Corrosion of Reinforced A630-420H Steel in Direct Contact with NaCl Solution
Abstract
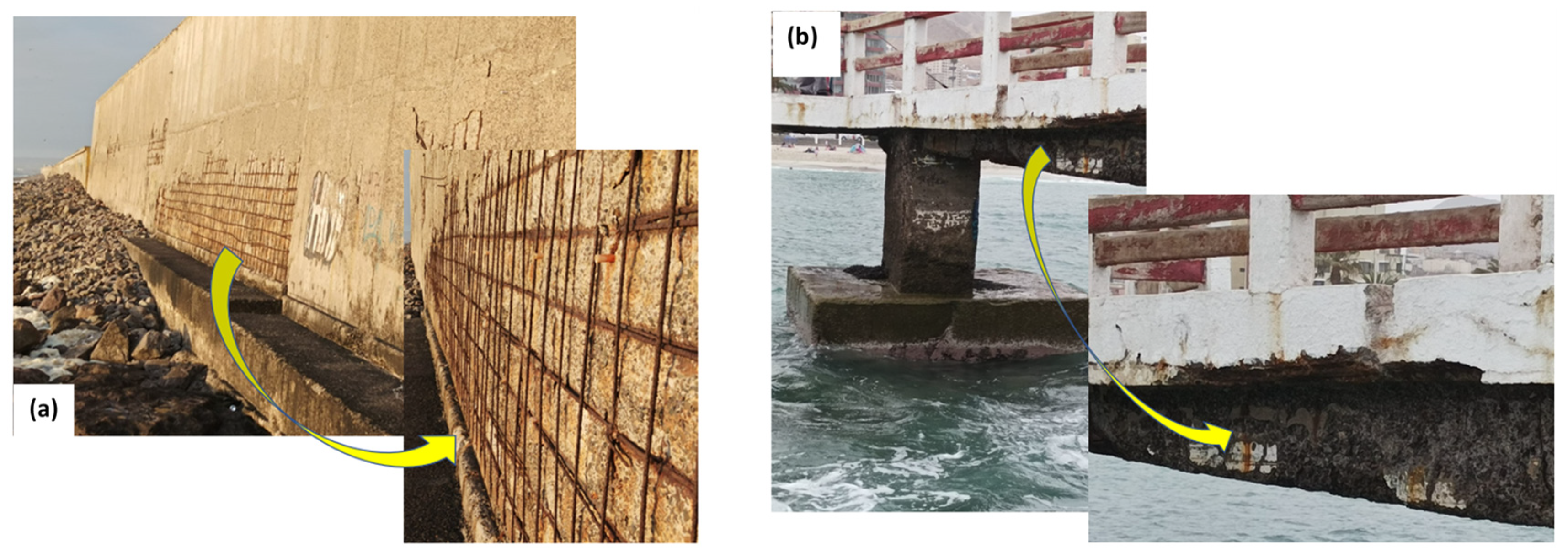
:1. Introduction
2. Experimental Section
2.1. Electrochemical Measurements
- (i)
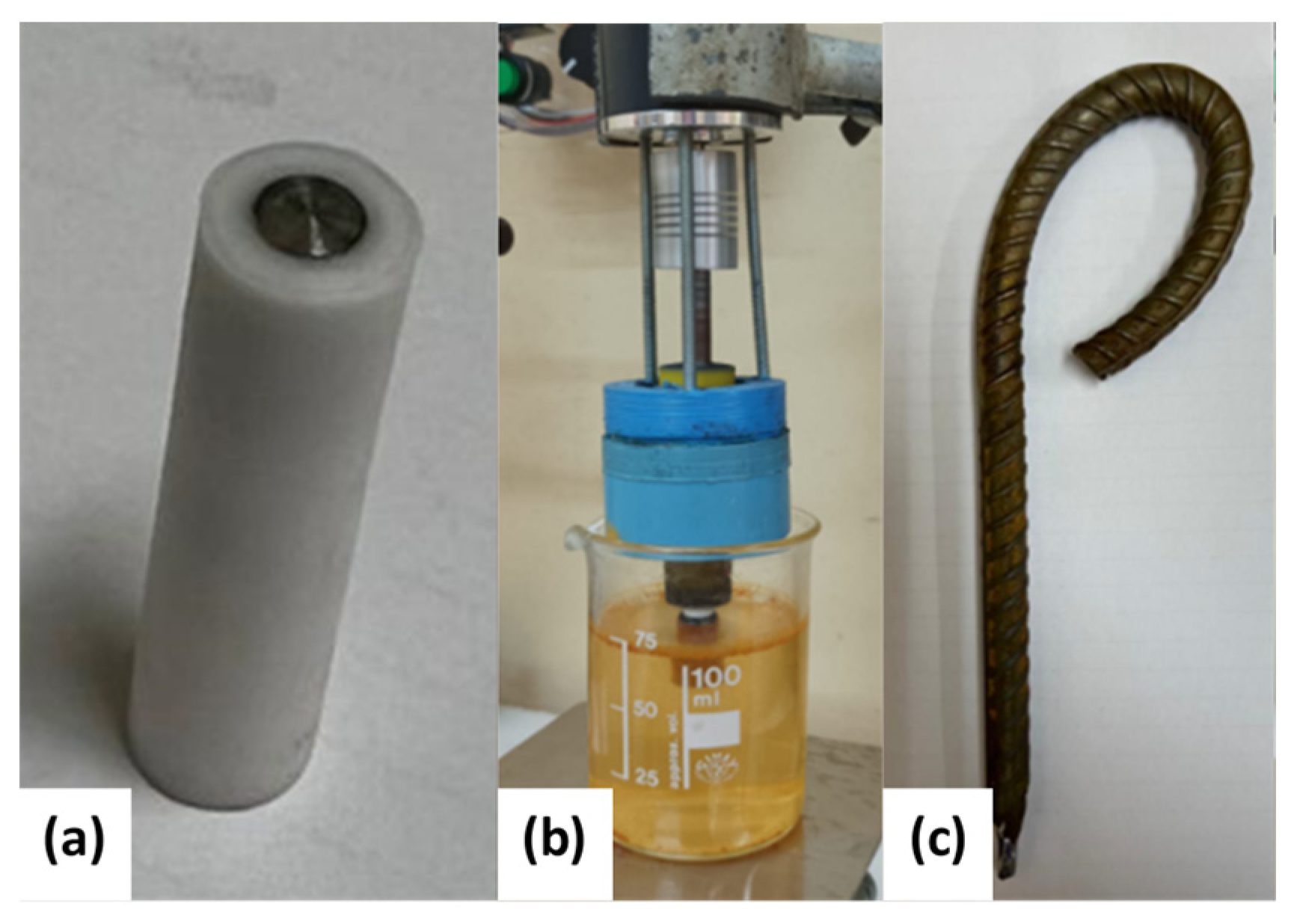
- Electrodes for linear sweep voltammetry (LSV) measurements: The electrodes were fabricated from a cylindrical rod of the specimen with a diameter of 4 mm and a length of 10 mm. The electrodes were concentrically inserted into a PTFE tube with a diameter of 8 mm and sealed with epoxy resin (Figure 2a). The electrochemical measurements were performed using a rotating disc electrode (RDE) interface at a rotation rate of 1200 rpm.
- (ii)
- Electrodes for weight-loss (WL) measurements: The electrodes were cylindrical bars measuring 10 cm in length and 10 mm in diameter. The electrodes were immersed in the test solution under open circuit potential (OCP) conditions for 48 h while rotating at 1200 rpm (Figure 2b). The electrodes were designed with a threaded extension to be coupled to a motor shaft adaptor, and an O-ring seal was used to keep the threaded section dry while the steel specimen was immersed in the electrolyte. This electrode design prevents the occurrence of crevice corrosion and ensures that reliable weight-loss measurements can be obtained.
- (iii)
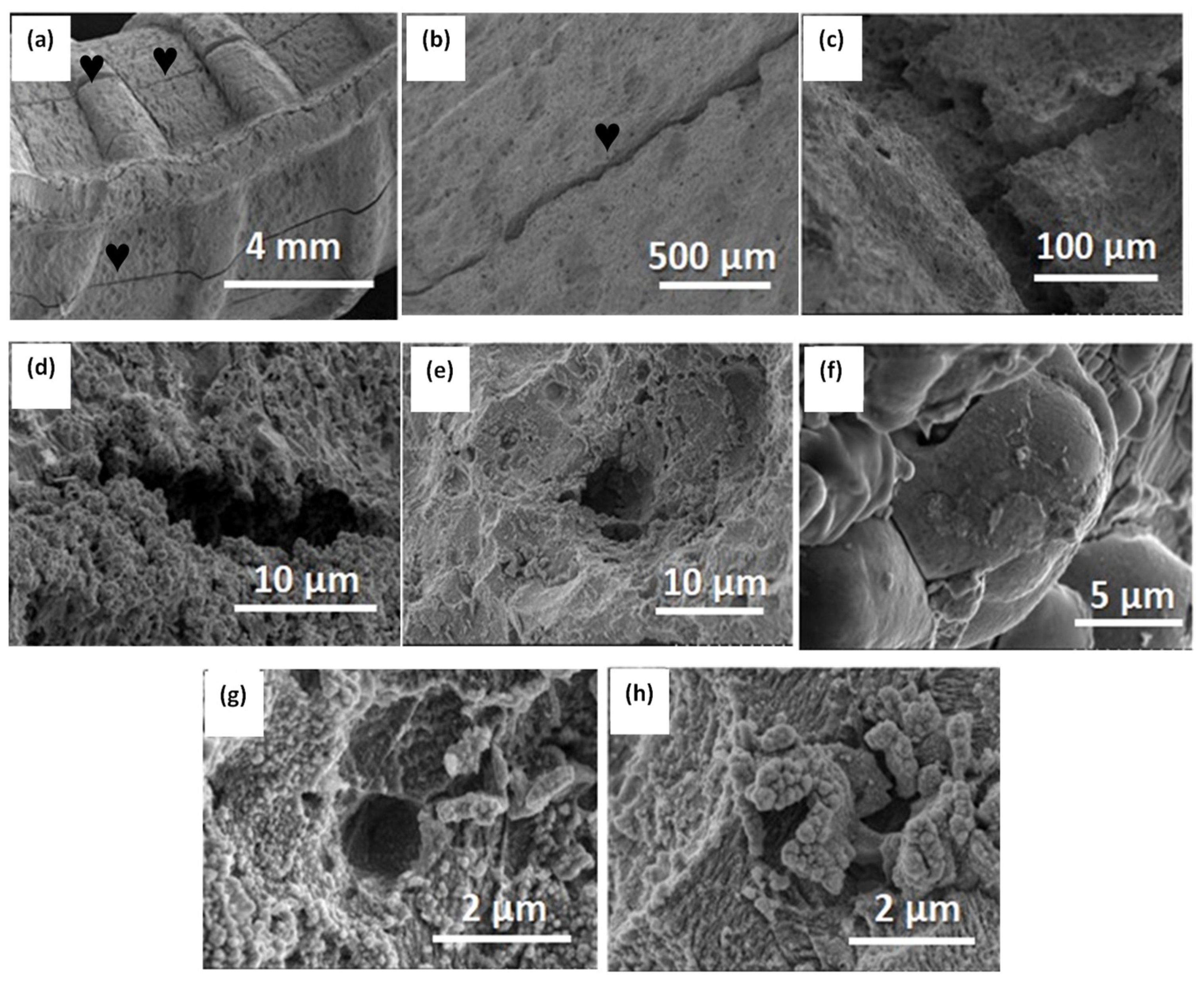
- Electrodes to simulate mechanical stress conditions: The electrodes were fabricated using a 160 mm long and 6 mm diameter bar. The upper parts of these electrodes were bent to simulate the mechanical stress conditions experienced by construction bars (Figure 2c). The curved electrodes were exposed to the test solution for 288 h under OCP and stationary hydrodynamic conditions. Several LSV measurements were conducted during this period.
