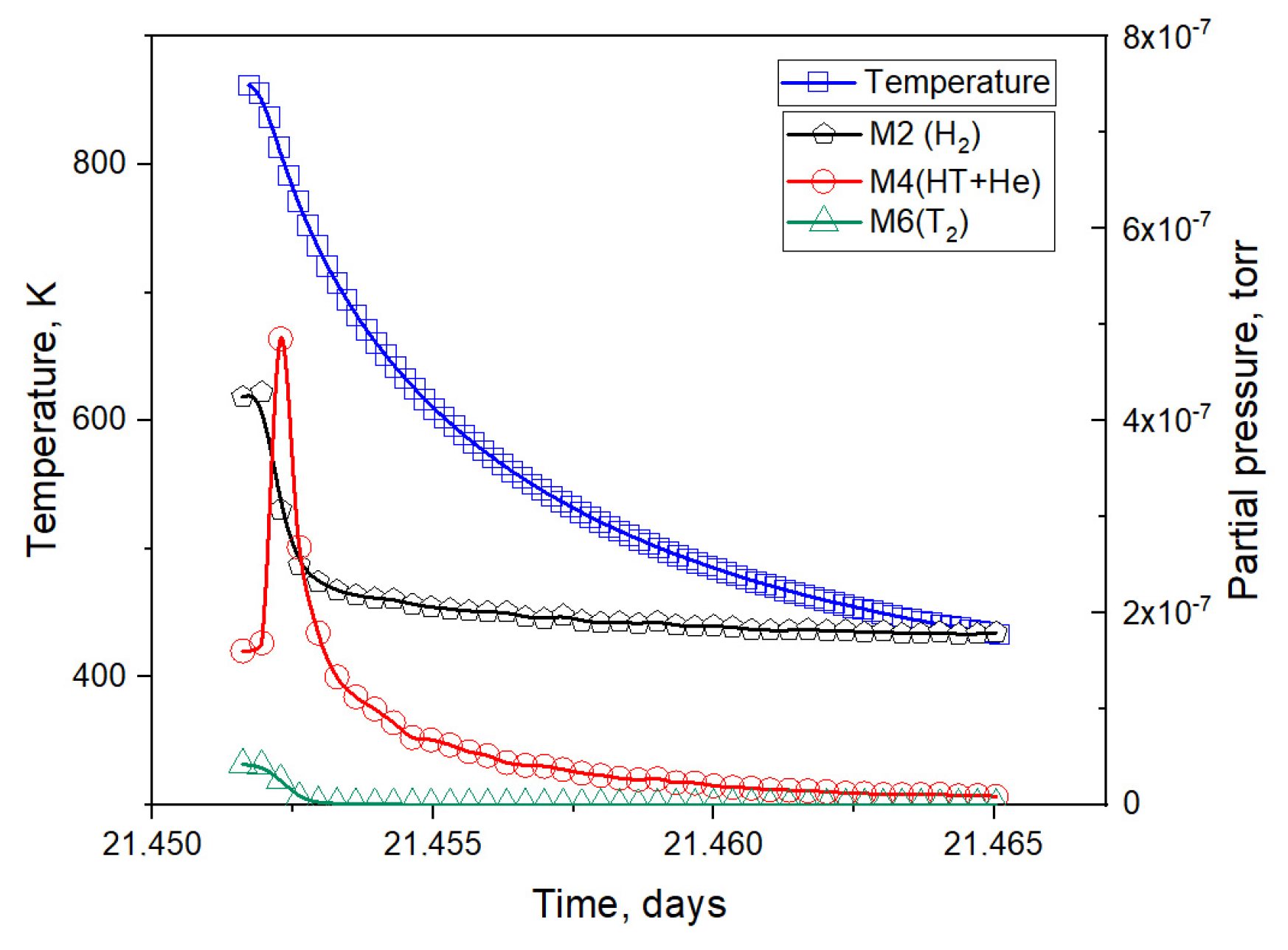
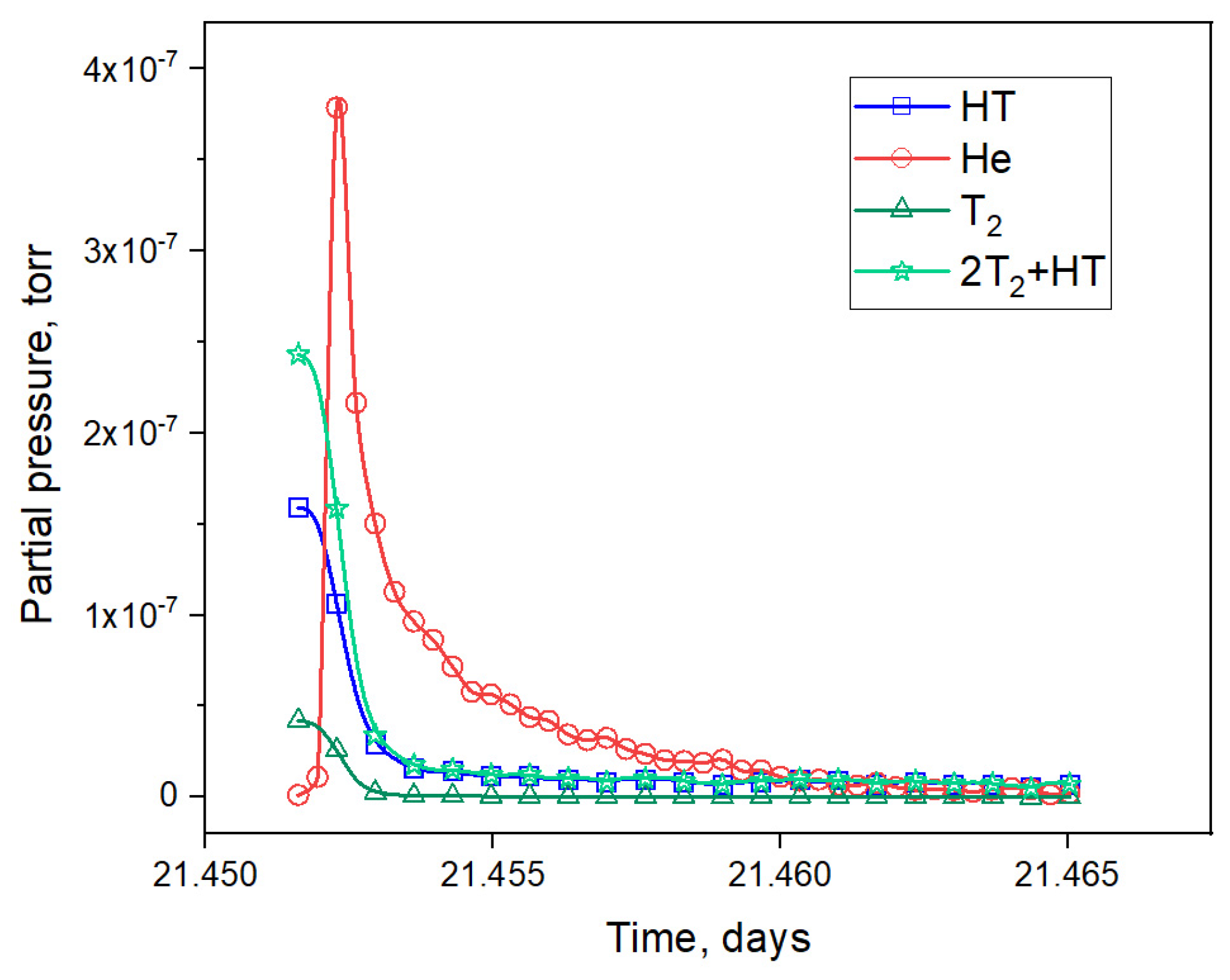
3.1. Results of the Reactor Experiment
3.2. Analysis of Helium Release from a Ceramic Pebble
- (1)
- The greatest number of observed helium release peaks ranged from 20% to 60% of the trend release level; their release rate increased with the irradiation time up to 2–3 days of irradiation, and then decreased;
- (2)
- Peaks with a release of more than 80% of the trend release level were mostly observed at the beginning of the experiment;
- (3)
- After the 7th day of irradiation, the release of helium in the form of peaks practically stopped.
