Synthesis and Characterization of a Novel Two-Dimensional Copper p-Aminophenol Metal–Organic Framework and Investigation of Its Tribological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cu(PAP)2
2.3. Preparation of PAO6 or Deionized Water-Based Cu(PAP)2
2.4. Characterization
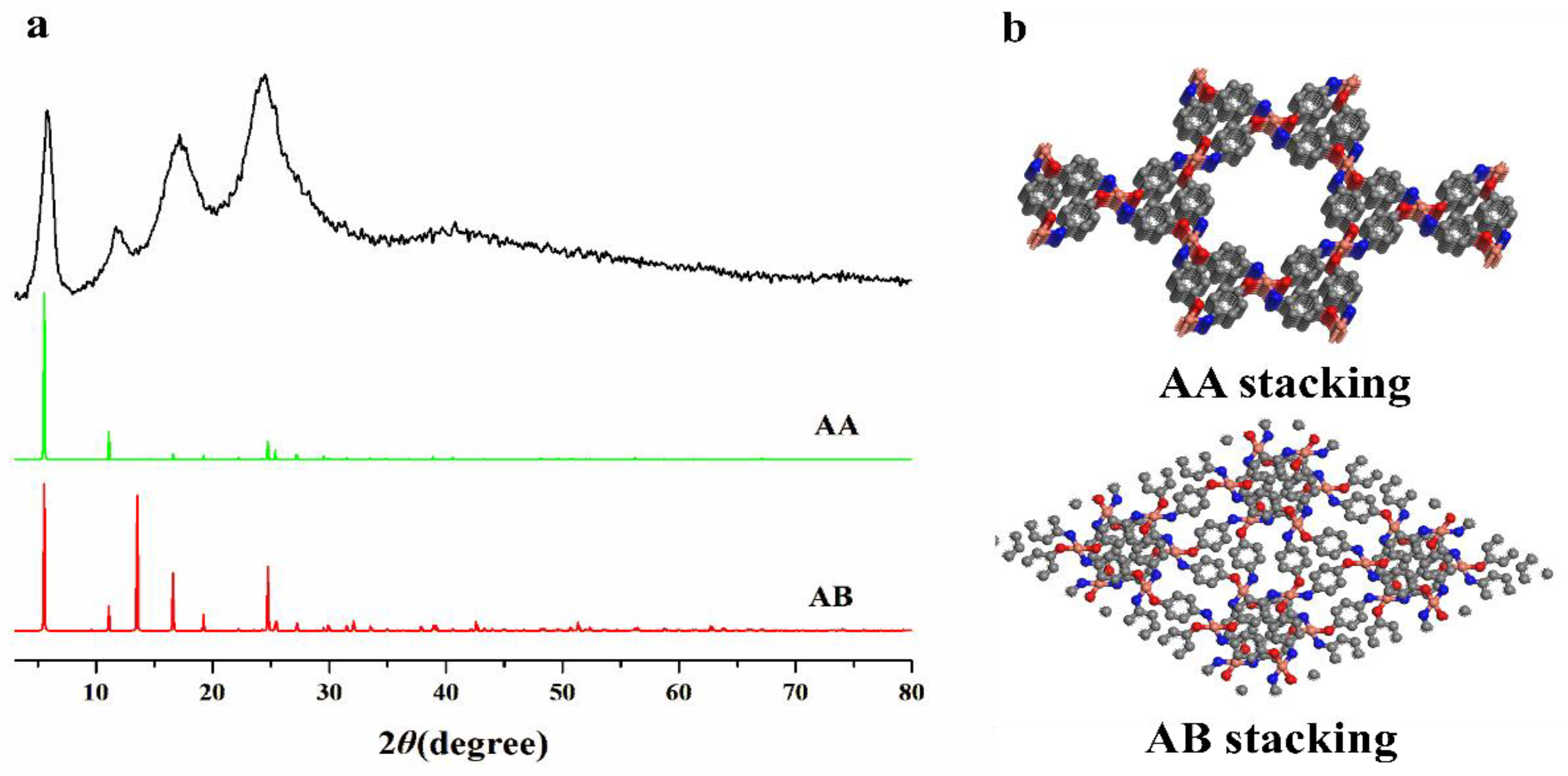
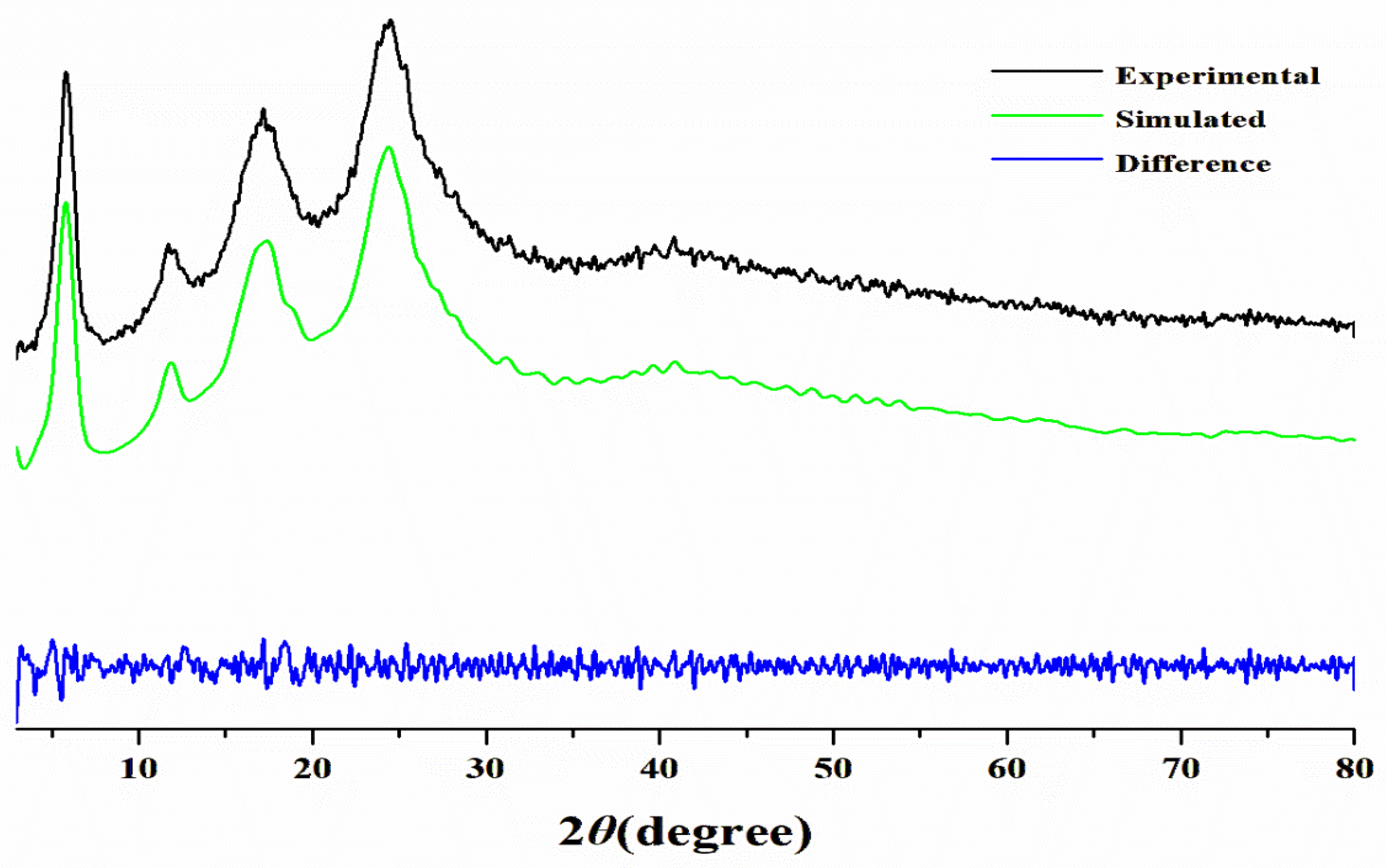
2.5. Structure Simulation
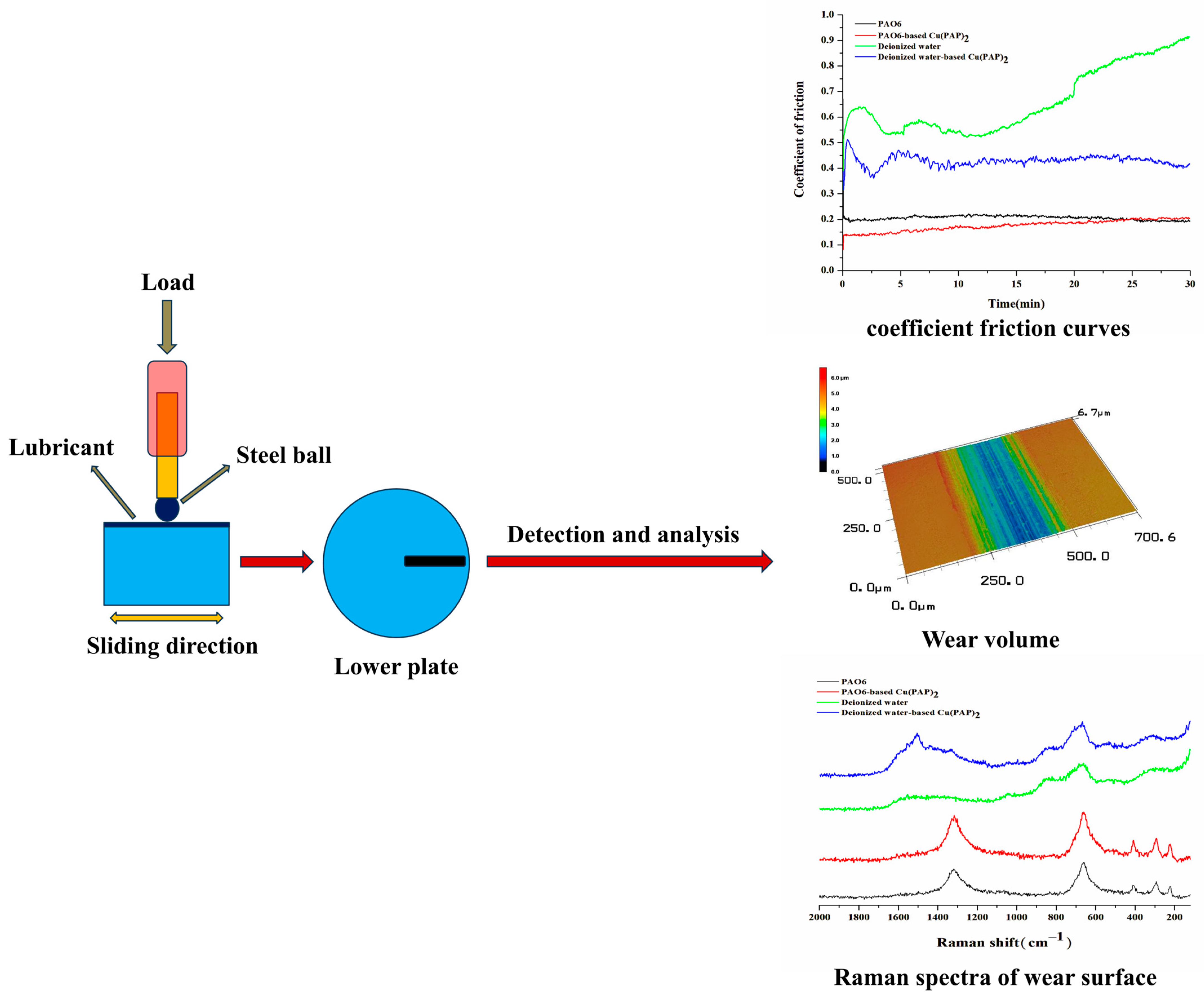
2.6. Tribological Tests
3. Results and Discussion
3.1. Structural Analysis of Cu(PAP)2
3.1.1. PXRD Analysis and Structure Simulation
3.1.2. FTIR and Raman Spectra Analysis
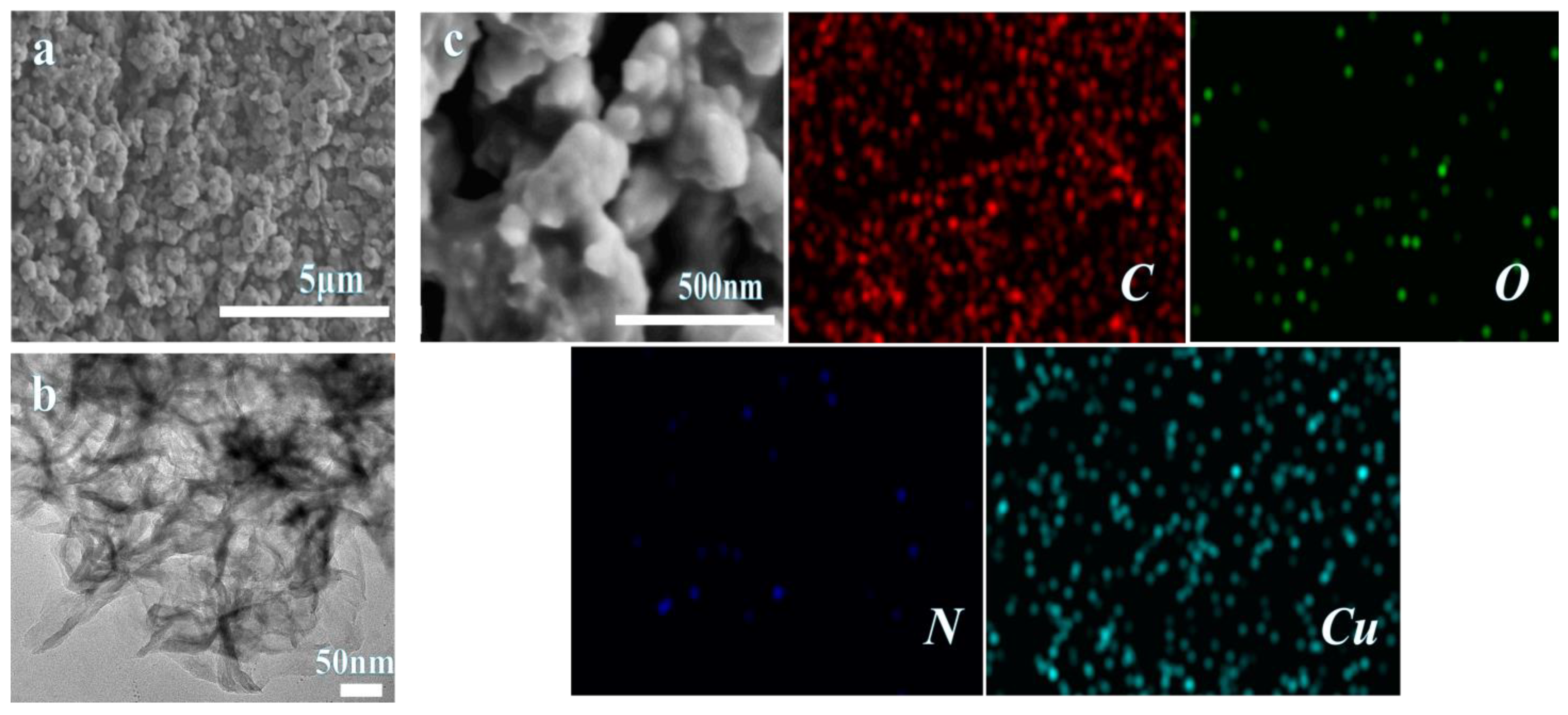
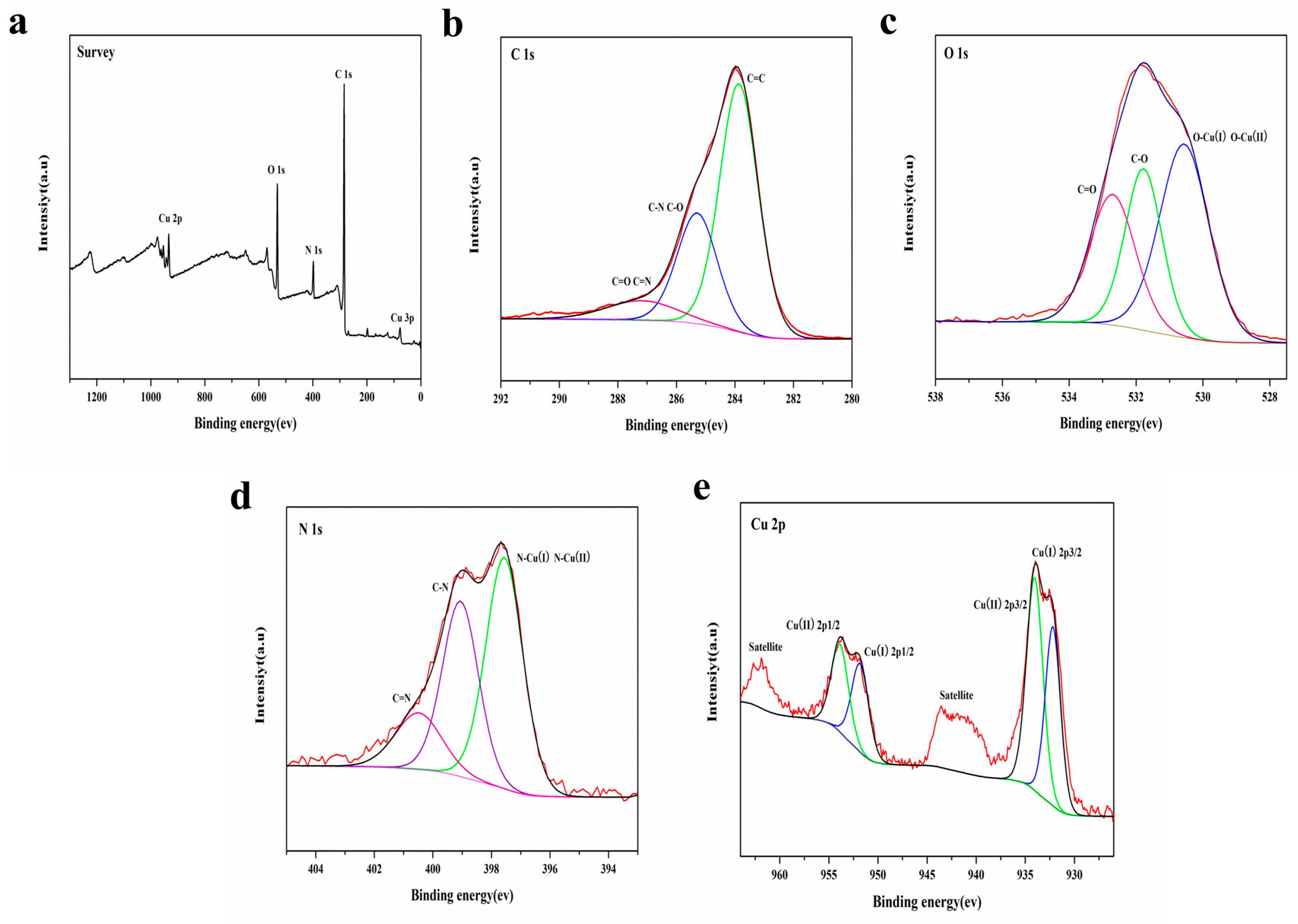
3.1.3. Morphology and XPS Analysis
3.1.4. Thermal Stability Analysis
3.2. Tribological Properties of Cu(PAP)2
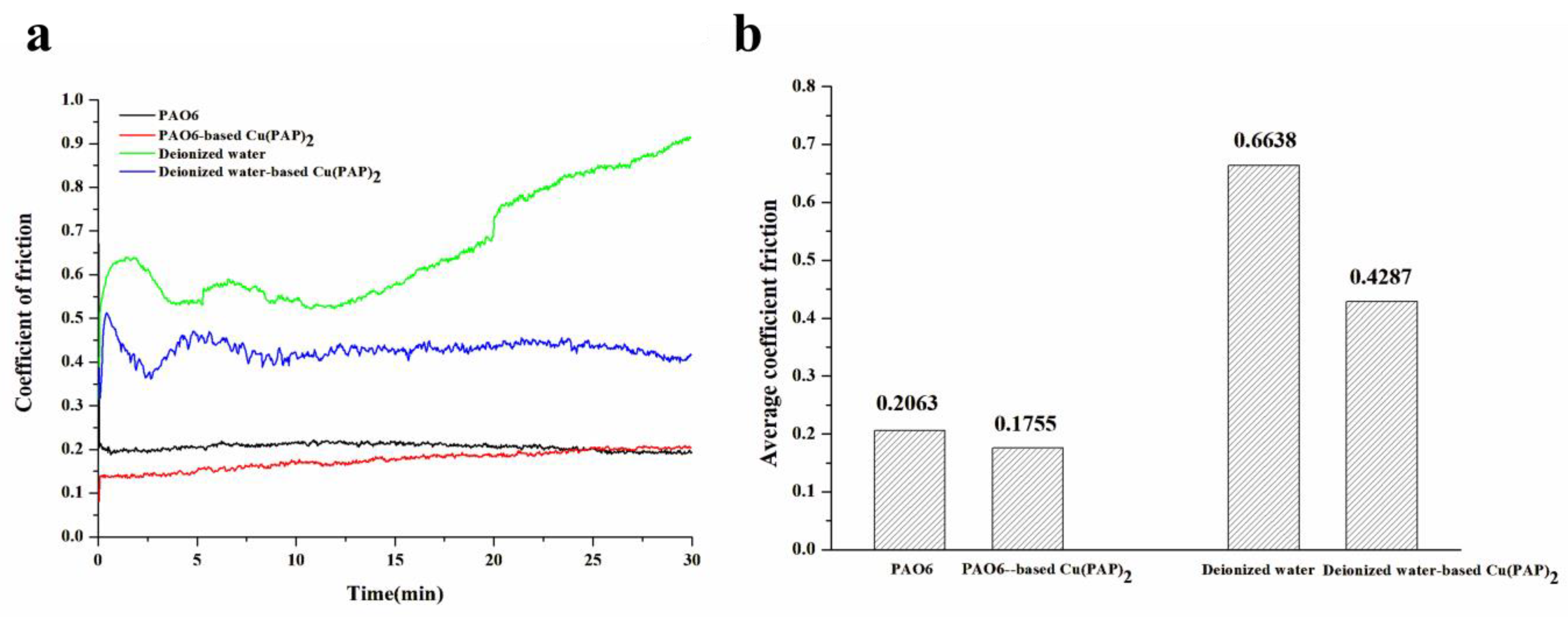
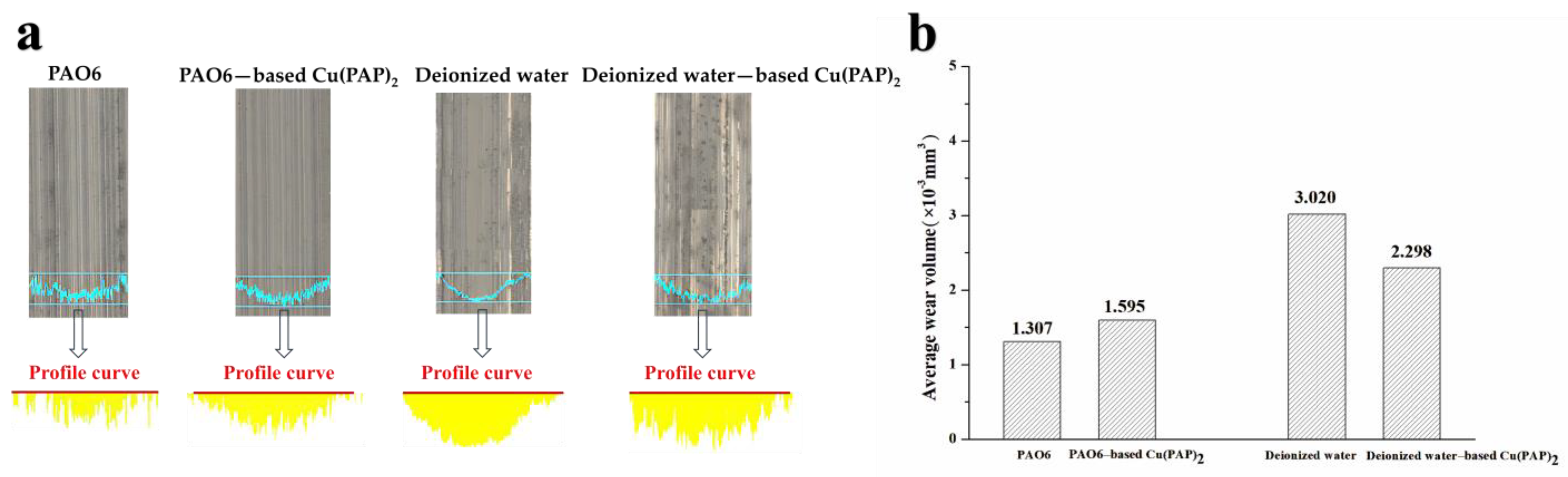
3.2.1. Results of Friction and Wear Performance Tests
3.2.2. Worn Surface Analysis of Raman Spectra
4. Conclusions
- First, a novel copper–organic framework was reported. The characterizations and DFT calculation indicated that Cu(PAP)2 is a typical 2D material with a staggered structure analogous to that of graphite.
