Process Optimization, Morphology, Structure, and Adhesive Strength of Electrodeposited Ni–Fe–Graphene Composite Coating on the 7075 Aluminum Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Pretreatment of Substrate
2.2. Preparation of Ni–Fe–Graphene Composite Coating
2.3. Characterization and Measurement of Coatings
3. Results and Discussion
3.1. Process Optimization
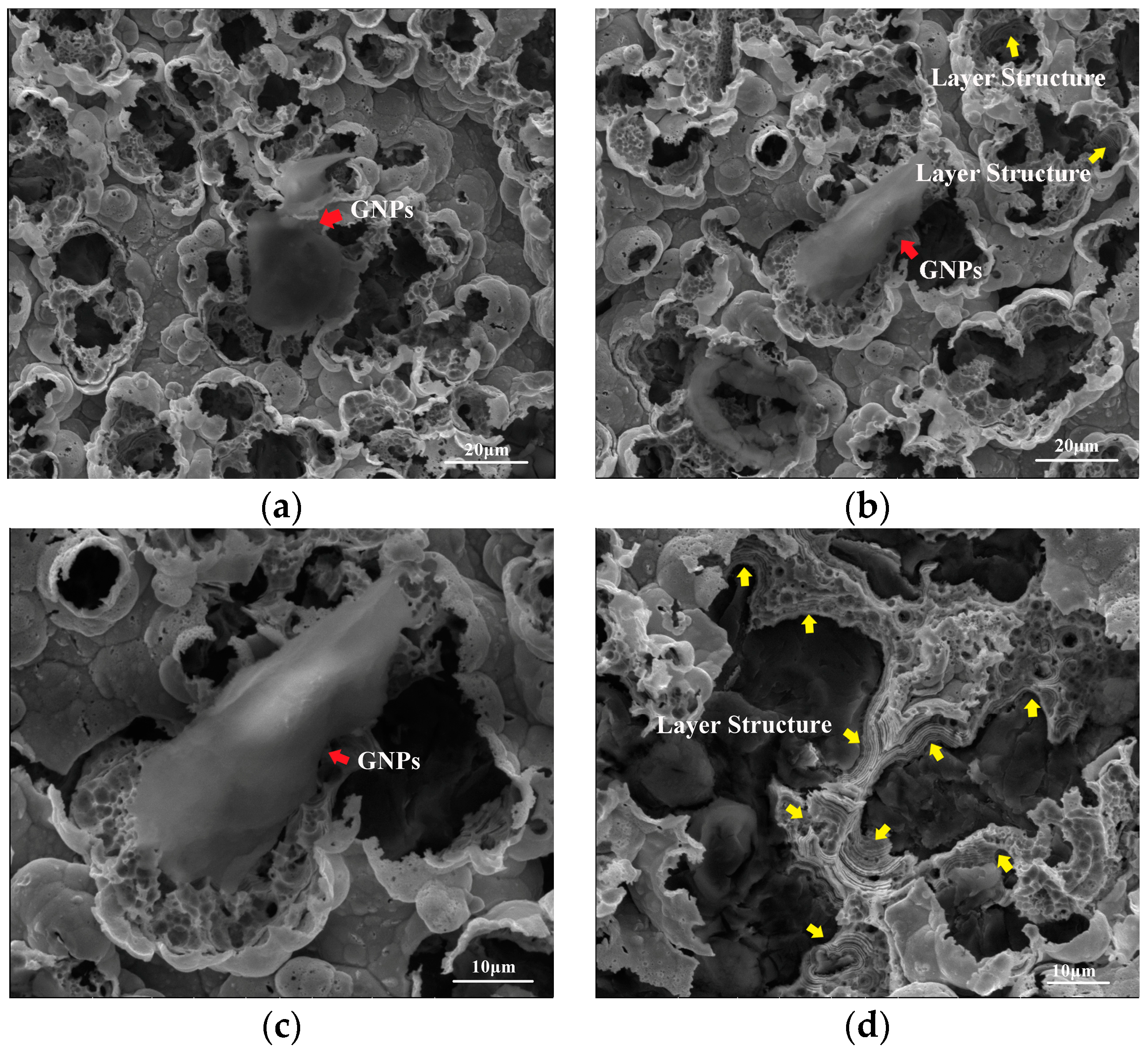
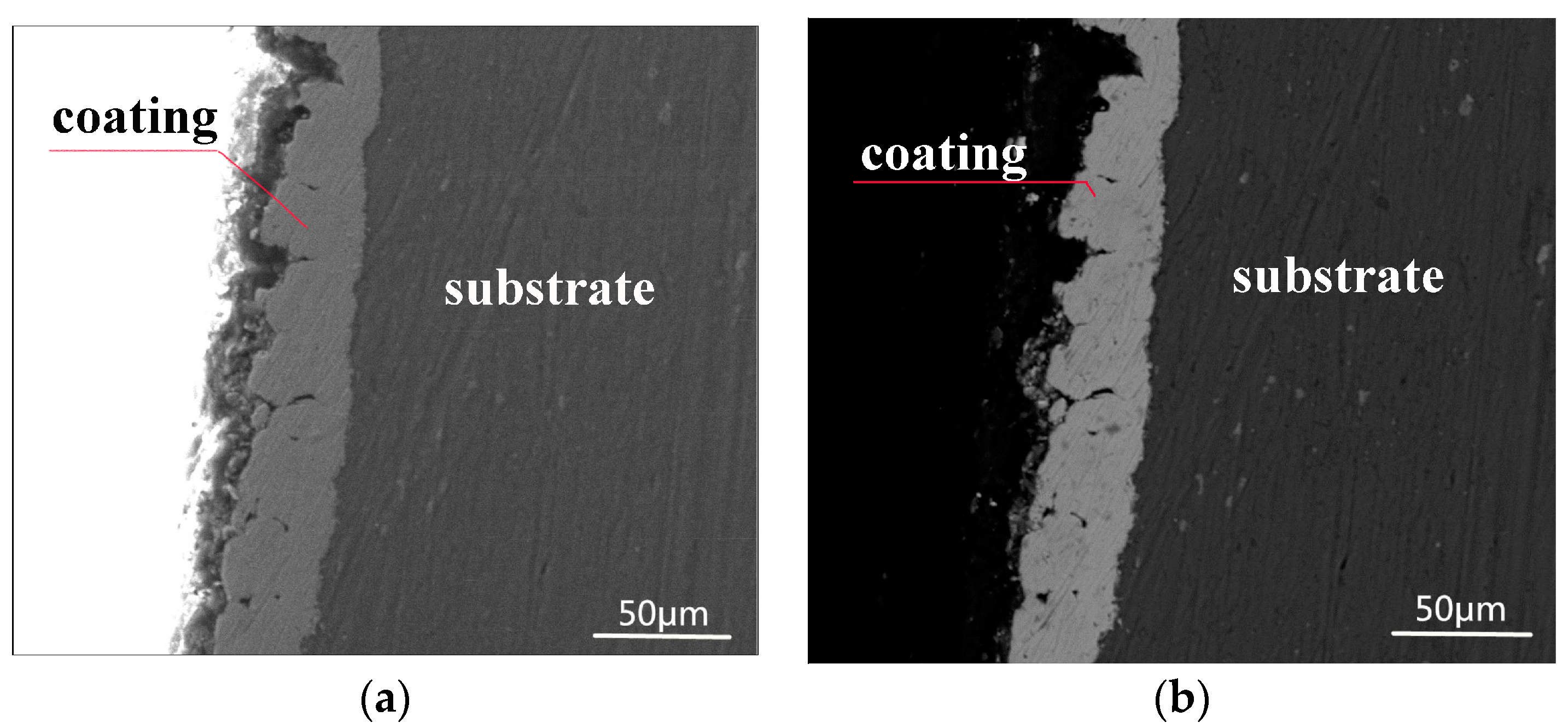
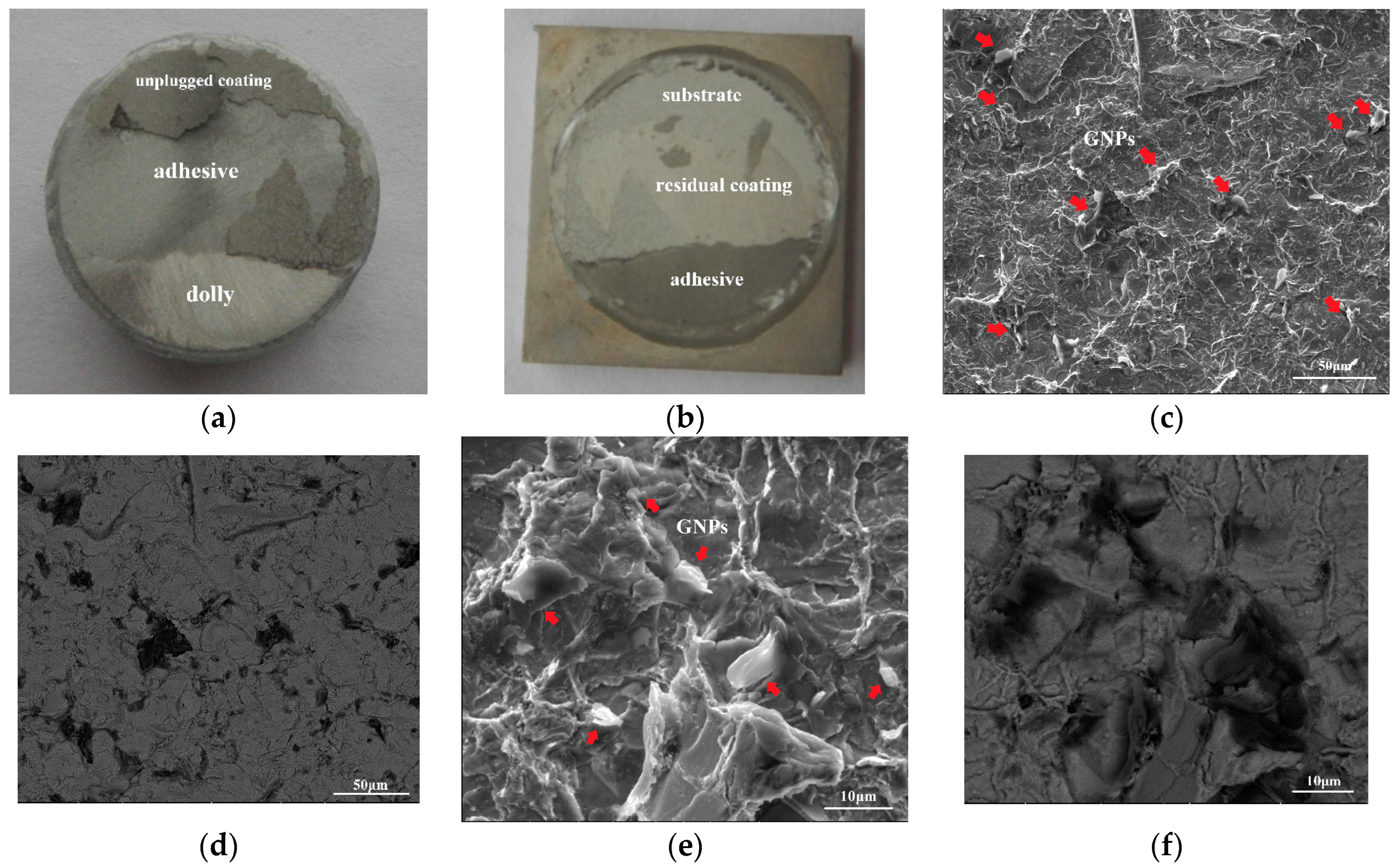
3.2. Morphology and Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagaria, S.K.; Periasamy, C. Magnetic Properties of Electroformed Ni and Ni-Fe for Micromagnetic MEMS Applications. J. Supercond. Nov. Magn. 2015, 28, 3357–3363. [Google Scholar] [CrossRef]
- Cheng, H.; Guo, H.X.; Wang, Q.; Wang, C.; Tang, Y.H. Electrodeposition technology and corrosion resistance of Ni-Fe alloy coating. Plat. Finish. 2011, 33, 1–4. [Google Scholar]
- Vicenzo, A. Structure and Mechanical Properties of Electrodeposited Nanocrystalline Ni-Fe Alloys. J. Electrochem. Soc. 2013, 160, D570–D577. [Google Scholar] [CrossRef]
- Du, J.; Yu, Z.; Guo, B.; Li, H.; He, Y. Friction and wear properties of Ni-Fe alloy coatings with different Fe content. Mater. Mech. Eng. 2009, 33, 81–85. [Google Scholar]
- Li, H.; Ebrahimi, F. Synthesis and characterization of electrodeposited nanocrystalline nickel–iron alloys. Mater. Sci. Eng. A-Struct. 2003, 347, 93–101. [Google Scholar] [CrossRef]
- Giallonardo, J.; Erb, U.; Aust, K.; Palumbo, G. The influence of grain size and texture on the Young’s modulus of nanocrystalline nickel and nickel–iron alloys. Philos. Mag. 2011, 91, 4594–4605. [Google Scholar] [CrossRef]
