Influence of Ag and/or Sr Dopants on the Mechanical Properties and In Vitro Degradation of β-Tricalcium Phosphate-Based Ceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder Synthesis
2.2. Synthesis of Bone-like Porous Ceramics
2.3. Specimen Characterization
2.4. In Vitro Degradation
3. Results and Discussion
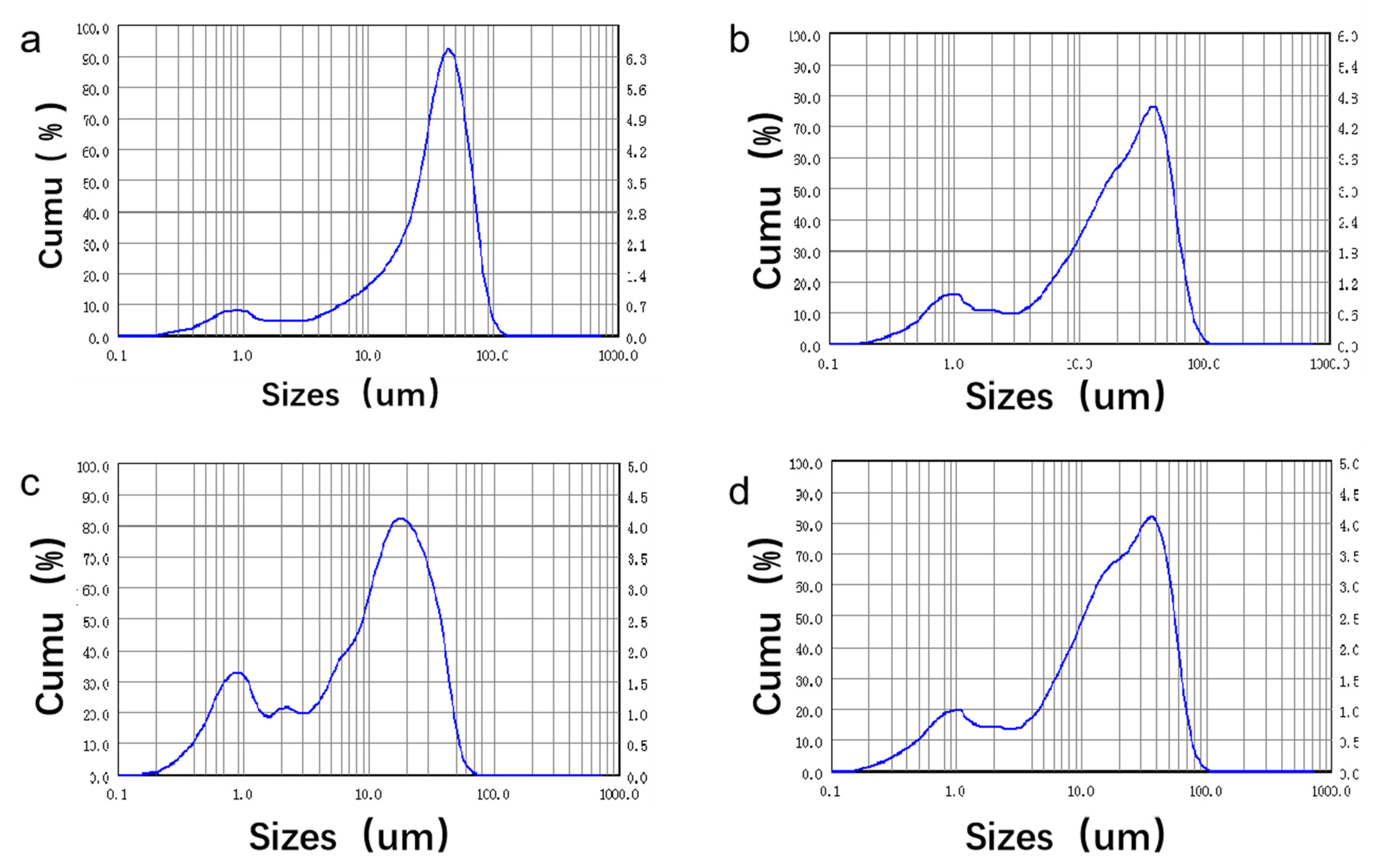
3.1. Specimen Characterization
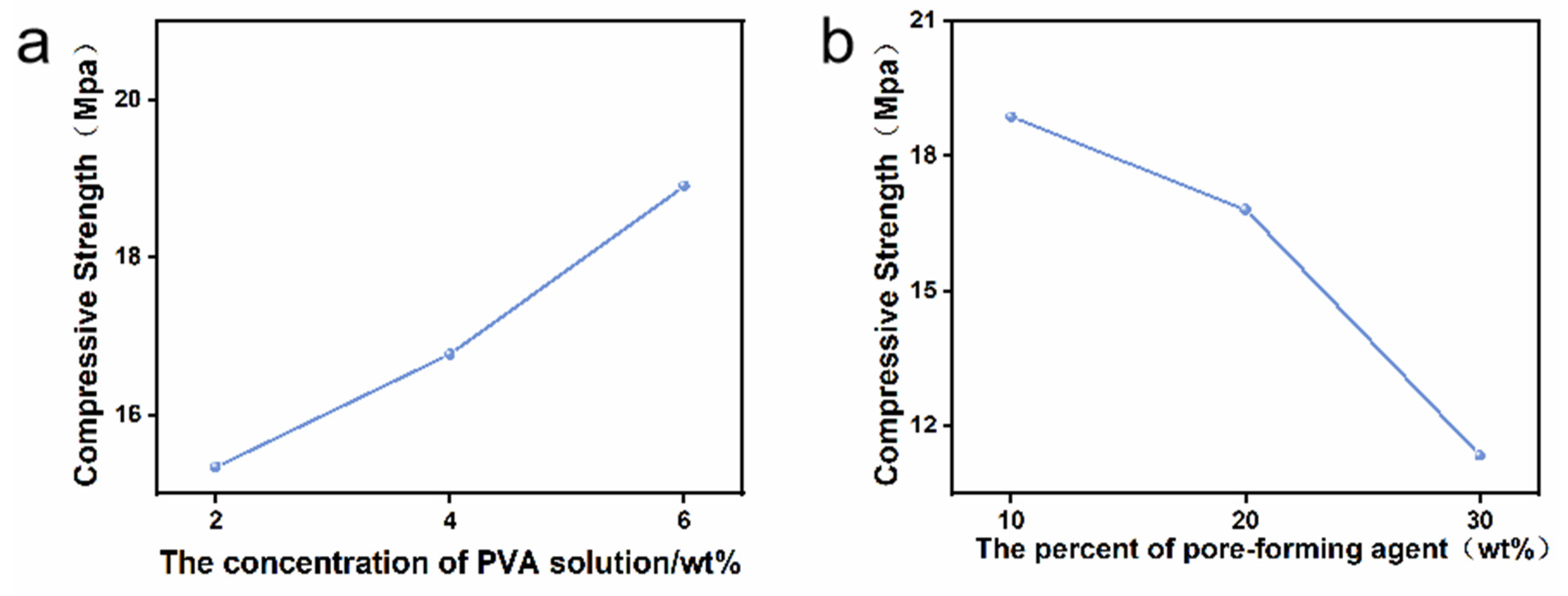
3.2. Compressive Strength
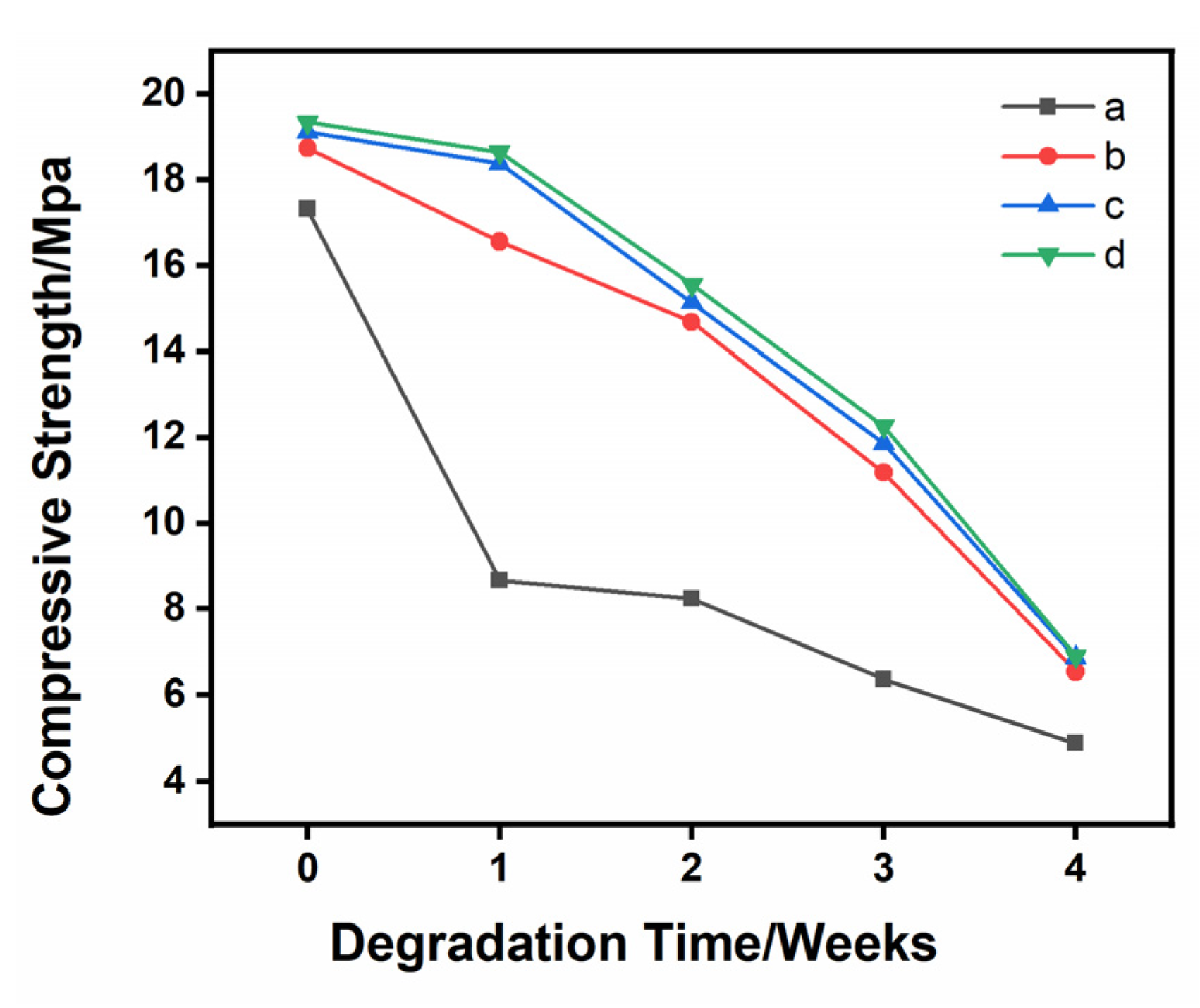
3.3. Specimen Characterization after Degradation
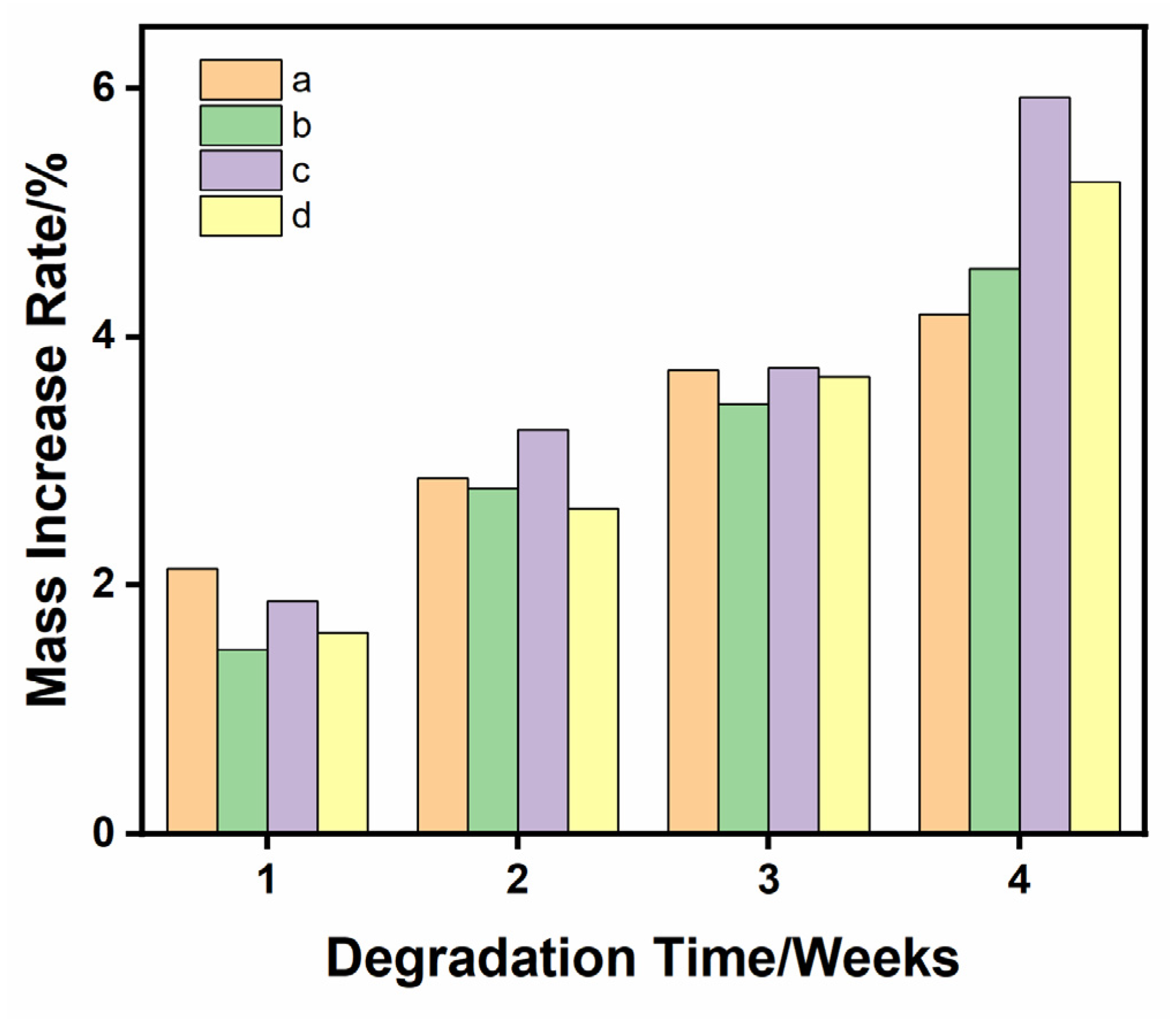
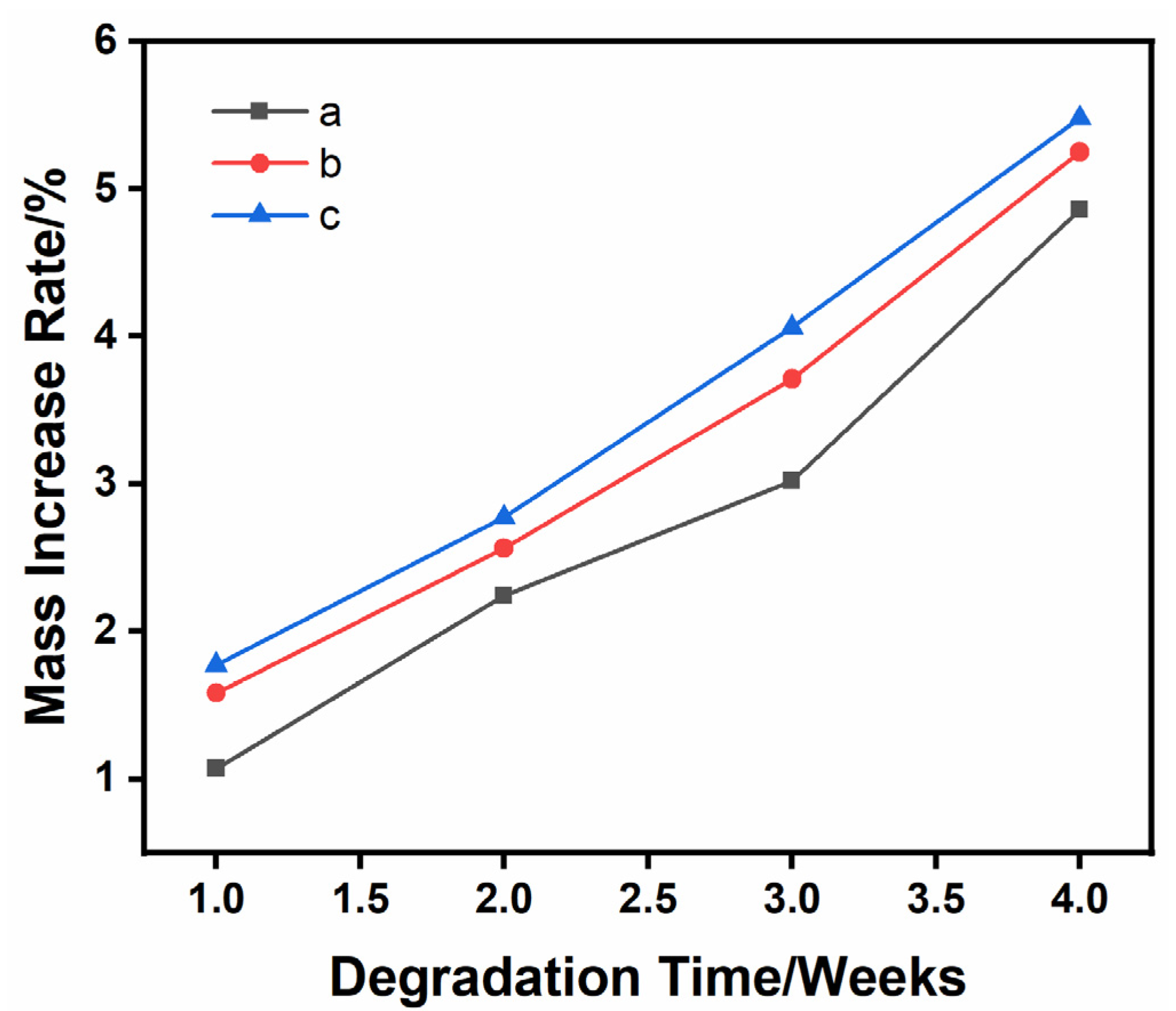
3.4. Material Quality Increases after Degradation
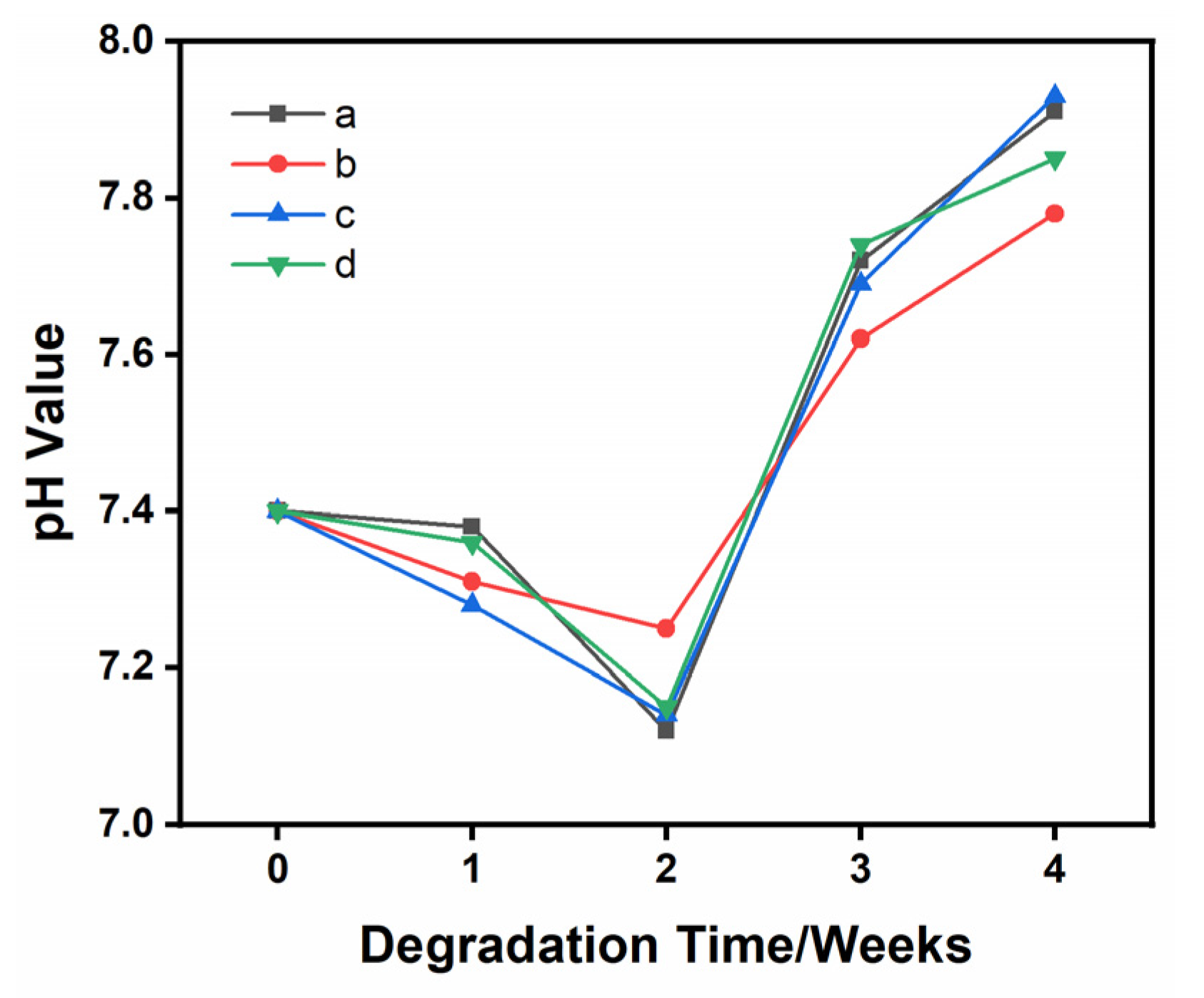
3.5. pH Changes during Degradation
3.6. SEM Observations after Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arpak, M.C.; Daglilar, S.; Kalkandelen, C.; Balescu, L.-M.; Sasmazel, H.T.; Pasuk, I.; Stan, G.E.; Durukan, K.; Gunduz, O. Physico-chemical characterization and in vitro biological study of manganese doped beta-tricalcium phosphate-based ceramics for bone regeneration applications. J. Aust. Ceram. Soc. 2023. [Google Scholar] [CrossRef]
- Deng, L.; Huang, L.; Pan, H.; Zhang, Q.; Que, Y.; Fan, C.; Chang, J.; Ni, S.; Yang, C. 3D printed strontium-zinc-phosphate bioceramic scaffolds with multiple biological functions for bone tissue regeneration. J. Mater. Chem. B 2023, 11, 5469–5482. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Liu, L.; Cheng, L.; Chen, X.; Xi, S.; Jiang, T. Calcium-to-phosphorus releasing ratio affects osteoinductivity and osteoconductivity of calcium phosphate bioceramics in bone tissue engineering. Biomed. Eng. Online 2023, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Le Ferrand, H.; Athanasiou, C.E. A Materials Perspective on the Design of Damage-Resilient Bone Implants Through Additive/Advanced Manufacturing. JOM 2020, 72, 1195–1210. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Demirel, M.; Kaya, A.I. Effect of strontium-containing compounds on bone grafts. J. Mater. Sci. 2020, 55, 6305–6329. [Google Scholar] [CrossRef]