2.2. Kinetic Analysis
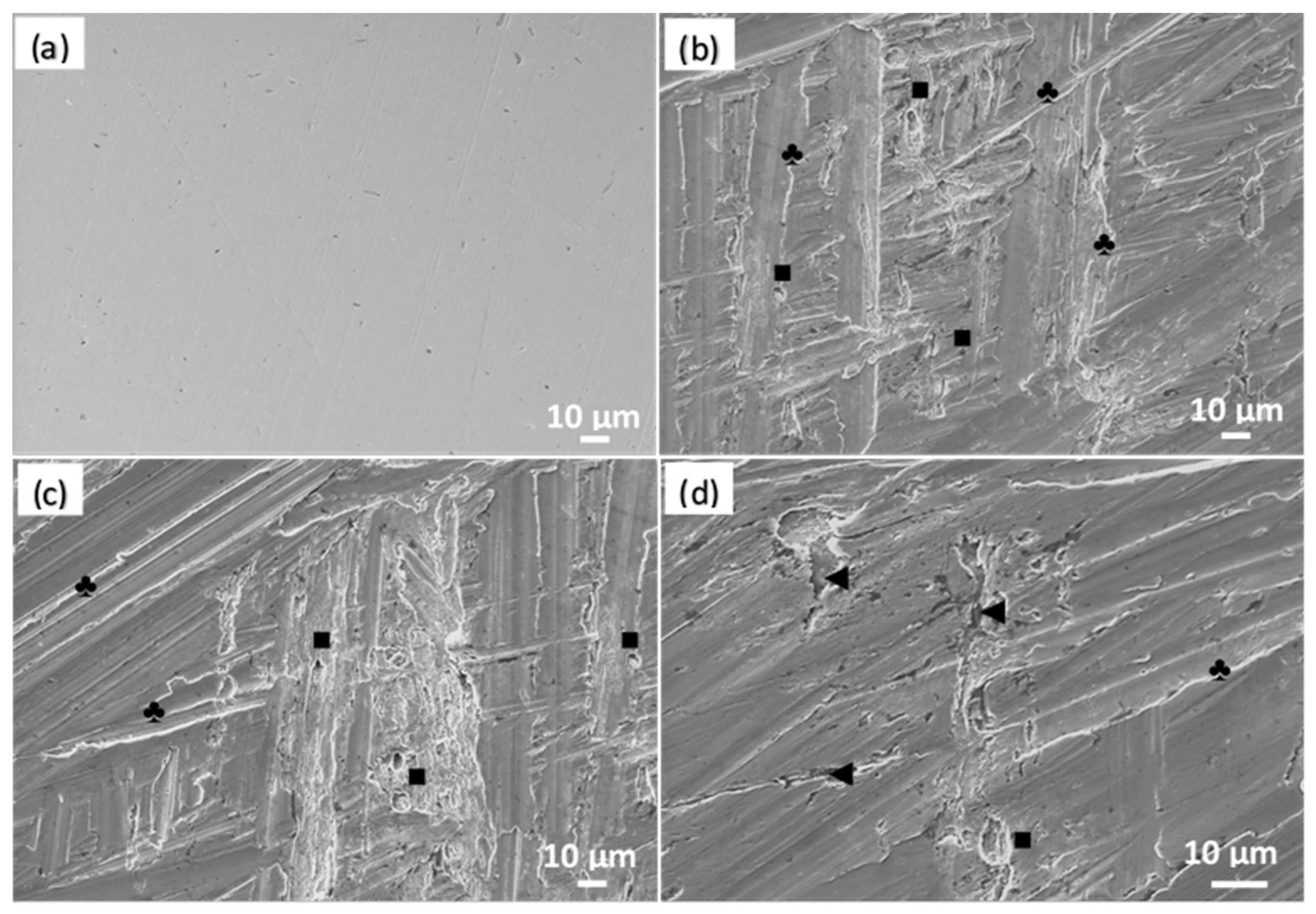
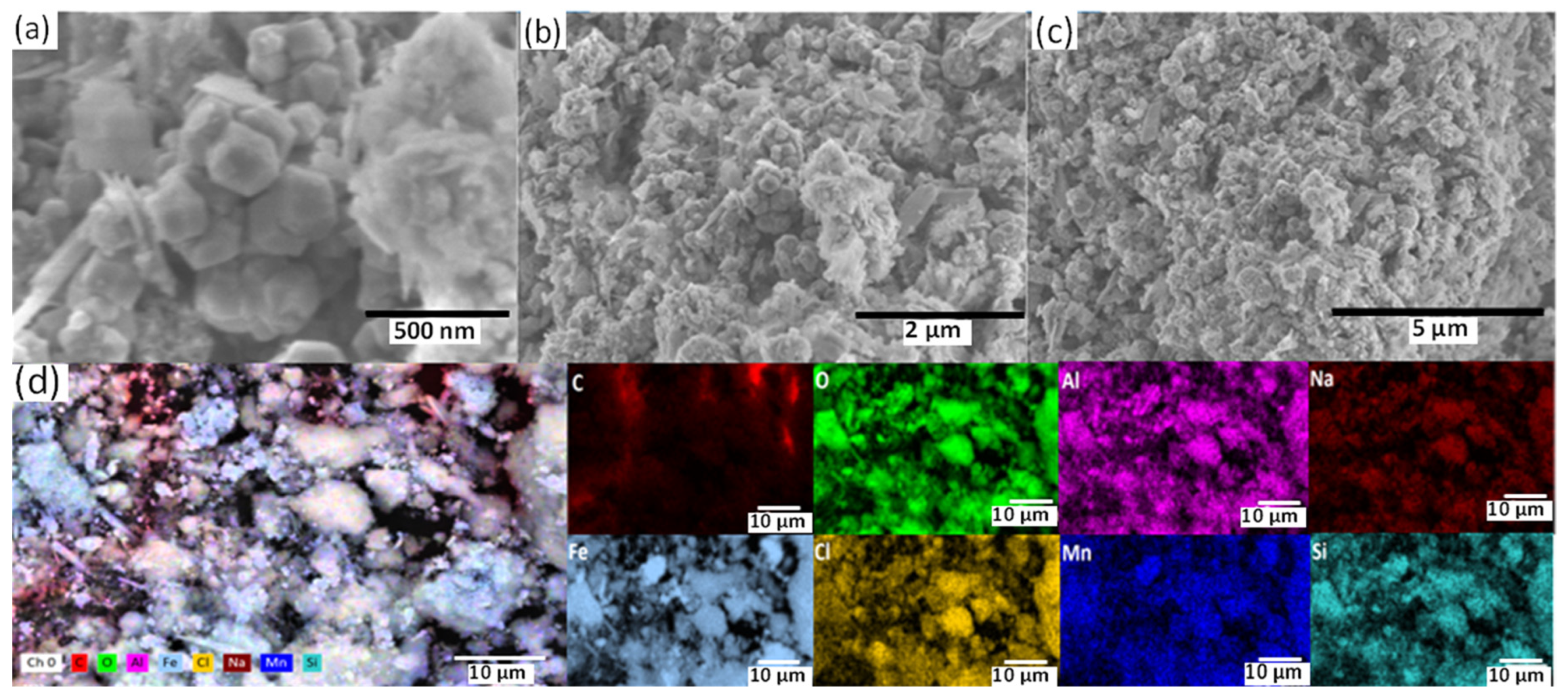
2.3. Surface Analysis
3. Result and Discussion
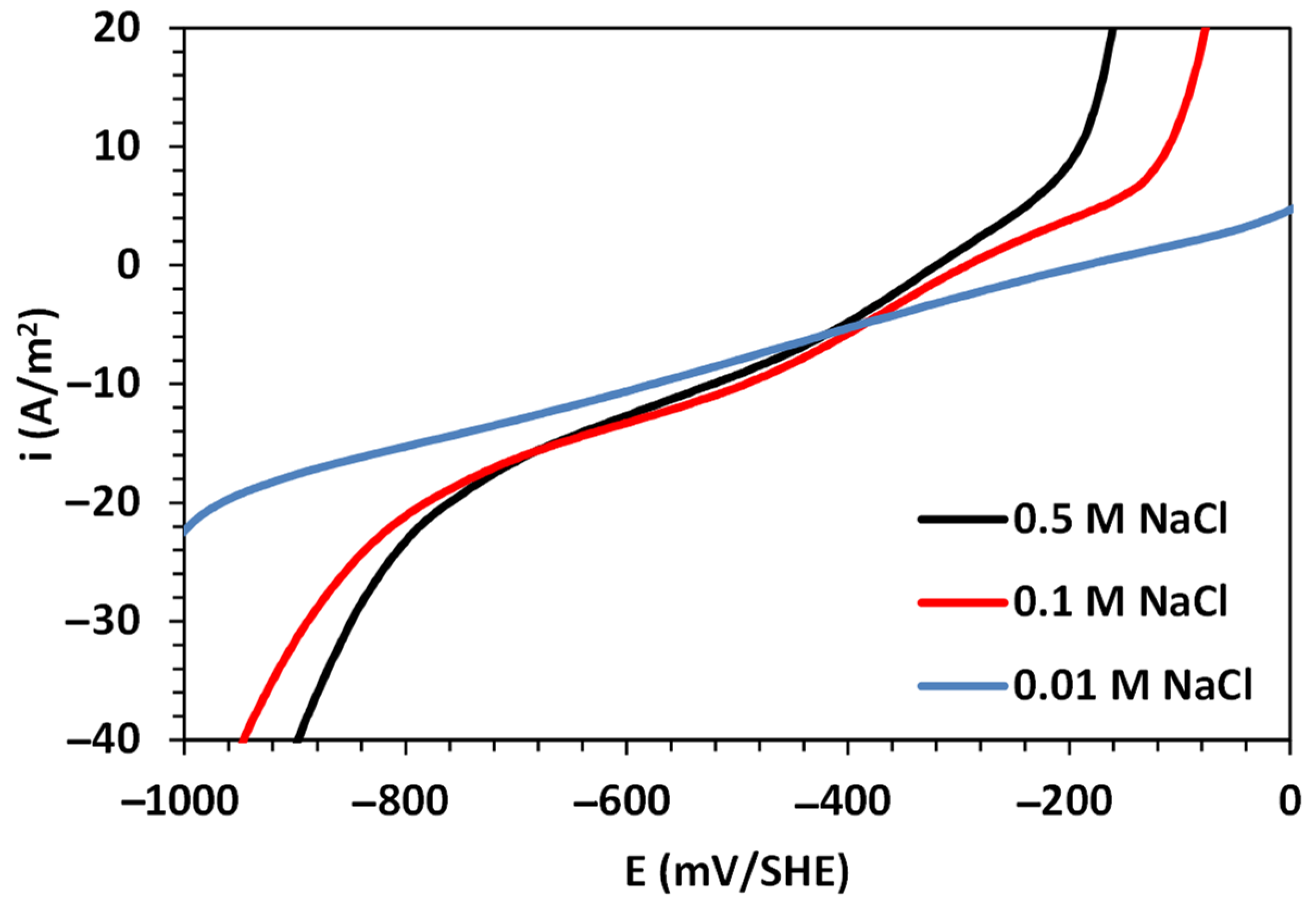
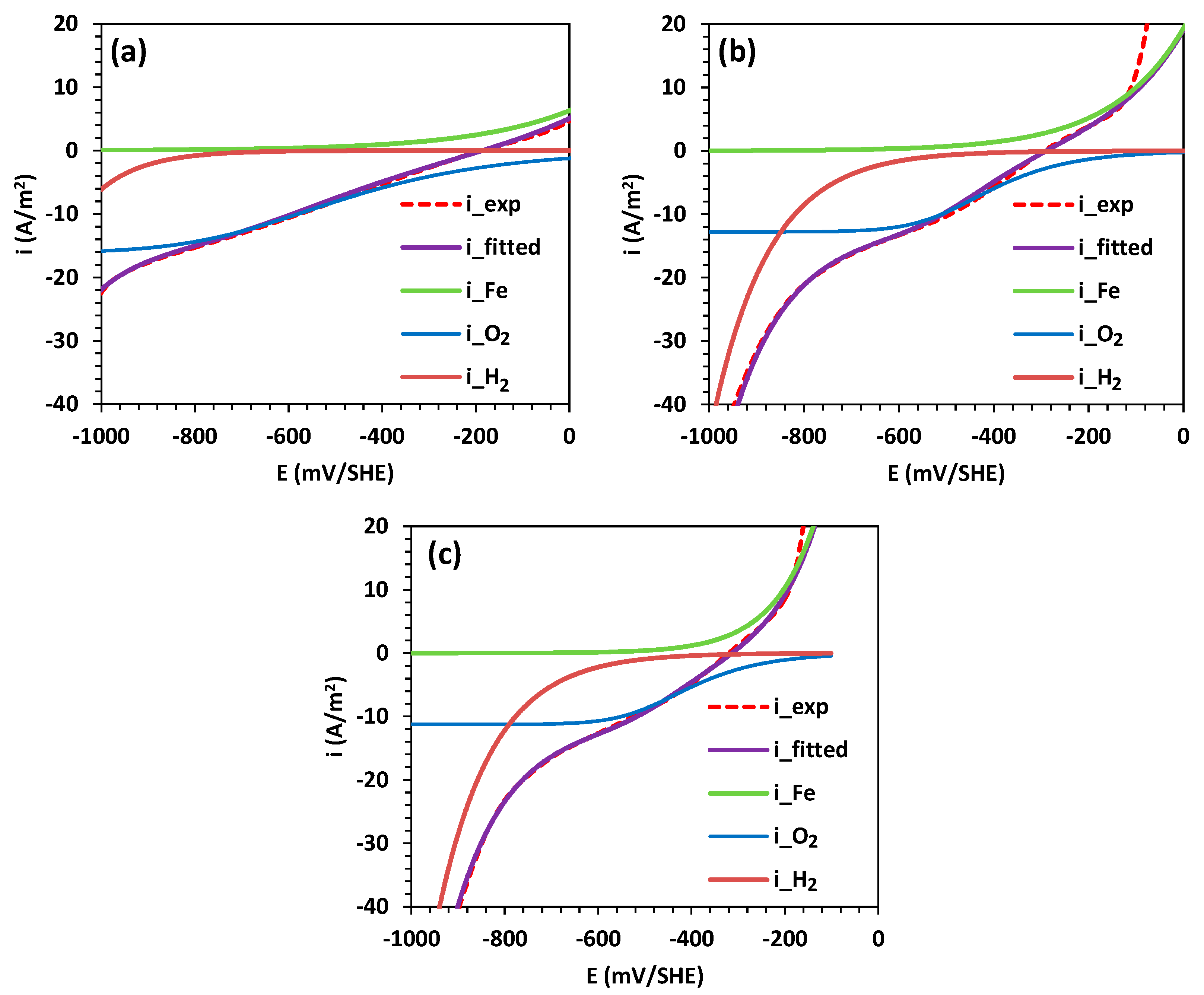
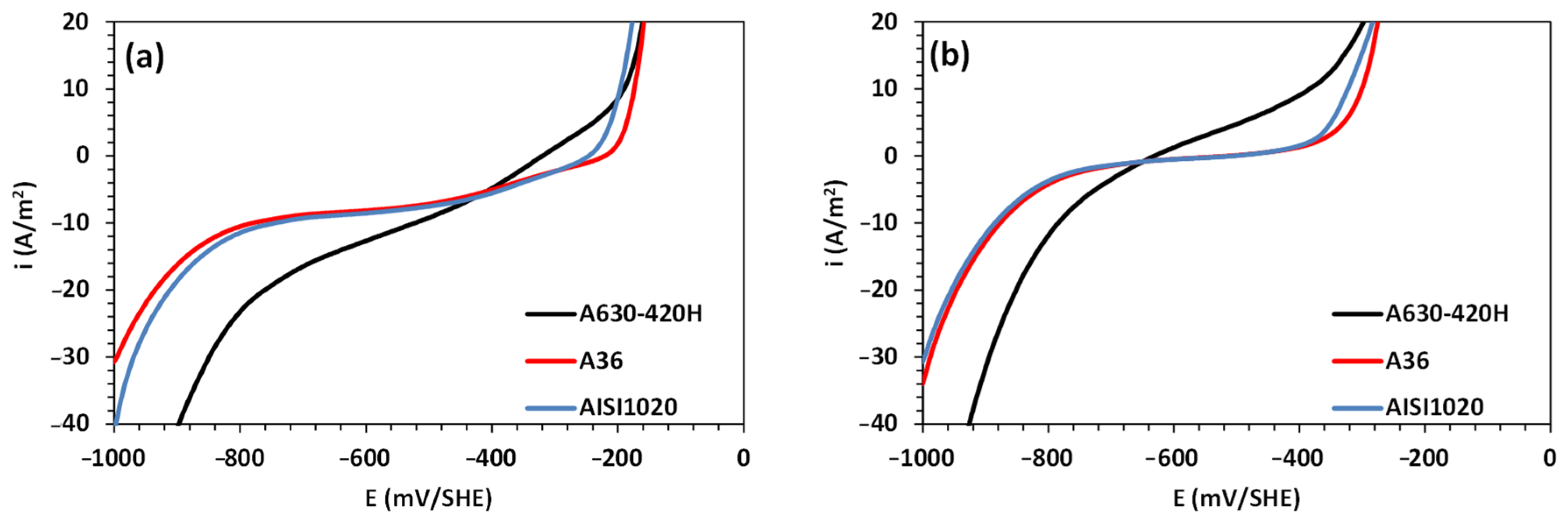
3.1. Corrosion Analysis via Electrochemical Polarization
3.2. Corrosion Analysis via Weight-Loss Measurements
3.3. Corrosion Analysis under Mechanical Stress Conditions of the Electrode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, H.; Saraswathy, V. Corrosion monitoring of reinforced concrete structures—A review. Int. J. Electrochem. Sci. 2007, 2, 1–28. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Matthews, J.D. Performance of Pfa concrete in a marine environment–10-Year results. Cem. Concr. Compos. 2004, 26, 5–20. [Google Scholar] [CrossRef]
- Kenshel, O.; O’Connor, A. Assessing chloride induced deterioration in condition and safety of concrete structures in marine environments. Eur. J. Environ. Civ. Eng. 2009, 13, 593–613. [Google Scholar] [CrossRef]
- Gaidis, J.M. Chemistry of corrosion inhibitors. Cem. Concr. Compos. 2004, 26, 181–189. [Google Scholar] [CrossRef]
- Val, D.V.; Stewart, M.G. Life-cycle cost analysis of reinforced concrete structures in marine environments. Struct. Saf. 2003, 25, 343–362. [Google Scholar] [CrossRef]
- Angst, U.M.; Geiker, M.R.; Alonso, M.C.; Polder, R.; Isgor, O.B.; Elsener, B.; Wong, H.; Michel, A.; Hornbostel, K.; Gehlen, C.; et al. The effect of the steel–concrete interface on chloride-induced corrosion initiation in concrete: A critical review by RILEM TC 262-SCI. Mater. Struct. Constr. 2019, 52, 88. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Z.; Zhao, Y.; Zhou, H.; Wang, X.; Zhou, H.; Xing, F. Chloride distribution and steel corrosion in a concrete bridge after long-term exposure to natural marine environment. Materials 2020, 13, 3900. [Google Scholar] [CrossRef]
