Electromechanical Coupling in Collagen Measured under Increasing Relative Humidity †
Abstract
:1. Introduction
2. Methods and Materials
2.1. Preparation of Collagen Fibrils
2.2. Preparation of Rat Tail Tendon
2.3. AFM and LPFM Experiments
2.4. Humidity Measurements
3. Results
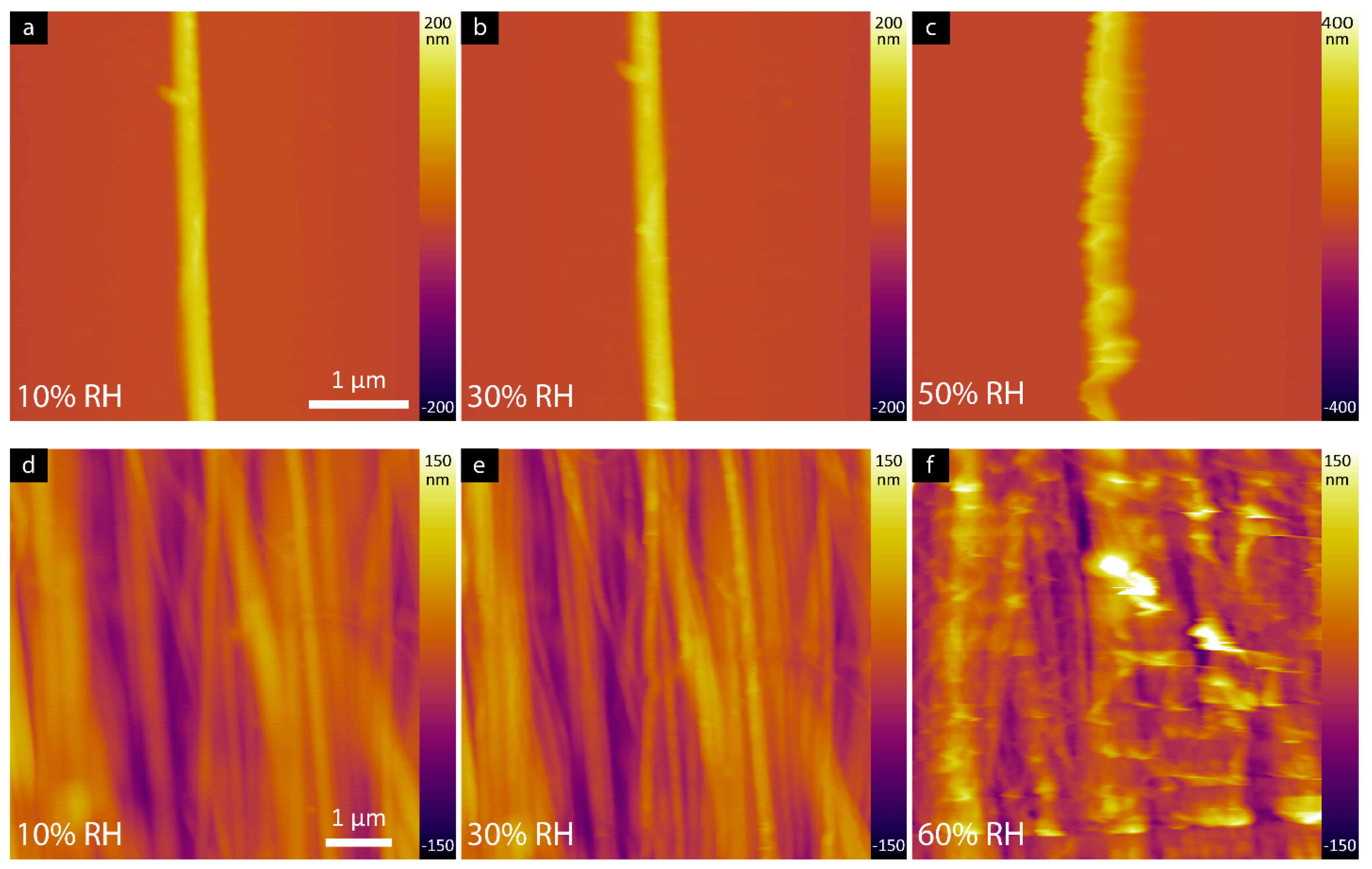
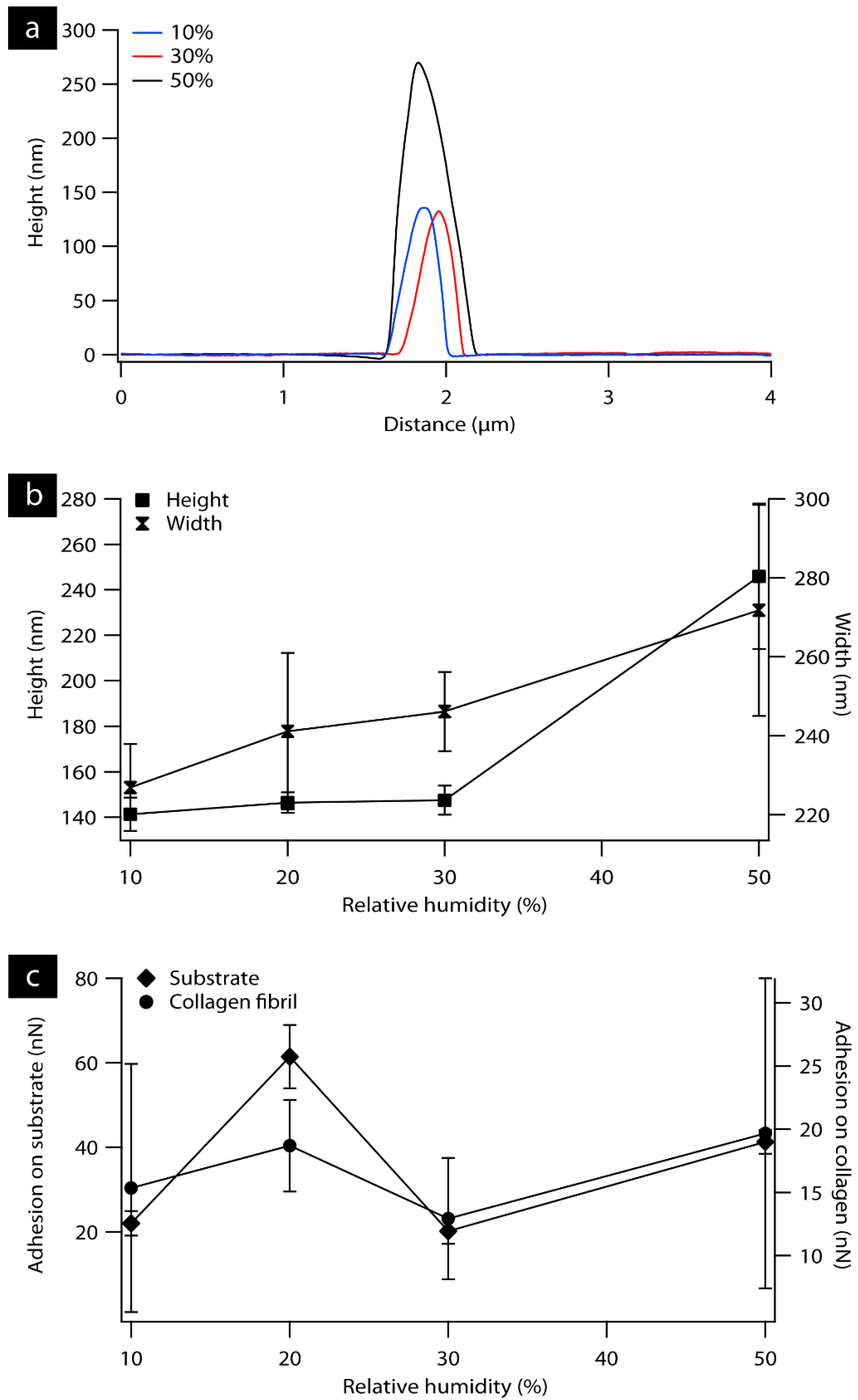
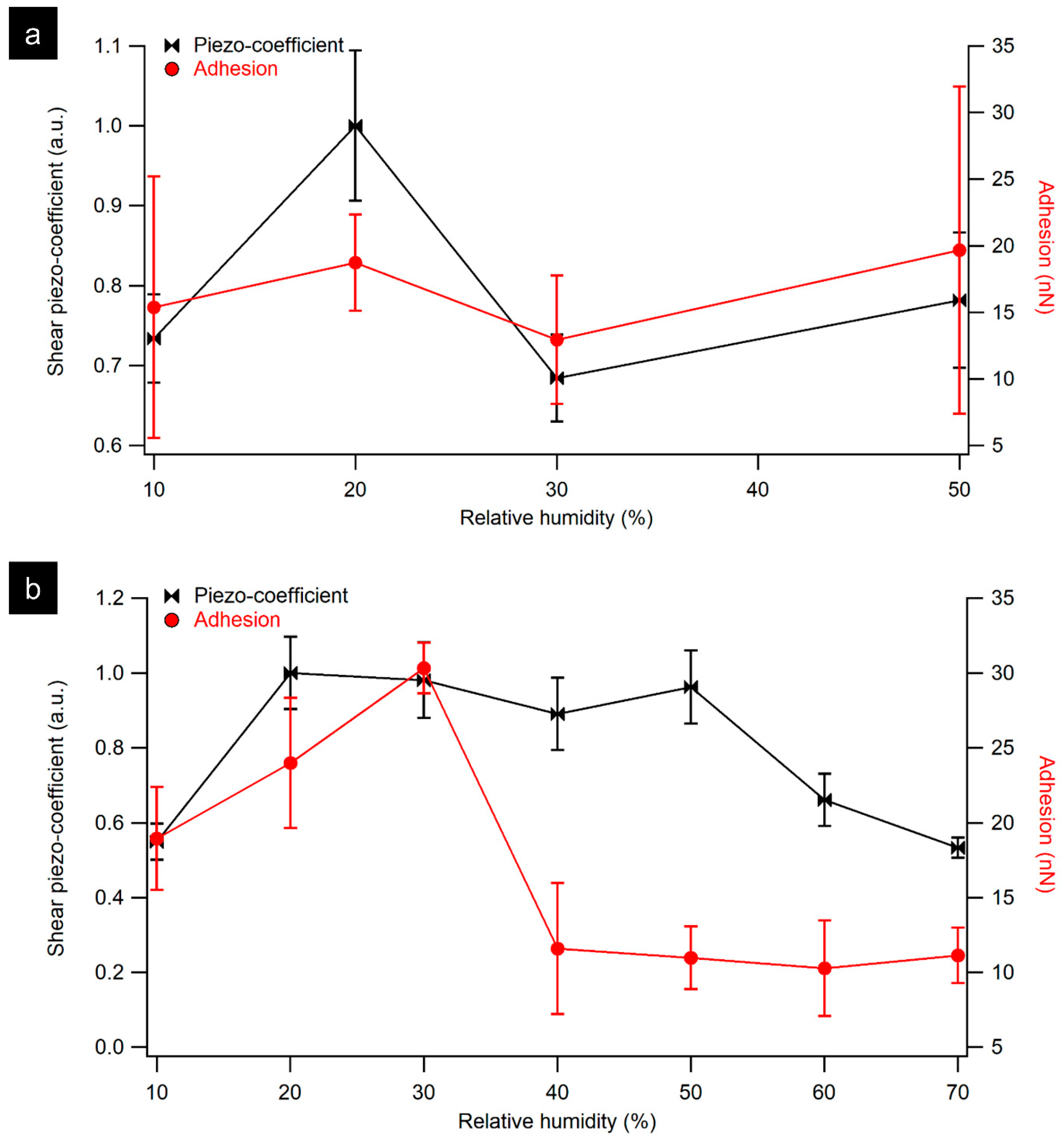
3.1. AFM Characterization of Collagen Fibril and Rat Tail Tendon as a Function of Relative Humidity
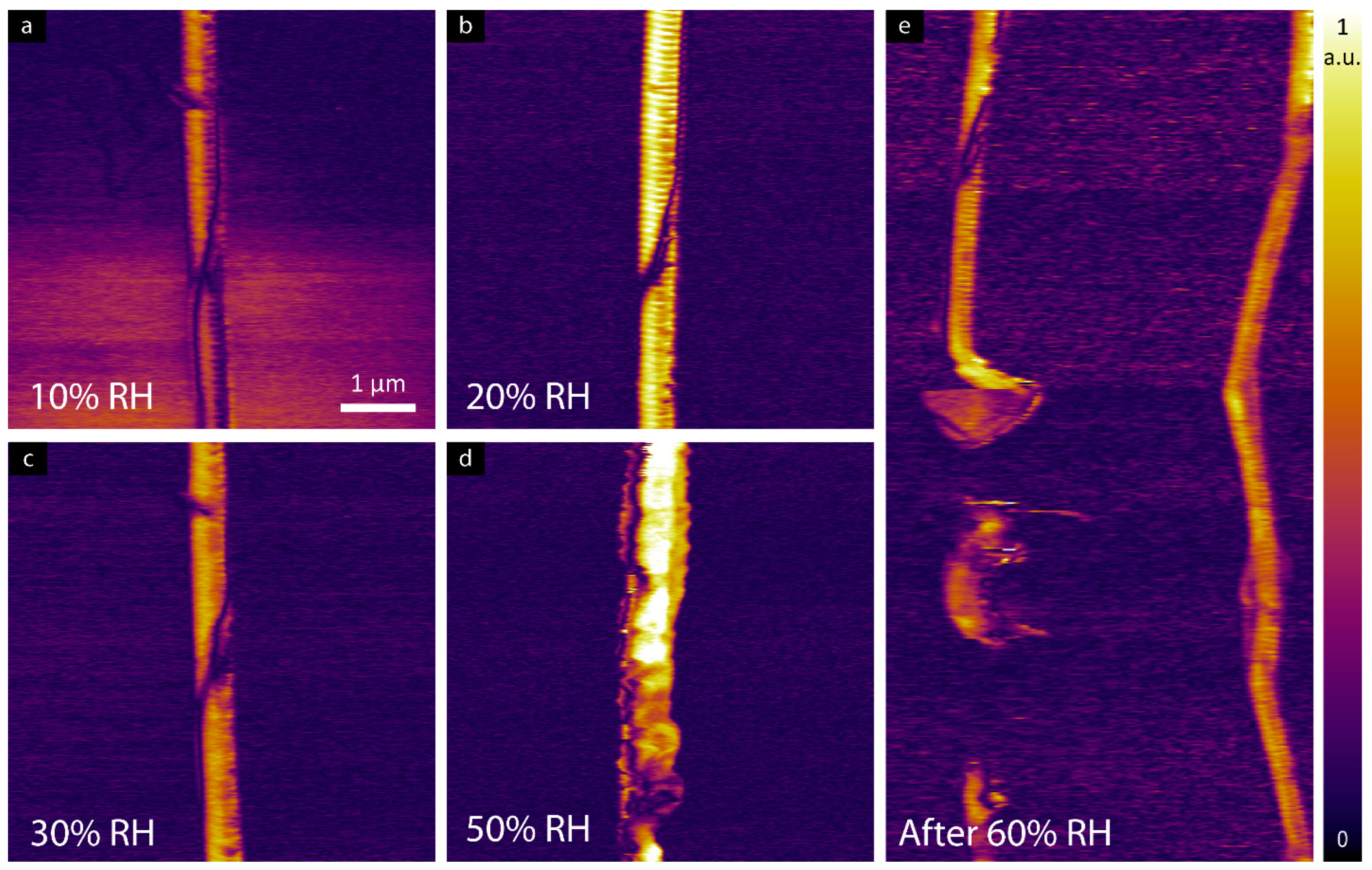
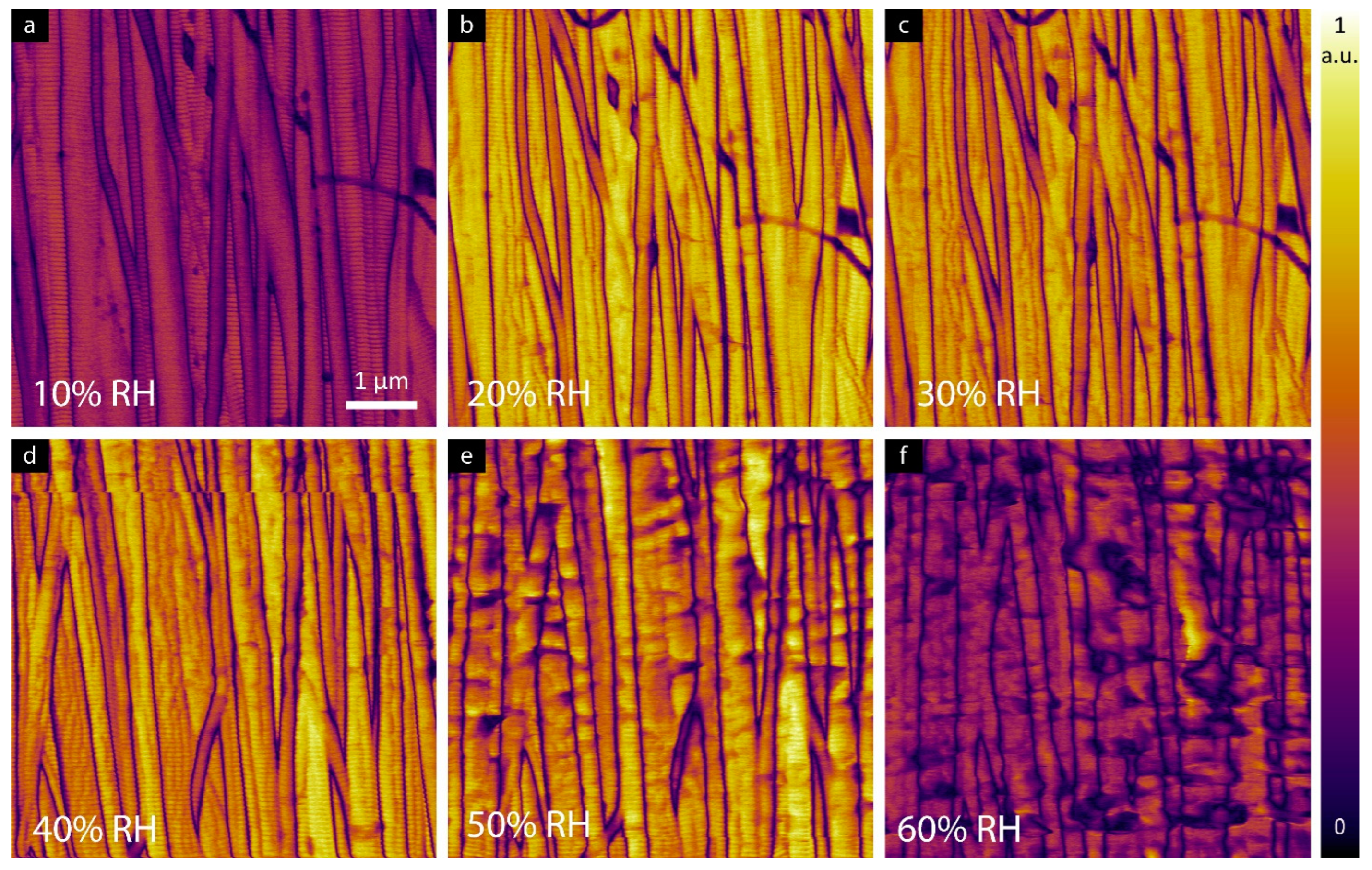
3.2. LPFM Analysis as a Function of Relative Humidity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Gross, D.; Williams, W.S. Streaming Potential and the Electromechanical Response of Physiologically-Moist Bone. J. Biomech. 1982, 15, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Sancho, F.; Abdollahi, A.; Damjanovic, D.; Catalan, G. Flexoelectricity in Bones. Adv. Mater. 2018, 30, 1705316. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Eriksson, C. Electrical Properties of Wet Collagen. Nature 1968, 218, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Eriksson, C. Piezoelectric Properties of Dry and Wet Bone. Nature 1970, 227, 491–492. [Google Scholar] [CrossRef]
