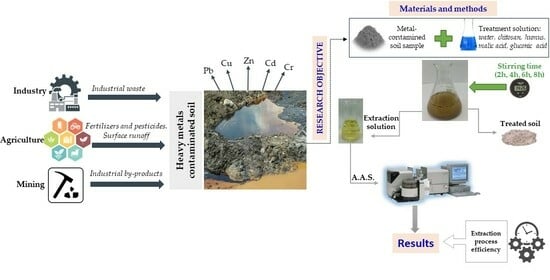
Influence of the Extraction Solution on the Removal of Heavy Metals from Polluted Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Soil Analysis Methods
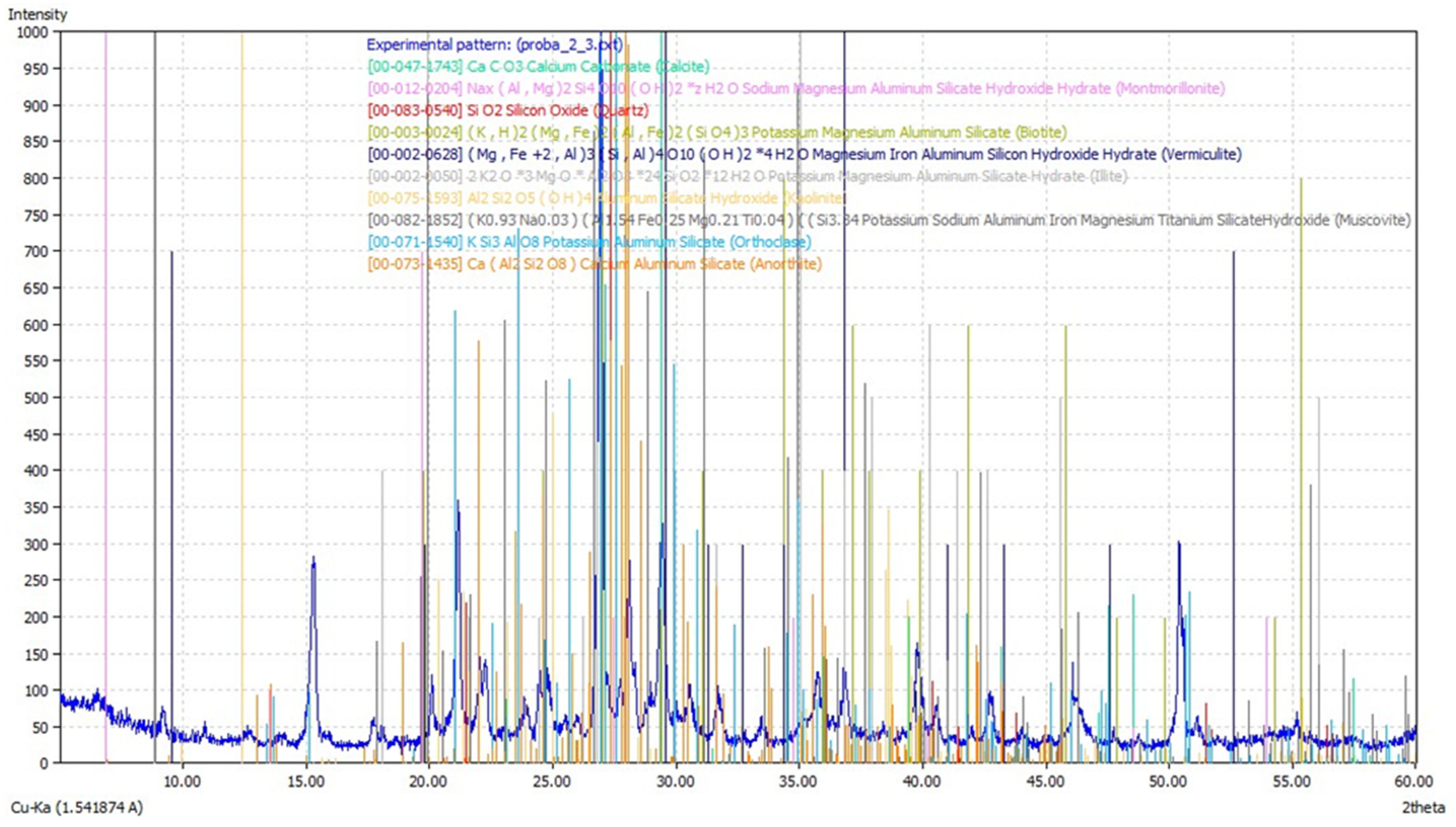
2.3. Determination of Soil Mineral Phases
2.4. Determination of Soil Metal Concentration
2.5. Preparation of Extraction Solutions
2.6. Metal Extraction
2.7. Extraction Process Efficiency
3. Results and Discussions
3.1. Soil Characterization
3.2. Determination of Mineral Phases
3.3. Determination of Soil Metal Concentration
3.4. Metal Extraction from Soil
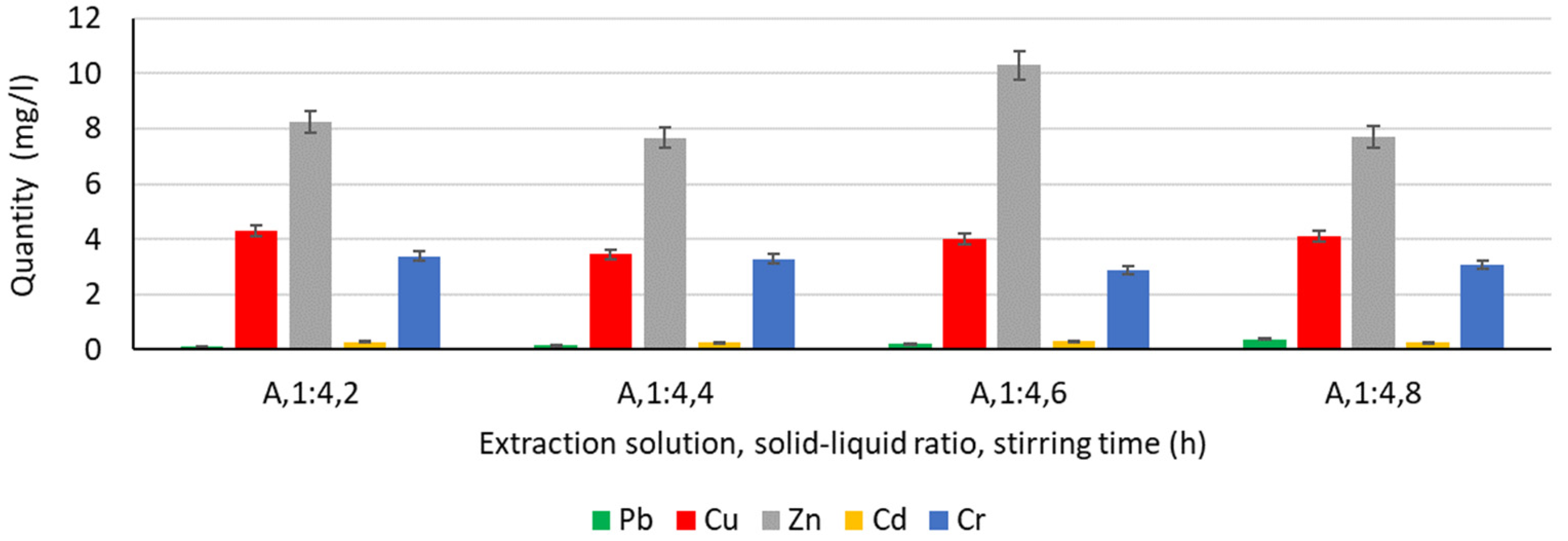
3.4.1. Extraction of Heavy Metals from Water
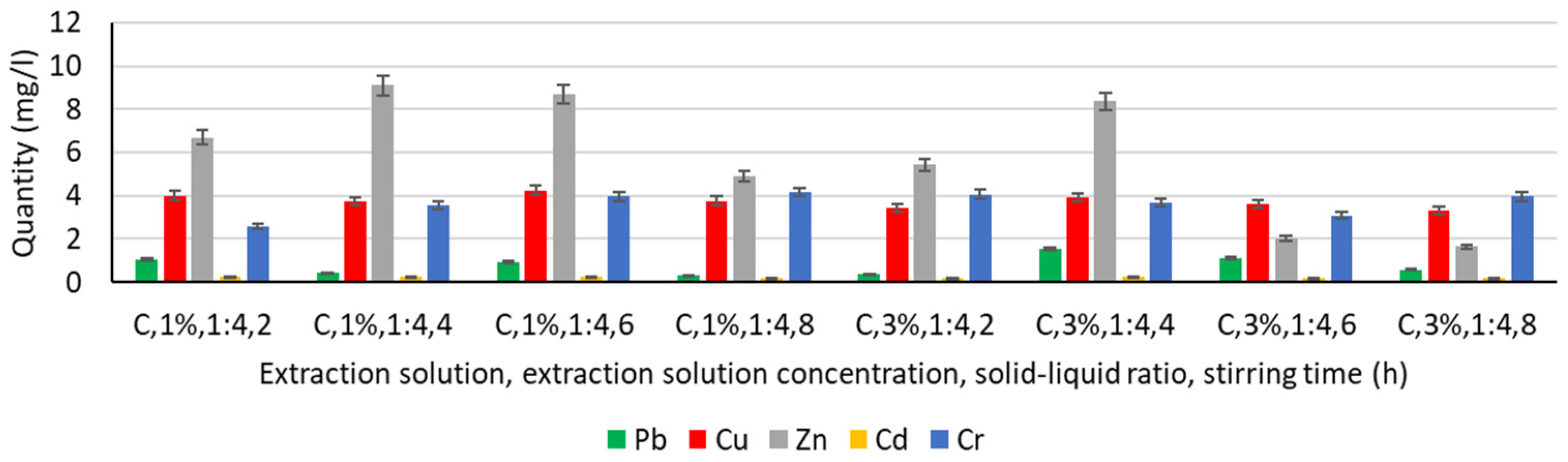
3.4.2. Extraction of Heavy Metals from Chitosan Solution
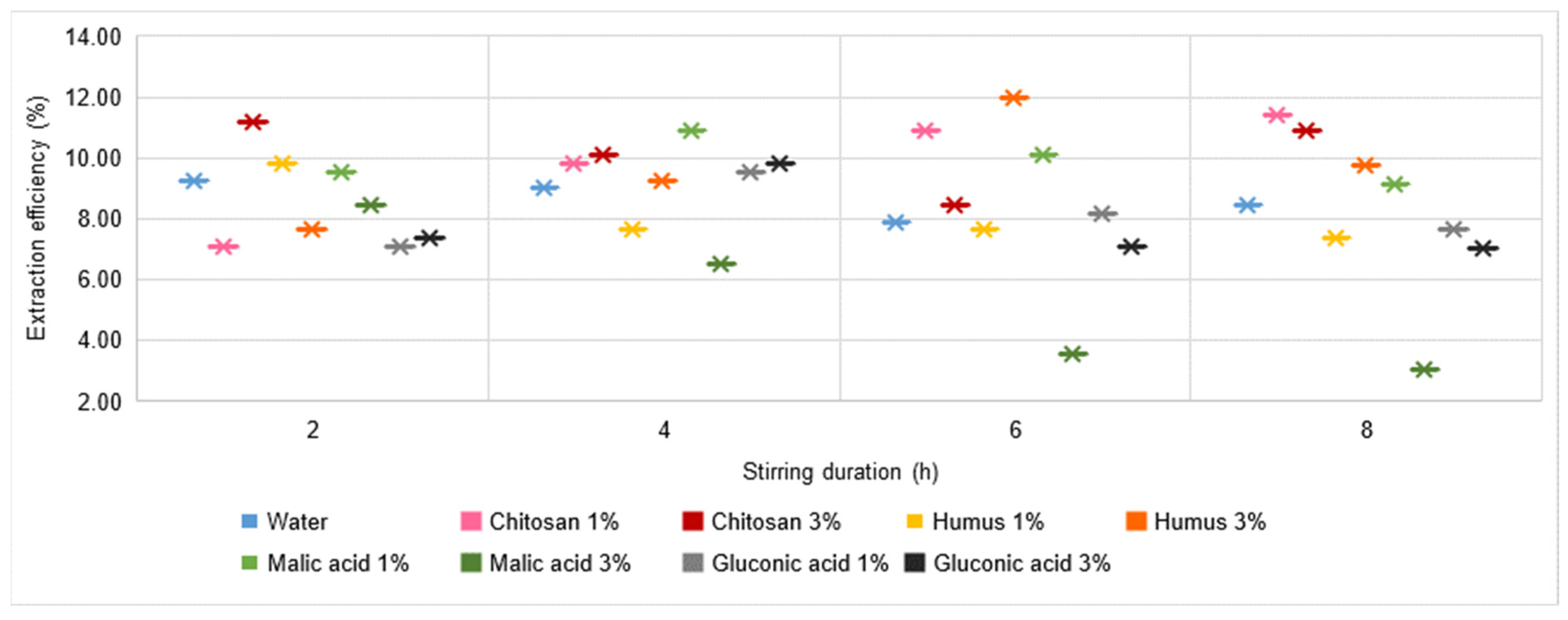
- In the case of Pb, for stirring times of 4 h, 6 h, and 8 h, increasing the concentration of the chitosan solution induces an increase in the amount extracted even by 200% (stirring time 4 h);
