Comparison of Thermal Deformation Behavior and Characteristics of Mg-Gd-Y-Zn Alloys with and without Bulk LPSO Phase
Abstract
:1. Introduction
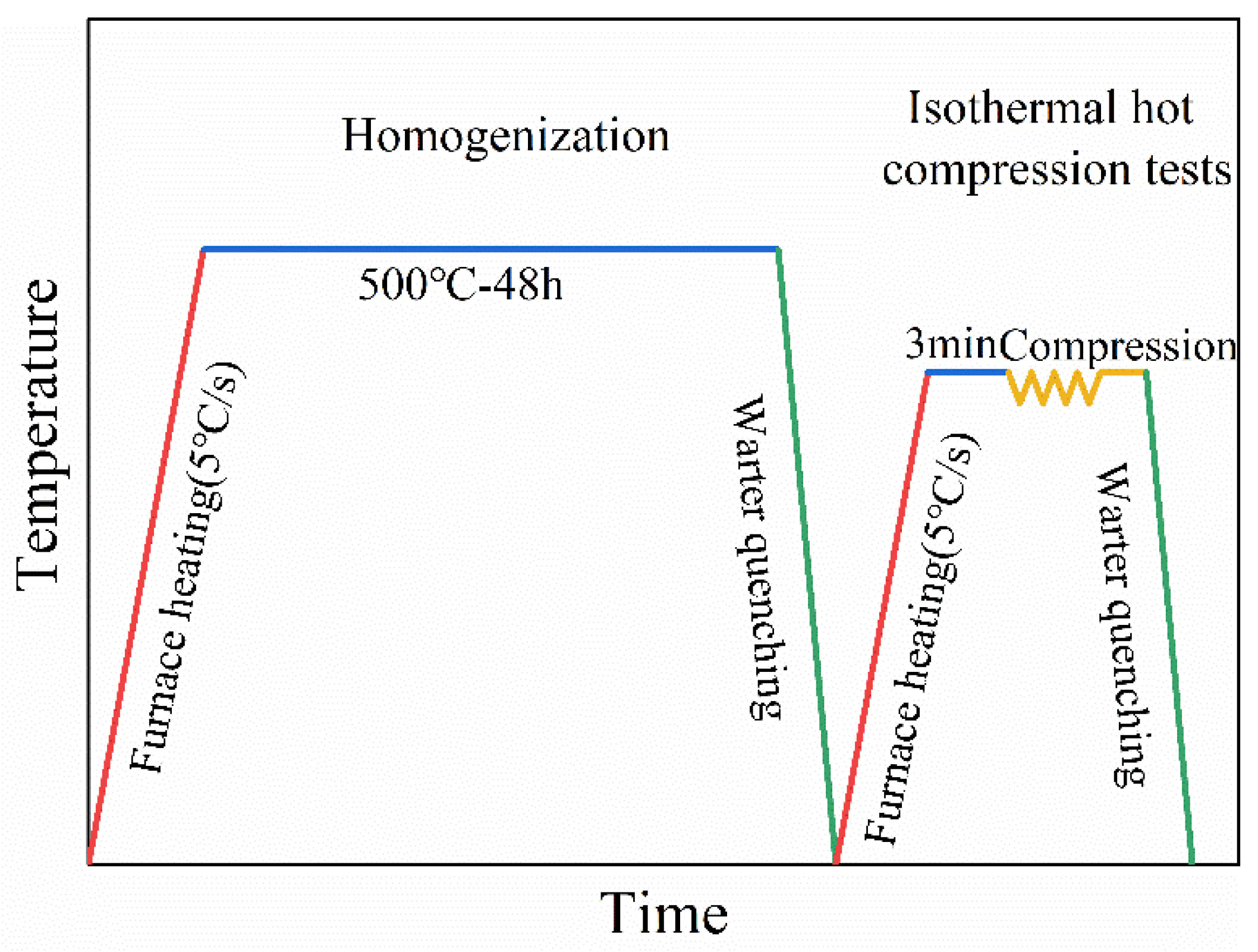
2. Materials and Experiments
3. Results and Discussion
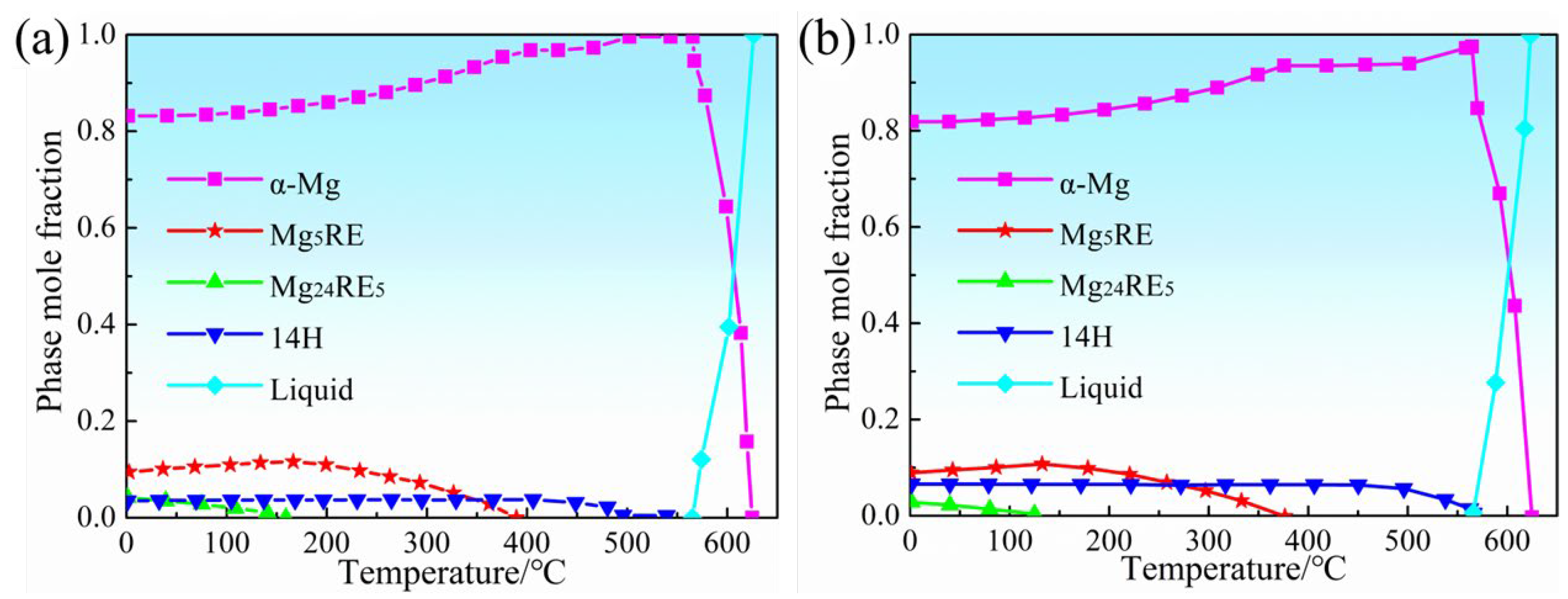
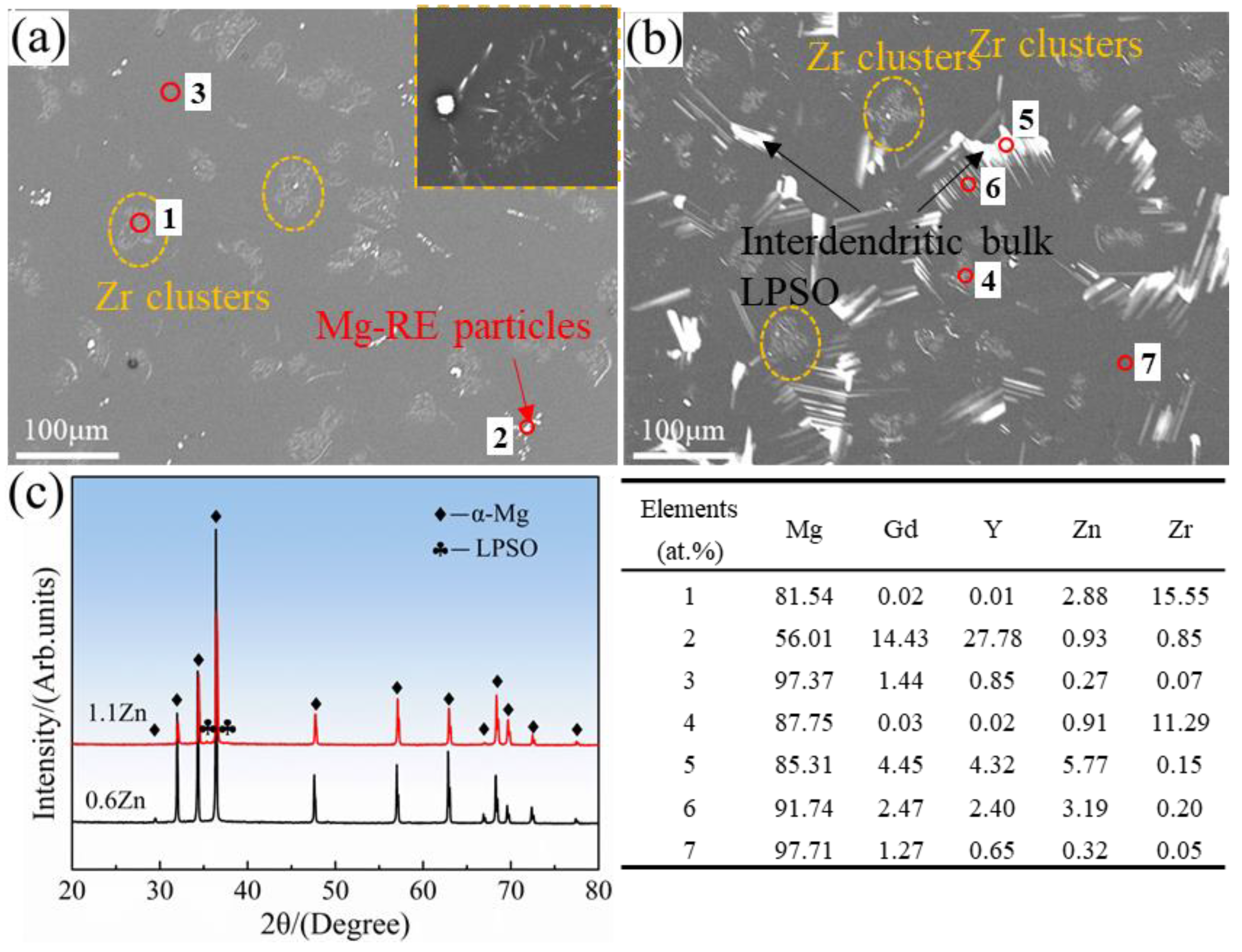
3.1. Initial Microstructure
3.2. Thermal Compression Deformation Behavior
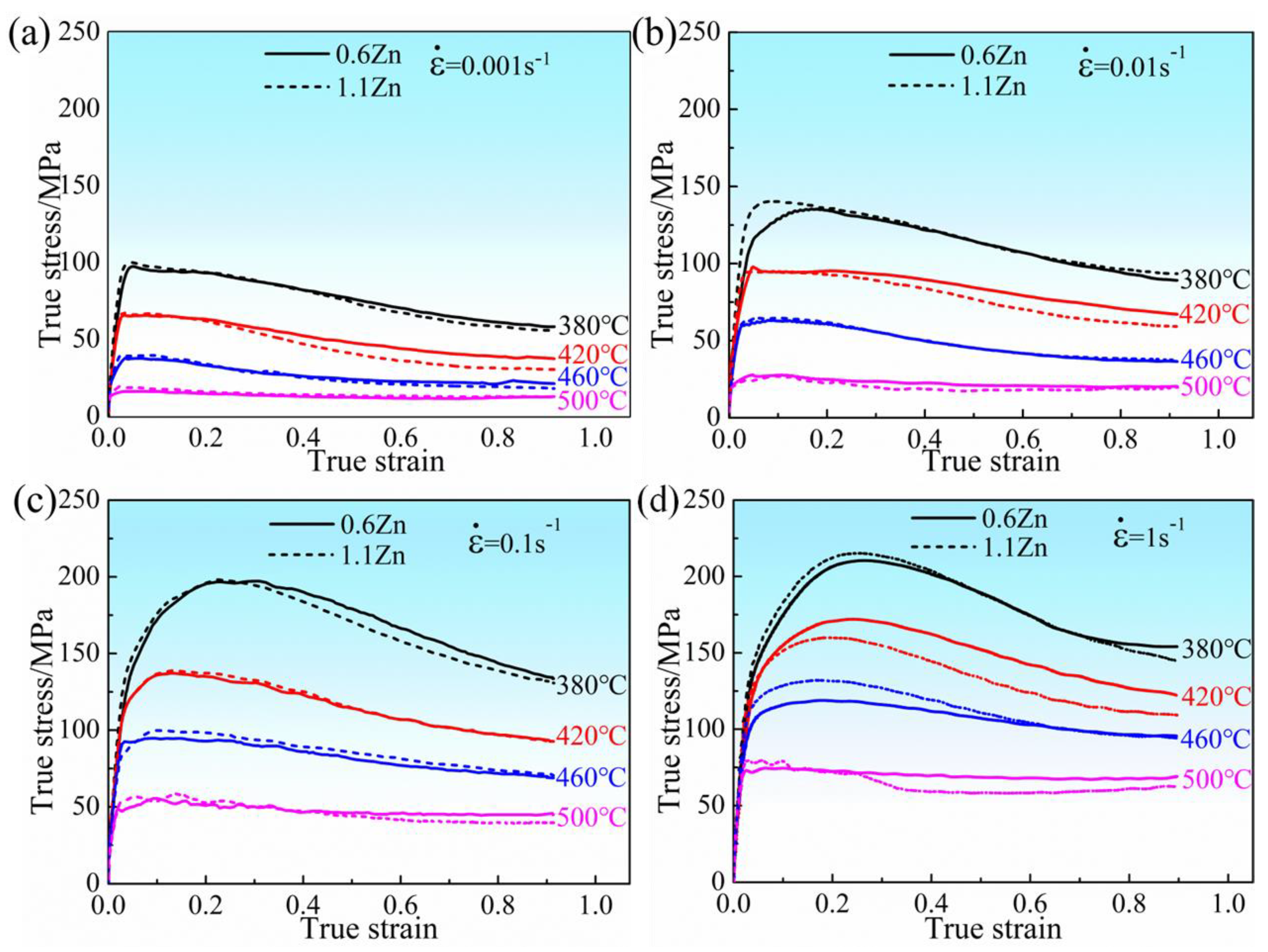
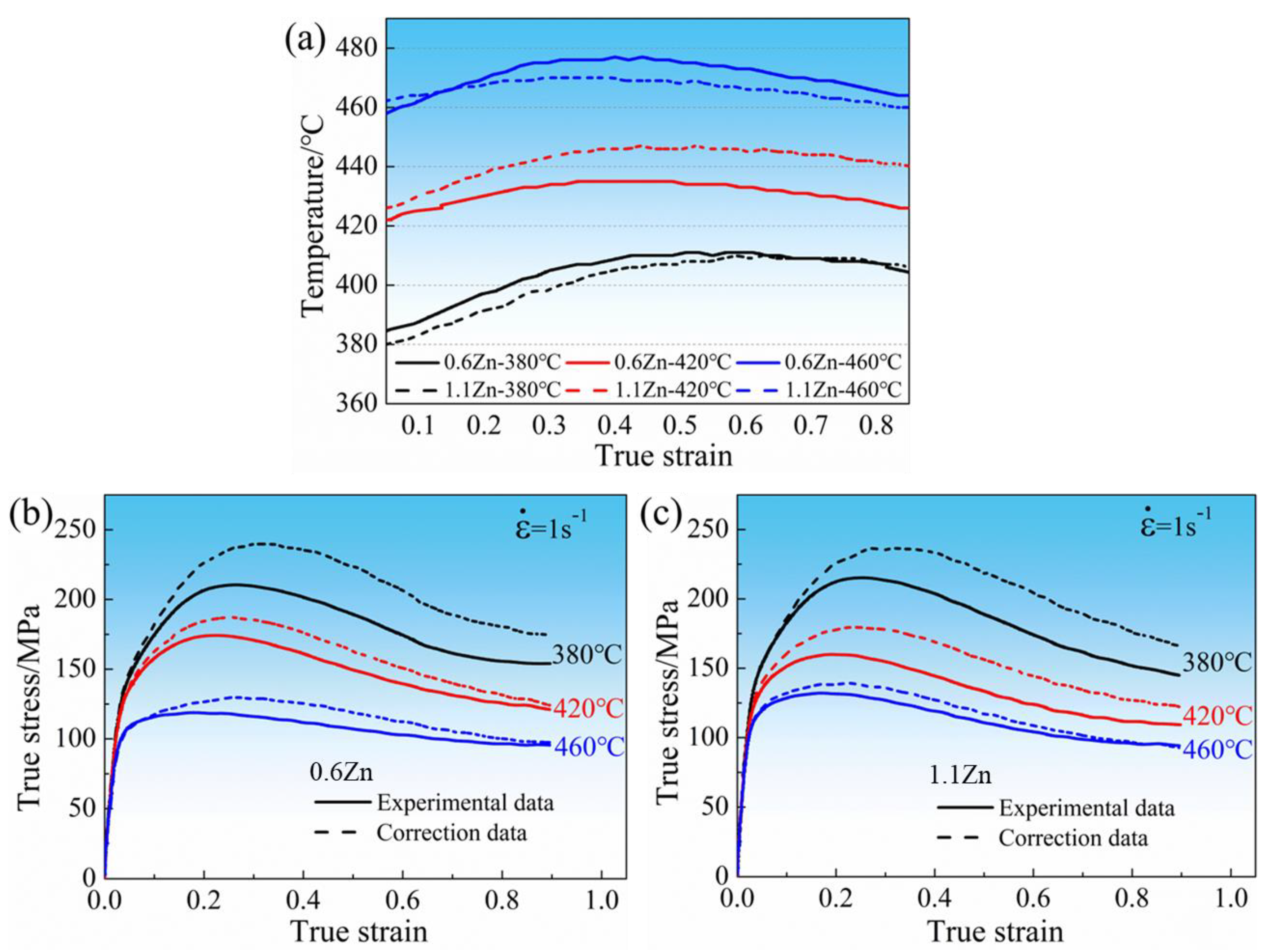
3.2.1. Flow Stress Curves
3.2.2. Activation Energy
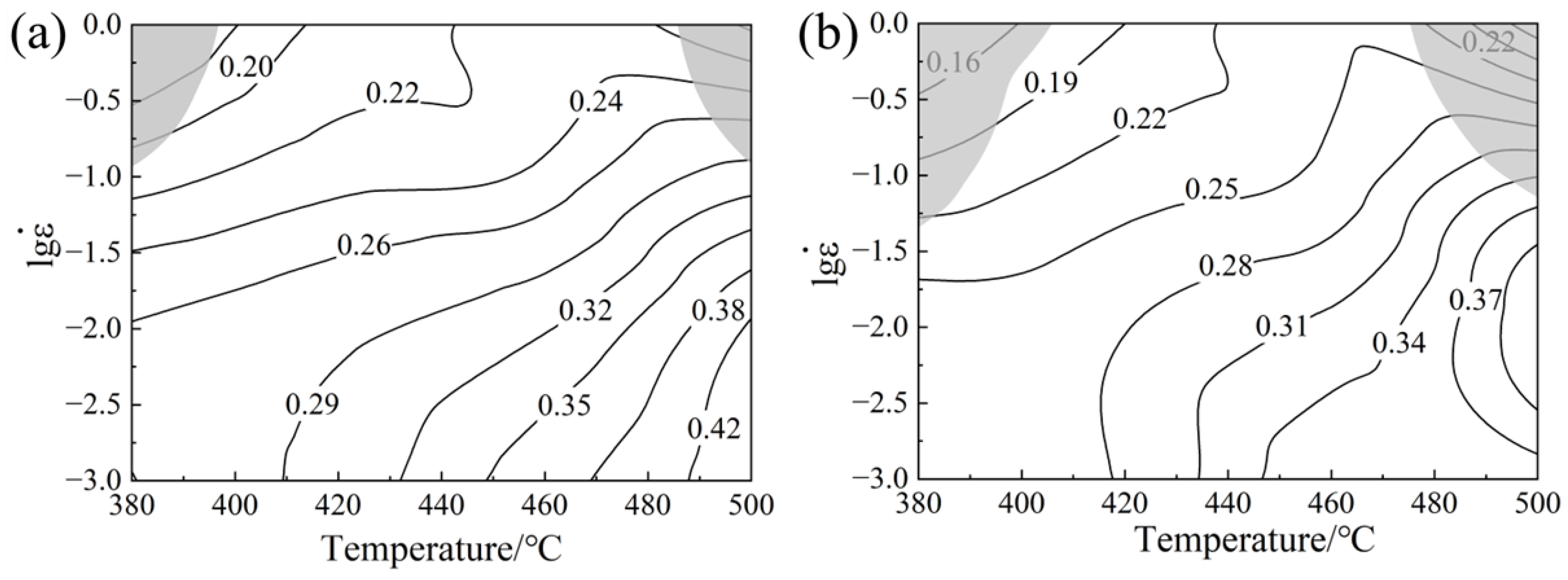
3.3. Processing Maps
3.4. Microstructure Analysis of Characteristic Regions
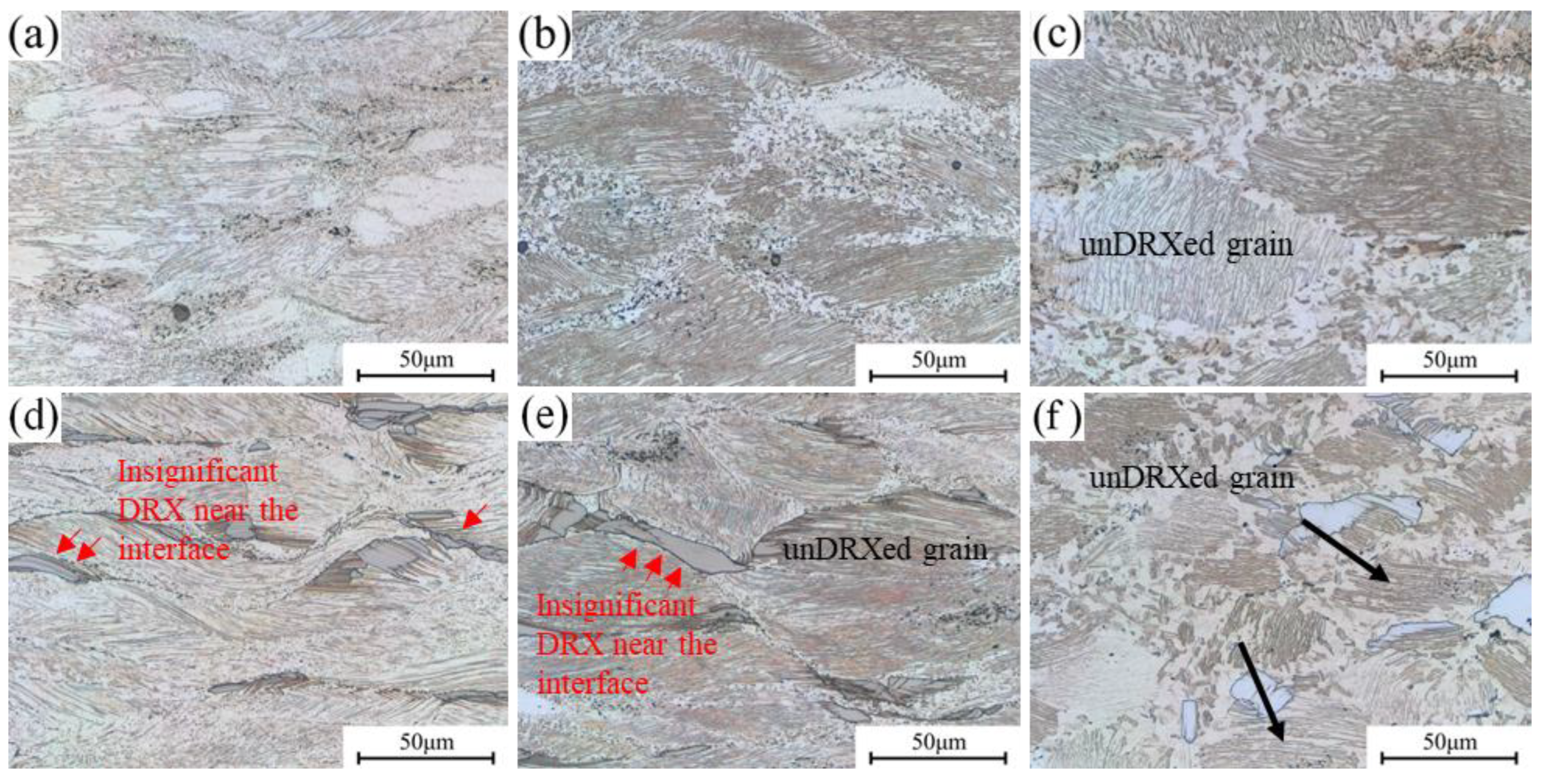
3.4.1. Microstructures with Variation in η Value
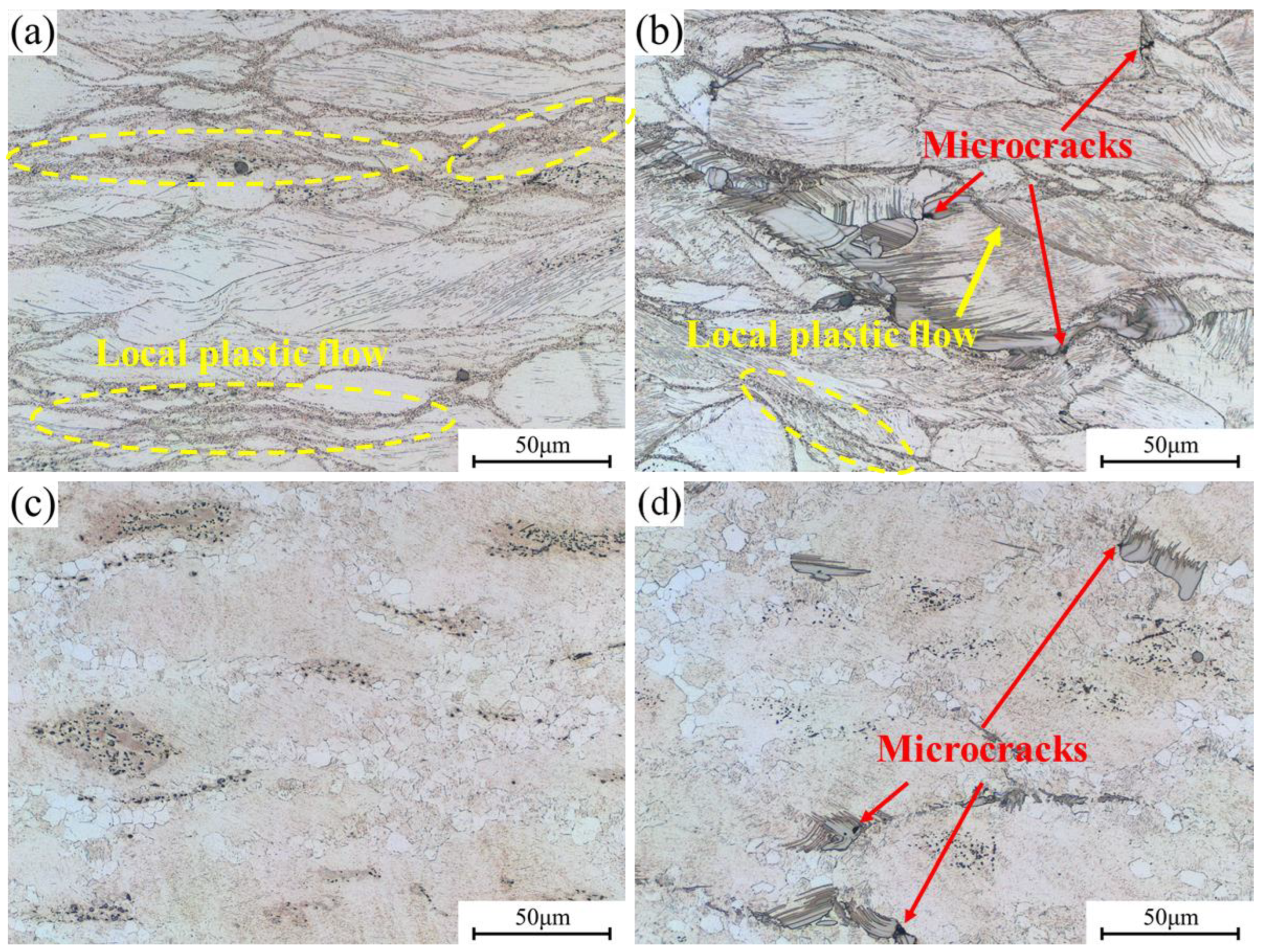
3.4.2. Microstructure Analysis in Instability Regions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Šašek, S.; Minárik, P.; Stráská, J.; Hosová, K.; Veselý, J.; Kubásek, J.; Král, R.; Krajňák, T.; Vojtěch, D. Novel Ultrafine-Grain Mg-Gd/Nd-Y-Ca Alloys with an Increased Ignition Temperature. Materials 2023, 16, 1299. [Google Scholar] [CrossRef]