- Significantly different tribological performances were observed when using Cu(PAP)2 as a lubricating additive in PAO6 or deionized water, and deionized water-based Cu(PAP)2 showed much better friction reduction (35.6% decrease) and anti-wear (23.9% decrease) behaviors than those of PAO6-based Cu(PAP)2.
- The Raman spectral analysis of the worn surface showed that Cu(PAP)2 can penetrate the interface between friction pairs in deionized water, but not in PAO6, which results in the significantly different tribological properties of PAO6-based Cu(PAP)2 and deionized water-based Cu(PAP)2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Dong, R.; Feng, X. Two-dimensional conjugated metal-organic frameworks (2D c-MOFs): Chemistry and function for MOFtronics. Chem. Soc. Rev. 2021, 50, 2764–2793. [Google Scholar] [CrossRef] [PubMed]
- Hmadeh, M.; Lu, Z.; Liu, Z.; Gándara, F.; Furukawa, H.; Wan, S.; Augustyn, V.; Chang, R.; Liao, L.; Zhou, F.; et al. New Porous Crystals of Extended Metal-Catecholates. Chem. Mater. 2012, 24, 3511–3513. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, T.; Feng, X. Interface-Assisted Synthesis of 2D Materials: Trend and Challenges. Chem. Rev. 2018, 118, 6189–6235. [Google Scholar] [CrossRef] [PubMed]
- Mendecki, L.; Ko, M.; Zhang, X.; Meng, Z.; Mirica, K. Porous Scaffolds for Electrochemically Controlled Reversible Capture and Release of Ethylene. J. Am. Chem. Soc. 2017, 139, 17229–17232. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Si, D.; Xie, R.; Yin, Q.; Zhang, M.; Wu, Q.; Chai, G.; Huang, Y.; Cao, R. Conductive Two-Dimensional Phthalocyanine-based Metal-Organic Framework Nanosheets for Efficient Electroreduction of CO2. Angew. Chem. Int. Ed. 2021, 60, 17108–17114. [Google Scholar] [CrossRef] [PubMed]
- Clough, A.; Yoo, J.; Mecklenburg, M.; Marinescu, S. Two-Dimensional Metal-Organic Surfaces for Efficient Hydrogen Evolution from Water. J. Am. Chem. Soc. 2015, 137, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Sheberla, D.; Bachman, J.; Elias, J.S.; Sun, C.; Shao-Horn, Y.; Dinca, M. Conductive MOF Electrodes for Stable Supercapacitors with High Areal Capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef]
- Meng, Z.; Aykanat, A.; Mirica, K. Welding Metallophthalocyanines into Bimetallic Molecular Meshes for Ultrasensitive, Low-Power Chemiresistive Detection of Gases. J. Am. Chem. Soc. 2019, 141, 2046–2053. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Q.; Xu, H.; Dong, J. The synthesis and tribological properties of small-and large-sized crystals of zeolitic imidazolate framework-71. RSC Adv. 2016, 6, 18052–18059. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, Z.; Song, Z.; Li, J.; Dong, J. Synthesis of ZIF-8 and ZIF-67 by Steam-Assisted Conversion and an Investigation of Their Tribological Behaviors. Angew. Chem. Int. Ed. 2011, 50, 672–675. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Z.; Cheng, Z. High performance of MOF-structured lubricating material with nano- and micro-sized morphologies. Mater. Lett. 2019, 248, 222–226. [Google Scholar] [CrossRef]
- Wang, F.; Fan, L.; Liu, Z.; Cheng, Z. Surfactant-mediated preparation and tribological behaviors of few-layer ZnBDC. Mater. Lett. 2019, 257, 126757–126761. [Google Scholar] [CrossRef]
- Wang, F.; Tan, G.; Ding, H.; Liu, Z.; Cheng, Z. Novel fabrication for 2D MOFs-based oil-in-water lubricating emulsion via the self-assembly interface. Mater. Lett. 2021, 297, 129981–129985. [Google Scholar] [CrossRef]
- Saito, T.; Honda, F. Chemical contribution to friction behavior of sintered hexagonal boron nitride in water. Wear 2000, 237, 253–260. [Google Scholar] [CrossRef]
- Pu, J.; Mo, Y.; Wan, S.; Wang, L. Fabrication of novel graphene–fullerene hybrid lubricating films based on self-assembly for MEMS applications. Chem. Commun. 2014, 50, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xie, G.; Luo, J. Black phosphorus as a new lubricant. Friction 2018, 6, 116–142. [Google Scholar] [CrossRef]
- Watanabe, S.; Noshiro, J.; Miyake, S. Tribological characteristics of WS2/MoS2 solid lubricating multilayer films. Surf. Coat. Technol. 2004, 183, 347–351. [Google Scholar] [CrossRef]