- Cu extraction is slightly reduced by 5–15% with increasing chitosan solution concentration for all stirring times;
- In the case of Zn, increasing the concentration of the chitosan solution reduces the extraction process, regardless of the stirring time. In the case of longer stirring, there is even a significant reduction of Zn, i.e., by more than 76% for a stirring time of 6 h and more than 66% for a stirring time of 8 h;
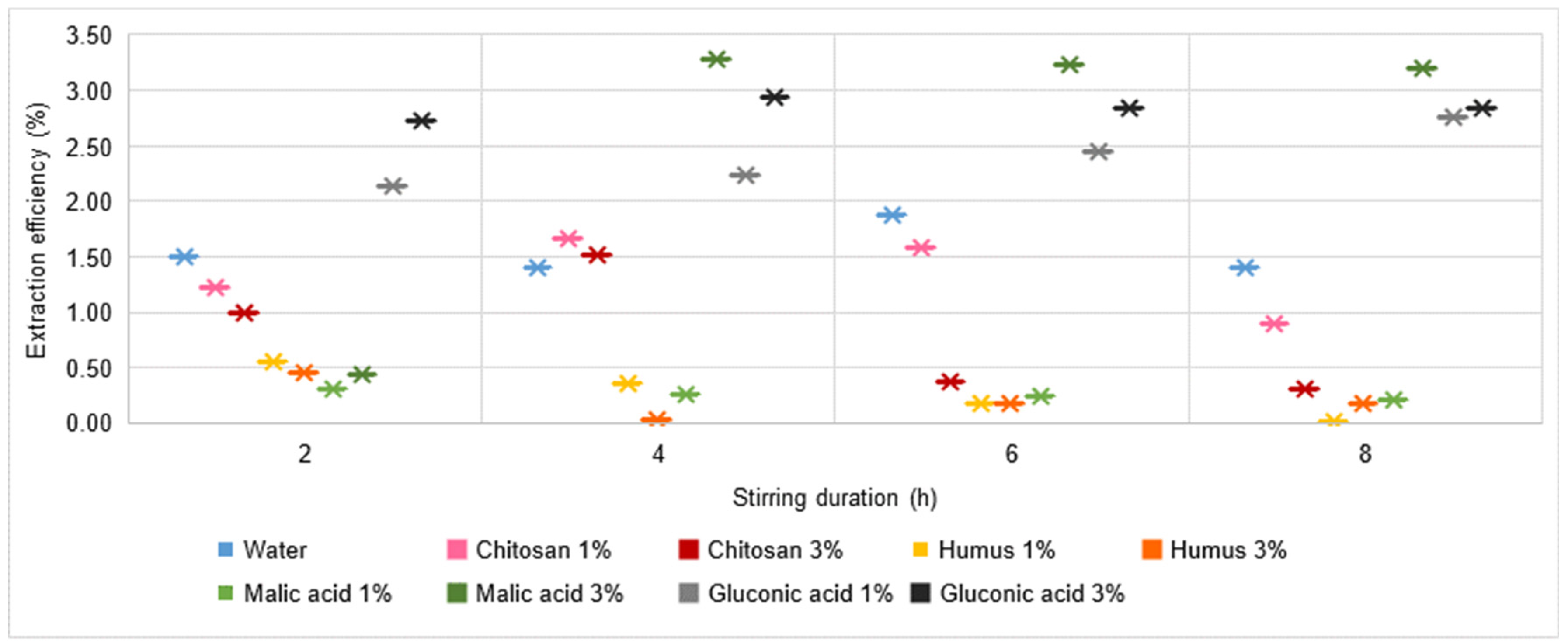
- Cd extraction is reduced in both chitosan solutions and is not significantly influenced by either increasing chitosan solution concentration or increasing stirring time;
- In Cr, increasing the concentration of the chitosan solution improves the extraction by more than 50% at a stirring time of 2 h but does not change significantly when increasing the stirring time to 4 h, 6 h, or 8 h.