- Fukada, E.; Yasuda, I. Piezoelectric Effects in Collagen. Jpn J. Appl. Phys. 1964, 3, 502B. [Google Scholar] [CrossRef]
- Eriksson, C. Streaming Potentials and Other Water-Dependent Effects in Mineralized Tissue. N. Y. Acad. Sci. USA 1974, 238, 321–333. [Google Scholar] [CrossRef]
- Pienkowski, D.; Pollack, S.R. The Origin of Stress-Generated Potentials in Fluid-Saturated Bone. J. Orthop. Res. 1983, 1, 30–41. [Google Scholar] [CrossRef]
- Bassett, C.A.L.; Becker, R.O. Generation of Electric Potentials by Bone in Response to Mechanical Stress. Science 1962, 137, 1063–1064. [Google Scholar] [CrossRef]
- Reinish, G.B. Piezoelectric Properties of Bone as Functions of Moisture Content. Nature 1975, 253, 626–627. [Google Scholar] [CrossRef]
- Marino, A.A.; Becker, R.O. Piezoelectricity in Hydrated Frozen Bone and Tendon. Nature 1975, 253, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Ahn, A.C.; Grodzinsky, A.J. Relevance of Collagen Piezoelectricity to “Wolff’s Law”: A Critical Review. Med. Eng. Phys. 2009, 31, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Halperin, C.; Mutchnik, S.; Agronin, A.; Molotskii, M.; Urenski, P.; Salai, M.; Rosenman, G. Piezoelectric Effect in Human Bones Studied in Nanometer Scale. Nano Lett. 2004, 4, 1253–1256. [Google Scholar] [CrossRef]