- Guo, T.; Geng, Y.; Song, X.; Rui, X.; Ge, Z. Tracing magnesium flows in China: A dynamic material flow analysis. Resour. Policy 2023, 83, 103627. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, W.; Ma, K.; Dai, C.; Wang, D.; Wang, J. A new design strategy for the crack-free composite CaHPO4·2H2O/CaCO3 coating on AZ41 Mg alloy for magnesium concrete formwork. Surf. Coat. Technol. 2023, 468, 129784. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, X.; Sun, Y.; Guan, S.; Chen, Y. Exploring a balance between strength and ductility of hexagonal BN nanoplatelet reinforced ZK61 magnesium composite. J. Magnes. Alloys 2022. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Zhang, K.; Li, X.; Ma, M.; Shi, G.; Li, T. Phase constitutions, growth pattern and mechanical properties of Mg-1.4Gd-1.2Y-xZn-0.15Zr (at%) alloys. J. Rare Earths 2020, 38, 315–323. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, H.; Li, Q.; Chen, J.; Xia, W.; Su, B.; Song, M. Enhancing strength and ductility of low RE content Mg-Gd-Y-Zr alloy via a novel thermomechanical treatment based on multi-directional forging. J. Alloys Compd. 2023, 958, 170535. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, D.; Zhang, Y.; Zhou, J.; Sun, D.; Li, H. Research advances on weldability of Mg alloy and other metals worldwide in recent 20 years. J. Mater. Res. Technol. 2023, 25, 3458–3481. [Google Scholar] [CrossRef]