- Zwingenberger, S.; Nich, C.; Valladares, R.D.; Yao, Z.; Stiehler, M.; Goodman, S.B. Recommendations and Considerations for the Use of Biologics in Orthopedic Surgery. Biodrugs 2012, 26, 245–256. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Khayrutdinova, D.R.; Goldberg, M.A.; Antonova, O.S.; Krokhicheva, P.A.; Fomin, A.S.; Obolkina, T.O.; Konovalov, A.A.; Akhmedova, S.A.; Sviridova, I.K.; Kirsanova, V.A.; et al. Effects of Heat Treatment on Phase Formation in Cytocompatible Sulphate-Containing Tricalcium Phosphate Materials. Minerals 2023, 13, 147. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Yang, Y.; He, X.; Wei, X.; Tan, Q.; Wang, Y.; Xu, S.; Chang, S.; Liu, W. Biocompatibility and osteointegration capability of beta-TCP manufactured by stereolithography 3D printing: In vitro study. Open Life Sci. 2023, 18, 20220530. [Google Scholar] [CrossRef]
- Mommer, A.; Tabatabaei, F.; Tayebi, L.; Vahabzadeh, S. Role of magnesium on phase composition of tricalcium phosphate and its interaction with human dental pulp stem cells. J. Mater. Res. 2023, 38, 228–236. [Google Scholar] [CrossRef]
- Spirandeli, B.R.; Martins, E.F.; Dona, L.R.M.; Ribas, R.G.; Campos, T.M.B.; Esposito, E.; Thim, G.P.; Tada, D.B.; Trichês, E.S. Synergistic Effect of Incorporation of BG 45S5 and Silver Nanoparticles on β-TCP Scaffolds: Structural Characterization and Evaluation of Antimicrobial Activity and Biocompatibility. Mater. Res. 2023, 26, e20230137. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, X.; Zhang, F.; Hong, Y.; Qiu, Y.; Yang, X.; Li, Y.; Zhong, C.; Yang, H.; Gou, Z. Customized bioceramic scaffolds and metal meshes for challenging large-size mandibular bone defect regeneration and repair. Regen. Biomater. 2023, 10, rbad057. [Google Scholar] [CrossRef] [PubMed]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Hench, L.L.; Wilson, J. Surface-active biomaterials. Science 1984, 226, 630–636. [Google Scholar] [CrossRef]