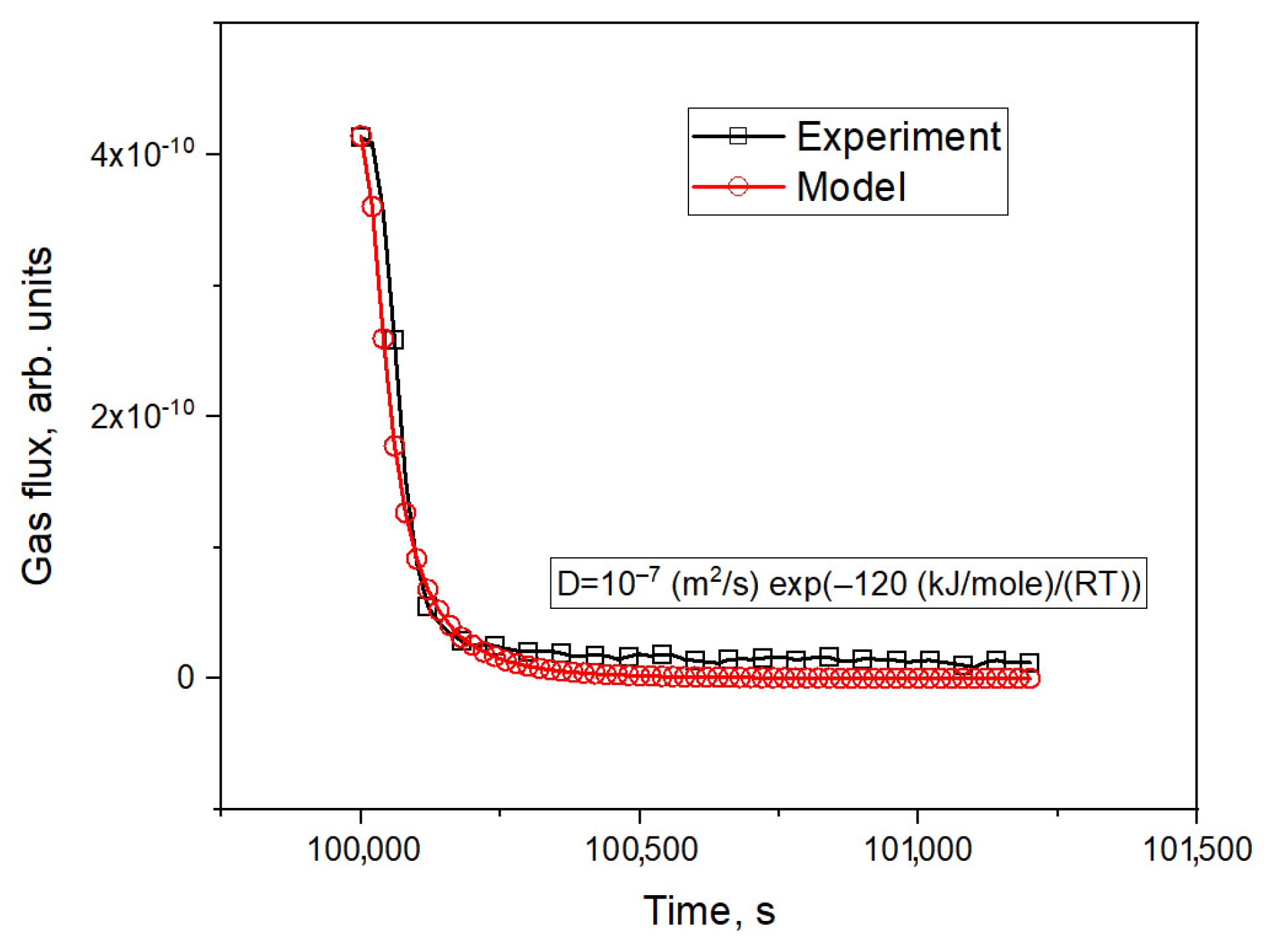
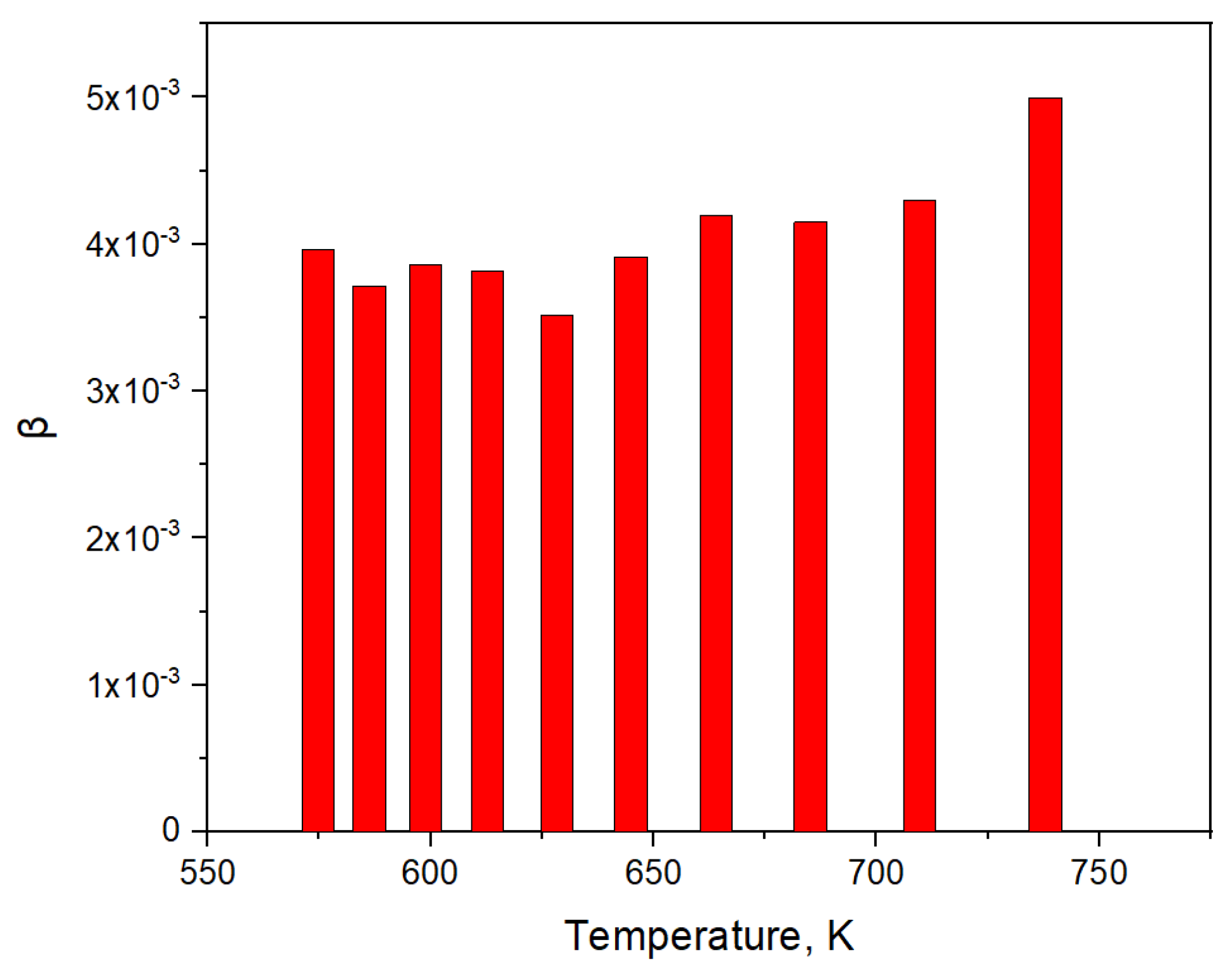
3.3. Simulation of Tritium Release and Estimation of the Effusion Parameters of Helium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giancarli, L.M.; Bravo, X.; Cho, S.; Ferrari, M.; Hayashi, T.; Kim, B.; Leal-Pereira, A.; Martins, J.P.; Merola, M.; Pascal, R.; et al. Overview of recent ITER TBM Program activities. Fusion Eng. Des. 2020, 158, 111674. [Google Scholar] [CrossRef]
- Danani, C.; Swami, H.L.; Chaudhuri, P.; Mutzke, A.; Schneider, R.; Warrier, M. Multi-model quantification of defects in irradiated lithium titanate. Fusion Eng. Des. 2019, 140, 92–96. [Google Scholar] [CrossRef]
- Giancarli, L.M.; Abdou, M.; Campbell, D.J.; Chuyanov, V.A.; Ahn, M.Y.; Enoeda, M.; Pane, C.; Poitevin, Y.; Rajendra Kumar, E.; Ricapito, I.; et al. Overview of the ITER TBM Program. Fusion Eng. Des. 2012, 87, 395–402. [Google Scholar] [CrossRef]
- Blynskiy, P.; Chikhray, Y.; Kulsartov, T.; Gabdullin, M.; Zaurbekova, Z.; Kizane, G.; Shaimerdenov, A. Experiments on tritium generation and yield from lithium ceramics during neutron irradiation. Int. J. Hydrogen Energy 2021, 46, 9186–9192. [Google Scholar] [CrossRef]
- Kulsartov, T.; Shaimerdenov, A.; Zaurbekova, Z.; Kenzhina, I.; Chikhray, Y.; Kizane, G.; Ponkratov, Y. Features of the in-situ experiments on studying of tritium release from lithium ceramic Li2TiO3 using vacuum extraction method. Fusion Eng. Des. 2021, 172, 112703. [Google Scholar] [CrossRef]
- Kulsartov, T.; Kenzhina, I.; Chikhray, Y.; Zaurbekova, Z.; Kenzhin, Y.; Aitkulov, M.; Dyussambayev, D. Determination of the activation energy of tritium diffusion in ceramic breeders by reactor power variation. Fusion Eng. Des. 2021, 172, 112783. [Google Scholar] [CrossRef]