- Senga-Kiesse, T.; Bonnet, S.; Amiri, O.; Ventura, A. Analysis of corrosion risk due to chloride diffusion for concrete structures in marine environment. Mar. Struct. 2020, 73, 102804. [Google Scholar] [CrossRef]
- Yi, Y.; Zhu, D.; Guo, S.; Zhang, Z.; Shi, C. A review of the deterioration and approaches to enhance the durability of concrete in the marine environment. Cem. Concr. Compos. 2020, 113, 103695. [Google Scholar] [CrossRef]
- Costa, A.; Appleton, J. Chloride penetration into concrete in marine environment-Part I: Main parameters affecting chloride penetration. Mater. Struct. Constr. 1999, 32, 252–259. [Google Scholar] [CrossRef]
- Bolzoni, F.; Brenna, A.; Fumagalli, G.; Goidanish, S.; Lazzari, L.; Ormellese, M.; Pedeferri, M.P. Experiences on corrosion inhibitors for reinforced concrete. Int. J. Corros. Scale Inhib. 2014, 3, 254–278. [Google Scholar] [CrossRef]
- James, A.; Bazarchi, E.; Chiniforush, A.A.; Panjebashi, P.; Hosseini, M.R.; Akbarnezhad, A.; Martek, I.; Ghodoosi, F.R. Rebar corrosion detection, protection, and rehabilitation of reinforced concrete structures in coastal environments: A Review. Constr. Build. Mater. 2019, 224, 1026–1039. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wang, Y.; Li, Q.; Yan, B. Semi-empirical prediction model of chloride-induced corrosion rate in uncracked reinforced concrete exposed to a marine environment. Electrochim. Acta 2020, 331, 135376. [Google Scholar] [CrossRef]
- Pyo, S.; Koh, T.; Tafesse, M.; Kim, H.K. Chloride-induced corrosion of steel fiber near the surface of ultra-high performance concrete and its effect on flexural behavior with various thicknesses. Constr. Build. Mater. 2019, 224, 206–213. [Google Scholar] [CrossRef]
- Haque, M.N.; Al-Khaiat, H. Carbonation of concrete structures in hot dry coastal regions. Cem. Concr. Compos. 1997, 19, 123–129. [Google Scholar] [CrossRef]