- Kondo, N.; Ogose, A.; Tokunaga, K.; Ito, T.; Arai, K.; Kudo, N.; Inoue, H.; Irie, H.; Endo, N. Bone formation and resorption of highly purified beta-tricalcium phosphate in the rat femoral condyle. Biomaterials 2005, 26, 5600–5608. [Google Scholar] [CrossRef]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/beta-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef]
- Miranda, P.; Pajares, A.; Saiz, E.; Tomsia, A.P.; Guiberteau, F. Mechanical properties of calcium phosphate scaffolds fabricated by robocasting. J. Biomed. Mater. Res. Part A 2008, 85, 218–227. [Google Scholar] [CrossRef]
- Johnson, A.J.W.; Herschler, B.A. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef]
- Bornapour, M.; Muja, N.; Shum-Tim, P.; Cerruti, M.; Pekguleryuz, M. Biocompatibility and biodegradability of Mg-Sr alloys: The formation of Sr-substituted hydroxyapatite. Acta Biomater. 2013, 9, 5319–5330. [Google Scholar] [CrossRef]
- Kaygili, O.; Tatar, C.; Yakuphanoglu, F.; Keser, S. Nano-crystalline aluminum-containing hydroxyapatite based bioceramics: Synthesis and characterization. J. Sol-Gel Sci. Technol. 2013, 65, 105–111. [Google Scholar] [CrossRef]
- Gopi, D.; Shinyjoy, E.; Kavitha, L. Synthesis and spectral characterization of silver/magnesium co-substituted hydroxyapatite for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 127, 286–291. [Google Scholar] [CrossRef]
- Kaygili, O.; Dorozhkin, S.V.; Ates, T.; Al-Ghamdi, A.A.; Yakuphanoglu, F. Dielectric properties of Fe doped hydroxyapatite prepared by sol-gel method. Ceram. Int. 2014, 40, 9395–9402. [Google Scholar] [CrossRef]
- Kaygili, O.; Keser, S. Sol-gel synthesis and characterization of Sr/Mg, Mg/Zn and Sr/Zn co-doped hydroxyapatites. Mater. Lett. 2015, 141, 161–164. [Google Scholar] [CrossRef]
- Ates, T.; Dorozhkin, S.V.; Kaygili, O.; Kom, M.; Ercan, I.; Bulut, N.; Firdolas, F.; Keser, S.; Gursoy, N.C.; Ozercan, I.H.; et al. The effects of Mn and/or Ni dopants on the in vitro/in vivo performance, structural and magnetic properties of β-tricalcium phosphate bioceramics. Ceram. Int. 2019, 45, 22752–22758. [Google Scholar] [CrossRef]