- Tanjil, M.; Jeong, Y.; Yin, Z.; Panaccione, W.; Wang, C. Angstrom-Scale, Atomically Thin 2D Materials for Corrosion Mitigation and Passivation. Coatings 2019, 9, 133. [Google Scholar] [CrossRef]
- Goto, M.; Kasahara, A.; Tosa, M. Low-friction coatings of zinc oxide synthesized by optimization of crystal preferred orientation. Tribol. Lett. 2011, 43, 155–162. [Google Scholar] [CrossRef]
- Lin, G.; Ding, H.; Yuan, D.; Wang, B.; Wang, C. A Pyrene-Based, Fluorescent Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2016, 138, 3302–3305. [Google Scholar] [CrossRef]
- Jiang, Y.; Oh, I.; Joo, S.; Seo, Y.; Lee, S.; Seong, W.; Kim, J.; Hwang, J.; Kwak, S.; Yoo, J.; et al. Synthesis of a Copper 1,3,5-Triamino-2,4,6-benzenetriol Metal-Organic Framework. J. Am. Chem. Soc. 2020, 142, 18346–18354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, C.; Li, Q.; Xiong, L.; Chen, R.; Wan, X.; Wang, Z.; Chen, W.; Deng, Z.; Peng, Y. Selective reduction of CO2 by conductive MOF nanosheets as an efficient co-catalyst under visible light illumination. Appl. Catal. B-Envron. 2018, 238, 339–345. [Google Scholar] [CrossRef]