- Du, F.; Jin, Z.; She, W.; Xiong, C.; Feng, G.; Fan, J. Chloride Ions Migration and Induced Reinforcement Corrosion in Concrete with Cracks: A Comparative Study of Current Acceleration and Natural Marine Exposure. Constr. Build. Mater. 2020, 263, 120099. [Google Scholar] [CrossRef]
- Rodrigues, R.; Gaboreau, S.; Gance, J.; Ignatiadis, I.; Betelu, S. Reinforced concrete structures: A review of corrosion mechanisms and advances in electrical methods for corrosion monitoring. Constr. Build. Mater. 2021, 269, 121240. [Google Scholar] [CrossRef]
- Capozucca, R. Damage to reinforced concrete due to reinforcement corrosion. Constr. Build. Mater. 1995, 9, 295–303. [Google Scholar] [CrossRef]
- Raupach, M. Chloride-induced macrocell corrosion of steel in concrete—Theoretical background and practical consequences. Constr. Build. Mater. 1996, 10, 329–338. [Google Scholar] [CrossRef]
- Joycee, S.C.; Vidya, T.; Rattihka, S.A.; Prabha, B.S.; Dorothy, R.; Sasilatha, T.; Rajendran, S. Corrosion resistance of mild steel in simulated concrete pore solution before and after a paint coating. Int. J. Corros. Scale Inhib. 2021, 10, 1323–1335. [Google Scholar]
- Brenna, A.; Ormellese, M.; Pedeferri, M.P.; Bolzoni, F. Effect of binary mixtures on chloride induced corrosion of rebars in concrete. Int. J. Corros. Scale Inhib. 2018, 7, 151–164. [Google Scholar]
- Alhozaimy, A.; Hussain, R.R.; Al-Zaid, R.; Al-Negheimish, A. Coupled effect of ambient high relative humidity and varying temperature marine environment on corrosion of reinforced concrete. Constr. Build. Mater. 2012, 28, 670–679. [Google Scholar] [CrossRef]
- Tatematsu, H.; Sasaki, T. Repair materials system for chloride-induced corrosion of reinforcing bars. Cem. Concr. Compos. 2003, 25, 123–129. [Google Scholar] [CrossRef]