- Starosta, R.; Zielinski, A. Effect of chemical composition on corrosion and wear behavior of the composite Ni-Fe-Al2O3 coatings. J. Mater. Process Technol. 2004, 157, 434–441. [Google Scholar] [CrossRef]
- Ataee-Esfahani, H.; Vaezi, M.R.; Nikzad, L.; Yazdani, B.; Sadrnezhaad, S.K. Influence of SiC nanoparticles and saccharin on the structure and properties of electrodeposited Ni–Fe/SiC nanocomposite coatings. J. Alloys Compd. 2009, 484, 540–544. [Google Scholar] [CrossRef]
- Soltani, H.; Tavoosi, M.; Loghman-Estarki, M.R. Fe–Ni composite coatings reinforced with SiC nanoparticles and carbon nanotubes: Corrosion and tribological performance. Mater. Res. Express 2019, 6, 115106. [Google Scholar] [CrossRef]
- Yousefi, E.; Sharafi, S.; Irannejad, A. The structural, magnetic, and tribological properties of nanocrystalline Fe-Ni permalloy and Fe-Ni-TiO2 composite coatings produced by pulse electro co-deposition. J. Alloys Compd. 2018, 753, 308–319. [Google Scholar]
- Ledwig, P.; Ratajski, T.; Indyka, P.; Kalemba-Rec, I.; Kopia, A.; Kąc, M.; Dubiel, B. Microstructure and properties of electrodeposited nc-TiO2/Ni-Fe and Ni-Fe Coatings. Met. Mater. Int. 2020, 26, 812–826. [Google Scholar]
- Yousefi, E.; Sharafi, S.; Irannejad, A. Microstructure, tribological behavior and magnetic properties of Fe-Ni-TiO2 composite coatings synthesized via pulse frequency variation. Trans. Nonferr. Metal. Soc. 2021, 31, 3800–3813. [Google Scholar]
- Tripathi, M.K.; Singh, V.B.; Singh, H.K. Structure and properties of electrodeposited functional Ni–Fe/TiN nanocomposite coatings. Surf. Coat. Technol. 2015, 278, 146–156. [Google Scholar] [CrossRef]
- Jin, H.; Ji, R.; Dong, T.; Liu, S.; Liu, Y.; Zhang, F.; Ma, C.; Liu, S. Fabrication and characterization of WC particles reinforced Ni Fe composite coating by jet electrodeposition. Surf. Coat. Technol. 2021, 421, 127368. [Google Scholar] [CrossRef]