- Mohamed, S.; Kamal, H.; Moustafa, Y.M.; Abdelghany, M.I. Effect of Ag-doping on the thermal features of hydroxyapatite. Egypt. J. Chem. 2022, 65, 139–153. [Google Scholar] [CrossRef]
- Qi, D.; Su, J.; Li, S.; Zhu, H.; Cheng, L.; Hua, S.; Yuan, X.; Jiang, J.; Shu, Z.; Shi, Y.; et al. 3D printed magnesium-doped ?-TCP gyroid scaffold with osteogenesis, angiogenesis, immunomodulation properties and bone regeneration capability in vivo. Biomater. Adv. 2022, 136, 212759. [Google Scholar] [CrossRef] [PubMed]
- Santoni, B.L.G.; Niggli, L.; Dolder, S.; Loeffel, O.; Sblendorio, G.A.; Heuberger, R.; Maazouz, Y.; Staehli, C.; Doebelin, N.; Bowen, P.; et al. Effect of minor amounts of beta-calcium pyrophosphate and hydroxyapatite on the physico-chemical properties and osteoclastic resorption of beta-tricalcium phosphate cylinders. Bioact. Mater. 2022, 10, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, X.; Ye, J.; He, F. Effects of zinc/gallium dual doping on the physicochemical properties and cell response of 3D printed 13-tricalcium phosphate ceramic scaffolds. Ceram. Int. 2022, 48, 28557–28564. [Google Scholar] [CrossRef]
- Mestres, G.; Le Van, C.; Ginebra, M.-P. Silicon-stabilized alpha-tricalcium phosphate and its use in a calcium phosphate cement: Characterization and cell response. Acta Biomater. 2012, 8, 1169–1179. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Lin, D.; Shi, H.; Yuan, Y.; Tang, W.; Zhou, H.; Guo, H.; Qian, J.; Liu, C. Magnesium modification of a calcium phosphate cement alters bone marrow stromal cell behavior via an integrin-mediated mechanism. Biomaterials 2015, 53, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.T.; Ling, L.S.; Hussain, R. Injectable magnesium-doped brushite cement for controlled drug release application. J. Mater. Sci. 2016, 51, 7427–7439. [Google Scholar] [CrossRef]