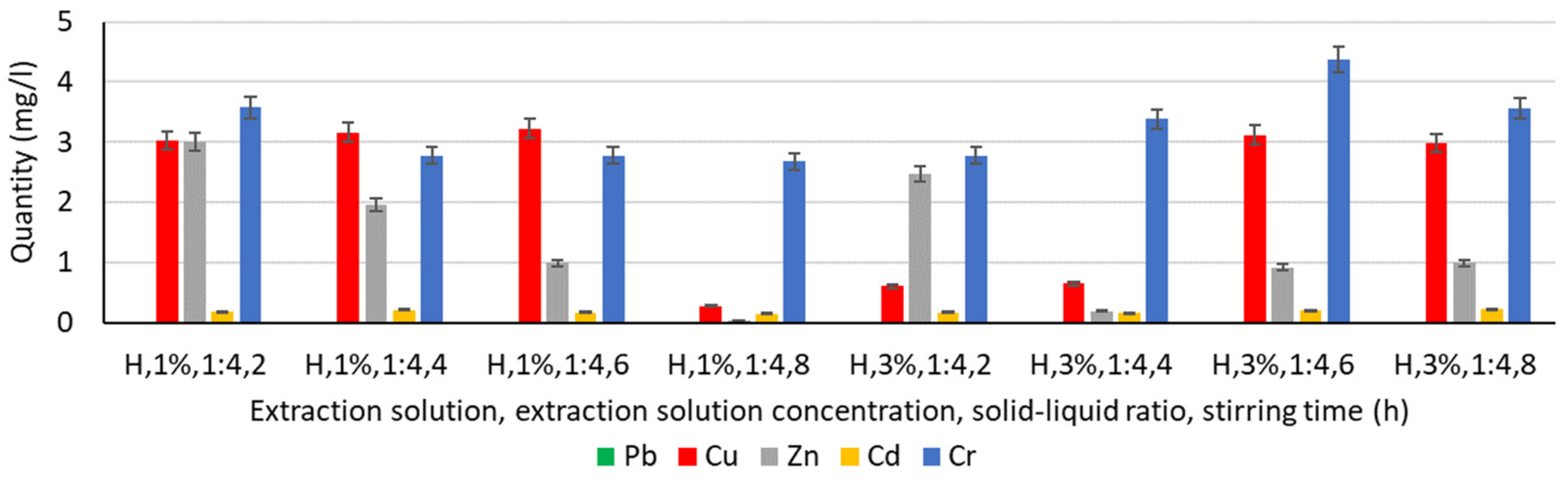
3.4.3. Extraction of Heavy Metals from Humus Solution
3.4.4. Extraction of Heavy Metals from Malic Acid Solution
- Cu extraction increases by up to 10 times compared to using water, chitosan solution, or humus solution if 1% malic acid solution is used, and by more than 20 times if 3% malic acid solution is used. Increasing the concentration of the malic acid solution from 1% to 3% improves the Cu extraction process by 75–165%; this improvement is also influenced by the stirring time. It is noted, however, that in the 1% malic acid solution, as the stirring time increases, there is a continuous reduction in the amount of Cu by 10–24%, compared to the amount recorded with a stirring time of 2 h. In contrast to this downward trend for the 1% malic acid solution, when using the 3% malic acid solution, increasing the stirring time results in a uniform-continuous increase in the amount of Cu extracted by 10–15% compared to the amount extracted with a 2 h stirring time;
- Pb extraction is also dramatically improved, more so as the concentration of the malic acid solution increases from 1% to 3%. Thus, while in the case of water, chitosan, or humus solutions, the quantities of Pb extracted were in the range of unit or subunit values (maximum 1.55 mg L−1 or even 0 for humus solutions), under these conditions, values in the range 3–7.4 mg L−1 are recorded for the 1% malic acid solution and in the range 25.27–34.98 mg L−1 for the 3% malic acid solution. Analysing the influence of stirring time on Pb extraction, two different tendencies are observed, similar to the Cu case, depending on the solution concentration. In the 1% solution, the increase in stirring time has a negative effect, with the amounts of Pb decreasing continuously from 7.4 mgL−1 (stirring time of 2 h) to 3.02 mg L−1 (stirring time of 8 h), while in the 3% malic acid solution, the increase in stirring time causes a continuous increase of 15–38% compared to the amount extracted with a stirring time of 2 h;
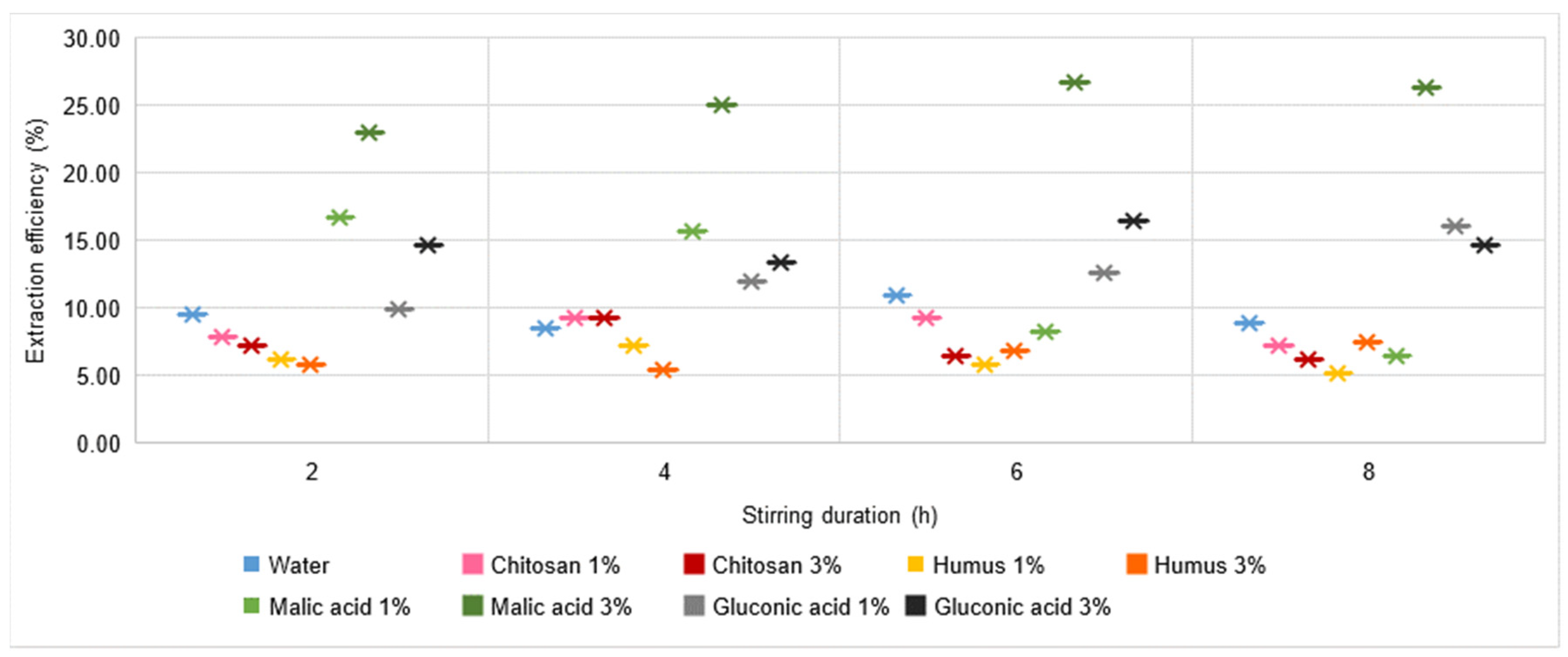
- Zn extraction in malic acid solutions is lower compared to extraction in water, chitosan solution, or humus solution. However, it can be appreciated that the use of a more concentrated malic acid solution (3%) is beneficial in terms of Zn extraction possibilities;
- Cr extraction in malic acid solutions is not significantly different from the cases using water, chitosan, or humus solutions, with values in the range of 1.11–3.97 mg L−1. For this element, a negative effect of increasing the concentration of the malic acid solution and increasing the stirring time can be appreciated;
- In the case of Cd, an improvement in the process is appreciated with increasing concentration of malic acid solution, but the recorded values (mg L−1) remain subunit. It should be noted, however, that in the case of the use of 3% malic acid solution, the values recorded exceed 0.5 mg L−1, falling within the range 0.67–0.78 mg L−1, values which were not recorded in the previous cases of the use of other extraction solutions, the maximum value being 0.32 mg L−1 in the case of extraction in water with a stirring time of 6 h. Even for the case of using 1% malic acid solution, slightly improved values are recorded, i.e., 0.49 mg L−1 for 2 h stirring time. A similarity with the previous situations can be appreciated in the tendency for reduction of Cd extraction with increasing stirring time in a 1% malic acid solution, as opposed to the positive influence of increasing Cd quantity with increasing stirring time in a 3% solution.