- Luescher, M.; Rüegg, M.; Schindler, P. Effect of Hydration upon the Thermal Stability of Tropocollagen and Its Dependence on the Presence of Neutral Salts. Biopolymers 1974, 13, 2489–2503. [Google Scholar] [CrossRef]
- Yang, L.; van der Werf, K.O.; Koopman, B.F.J.M.; Subramaniam, V.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Micromechanical Bending of Single Collagen Fibrils Using Atomic Force Microscopy. J. Biomed. Mater. Res. A 2007, 82, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Rajan, N.; Habermehl, J.; Coté, M.-F.; Doillon, C.J.; Mantovani, D. Preparation of Ready-to-Use, Storable and Reconstituted Type I Collagen from Rat Tail Tendon for Tissue Engineering Applications. Nat. Protoc. 2006, 1, 2753–2758. [Google Scholar] [CrossRef]
- Denning, D.; Guyonnet, J.; Rodriguez, B.J. Applications of Piezoresponse Force Microscopy in Materials Research: From Inorganic Ferroelectrics to Biopiezoelectrics and Beyond. Int. Mater. Rev. 2016, 61, 46–70. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity Fixed Points of Binary Saturated Aqueous Solutions. J. Res. Natl. Bur. Stand. A Phys. Chem. 1977, 81A, 89. [Google Scholar] [CrossRef]
- Xu, L.; Lio, A.; Hu, J.; Ogletree, D.F.; Salmeron, M. Wetting and Capillary Phenomena of Water on Mica. J. Phys. Chem. B 1998, 102, 540–548. [Google Scholar] [CrossRef]