- Rasooli, A.; Safavi, M.S.; Babaei, F.; Ansarian, A. Electrodeposited Ni–Fe–Cr2O3 nanocomposite coatings: A survey of influences of Cr2O3 nanoparticles loadings in the electrolyte. J. Alloys Compd. 2020, 822, 153725. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Singh, V.B. Properties of electrodeposited functional Ni-Fe/AlN nanocomposite coatings. Arab. J. Chem. 2019, 12, 3601–3610. [Google Scholar]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar]
- Han, T.-W.; He, P.-F.; Wang, J.; Wu, A.-H. Molecular dynamics simulation of a single graphene sheet under tension. Carbon 2010, 25, 261–265. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar]
- Sandoz-Rosado, E.J.; Tertuliano, O.A.; Terrell, E.J. An atomistic study of the abrasive wear and failure of graphene sheets when used as a solid lubricant and a comparison to diamond-like-carbon coatings. Carbon 2012, 50, 4078–4084. [Google Scholar] [CrossRef]
- Lin, J.; Wang, L.; Chen, G. Modification of Graphene Platelets and their Tribological Properties as a Lubricant Additive. Tribol. Lett. 2011, 41, 209–215. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, T.; Wang, J.; Ni, J.; Li, H.; Shao, X. Synthesis, characterization and tribological properties of Cu/reduced graphene oxide composites. Tribol. Int. 2015, 88, 17–24. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Liu, C.; Su, F.; Liang, J. Producing cobalt–graphene composite coating by pulse electrodeposition with excellent wear and corrosion resistance. Appl. Surf. Sci. 2015, 351, 889–896. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Xiong, D.; He, Y.; Ji, Y.; Qin, Y. Preparation and tribological behavior of Ni-graphene composite coating under room temperature. Appl. Surf. Sci. 2016, 361, 49–56. [Google Scholar] [CrossRef]