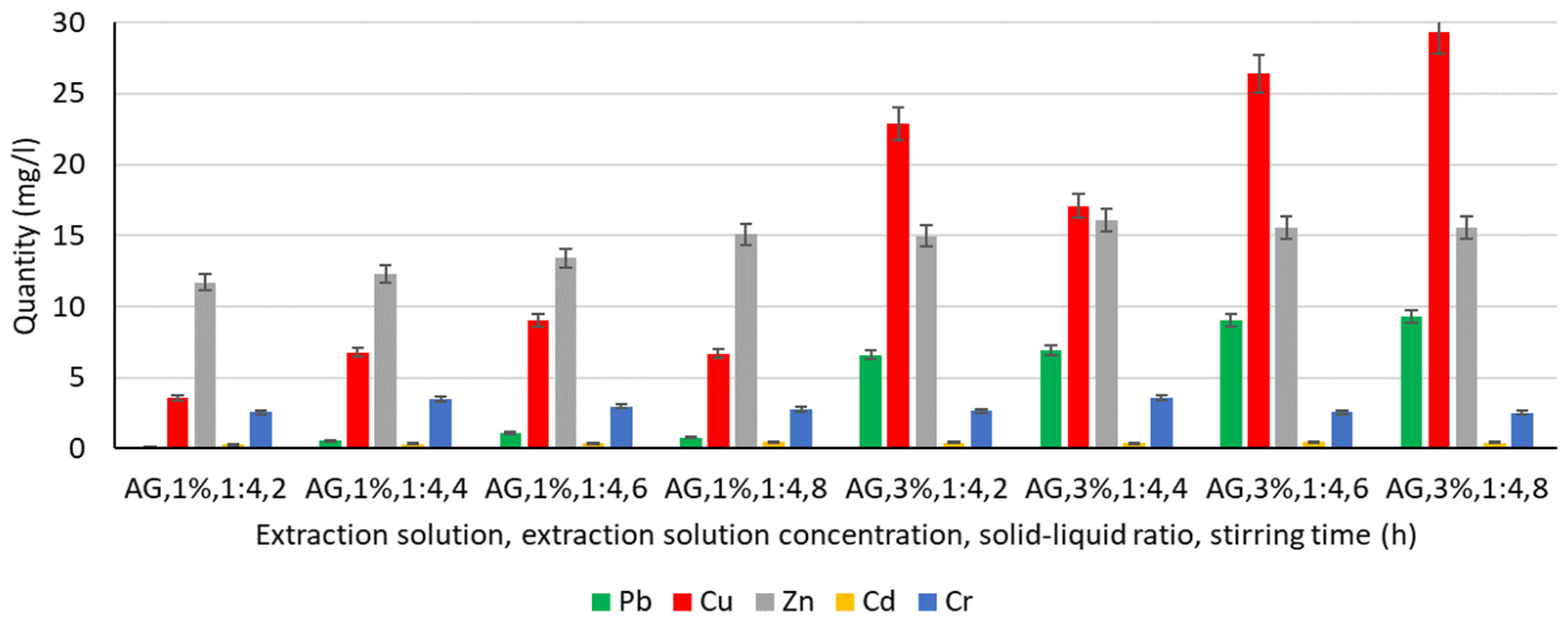
3.4.5. Extraction of Heavy Metals from Gluconic Acid Solution
- The amount of Cu and Zn extracted represents the highest values compared to the other metals analysed, and increasing the concentration of the gluconic acid solution increases the amount of Zn extracted, even by 50%;
- A positive influence is the increase in stirring time in both 1% and 3% gluconic acid solutions. Overall, by analysing the values obtained experimentally after the 3% malic acid solution under conditions of 4–8 h stirring time, gluconic acid solutions are, of all the solutions analysed, the most favourable medium for Zn extraction. So far, the values recorded were 18.03 mg L−1 (malic acid solution 3%, 4 h stirring time) and 17.75 mg L−1, respectively, for the situations using malic acid solution 3%, 6 h, and 8 h stirring time, respectively, in the other solutions (water). In the case of a 3% gluconic acid solution, the values recorded reach and exceed 14.98 mg L−1, increasing with increasing stirring time;
- Except for favourable cases of Pb extraction in malic acid solution and in 3% gluconic acid solution, the values recorded show better extraction compared to the results obtained for water, chitosan, or humus solution, for which subunit values were generally recorded, with a maximum of 1.55 mg L−1 (3% chitosan solution, 4 h stirring time). In a 3% gluconic acid solution, values of 6.61–9.30 mg L−1 are recorded, with a tendency to increase with increasing stirring time;
- A similar behaviour is recorded for Cu: quantitatively, the recorded values, although lower compared to malic acid solution, are higher than for water, chitosan solution, or humus solution. The extraction process is favoured by a higher concentration of gluconic acid solution (3%) resulting in an amount of Cu extracted 2–5 times higher compared to the amount of metal extracted when using a 1% concentration solution. However, it is not possible to assess the continuously favourable or unfavourable influence of increasing the stirring time on the amount of Cu extracted from the soil;
- Quantitatively, Cr and Cd keep approximately similar range limits to those recorded for all the other solutions analysed, the values recorded being subunitary, with a maximum of 0.48 mg L−1 (gluconic acid solution 3%, 6 h stirring time) in the case of Cd and unitary in the case of Cr, with a maximum of 3.58 mg L−1 (gluconic acid solution 3%, 4 h stirring time).