- Zarate, N.V.; Harrison, A.J.; Litster, J.D.; Beaudoin, S.P. Effect of Relative Humidity on Onset of Capillary Forces for Rough Surfaces. J. Colloid. Interface Sci. 2013, 411, 265–272. [Google Scholar] [CrossRef]
- Bazaid, A.; Neumayer, S.M.; Sorushanova, A.; Guyonnet, J.; Zeugolis, D.; Rodriguez, B.J. Non-Destructive Determination of Collagen Fibril Width in Extruded Collagen Fibres by Piezoresponse Force Microscopy. Biomed. Phys. Eng. Express 2017, 3, 055004. [Google Scholar] [CrossRef]
- Pineri, M.H.; Escoubes, M.; Roche, G. Water–Collagen Interactions: Calorimetric and Mechanical Experiments. Biopolymers 1978, 17, 2799–2815. [Google Scholar] [CrossRef]
- Bella, J.; Brodsky, B.; Berman, H.M. Hydration Structure of a Collagen Peptide. Structure 1995, 3, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Kopp, J.; Bonnet, M.; Renou, J.P. Effect of Collagen Crosslinking on Collagen-Water Interactions (A DSC Investigation). Matrix 1990, 9, 443–450. [Google Scholar] [CrossRef]
- Melacini, G.; Bonvin, A.M.J.J.; Goodman, M.; Boelens, R.; Kaptein, R. Hydration Dynamics of the Collagen Triple Helix by NMR11Edited by P.E. Wright. J. Mol. Biol. 2000, 300, 1041–1048. [Google Scholar] [CrossRef]
- Grant, C.A.; Brockwell, D.J.; Radford, S.E.; Thomson, N.H. Effects of Hydration on the Mechanical Response of Individual Collagen Fibrils. Appl. Phys. Lett. 2008, 92, 233902. [Google Scholar] [CrossRef]