- Pang, L.; Li, Q. Service life prediction of RC structures in marine environment using long term chloride ingress data: Comparison between exposure trials and real structure surveys. Constr. Build. Mater. 2016, 113, 979–987. [Google Scholar] [CrossRef]
- Real, S.; Bogas, J.A.; Ferrer, B. Service life of reinforced structural lightweight aggregate concrete under chloride-induced Corrosion. Mater. Struct. Constr. 2017, 50, 101. [Google Scholar] [CrossRef]
- Chalhoub, C.; François, R.; Carcasses, M. Effect of cathode–anode distance and electrical resistivity on macrocell corrosion currents and cathodic response in cases of chloride induced corrosion in reinforced concrete structures. Constr. Build. Mater. 2020, 245, 118337. [Google Scholar] [CrossRef]
- Glass, G.K.; Buenfeld, N.R. Chloride-induced corrosion of steel in concrete. Prog. Struct. Eng. Mater. 2000, 2, 448–458. [Google Scholar] [CrossRef]
- Brenna, A.; Beretta, S.; Bolzoni, F.; Pedeferri, M.; Ormellese, M. Effects of AC-interference on chloride-induced corrosion of reinforced concrete. Constr. Build. Mater. 2017, 137, 76–84. [Google Scholar] [CrossRef]
- Vu, K.A.T.; Stewart, M.G. Structural reliability of concrete bridges including improved chloride-induced corrosion models. Struct. Saf. 2000, 22, 313–333. [Google Scholar] [CrossRef]
- Sumra, Y.; Payam, S.; Zainah, I. The pH of cement-based materials: A review. J. Wuhan Univ. Technol. Mat. Sci. Edit. 2020, 35, 908–924. [Google Scholar] [CrossRef]
- Cáceres, L.; Vargas, T.; Herrera, L. Influence of pitting and iron oxide formation during corrosion of carbon steel in unbuffered NaCl solutions. Corros. Sci. 2009, 51, 971–978. [Google Scholar] [CrossRef]
- Soliz, A.; Mayrhofer, K.J.J.; Cáceres, L. Influence of hydrodynamic flow patterns on the corrosion behavior of carbon steel in a neutral LiBr solution. Int. J. Electrochem. Sci. 2018, 13, 10050–10075. [Google Scholar] [CrossRef]