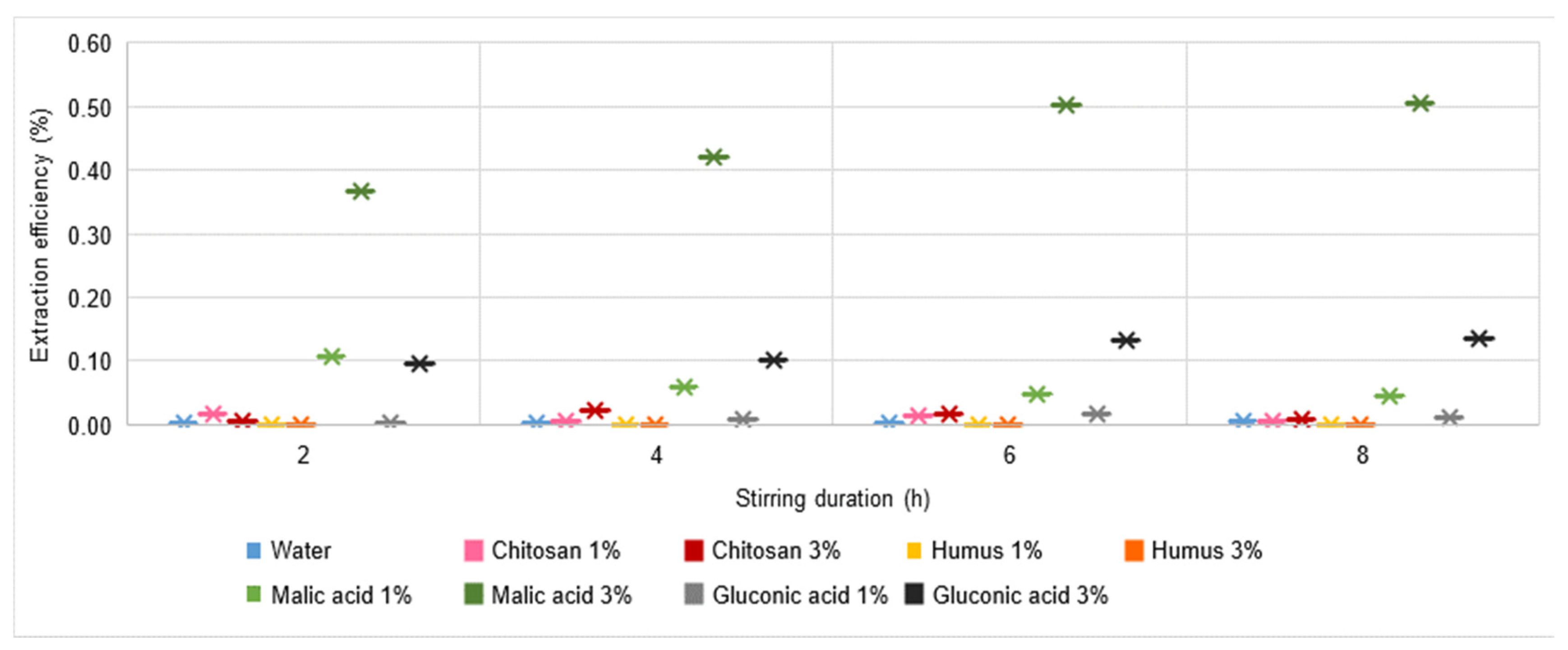
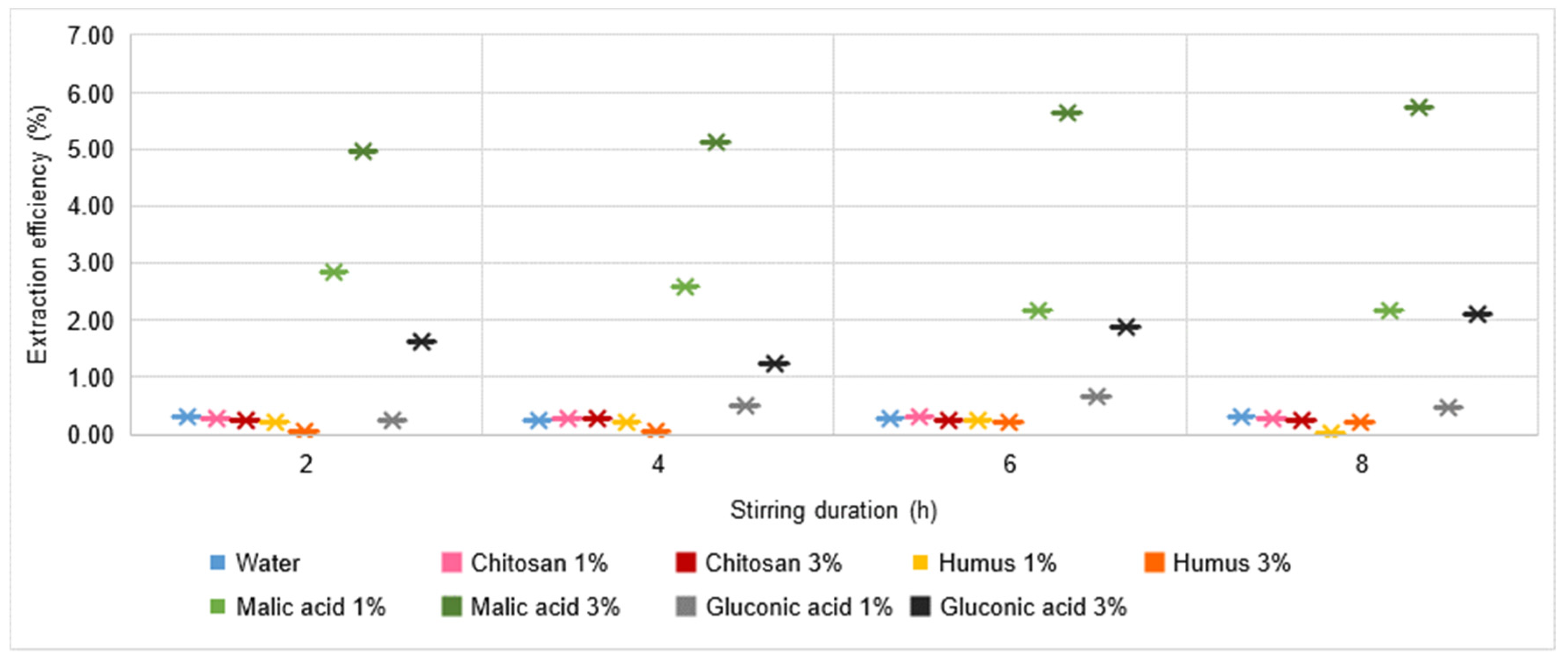
3.5. Extraction Process Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.; Yan, H.; Liu, C.; Li, C.; Li, S.; Gao, H. Heavy metal lead (Pb) removal in contaminated soils using citric acid and malic acid as washing agents. Res. Sq. 2022, 1, 1–24. [Google Scholar] [CrossRef]
- Liu, H.; Chen, P.; Wang, H.; Yang, Y.; Wu, Y. Remediation of Cu-, Zn-, and Pb-Contaminated Soil Using Different Soil Washing Agents: Removal Efficiencies and Mechanisms. Water Air Soil Pollut. 2023, 234, 476. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.X. Modified-biochar adsorbents (MBAs) for heavy-metal ions adsorption: A critical review. J. Environ. Chem. Eng. 2022, 10, 107393. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Z.; Zhao, Y.; Gu, Y.; Wang, Y.; Yu, J.; Xu, H. The performance of biochar-microbe multiple biochemical material on bioremediation and soil micro-ecology in the cadmium aged soil. Sci. Total Environ. 2019, 686, 719–728. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Wei, M.; Zeng, G.; Hou, S.; Li, D.; Xu, H. Elucidation of the mechanisms into effects of organic acids on soil fertility, cadmium speciation and ecotoxicity in contaminated soil. Chemosphere 2020, 239, 124706. [Google Scholar] [CrossRef] [PubMed]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and environmental aspects of slag: A review. J. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Wang, J.P.; Erdenebold, U. A study on reduction of copper smelting slag by carbon for recycling into metal values and cement raw material. Sustainability 2020, 12, 1421. [Google Scholar] [CrossRef]
- Moon, D.H.; Lee, J.-R.; Wazne, M.; Park, J.-H. Assessment of soil washing for Zn contaminated soils using various washing solutions. J. Ind. Eng. Chem. 2012, 18, 822–825. [Google Scholar] [CrossRef]
- Lajayer, B.A.; Ghorbanpour, M.; Nikabadi, S. Heavy metals in a contaminated environmenta: Destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Gorospe, J. Growing Greens and Soiled Soil; San Jose State University: San Jose, CA, USA, 2012. [Google Scholar]
- EEA Progress in Management of Contaminated Sites. Available online: https://www.eea.europa.eu/data-and-maps/indicators/progress-in-management-of-contaminated-sites-3/assessment (accessed on 28 August 2023).