- Wallace, J.M.; Erickson, B.; Les, C.M.; Orr, B.G.; Banaszak Holl, M.M. Distribution of Type I Collagen Morphologies in Bone: Relation to Estrogen Depletion. Bone 2010, 46, 1349–1354. [Google Scholar] [CrossRef]
- Wallace, J.M.; Orr, B.G.; Marini, J.C.; Holl, M.M.B. Nanoscale Morphology of Type I Collagen Is Altered in the Brtl Mouse Model of Osteogenesis Imperfecta. J. Struct. Biol. 2011, 173, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Liroff, K.G.; Turner, A.S.; Les, C.M.; Orr, B.G.; Holl, M.M.B. Estrogen Depletion Results in Nanoscale Morphology Changes in Dermal Collagen. J. Investig. Dermatol. 2012, 132, 1791–1797. [Google Scholar] [CrossRef]
- Erickson, B.; Fang, M.; Wallace, J.M.; Orr, B.G.; Les, C.M.; Banaszak Holl, M.M. Nanoscale Structure of Type I Collagen Fibrils: Quantitative Measurement of D-Spacing. Biotechnol. J. 2012, 8, 117–126. [Google Scholar] [CrossRef]
- Andriotis, O.G.; Elsayad, K.; Smart, D.E.; Nalbach, M.; Davies, D.E.; Thurner, P.J. Hydration and Nanomechanical Changes in Collagen Fibrils Bearing Advanced Glycation End-Products. Biomed. Opt. Express 2019, 10, 1841. [Google Scholar] [CrossRef]