- Cáceres, L.; Frez, Y.; Galleguillos, F.; Soliz, A.; Gómez-Silva, B.; Borquez, J. Aqueous dried extract of skytanthus acutus meyen as corrosion inhibitor of carbon steel in neutral chloride solutions. Metals 2021, 11, 1992. [Google Scholar] [CrossRef]
- Soliz, A.; Guzmán, D.; Cáceres, L.; Galleguillos-Madrid, F. Electrochemical kinetic analysis of carbon steel powders produced by high-energy ball milling. Metals 2022, 12, 665. [Google Scholar] [CrossRef]
- Flitt, H.J.; Schweinsberg, D.P. Synthesis, matching and deconstruction of polarization curves for the active corrosion of zinc in aerated near-neutral NaCl solutions. Corros. Sci. 2010, 52, 1905–1914. [Google Scholar] [CrossRef]
- Sun, R.; Yu, Q.; Zhang, Y.; Yan, X.; Lu, Y.; Zhang, C.; Wang, Q. Effect of Si content on the corrosion behavior of 420 MPa weathering steel. Metals 2019, 9, 486. [Google Scholar] [CrossRef]
- Guzmán, D.; Muranda, D.; Soliz, A.; Aguilar, C.; Guzmán, A.; Sancy, M.; Pineda, F.; Rojas, P. Powder Metallurgy production of Ti-2 wt pct Si alloy: Structural, mechanical, and electrochemical characterization of the sintered material. Metall. Mat. Trans. A 2020, 51, 6461–6469. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B. Hydrogen embrittlement of low carbon structural steel at macro-, micro- and nano-levels. Int. J. Hydrogen Energy 2020, 45, 2145–2156. [Google Scholar] [CrossRef]
- Grousset, S.; Kergourlay, F.; Neff, D.; Foy, E.; Gallias, J.L.; Reguer, S.; Dillmanna, P.; Noumowe, A. In Situ monitoring of corrosion processes by coupled micro-XRF/micro-XRD mapping to understand the degradation mechanisms of reinforcing bars in hydraulic binders from historic monuments. J. Anal. At. Spectrom. 2015, 30, 721. [Google Scholar] [CrossRef]


| Parameter | NaCl, M | ||
|---|---|---|---|
| 0.01 | 0.1 | 0.5 | |
| , A/m2 | 0.34 | 0.32 | 0.10 |
| , mV/SHE | 490 | 349 | 212 |
| , A/m2 | −3.27 × 10−2 | −1.62 × 10−4 | −7.35 × 10−5 |
| , mV/SHE | −525 | −263 | −247 |