- Order No. 756 of 3 November 1997 for the Approval of the Regulation on Environmental Pollution, Assesment. In Official Gazzate No. 303 Bis of 6 November 1997; Ministry of Waters, Forests and Environmental Protection: Bucharest, Romania, 1997; Available online: https://legislatie.just.ro/Public/DetaliiDocument/13572 (accessed on 28 August 2023). (In Romanian)
- Tangahu, B.V.; Abdullah, S.R.S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- NEPA. Annual Report on the State of the Environment in Romania in 2020; The National Agency for Environmental Protection: Bucharest, Romania, 2020. Available online: www.anpm.ro (accessed on 28 August 2023). (In Romanian)
- Elekes, C.C. Assessment of Historical Heavy Metal Pollution of Land in the Proximity of Industrial Area of Targoviste, Romania, Environmental Risk Assessment of Soil Contamination; Hernandez Soriano, M.C., Ed.; University of Queensland: Brisbane, Australia, 2014. [Google Scholar] [CrossRef]
- Vrînceanu, N.O.; Motelică, D.M.; Dumitru, M.; Toti, M.; Gamenţ, E.; Tănase, V. Aspects Concerning soil Pollution with Heavy Metals in Copşa Mică Area. In Proceedings of the International Conference “Soil under Global Change”, Constanţa, Romania, 3–6 September 2002; pp. 357–365. [Google Scholar]
- Lăcătuşu, R.; Cârstea, S.; Lungu, M.; Kovacsovics, B.; Lazăr, R. Soil pollution with cyanides and heavy metals in the Baia Mare area; ecological reconstruction. Soil Sci. 2002, 36, 77–87. [Google Scholar]
- Sun, Y.; Zhang, P.; Li, Z.; Chen, J.; Ke, Y.; Hu, J.; Liu, B.; Yang, J.; Liang, S.; Su, X.; et al. Iron-calcium reinforced solidification of arsenic alkali residue in geopolymer composite: Wide pH stabilization and its mechanism. Chemosphere 2022, 312, 137063. [Google Scholar] [CrossRef]
- Scanferla, P.; Marcomini, A.; Pellay, R.; Girotto, P.; Zavan, D.; Fabris, M.; Collina, A. Remediation of a Heavy Metals Contaminated Site with a Botanical Garden: Monitoring Results of the Application of an Advanced S/S Technique. Chem. Eng. Trans. 2012, 28, 235–240. [Google Scholar]
- Sun, L.; Wu, Q.; Liao, K.; Yu, P.; Cui, Q.; Rui, Q.; Wang, D. Contribution of heavy metals to toxicity of coal combustion related fine particulate matter (PM2.5) in Caenorhabditis elegans with wild-type or susceptible genetic background. Chemosphere 2016, 144, 2392–2400. [Google Scholar] [CrossRef]
- Sinha, S.; Mishra, R.K.; Sinam, G.; Mallick, S.; Gupta, A.K. Comparative Evaluation of Metal Phytoremediation Potential of Trees, Grasses, and Flowering Plants from Tannery-Wastewater-Contaminated Soil in Relation with Physicochemical Properties. Soil Sediment Contam. Int. J. 2013, 22, 958–983. [Google Scholar] [CrossRef]
- Bahemmat, M.; Farahbakhsh, M.; Kianirad, M. Humic substances-enhanced electroremediation of heavy metals contaminated soil. J. Hazard. Mater. 2016, 312, 307–318. [Google Scholar] [CrossRef]
- Golia, E.E.; Angelaki, A.; Giannoulis, K.D.; Skoufogianni, E.; Bartzialis, D.; Cavalaris, C.; Vleiora, S. Evaluation of soil properties, irrigation and solid waste application levels on Cu and Zn uptake by industrial hemp. Agron. Res. 2021, 19, 92–99. [Google Scholar]
- Soleimani, M.; Hajabbasi, M.A.; Afyuni, M.; Akbar, S. Comparison of Natural Humic Substances and Synthetic Ethylenediaminetetraacetic Acid and Nitrilotriacetic Acid as Washing Agents of a Heavy Metal–Polluted Soil. J. Environ. Qual. 2010, 39, 855–862. [Google Scholar] [CrossRef]
- Lestan, D.; Luo, C.; Li, X. The use of chelating agents in the remediation of metal-contaminated soils: A review. Environ. Pollut. 2008, 153, 3–13. [Google Scholar] [CrossRef]
- Marchiol, L.; Assolari, S.; Sacco, P.; Zerbi, G. Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ. Pollut. 2004, 132, 21–27. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.P.S. Heavy metal toxicity in soils: Sources, remediation technologies and challenges. Adv. Plants Agric. Res. 2016, 5, 445–446. [Google Scholar]
- Gong, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef]
- Chen, X.; Achal, V. Biostimulation of carbonate precipitation process in soil for copper immobilization. J. Hazard. Mater. 2019, 368, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Gusiatin, Z.M. Novel and Eco-Friendly Washing Agents to Remove Heavy Metals from Soil by Soil Washing. Environ. Anal. Ecol. Stud. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Kim, M.-S.; Koo, N.; Kim, J.-G.; Lee, S.-H. Effects of Washing Solution, Washing Time, and Solid-Solution Rate on the Maximum Heavy Metals Removal Efficiency. Appl. Sci. 2021, 11, 6398. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Hansen, H.C.B.; Holm, P.E.; Jensen, J.K.; Rasmussen, S.B.; Sabiene, N.; Steponkaite, L.; Strobel, B.W. Experimental assessment of using soluble humic substances for remediation of heavy metal polluted soils. Soil Sediment Contam. 2009, 18, 369–382. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Holm, P.E.; Jensen, J.K.; Soleimani, M.; Strobel, B.W. Cleaning heavy metal contaminated soil with soluble humic substances instead of synthetic polycarboxylic acids. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2011, 61, 577. [Google Scholar]
- Borggaard, O.K.; Holm, P.E.; Strobel, B.W. Potential of dissolved organic matter (DOM) to extract As, Cd, Co, Cr, Cu, Ni, Pb and Zn from polluted soils: A review. Geoderma 2019, 343, 235–246. [Google Scholar] [CrossRef]
- Yang, T.; Hodson, M.E. Investigating the use of synthetic humic-like acid as a soil washing treatment for metal contaminated soil. Sci. Total Environ. 2019, 647, 290. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lin, Q.; Wang, Y.; Luo, H.; Huang, Z.; Fu, H.; Chen, H.; Xiao, R. The removal of Cu, Ni, and Zn in industrial soil by washing with EDTA-organic acids. Arab. J. Chem. 2020, 13, 5160–5170. [Google Scholar] [CrossRef]
- Race, M.; Marotta, R.; Fabbricino, M.; Pirozzi, F.; Andreozzi, R.; Cortese, L.; Giudicianni, P. Copper and zinc removal from contaminated soils through soil washing process using ethylenediaminedisuccinic acid as a chelating agent: A modeling investigation. J. Environ. Chem. Eng. 2016, 4, 2878–2891. [Google Scholar] [CrossRef]
- Mohanty, B.; Mahindrakar, A.B. Removal of Heavy Metal by Screening Followed by Soil Washing from Contaminated Soil. IJTES 2011, 2, 290–293. [Google Scholar]
- Hydari, S.; Sharififard, H.; Nabavinia, M.; Reza Parvizi, M. A comparative investigation on removal performances of commercial activated carbon, chitosan biosorbent and chitosan/activated carbon composite for cadmium. Chem. Eng. J. 2012, 193, 276–282. [Google Scholar] [CrossRef]