- Sedin, D.L.; Rowlen, K.L. Adhesion Forces Measured by Atomic Force Microscopy in Humid Air. Anal. Chem. 2000, 72, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Schatz, G.C.; Ratner, M.A. Capillary Force in Atomic Force Microscopy. J. Chem. Phys. 2004, 120, 1157–1160. [Google Scholar] [CrossRef]
- Farshchi-Tabrizi, M.; Kappl, M.; Cheng, Y.; Gutmann, J.; Butt, H.J. On the Adhesion between Fine Particles and Nanocontacts: An Atomic Force Microscope Study. Langmuir 2006, 22, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Qian, L. Investigation of Humidity-Dependent Capillary Force. Langmuir 2000, 16, 8153–8158. [Google Scholar] [CrossRef]
- Kim, H.; Smit, B.; Jang, J. Monte Carlo Study on the Water Meniscus Condensation and Capillary Force in Atomic Force Microscopy. J. Phys. Chem. C 2012, 116, 21923–21931. [Google Scholar] [CrossRef]
- Bewig, K.W.; Zisman, W.A. The Wetting of Gold and Platinum by Water. J. Phys. Chem. 1965, 69, 4238–4242. [Google Scholar] [CrossRef]
- Gardner, J.R.; Woods, R. The Hydrophilic Nature of Gold and Platinum. J. Electroanal. Chem. 1977, 81, 285–290. [Google Scholar] [CrossRef]
- Fukada, E.; Ueda, H.; Rinaldi, R. Piezoelectric and Related Properties of Hydrated Collagen. Biophys. J. 1976, 16, 911–918. [Google Scholar] [CrossRef]