- Shi, G.; Yuan, J.; Li, T.; Zhang, K.; Li, X.; Li, Y.; Ma, M. Enhanced precipitation strengthening of extruded Mg-8wt.% Al-0.5wt.%Zn (AZ80) magnesium alloy by extension twinning. Mater. Sci. Eng. A 2020, 774, 138906. [Google Scholar] [CrossRef]
- Liao, H.B.; Mo, L.L.; Li, C.B.; Zhan, M.Y.; Jun, D.U. Grain refinement of Mg-Al binary alloys inoculated by in-situ oxidation. Trans. Nonferrous Met. Soc. China 2022, 32, 3212–3221. [Google Scholar] [CrossRef]
- Najafi, S.; Sheikhani, A.; Sabbaghian, M.; Nagy, P.; Fekete, K.; Gubicza, J. Modification of the Tensile Performance of an Extruded ZK60 Magnesium Alloy with the Addition of Rare Earth Elements. Materials 2023, 16, 2828. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, K.; Zhang, X.; Li, X.; Li, T.; Li, Y.; Shi, G. Thermal characteristics of Mg–Zn–Mn alloys with high specific strength and high thermal conductivity. J. Alloys Compd. 2013, 578, 32–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Wei, S.; Wang, Y.; Li, L. Estimation of mechanical properties of Mg-5Zn-0.5Al-xSn alloy based on virtual crystal approximation. Heliyon 2022, 8, e11224. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, K.; Shi, G.; Wang, K.; Li, Y.; Li, X.; Yuan, J. Microstructure evolution, mechanical properties and creep mechanisms of Mg-12Gd-1MM-0.6Zr (wt%) magnesium alloy. J. Rare Earths 2021, 39, 600–608. [Google Scholar] [CrossRef]