- Cama, G.; Nkhwa, S.; Gharibi, B.; Lagazzo, A.; Cabella, R.; Carbone, C.; Dubruel, P.; Haugen, H.; Di Silvio, L.; Deb, S. The role of new zinc incorporated monetite cements on osteogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zeng, S.; Liu, X.; Yu, T.; Zhou, C. Effects of strontium doping on the degradation and Sr ion release behaviors of -tricalcium phosphate bone cement. J. Am. Ceram. Soc. 2018, 101, 502–508. [Google Scholar] [CrossRef]
- Wu, T.; Shi, H.; Liang, Y.; Lu, T.; Lin, Z.; Ye, J. Improving osteogenesis of calcium phosphate bone cement by incorporating with manganese doped beta-tricalcium phosphate. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110481. [Google Scholar] [CrossRef]
- Ahmadi, M.; Dini, G.; Afshar, M.; Ahmadpour, F. Synthesis, characterization, and bioactivity evaluation of biphasic calcium phosphate nanopowder containing 5.0 mol% strontium, 0.6 mol% magnesium, and 0.2 mol% silicon for bone regeneration. J. Mater. Res. 2022, 37, 1916–1928. [Google Scholar] [CrossRef]
- Gu, X.N.; Xie, X.H.; Li, N.; Zheng, Y.F.; Qin, L. In vitro and in vivo studies on a Mg-Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Cannillo, V. Hydroxyapatite and tricalcium phosphate composites with bioactive glass as second phase: State of the art and current applications. J. Biomed. Mater. Res. Part A 2016, 104, 1030–1056. [Google Scholar] [CrossRef]
- Hu, B.; Meng, Z.D.; Zhang, Y.Q.; Ye, L.Y.; Wang, C.J.; Guo, W.C. Sr-HA scaffolds fabricated by SPS technology promote the repair of segmental bone defects. Tissue Cell 2020, 66, 101386. [Google Scholar] [CrossRef]
- Blake, G.M.; Zivanovic, M.A.; McEwan, A.J.; Ackery, D.M. Sr-89 Therapy: Strontium kinetics in disseminated carcinoma of the prostate. Eur. J. Nucl. Med. 1986, 12, 447–454. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Deyneko, D.V.; Forysenkova, A.A.; Morozov, V.A.; Akhmedova, S.A.; Kirsanova, V.A.; Sviridova, I.K.; Sergeeva, N.S.; Rodionov, S.A.; Udyanskaya, I.L.; et al. Strontium Substituted beta-Tricalcium Phosphate Ceramics: Physiochemical Properties and Cytocompatibility. Molecules 2022, 27, 6085. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Fadeeva, I.V.; Forysenkova, A.A.; Davydova, G.A.; Fosca, M.; Filippov, Y.Y.; Antoniac, I.V.; Antoniac, A.; D’Arco, A.; Di Fabrizio, M.; et al. Strontium Substituted Tricalcium Phosphate Bone Cement: Short and Long-Term Time-Resolved Studies and In Vitro Properties. Adv. Mater. Interfaces 2022, 9, 2200803. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Kuo, B.-T.; Ho, W.-F. Effects of cooling conditions and chitosan coating on the properties of porous calcium phosphate granules produced from hard clam shells. Adv. Powder Technol. 2022, 33, 103774. [Google Scholar] [CrossRef]
- Guo, D.; Xu, K.; Liu, Y. Physicochemical properties and cytotoxicities of Sr-containing biphasic calcium phosphate bone scaffolds. J. Mater. Sci. Mater. M. 2010, 21, 1927–1936. [Google Scholar] [CrossRef]
- Ewald, A.; Hoesel, D.; Patel, S.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Silver-doped calcium phosphate cements with antimicrobial activity. Acta Biomater. 2011, 7, 4064–4070. [Google Scholar] [CrossRef]
- Rau, J.V.; Fosca, M.; Graziani, V.; Egorov, A.A.; Zobkov, Y.V.; Fedotov, A.Y.; Ortenzi, M.; Caminiti, R.; Baranchikov, A.E.; Komlev, V.S. Silver-Doped Calcium Phosphate Bone Cements with Antibacterial Properties. J. Funct. Biomater. 2016, 7, 10. [Google Scholar] [CrossRef]
- Song, W.-H.; Ryu, H.S.; Hong, S.-H. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coating formed by micro-arc oxidation. J. Biomed. Mater. Res. Part A 2009, 88A, 246–254. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. In vitro antimicrobial and biological properties of laser assisted tricalcium phosphate coating on titanium for load bearing implant. Mater. Sci. Eng. C Mater. Biol. Appl. 2009, 29, 1965–1968. [Google Scholar] [CrossRef]
- Turkoz, M.; Atilla, A.O.; Evis, Z. Silver and fluoride doped hydroxyapatites: Investigation by microstructure, mechanical and antibacterial properties. Ceram. Int. 2013, 39, 8925–8931. [Google Scholar] [CrossRef]