- Price, R.I.; Lees, S.; Kirschner, D.A. X-Ray Diffraction Analysis of Tendon Collagen at Ambient and Cryogenic Temperatures: Role of Hydration. Int. J. Biol. Macromol. 1997, 20, 23–33. [Google Scholar] [CrossRef]
- Andriotis, O.G.; Chang, S.W.; Vanleene, M.; Howarth, P.H.; Davies, D.E.; Shefelbine, S.J.; Buehler, M.J.; Thurner, P.J. Structure-Mechanics Relationships of Collagen Fibrils in the Osteogenesis Imperfecta Mouse Model. J. R. Soc. Interface 2015, 12, 20150701. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Cho, H. Collagen Piezoelectricity in Osteogenesis Imperfecta and Its Role in Intrafibrillar Mineralization. Commun. Biol. 2022, 5, 1229. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hu, X.; Zhang, Y.; Li, X.; Chen, B.; An, Q.; Zhao, Y.; Zhang, Y. Biomineralized Piezoelectrically Active Scaffolds for Inducing Osteogenic Differentiation. Chem. A Eur. J. 2023, 29, e202203166. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.J.; Jesse, S.; Baddorf, A.P.; Kalinin, S.V. High Resolution Electromechanical Imaging of Ferroelectric Materials in a Liquid Environment by Piezoresponse Force Microscopy. Phys. Rev. Lett. 2006, 96, 237602. [Google Scholar] [CrossRef] [PubMed]
| Salt Solution | Salt Concentration (g/mL) | RH (%) | |
|---|---|---|---|
| Literature [18] | Achieved | ||
| Magnesium chloride (MgCl2) | 0.5 | 33% | 31% |
| Potassium carbonate (K2CO3) | 1.1 | 43% | 43% |