- Kulsartov, T.; Kenzhina, I.; Tolenova, A.; Kenzhin, Y.; Shaimerdenov, A.; Nesterov, Y.; Gluchshenko, A. Modeling of hydrogen isotopes release from lithium ceramics Li2TiO3 during in-situ experiments using vacuum extraction method. Fusion Eng. Des. 2021, 170, 112705. [Google Scholar] [CrossRef]
- Kenzhina, I.; Kulsartov, T.; Chikhray, Y.; Kenzhin, Y.; Zaurbekova, Z.; Shaimerdenov, A.; Kizane, G.; Zarins, A.; Kozlovskiy, A.; Gabdullin, M.; et al. Analysis of the reactor experiments results on the study of gas evolution from two-phase Li2TiO3-Li4SiO4 lithium ceramics. Nucl. Mater. Energy 2022, 30, 101132. [Google Scholar] [CrossRef]
- Shaimerdenov, A.; Gizatulin, S.; Dyussambayev, D.; Askerbekov, S.; Kenzhina, I. The WWR-K Reactor Experimental Base for Studies of the Tritium Release from Materials Under Irradiation. Fusion Sci. Technol. 2020, 76, 304–313. [Google Scholar] [CrossRef]
- Kulsartov, T.; Zaurbekova, Z.; Knitter, R.; Shaimerdenov, A.; Chikhray, Y.; Askerbekov, S.; Akhanov, A.; Kenzhina, I.; Kizane, G.; Kenzhin, Y.; et al. Studies of two-phase lithium ceramics Li4SiO4-Li2TiO3 under conditions of neutron irradiation. Nucl. Mater. Energy 2022, 30, 101129. [Google Scholar] [CrossRef]
- Madhusudana, C.V. Special Topics in Thermal Contact Conductance. In Thermal Contact Conductance; Mechanical Engineering Series; Springer: New York, NY, USA, 1996. [Google Scholar]
- Gan, Y. Thermo-Mechanics of Pebble Beds in Fusion Blankets. Ph.D. Thesis, Kalsruhe Institute of Technology, Karlsruhe, Germany, 2008. [Google Scholar] [CrossRef]
- Moriyama, H.; Tanaka, S.; Noda, K. Irradiation effects in ceramic breeder materials. J. Nucl. Mater. 1998, 258–263, 587–594. [Google Scholar] [CrossRef]