- Lei, B.; Wang, C.; Jiang, B.; Bai, S.; Dong, Z.; Qian, X.; Pan, F. Role of Y on the microstructure and mechanical properties of Mg-Gd-Zr alloy. Mater. Sci. Eng. A 2022, 861, 144371. [Google Scholar] [CrossRef]
- Peng, S.; Wu, Y.; Zhang, T.; Xie, Q.; Yuan, Z.; Yin, L. Dynamic Constitutive Relationship of Mg–Gd–Y–Zr–Ag Alloy during High Temperature Deformation Process. Materials 2023, 16, 2587. [Google Scholar] [CrossRef]
- Xu, C.; Fan, G.H.; Nakata, T.; Liang, X.; Chi, Y.Q.; Qiao, X.G.; Xie, H.L. Deformation behavior of ultra-strong and ductile Mg-Gd-Y-Zn-Zr alloy with bimodal microstructure. Metall. Mater. Trans. A 2018, 49, 1931–1947. [Google Scholar] [CrossRef]
- Wu, G.; Li, Z.; Yu, J.; Liu, Y.; Zhang, Z.; Dong, B.; Huang, H. Optimization in strength-ductility of Mg-RE-Zn alloy based on different repetitive upsetting extrusion deformation paths. Mater. Des. 2023, 232, 112114. [Google Scholar] [CrossRef]
- Li, L.; Bao, J.; Qiao, M.; Tian, J.; Yang, Y.; Sha, J.; Zhang, Z. Improvement of strength-ductility balance and corrosion resistance in as-extruded Mg-Gd-Zn-Zr alloys by Zn/Gd ratio. Mater. Sci. Eng. A 2023, 872, 144979. [Google Scholar] [CrossRef]