- Lian, Y.; Yang, W.; Zhang, C.; Sun, H.; Deng, Z.; Xu, W.; Li Song, L.; Ouyang, Z.; Wang, Z.; Guo, J.; et al. Unpaired 3d Electron on Atomically Dispersed Cobalt Centre in Coordination Polymers to Regulate both ORR Activity and Selectivity. Angew. Chem. Int. Ed. 2020, 59, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Xiong, P.; Liu, J.; Xie, Z.; Wang, Q.; Yang, X.; Hu, E.; Cao, Y.; Sun, J.; Xu, Y.; et al. Redox-Active 2D Metal-Organic Framework for Efficient Lithium Storage with Extraordinary High Capacity. Angew. Chem. Int. Ed. 2023, 59, 5273–5277. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Fenghua Su, F.; Chen, Y. Supercritical Fluid Synthesis and Tribological Applications of Silver Nanoparticle-decorated Graphene in Engine Oil Nanofluid. Sci. Rep. 2016, 6, 31246–31258. [Google Scholar] [CrossRef] [PubMed]
- Wei Dai, W.; Kheireddin, B.; Gao, H.; Kan, Y.; Clearfield, A.; Liang, H. Formation of Anti-Wear Tribofilms via α-ZrP Nanoplatelet as Lubricant Additives. Lubricants 2016, 4, 28. [Google Scholar] [CrossRef]
- Jaiswal, V.; Kalyani; Umrao, S.; Rastogi, R.; Kumar, R.; Srivastava, A. Synthesis, Characterization, and Tribological Evaluation of TiO2-Reinforced Boron and Nitrogen co-Doped Reduced Graphene Oxide Based Hybrid Nanomaterials as Efficient Antiwear Lubricant Additives. ACS Appl. Mater. Interfaces 2016, 8, 11698–11710. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Z.; Cheng, Z. Ultrasonic-assisted exfoliation for 2D Zn(Bim)(OAc) nanosheets used as an oil-soluble additive in lubricants. Appl. Organomet. Chem. 2020, 34, 5950–5958. [Google Scholar] [CrossRef]