- Algul, H.; Tokur, M.; Ozcan, S.; Uysal, M.; Cetinkaya, T.; Akbulut, H.; Alp, A. The effect of graphene content and sliding speed on the wear mechanism of nickel–graphene nanocomposites. Appl. Surf. Sci. 2015, 359, 340–348. [Google Scholar] [CrossRef]
- Li, N.; Zhang, L.; Xu, M.; Xia, T.; Ruan, X.; Song, S.; Ma, H. Preparation and mechanical property of electrodeposited Al-graphene composite coating. Mater. Des. 2016, 111, 522–527. [Google Scholar] [CrossRef]
- Pavithra, C.L.P.; Sarada, B.V.; Rajulapati, K.V.; Rao, T.N.; Sundararajan, G. A new electrochemical approach for the synthesis of copper-graphene nanocomposite foils with high hardness. Sci. Rep. 2014, 4, 4049. [Google Scholar] [PubMed]
- Li, Y.; Wang, G.; Liu, S.; Zhao, S.; Zhang, K. The preparation of Ni/GO composite foils and the enhancement effects of GO in mechanical properties. Compos. Part B Eng. 2018, 135, 43–48. [Google Scholar] [CrossRef]
- Aliyu, A.; Srivastava, C. Morphology and corrosion properties of FeMn-Graphene oxide composite coatings. J. Alloys Compd. 2020, 821, 153560. [Google Scholar] [CrossRef]
- Badrayyana, S.; Bhat, D.K.; Shenoy, S.; Ullal, Y.; Hegde, A.C. Novel Fe–Ni-Graphene composite electrode for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 10453–10462. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Xia, H.; Zhang, J.; Zhang, P.; Xu, M.; Ma, H. Preparation and Mechanical Properties of (Ni-Fe)-Graphene Composite Coating. Adv. Eng. Mater. 2016, 10, 1716–1719. [Google Scholar] [CrossRef]
- Li, N.; Zhang, L.; Zhu, Y.; Xu, M.; Xu, Y.; Ruan, X.; Ma, H. The Effect of Graphene on the Deposition and Mechanical Property of Ni-Fe-Graphene Composite Coating. J. Electrochem. Soc. 2018, 165, D215–D222. [Google Scholar] [CrossRef]