- Ding, Z.; Zhi, X.; Liu, B.; Hou, H.; Zhang, S.; Guo, W.; Zhao, Y. Enhancement of strength and elastic modulus of Mg-Gd-Y-Zn-Zr alloy by Sn addition. Mater. Sci. Eng. A 2022, 854, 143885. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, M.; Yang, X.; Lei, G.; Jia, J.; Wu, G.; Yu, J. Hot tensile deformation behavior and a fracture damage model of the wrought Mg–Gd− Y–Zn–Zr alloy. J. Mater. Res. Technol. 2023, 22, 2093–2110. [Google Scholar] [CrossRef]
- Wang, G.; Mao, P.; Wang, Z.; Zhou, L.; Wang, F.; Liu, Z. Hot Deformation Behavior of an As-Extruded Mg-2.5Zn-4Y Alloy Containing LPSO Phases. Metals 2022, 12, 674. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, Z.; Huo, Q.; Xiao, Z.; Fang, Z.; Yang, X. Dynamic recrystallization behaviors of Mg-Gd-Y-Zn-Zr alloy with different morphologies and distributions of LPSO phases. Mater. Sci. Eng. A 2018, 715, 389–403. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, S.F.; Wang, R.X.; Zhang, S.H.; Cheng, M. Effect of hydrostatic pressure on LPSO kinking and microstructure evolution of Mg–11Gd–4Y–2Zn–0.5Zr alloy. Acta Metall. Sin. 2021, 34, 248–264. [Google Scholar] [CrossRef]
- Li, B.; Teng, B.; Xu, W. Hot deformation characterization of homogenized Mg-Gd-Y-Zn-Zr alloy during isothermal compression. JOM 2019, 71, 4059–4070. [Google Scholar] [CrossRef]