| Magnesium nitrate (Mg (NO3)2) | 1.2 | 53% | 54% |
| Sodium chloride (NaCl) | 0.3 | 75% | 60% |
| Potassium chloride (KCl) | 0.4 | 84% | 73% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazaid, A.; Zhang, F.; Zhang, Q.; Neumayer, S.; Denning, D.; Habelitz, S.; Marina Ferreira, A.; Rodriguez, B.J. Electromechanical Coupling in Collagen Measured under Increasing Relative Humidity. Materials 2023, 16, 6034. https://doi.org/10.3390/ma16176034
Bazaid A, Zhang F, Zhang Q, Neumayer S, Denning D, Habelitz S, Marina Ferreira A, Rodriguez BJ. Electromechanical Coupling in Collagen Measured under Increasing Relative Humidity. Materials. 2023; 16(17):6034. https://doi.org/10.3390/ma16176034
Chicago/Turabian StyleBazaid, Arwa, Fengyuan Zhang, Qiancheng Zhang, Sabine Neumayer, Denise Denning, Stefan Habelitz, Ana Marina Ferreira, and Brian J. Rodriguez. 2023. "Electromechanical Coupling in Collagen Measured under Increasing Relative Humidity" Materials 16, no. 17: 6034. https://doi.org/10.3390/ma16176034
APA StyleBazaid, A., Zhang, F., Zhang, Q., Neumayer, S., Denning, D., Habelitz, S., Marina Ferreira, A., & Rodriguez, B. J. (2023). Electromechanical Coupling in Collagen Measured under Increasing Relative Humidity. Materials, 16(17), 6034. https://doi.org/10.3390/ma16176034