- Faria, D.; Silva, S.; Oliveira, M. Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides. J. Raman. Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Xie, H.; Dang, S.; Jiang, B.; Xiang, L.; Zhou, S.; Sheng, H.; Yang, T.; Pan, F. Tribological performances of SiO2/graphene combinations as water-based lubricant additives for magnesium alloy rolling. Appl. Surf. Sci. 2019, 475, 847–856. [Google Scholar] [CrossRef]
- Li, C.; Wu, B.; Chen, X.; Li, L.; Wang, X.; Gao, X.; Wang, X.; Hu, K.; Hu, X. Synergistic Lubricating Performance of Graphene Oxide and Modified Biodiesel Soot as Water Additives. Lubricants 2022, 10, 175. [Google Scholar] [CrossRef]
- Cho, D.; Kim, J.; Kwon, S.; Lee, C.; Lee, Y. Evaluation of hexagonal boron nitride nano-sheets as a lubricant additive in water. Wear 2013, 302, 981–986. [Google Scholar] [CrossRef]
- Wei Wang, W.; Dong, S.; Gao, Y.; Zhang, G.; Wang, K. Tribological behaviors of black phosphorus/MoS2 composites as water-based lubrication additives. Lubr. Sci. 2021, 33, 404–416. [Google Scholar] [CrossRef]




| 2D Materials | Testing Conditions | Tribological Performance | Reference | |
|---|---|---|---|---|
| Friction Coefficients | Wear | |||