- Zhou, X.; Yao, Y.; Zhang, J.; Chen, X.; Huang, W.; Pan, J.; Weng, M. A high-performance Mg-4.9 Gd-3.2 Y-1.1Zn-0.5Zr alloy via multidirectional forging after analyzing its compression behavior. J. Mater. Sci. Technol. 2021, 70, 156–167. [Google Scholar] [CrossRef]
- Tahreen, N.; Zhang, D.; Pan, F.; Jiang, X.; Li, C.; Li, D.; Chen, D. Characterization of hot deformation behavior of an extruded Mg–Zn–Mn–Y alloy containing LPSO phase. J. Alloys Compd. 2015, 644, 814–823. [Google Scholar] [CrossRef]
- Xu, W.; Jin, X.; Shan, D.; Chai, B. Study on the effect of solution treatment on hot deformation behavior and workability of Mg-7Gd-5Y-0.6Zn-0.8Zr magnesium alloy. J. Alloys Compd. 2017, 720, 309–323. [Google Scholar] [CrossRef]
- Tadić, M.; Panjan, M.; Čekada, M.; Jagličić, Z.; Pregelj, M.; Spreitzer, M.; Panjan, P. Perpendicular magnetic anisotropy at room-temperature in sputtered a-Si/Ni/a-Si layered structure with thick Ni (nickel) layers. Ceram. Int. 2023, 49, 32068–32077. [Google Scholar] [CrossRef]
- Fan, J.; Ma, M.; Zhang, K.; Li, Y.; Li, X.; Shi, G.; Yuan, J. Hot deformation behavior and finite element simulation of Mg-8.3-Gd-4.4-Y-1.5-Zn-0.8-Mn alloy. J. Rare Earths 2022, 40, 831–839. [Google Scholar] [CrossRef]
- Zhu, R.; Qing, L.; Li, J.; Chen, Y.; Zhang, X.; Zheng, Z. Flow curve correction and processing map of 2050 Al–Li alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 404–414. [Google Scholar] [CrossRef]