| , A/m2 | −16.2 | −12.8 | −11.3 |
| , A/m2 | −0.01 | −0.29 | −0.39 |
| , mV/SHE | −228 | −276 | −270 |
| , mV/SHE | −186 | −293 | −320 |
| , A/m2 | 2.63 | 2.80 | 3.02 |
| Parameter | Steel Type in Aerated 0.5 M NaCl | Steel Type in De-Aerated 0.5 M NaCl | ||||
|---|---|---|---|---|---|---|
| A36 | AISI 1020 | A360-420H | A36 | AISI 1020 | A360-420H | |
| , A/m2 | 1.46 × 10−7 | 2.96 × 10−6 | 0.10 | 9.41 × 10−3 | 0.01 | 2.33 |
| , mV/SHE | 57 | 65 | 212 | 107 | 104 | 358 |
| , A/m2 | −5.69 × 10−5 | −3.35 × 10−5 | −7.35 × 10−5 | - | - | - |
| , mV/SHE | −240 | −227 | −247 | - | - | - |
| , A/m2 | −8.1 | −8.3 | −11.3 | - | - | - |
| , A/m2 | −0.02 | −0.03 | −0.39 | −0.06 | −0.05 | −0.25 |
| , mV/SHE | −196 | −198 | −270 | −215 | −211 | −238 |
| , mV/SHE | −221 | −247 | −320 | −492 | −507 | −625 |
| , A/m2 | 1.36 | 1.74 | 3.02 | 0.15 | 0.16 | 2.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madrid, F.M.G.; Soliz, A.; Cáceres, L.; Salazar-Avalos, S.; Guzmán, D.; Gálvez, E. Corrosion of Reinforced A630-420H Steel in Direct Contact with NaCl Solution. Materials 2023, 16, 6017. https://doi.org/10.3390/ma16176017
Madrid FMG, Soliz A, Cáceres L, Salazar-Avalos S, Guzmán D, Gálvez E. Corrosion of Reinforced A630-420H Steel in Direct Contact with NaCl Solution. Materials. 2023; 16(17):6017. https://doi.org/10.3390/ma16176017
Chicago/Turabian StyleMadrid, Felipe M. Galleguillos, Alvaro Soliz, Luis Cáceres, Sebastian Salazar-Avalos, Danny Guzmán, and Edelmira Gálvez. 2023. "Corrosion of Reinforced A630-420H Steel in Direct Contact with NaCl Solution" Materials 16, no. 17: 6017. https://doi.org/10.3390/ma16176017
APA StyleMadrid, F. M. G., Soliz, A., Cáceres, L., Salazar-Avalos, S., Guzmán, D., & Gálvez, E. (2023). Corrosion of Reinforced A630-420H Steel in Direct Contact with NaCl Solution. Materials, 16(17), 6017. https://doi.org/10.3390/ma16176017