- Kuang, D.; Xu, L.; Liu, L.; Hu, W.; Wu, Y. Graphene-nickel composites. Appl. Surf. Sci. 2013, 273, 484–490. [Google Scholar] [CrossRef]
- Jiang, K.; Li, J.; Liu, J. Electrochemical codeposition of graphene platelets and nickel for improved corrosion resistant properties. RSC Adv. 2014, 4, 36245–36252. [Google Scholar] [CrossRef]


| Type of Coatings | Hardness (HV) | Friction Coefficient | Reference |
|---|---|---|---|
| Ni–Fe/SiC | 710 | — | [8] |
| — | 0.6 | [9] | |
| Ni–Fe–TiO2 | 638 | 0.52 | [10] |
| 526 | — | [11] | |
| 647 | — | [12] | |
| Ni–Fe–TiN | 660 | — | [13] |
| Ni–Fe–WC | 569.2 | 0.727 | [14] |
| Ni–Fe–Cr2O3 | 565 | 0.72 | [15] |
| Ni–Fe–AlN | 560 | — | [16] |
| Ni–Fe–MWCNT | — | 0.38 | [9] |
| Factors | Graphene Concentration (g/L) | Current Density (A dm−2) | Agitation Speed (r min−1) | Temperature (°C) | |
|---|---|---|---|---|---|
| Levels | |||||
| 1 | 1 | 3 | 100 | 30 | |
| 2 | 1 | 5 | 150 | 40 | |
| 3 | 1 | 7 | 200 | 50 | |
| 4 | 1 | 9 | 250 | 60 | |
| 5 | 3 | 3 | 150 | 50 | |
| 6 | 3 | 5 | 100 | 60 | |
| 7 | 3 | 7 | 250 | 30 | |
| 8 | 3 | 9 | 200 | 40 | |
| 9 | 5 | 3 | 200 | 60 | |
| 10 | 5 | 5 | 250 | 50 | |
| 11 | 5 | 7 | 100 | 40 | |
| 12 | 5 | 9 | 150 | 30 | |
| 13 | 7 | 3 | 250 | 40 | |
| 14 | 7 | 5 | 200 | 30 | |
| 15 | 7 | 7 | 150 | 60 | |
| 16 | 7 | 9 | 100 | 50 | |
| Composition | Content (g L−1) |
|---|---|
| NiSO4·6H2O | 16.8 |
| FeSO4·7H2O | 11.1 |
| H3BO3 | 15 |
| C6H8O6 | 1 |
| Na2SO4 | 10 |
| C6H5O7(NH4)3 | 45 |
| C10H16N2O8 | 2.4 |
| Factors | Graphene Concentration (g L−1) | Current Density (A dm−2) | Agitation Speed (r min−1) | Temperature (°C) | Hardness (HV) | COF | |
|---|---|---|---|---|---|---|---|