| Cu(PAP)2 | Ball-on-plate reciprocating tribometer, 10 N | 35.6% | 23.9% | This work |
| Graphene | Ball-on-plate reciprocating tribometer, 3 N | 21.9% | 13.5% | [30] |
| Graphene Oxide (GO) | Ball-on-plate reciprocating tribometer, 10 N | 55.6% | 41.6% | [31] |
| Hexagonal boron nitride (h-BN) | Ball-on-plate reciprocating tribometer, 3 N | 22.5% | 16.9% | [32] |
| Molybdenum disulfide (MoS2) | Ball-on-plate reciprocating tribometer, 8 N | 26.5% | 74.6% | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Liu, Z.; Li, C.; Wang, X.; Li, M. Synthesis and Characterization of a Novel Two-Dimensional Copper p-Aminophenol Metal–Organic Framework and Investigation of Its Tribological Properties. Materials 2023, 16, 6061. https://doi.org/10.3390/ma16176061
Li L, Liu Z, Li C, Wang X, Li M. Synthesis and Characterization of a Novel Two-Dimensional Copper p-Aminophenol Metal–Organic Framework and Investigation of Its Tribological Properties. Materials. 2023; 16(17):6061. https://doi.org/10.3390/ma16176061
Chicago/Turabian StyleLi, Lei, Zhijun Liu, Chuan Li, Xiaodong Wang, and Mingling Li. 2023. "Synthesis and Characterization of a Novel Two-Dimensional Copper p-Aminophenol Metal–Organic Framework and Investigation of Its Tribological Properties" Materials 16, no. 17: 6061. https://doi.org/10.3390/ma16176061