- Paul, S.; Pal, A.; Choudhury, A.R.; Bodhak, S.; Balla, V.K.; Sinha, A.; Das, M. Effect of trace elements on the sintering effect of fish scale derived hydroxyapatite and its bioactivity. Ceram. Int. 2017, 43, 15678–15684. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Kahar, N.N.F.N.M.N.; Ahmad, N.; Jaafar, M.; Yahaya, B.H.; Sulaiman, A.R.; Hamid, Z.A.A. A review of bioceramics scaffolds for bone defects in different types of animal models: HA and beta-TCP. Biomed. Phys. Eng. Expr. 2022, 8, 052002. [Google Scholar] [CrossRef]
- Phatai, P.; Prachumrak, N.; Kamonwannasit, S.; Kamcharoen, A.; Roschat, W.; Phewphong, S.; Morales Futalan, C.; Khemthong, P.; Butburee, T.; Youngjan, S.; et al. Zinc-Silver Doped Mesoporous Hydroxyapatite Synthesized via Ultrasonic in Combination with Sol-Gel Method for Increased Antibacterial Activity. Sustainability 2022, 14, 11756. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, T.; Wu, M.; Wang, C.; Wang, J.; Han, Z. Enhanced bioactivity and antimicrobial properties of α-tricalcium phosphate cement via PDA@Ag coating. Mater. Lett. 2023, 330, 133230. [Google Scholar] [CrossRef]
- Hashimoto, K.; Ii, A.; Fujimoto, T.; Shibata, H. Fabrication and characterization of beta-type tricalcium phosphate sintered body with phosphorus/sulphur-complex cations. J. Ceram. Soc. Jpn. 2022, 130, 88–93. [Google Scholar] [CrossRef]
- Larionov, D.S.; Bitanova, V.A.; Evdokimov, P.V.; Garshev, A.V.; Putlyaev, V.I. Sol-Gel Synthesis of Ca3(PO4)2 and Ca3−xNa2x(PO4)2 Powders for the Fabrication of Bioceramics by 3D Printing. Inorg. Mater. 2022, 58, 302–310. [Google Scholar] [CrossRef]
- Liu, K.; Sun, J.; Zhu, Q.; Jin, X.; Zhang, Z.; Zhao, Z.; Chen, G.; Wang, C.; Jiang, H.; Zhang, P. Microstructures and properties of polycaprolactone/tricalcium phosphate scaffolds containing polyethylene glycol fabricated by 3D printing. Ceram. Int. 2022, 48, 24032–24043. [Google Scholar] [CrossRef]
- He, F.; Qiu, C.; Lu, T.; Shi, X.; Ye, J. Conjunction of gallium doping and calcium silicate mediates osteoblastic and osteoclastic performances of tricalcium phosphate bioceramics. Biomed. Mater. 2022, 17, 015012. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Hiromoto, S.; Kobayashi, E.; Sato, T. Effects of Incorporating Beta-Tricalcium Phosphate with Reaction Sintering into Mg-Based Composites on Degradation and Mechanical Integrity. Metals 2021, 11, 227. [Google Scholar] [CrossRef]