| No. | |||||||
| 1 | 1 | 3 | 100 | 30 | 534.8 (±14.50) | 0.2334 (±0.018) | |
| 2 | 1 | 5 | 150 | 40 | 788.4 (±25.55) | 0.2118 (±0.015) | |
| 3 | 1 | 7 | 200 | 50 | 772.2 (±25.78) | 0.2146 (±0.016) | |
| 4 | 1 | 9 | 250 | 60 | 944.9 (±35.66) | 0.2221 (±0.012) | |
| 5 | 3 | 3 | 150 | 50 | 283.6 (±28.36) | 0.3080 (±0.010) | |
| 6 | 3 | 5 | 100 | 60 | 621.5 (±18.33) | 0.1567 (±0.015) | |
| 7 | 3 | 7 | 250 | 30 | 673.4 (±26.25) | 0.2167 (±0.009) | |
| 8 | 3 | 9 | 200 | 40 | 542.9 (±24.10) | 0.1687 (±0.009) | |
| 9 | 5 | 3 | 200 | 60 | 191.8 (±4.22) | 0.3549 (±0.016) | |
| 10 | 5 | 5 | 250 | 50 | 422.5 (±39.00) | 0.2401 (±0.019) | |
| 11 | 5 | 7 | 100 | 40 | 483.7 (±30.09) | 0.1856 (±0.017) | |
| 12 | 5 | 9 | 150 | 30 | 513.0 (±26.36) | 0.1668 (±0.012) | |
| 13 | 7 | 3 | 250 | 40 | 271.4 (±28.36) | 0.2777 (±0.004) | |
| 14 | 7 | 5 | 200 | 30 | 551.0 (±21.67) | 0.2397 (±0.012) | |
| 15 | 7 | 7 | 150 | 60 | 536.4 (±24.26) | 0.2651 (±0.029) | |
| 16 | 7 | 9 | 100 | 50 | 590.7 (±23.83) | 0.2326 (±0.018) | |
| Index | Hardness (HV) | COF | |
|---|---|---|---|
| No. | |||
| 4 | 944.9 | 0.2221 | |
| 6 | 621.5 | 0.1567 | |
| Variation | 34.2% decrease (harmful) | 29.4% decrease (beneficial) | |
| Factors | Graphene Concentration (g L−1) | Current Density (A dm−2) | Agitation Speed (r min−1) | Temperature (°C) | |
|---|---|---|---|---|---|
| Level | |||||
| A1 | 760.1 | 320.4 | 557.7 | 568.1 | |
| A2 | 530.3 | 595.9 | 530.4 | 521.6 | |
| A3 | 402.8 | 616.5 | 514.5 | 517.3 | |
| A4 | 487.4 | 647.9 | 578.1 | 573.7 | |
| Ri | 357.3 | 327.5 | 63.6 | 56.4 | |
| Factors | Graphene Concentration (g L−1) | Current Density (A dm−2) | Agitation Speed (r min−1) | Temperature (°C) | |
|---|---|---|---|---|---|
| Level | |||||
| B1 | 0.2205 | 0.2935 | 0.2021 | 0.2142 | |