- Yi, N.; Wu, Y.; Fan, L.; Hu, S. Remediating Cd-Contaminated Soils Using Natural and Chitosan-Introduced Zeolite, Bentonite, and Activated Carbon. Pol. J. Environ. Stud. 2019, 28, 1461–1468. [Google Scholar] [CrossRef]
- Desbrieres, J.; Guibal, E. Sorption Processes and Pollution: Conventional and Non-Conventional Sorbents for Pollutant Removal from Wastemasters; Presses Universitaires de Franche-Comté: Besançon, France, 2011. [Google Scholar]
- Mahaweero, T. Extraction of Heavy Metals from Aqueous Solutions Using Chitosan/Montmorillonite Hybrid Hydrogels. Master’s Thesis, Department of Chemical Engineering, Case Western Reserve University, Cleveland, OH, USA, 2013. [Google Scholar]
- Sewvandi, G.A.; Adikary, S.U. Removal of Heavy Metals from Wastewater Using Chitosan. Soc. Soc. Manag. Syst. Internet J. 2011, 1, 123–128. [Google Scholar]
- Perminova, I.V.; Hatfield, K. Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice; Springer: Dordrecht, The Netherlands, 2005; pp. 3–36. [Google Scholar]
- Boguta, P.; Sokolowska, Z. Interactions of humic acids with metals. Acta Agrophys. 2013, 2, 1–113. [Google Scholar]
- Rahman, M.A.; Hasan, M.A.; Rahim, A.; Alam, A.M.S. Characterization of Humic Acid from the River Bottom Sediments of Burigonga: Complexation Studies of Metals with Humic Acid. Pak. J. Anal. Environ. Chem. 2010, 11, 42–52. [Google Scholar]
- Pehlivan, E.; Arslan, G. Uptake of Metal Ions on Humic Acids. Energy Sources A Recovery Util. Environ. Eff. 2006, 28, 1099–1112. [Google Scholar] [CrossRef]
- Alberts, J.J.; Filip, Z. Metal binding in estuarine humic and fulvic acids: FTIR analysis of humic acids—Metal complexes. Environ. Technol. 1998, 19, 923–931. [Google Scholar] [CrossRef]
- Tang, H.; Shuai, W.; Wang, X.; Liu, Y. Extraction of rare earth elements from a contaminated cropland soil using nitric acid, citric acid, and EDTA. Environ. Technol. 2017, 38, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Jelusic, M.; Lestan, D. Effect of EDTA washing of metal polluted garden soils. Part I: Toxicity hazards and impact on soil properties. Sci. Total Environ. 2014, 475, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Jeon, E.K.; Baek, K. Role of reducing agent in extraction of arsenic and heavy metals from soils by use of EDTA. Chemosphere 2016, 152, 274–283. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Kulikowska, D.; Klik, B. New-generation washing agents in remediation of metal-polluted soils and methods for washing effluent treatment: A review. Int. J. Environ. Res. Public Health 2020, 17, 6220. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, S.; Wang, G.; Li, T.; Xu, X.; Deng, O.; Zhang, Y.; Pu, Y. Enhancing the soil heavy metals removal efficiency by adding HPMA and PBTCA along with plant washing agents. J. Hazard. Mater. 2017, 339, 33–42. [Google Scholar] [CrossRef]
- Zheng, X.-J.; Li, Q.; Peng, H.; Zhang, J.-X.; Chen, W.-J.; Zhou, B.-C.; Chen, M. Remediation of Heavy Metal-Contaminated Soils with SoilWashing: A Review. Sustainability 2022, 14, 13058. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, G.; Zhang, G.; He, Q.; Wei, Z.; Zheng, W.; Qian, T.; Wu, Q. Effect of mixed chelators of EDTA, GLDA, and citric acid on bioavailability of residual heavy metals in soils and soil properties. Chemosphere 2018, 209, 776–782. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Kanyerere, T.; Wang, Y.-S.; Sun, M. Washing Reagents for Remediating Heavy-Metal-Contaminated Soil: A Review. Front. Earth Sci. 2022, 10, 901570. [Google Scholar] [CrossRef]
- Ubner, M. Interaction of Humic Substances with Metal Cations; Tallinn University of Technology, TUT Press: Tallinn, Estonia, 2004. [Google Scholar]
- Gusiatin, Z.M.; Klimiuk, E. Metal (Cu, Cd and Zn) removal and stabilization during multiple soil washing by saponin. Chemosphere 2012, 86, 383–391. [Google Scholar] [CrossRef]
- Huang, G.Y.; You, J.W.; Zhou, X.P.; Ren, C.; Islam, M.S.; Hu, H.Q. Effects of low molecular weight organic acids on Cu accumulation by castor bean and soil enzyme activities. Ecotoxicol. Environ. Saf. 2020, 203, 110983. [Google Scholar] [CrossRef]
- Gomez-Garrido, M.; Navarro, J.M.; Navarro, F.J.M.; Cano, A.F. The chelating effect of citric acid, oxalic acid, amino acids and Pseudomonas fluorescens bacteria on phytoremediation of Cu, Zn, and Cr from soil using Suaeda vera. Int. J. Phytoremediat. 2018, 20, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.M.; Lu, H.F.; Zhang, X.X. Change in phytoextraction of Cd by rapeseed (Brassica napus L.) with application rate of organic acids and the impact of Cd migration from bulk soil to the rhizosphere. Environ. Pollut. 2020, 267, 115452. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Luo, T.; Zhong, S.; Zhou, F.; Zhang, Y.; Ma, Y.; Fu, Q. Long-term effects of low-molecular-weight organic acids on remobilization of Cd, Cr, Pb, and As in alkaline coastal wetland soil. Environ. Pollut. 2021, 33, 266–277. [Google Scholar] [CrossRef]
- Han, R.; Dai, H.; Skuza, L.; Wei, S. Comparative study on different organic acids for promoting Solanum nigrum L. hyperaccumulation of Cd and Pb from the contaminated soil. Chemosphere 2021, 278, 130446. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, H.X.; Gong, Z.Q.; Liu, S.; Yang, Y.T. Washing efficiency of Cd from contaminated lou soil by saponin and low-molecular-weight organic acids. J. Northwest A F Univ.-Nat. Sci. Ed. 2018, 46, 85–93. [Google Scholar]
- Zhong, L.; Yang, J. Reduction of Cr(VI) by Malic Acid in Aqueous Fe-Rich Soil Suspensions. Chemosphere 2011, 86, 973. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, F.J.; Zhou, Y. Removal of Cd, Pb, Zn, Cu in smelter soil by citric acid leaching. Chemosphere 2020, 255, 126690. [Google Scholar] [CrossRef]
- Jiang, H.; Li, T.; Han, X.; Yang, X.; He, Z. Effects of pH and low molecular weight organic acids on competitive adsorption and desorption of cadmium and lead in paddy soils. Environ Monit. 2012, 184, 6325–6335. [Google Scholar] [CrossRef]
- Parimal, P.; Ramesh, K.; Subhamay, B. Manufacture of gluconic acid: A review towards process intensification for green production. Chem. Eng. Process 2016, 104, 160–171. [Google Scholar]
- Scheglova, N.V.; Popova, T.V.; Druzhinina, A.V.; Smotrina, T.V. Spectrophotometric study of complexation of cobalt (II) with HEDP in aqueous solutions. J. Mol. Liq. 2019, 286, 110909. [Google Scholar] [CrossRef]
- Fischer, K.; Bipp, H.-P. Removal of Heavy Metals from Soil Components and Soils by Natural Chelating Agents. Part II. Soil Extraction by Sugar Acids. Water Air Soil Pollut. 2002, 138, 271–288. [Google Scholar] [CrossRef]
- Arafat, A.; Sabrin, A.S.; Shah, M.M.; Mohammad, M. Preparation and Characterization of Chitosan from Shrimp shell waste. Int. J. Sci. Eng. Res. 2015, 6, 538–541. [Google Scholar]