- Kotomin, E.A.; Popov, A.I. Radiation-induced point defects in simple oxides. Nucl. Instrum. Methods Phys. Res. 1998, 141, 1–15. [Google Scholar] [CrossRef]
- Hong, M.; Zhang, Y.C.; Liu, Y.H.; Fu, B.J. Fabrication of Li2TiO3 ceramic pebbles by gelcasting method. Key Eng. Mater. 2012, 512, 1717–1720. [Google Scholar] [CrossRef]
- Elliott, J.A.W.; Ward, C.A. Temperature programmed desorption. J. Chem. Phys. 1997, 106, 13–18. [Google Scholar] [CrossRef]



| Elements | Li | Ti | O | Ca | Na | K | Mg | B | Co | Al | Zr | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| composition (w%) | 11.4 | 44.4 | 44.1 | <0.01 | 0.012 | <0.0001 | 0.0006 | <0.01 | 0.0030 | 0.009 | 0.0003 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulsartov, T.; Zaurbekova, Z.; Chikhray, Y.; Kenzhina, I.; Askerbekov, S.; Shaimerdenov, A.; Akhanov, A.; Aitkulov, M.; Begentayev, M. Features of Helium and Tritium Release from Li2TiO3 Ceramic Pebbles under Neutron Irradiation. Materials 2023, 16, 5903. https://doi.org/10.3390/ma16175903
Kulsartov T, Zaurbekova Z, Chikhray Y, Kenzhina I, Askerbekov S, Shaimerdenov A, Akhanov A, Aitkulov M, Begentayev M. Features of Helium and Tritium Release from Li2TiO3 Ceramic Pebbles under Neutron Irradiation. Materials. 2023; 16(17):5903. https://doi.org/10.3390/ma16175903
Chicago/Turabian StyleKulsartov, Timur, Zhanna Zaurbekova, Yevgen Chikhray, Inesh Kenzhina, Saulet Askerbekov, Asset Shaimerdenov, Assyl Akhanov, Magzhan Aitkulov, and Meiram Begentayev. 2023. "Features of Helium and Tritium Release from Li2TiO3 Ceramic Pebbles under Neutron Irradiation" Materials 16, no. 17: 5903. https://doi.org/10.3390/ma16175903