| B2 | 0.2125 | 0.2121 | 0.2379 | 0.2110 | |
| B3 | 0.2369 | 0.2205 | 0.2445 | 0.2488 | |
| B4 | 0.2538 | 0.1976 | 0.2392 | 0.2497 | |
| Rj | 0.0413 | 0.0959 | 0.0424 | 0.0387 | |
| Factor | Graphene Concentration (g L−1) | Current Density (A dm−2) | Agitation Speed (r min−1) | Temperature (°C) | Hardness (HV) | COF | |
|---|---|---|---|---|---|---|---|
| No. | |||||||
| 17 | 1 | 9 | 250 | 60 | 912.6 | 0.1990 | |
| 18 | 3 | 9 | 100 | 40 | 592.4 | 0.2467 | |
| Composition | Fe (wt%) | Ni (wt%) | C (wt%) | O (wt%) |
|---|---|---|---|---|
| Ni–Fe | 40.21 | 56.43 | 2.32 | 1.03 |
| Ni–Fe–graphene | 41.62 | 52.08 | 5.33 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Zhang, L.; Ma, H.; Li, Q.; Sun, X. Process Optimization, Morphology, Structure, and Adhesive Strength of Electrodeposited Ni–Fe–Graphene Composite Coating on the 7075 Aluminum Alloy. Materials 2023, 16, 6062. https://doi.org/10.3390/ma16176062
Li N, Zhang L, Ma H, Li Q, Sun X. Process Optimization, Morphology, Structure, and Adhesive Strength of Electrodeposited Ni–Fe–Graphene Composite Coating on the 7075 Aluminum Alloy. Materials. 2023; 16(17):6062. https://doi.org/10.3390/ma16176062
Chicago/Turabian StyleLi, Na, Lan Zhang, Huizhong Ma, Qiao Li, and Xingke Sun. 2023. "Process Optimization, Morphology, Structure, and Adhesive Strength of Electrodeposited Ni–Fe–Graphene Composite Coating on the 7075 Aluminum Alloy" Materials 16, no. 17: 6062. https://doi.org/10.3390/ma16176062
APA StyleLi, N., Zhang, L., Ma, H., Li, Q., & Sun, X. (2023). Process Optimization, Morphology, Structure, and Adhesive Strength of Electrodeposited Ni–Fe–Graphene Composite Coating on the 7075 Aluminum Alloy. Materials, 16(17), 6062. https://doi.org/10.3390/ma16176062