- El Mehtedi, M.; Musharavati, F.; Spigarelli, S. Modelling of the flow behaviour of wrought aluminium alloys at elevated temperatures by a new constitutive equation. Mater. Des. 2014, 54, 869–873. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, C.; Gao, Y.; Jiang, S.; Liu, W.; Lu, L. Hot compression behavior of the Mg-Gd-Y-Zn-Zr alloy filled with intragranular long-period stacking ordered phases. J. Alloys Compd. 2017, 724, 528–536. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, K.; Ma, M.; Li, T. Constitutive modeling of flow behavior and processing maps of Mg–8.1 Gd–4.5 Y–0.3Zr alloy. J. Magnes. Alloys 2020, 8, 917–928. [Google Scholar] [CrossRef]
- Mu, T.; Zhang, K.; Li, Y.; Li, X.; Ma, M.; Shi, G.; Sun, Z. Study on thermal compression deformation behavior and constitutive model of homogenized Mg–5Zn–1Mn alloy. J. Mater. Res. Technol. 2023, 22, 838–852. [Google Scholar] [CrossRef]
- Malas, J.; Seetharaman, V. Using material behavior models to develop process control strategies. JOM 1992, 44, 8–13. [Google Scholar] [CrossRef]
- Ziegler, H. Progress in Solid Mechanics; Wiley Press: Hoboken, NJ, USA, 1963; p. 4e93. [Google Scholar]



| Alloy | n1 | α | β | N | S |