- Wang, M.; Ge, X.; Cui, Z.; Wu, S.; Zhu, S.; Liang, Y.; Li, Z.; Lu, W.W. Influences of strontium on the phase composition and lattice structure of biphasic calcium phosphate. Ceram. Int. 2021, 47, 16248–16255. [Google Scholar] [CrossRef]
- Joo, G.; Park, M.; Park, S.-s.; Tripathi, G.; Lee, B.-T. Tailored alginate/PCL-gelatin-beta-TCP membrane for guided bone regeneration. Biomed. Mater. 2022, 17, 045011. [Google Scholar] [CrossRef] [PubMed]





| Sample | Sr/(Sr + Ag + Ca), % | Ag/(Sr + Ag + Ca), % | (Sr + Ag + Ca)/P |
|---|---|---|---|
| TCP | - | - | 1.51 |
| 1Sr-TCP | 1.03 | - | 1.53 |
| 1Sr-0.8Ag-TCP | 1.06 | 0.83 | 1.51 |
| 1Sr-3Ag-TCP | 1.04 | 3.07 | 1.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, J.; Sun, T.; Lv, X.; Ma, Y.; Liu, G.; Li, L.; Yuan, S.; Fan, X. Influence of Ag and/or Sr Dopants on the Mechanical Properties and In Vitro Degradation of β-Tricalcium Phosphate-Based Ceramics. Materials 2023, 16, 6025. https://doi.org/10.3390/ma16176025
Che J, Sun T, Lv X, Ma Y, Liu G, Li L, Yuan S, Fan X. Influence of Ag and/or Sr Dopants on the Mechanical Properties and In Vitro Degradation of β-Tricalcium Phosphate-Based Ceramics. Materials. 2023; 16(17):6025. https://doi.org/10.3390/ma16176025
Chicago/Turabian StyleChe, Junjian, Tao Sun, Xueman Lv, Yunhai Ma, Guoqin Liu, Lekai Li, Shengwang Yuan, and Xueying Fan. 2023. "Influence of Ag and/or Sr Dopants on the Mechanical Properties and In Vitro Degradation of β-Tricalcium Phosphate-Based Ceramics" Materials 16, no. 17: 6025. https://doi.org/10.3390/ma16176025
APA StyleChe, J., Sun, T., Lv, X., Ma, Y., Liu, G., Li, L., Yuan, S., & Fan, X. (2023). Influence of Ag and/or Sr Dopants on the Mechanical Properties and In Vitro Degradation of β-Tricalcium Phosphate-Based Ceramics. Materials, 16(17), 6025. https://doi.org/10.3390/ma16176025