|---|---|---|---|---|---|
| 0.6Zn | 6.133 | 0.012 | 0.074 | 4.158 | 8.208 |
| 1.1Zn | 6.238 | 0.011 | 0.070 | 4.328 | 7.530 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Wang, Q.; Zhang, L.; Li, T.; Yuan, J.; Shi, G.; Wang, X.; Zhang, K.; Li, Y. Comparison of Thermal Deformation Behavior and Characteristics of Mg-Gd-Y-Zn Alloys with and without Bulk LPSO Phase. Materials 2023, 16, 5943. https://doi.org/10.3390/ma16175943
Chen D, Wang Q, Zhang L, Li T, Yuan J, Shi G, Wang X, Zhang K, Li Y. Comparison of Thermal Deformation Behavior and Characteristics of Mg-Gd-Y-Zn Alloys with and without Bulk LPSO Phase. Materials. 2023; 16(17):5943. https://doi.org/10.3390/ma16175943
Chicago/Turabian StyleChen, Dongjie, Qi Wang, Liang Zhang, Ting Li, Jiawei Yuan, Guoliang Shi, Xinyu Wang, Kui Zhang, and Yongjun Li. 2023. "Comparison of Thermal Deformation Behavior and Characteristics of Mg-Gd-Y-Zn Alloys with and without Bulk LPSO Phase" Materials 16, no. 17: 5943. https://doi.org/10.3390/ma16175943
APA StyleChen, D., Wang, Q., Zhang, L., Li, T., Yuan, J., Shi, G., Wang, X., Zhang, K., & Li, Y. (2023). Comparison of Thermal Deformation Behavior and Characteristics of Mg-Gd-Y-Zn Alloys with and without Bulk LPSO Phase. Materials, 16(17), 5943. https://doi.org/10.3390/ma16175943