- Agarwal, R.M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar]
- Kamari, A.; Pulford, I.D.; Hargreaves, J.S.J. Chitosan-assisted Phytoextraction of Heavy Metal from Lead/Zinc Tailings Using Lolium Perenne—A Preliminary Study. In Proceedings of the 15th International Conference on Heavy Metals in the Environment, Gdansk, Poland, 19–23 September 2010. [Google Scholar]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Lu, C.; Xu, Z.; Dong, B.; Zhang, Y.; Wang, M.; Zeng, Y.; Zhang, C. Machine learning for the prediction of heavy metal removal by chitosan-based flocculants. Carbohydr. Polym. 2022, 285, 119240. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Du, H.; Yang, Z.; Tian, Z.; Shen, S.; Shi, Y.; Zhang, L. Flocculation of different types of combined contaminants of antibiotics and heavy metals by thermo-responsive flocculants with various architectures. Sep. Purif. Technol. 2019, 223, 123–132. [Google Scholar] [CrossRef]
- Herath, B.G.; Crisler, A.; Bridges, G.; Patel, D.; Pittman, S.; Todd, C.M. Cadmium and Copper Removal from Aqueous Solutions Using Chitosan-Coated Gasifier Biochar. Front. Environ. Sci. 2020, 8, 541203. [Google Scholar]
- Wan, M.W.; Petrisor, I.G.; Lai, H.T.; Kim, D.; Yen, T.F. Copper adsorption through chitosan immobilized on sand to demonstrate the feasibility for in situ soil decontamination. Carbohydr. Polym. 2004, 55, 249–254. [Google Scholar] [CrossRef]
- Sur, I.M.; Micle, V.; Polyak, E.T.; Gabor, T. Assessment of Soil Quality Status and the Ecological Risk in the Baia Mare, Romania Area. Sustainability 2022, 14, 3739. [Google Scholar] [CrossRef]
- Damian, G.; Damian, F.; Nasui, D.; Pop, C.; Pricop, C. The soils quality from the southern–eastern part of Baia Mare zone affected by metallurgical industry. Carpathian J. Earth Environ. Sci. 2010, 5, 139–147. [Google Scholar]
- Aydinalp, C.; Marinova, S. Distribution and forms of heavy metals in some agricultural soils. Pol. J. Environ. Stud. 2003, 12, 629–633. [Google Scholar]
- STAS 7184/1-84; Soils. Sample Collection for Soil and Agrochemical Studies. ASRO: Bucharest, Romania, 1984. (In Romanian)
- SR ISO 11464:1998; Soil Quality. Pretreatment of Samples for Psysico-Chemical Analysis. ASRO: Bucharest, Romania, 1998. (In Romanian)
- STAS 7184/13-88; Soils. Determination of pH. ASRO: Bucharest, Romania, 1988. (In Romanian)
- SR ISO 10390:1999; Soil Quality. Determination of pH. ASRO: Bucharest, Romania, 1999. (In Romanian)
- Rusu, T.; Paulette, L.; Cacoveanu, H.; Turcu, V. Physics, Hydrophysics, Environmental Chemistry and Soil Respiration, Research Methods; Risoprint: Cluj-Napoca, Romania, 2007; pp. 1–189. (In Romanian) [Google Scholar]
- Dumitru, M.; Manea, A.; Ciobanu, C.; Dumitru, S.; Vrînceanu, N.; Calciu, I.; Tănase, V.; Preda, M.; Rîşnoveanu, I.; Mocanu, V.; et al. Soil Quality Monitoring in Romania; Sitech: Craiova, Romania, 2011; pp. 1–82. (In Romanian) [Google Scholar]
- Technical Agricultural Propaganda Editorial Office. Methodology of Soil Studies. Volumes I–III; Technical Agricultural Propaganda Editorial Office: Bucharest, Romania, 1987. (In Romanian) [Google Scholar]
- Ştefan, D.S.; Onose, C.; Apostol, D.G.; Bobirică, C. Environmental Quality Control—Practical Laboratory Work; Cartea Universitară: Bucharest, Romania, 2003. (In Romanian) [Google Scholar]
- Bumbu, I.; Bumbu, I.; Vîrlan, L. Environmental Control and Monitoring. Laboratory and Practical Course; Universitatea Tehnică A Moldovei: Chişinău, Moldova, 2006. (In Romanian) [Google Scholar]
- STAS 7148/6-78; Determination of Hygroscopicity Index. ASRO: Bucharest, Romania, 1978. (In Romanian)
- STAS 7184/21-82; Soils. Determination of Organic Carbon and Humus. ASRO: Bucharest, Romania, 1982. (In Romanian)
- de Oliveira, E. Sample preparation for atomic spectroscopy: Evolution and future trends. J. Braz. Chem. Soc. 2003, 14, 174–182. [Google Scholar] [CrossRef]
- STAS 7184/2-85; Soils. Determination of Nitrogen Content. ASRO: Bucharest, Romania, 1985. (In Romanian)
- SR ISO 11261/2001; Soil Quality. Determination of Nitrogen Content. ASRO: Bucharest, Romania, 2001. (In Romanian)
- STAS 7184/19-82; Soils. Determination of Phosphorus. ASRO: Bucharest, Romania, 1982. (In Romanian)
- STAS 7184/12-79; Soils. Determination of Cation Exchange Properties. ASRO: Bucharest, Romania, 1979. (In Romanian)
- Couillard, D.; Chartier, M.; Mercier, G. Étude de l’enlèvement du Cd, Cu, Mn et Zn par solubilisation biologique dans les sédiments lacustres fortement contaminés. J. Water Sci. 1994, 7, 251–268. [Google Scholar] [CrossRef][Green Version]
- Côme, B.; Barres-Lakkemand, A.; Ricour, J.; Martin, S. Applications comparatives de méthodes d’ évaluation de risques liés aux sites pollués: Premiers enseignements et perspectives. TSM 1993, 9, 447–451. [Google Scholar]
- Damian, F.; Damian, G. Detoxification of Heavy Metal Contaminated Soils. Am. J. Environ. Sci. 2007, 3, 193–198. [Google Scholar] [CrossRef]
- Luduşan, N. Efectele Acumulării Metalelor Grele în Soluri Asupra Componentei Biotice Din Depresiunea Zlatna. 2007. Available online: https://www.uab.ro/reviste_recunoscute/revcad/revcad_2007/27.ludusan_nicolae.pdf (accessed on 28 August 2023). (In Romanian).
- Damian, F.; Damian, G.; Lacătusu, R.; Macovei, G.; Iepure, G.; Năprădean, I.; Chira, R.; Kollar, L.; Rată, L.; Zaharia, C. Soils from the Baia Mare zone and the heavy metals pollution. Carpathian J. Earth Environ. Sci. 2008, 3, 85–98. [Google Scholar]
- Jiang, W.; Tao, T.; Liao, Z. Removal of Heavy Metal from Contaminated Soil with Chelating Agents. J. Soil Sci. 2011, 1, 70–76. [Google Scholar] [CrossRef]
- Hu, W.; Niu, Y.; Zhu, H.; Dong, K.; Wang, D.; Liu, F. Remediation of zinc-contaminated soils by using the two-step washing with citric acid and water-soluble chitosan. Chemosphere 2021, 282, 131092. [Google Scholar] [CrossRef]
- Wen, J.; Xing, L.; Wang, Y. Chemical and microbiological responses of heavy metal contaminated sediment subject to washing using humic substances. Environ. Sci. Pollut. Res. 2019, 26, 26696–26705. [Google Scholar] [CrossRef]
- Kulikowska, D.; Gusiatin, Z.M.; Bułkowska, K.; Kierklo, K. Humic substances from sewage sludge compost as washing agent effectively remove Cu and Cd from soil. Chemosphere 2015, 136, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, M.; Zhang, P.; Pan, Y.; Huang, Z.; Xiao, H. Remediation of Cd (II) ions in aqueous and soil phases using novel porous cellulose/chitosan composite spheres loaded with zero-valent iron nanoparticles. React. Funct. Polym. 2022, 173, 105210. [Google Scholar] [CrossRef]
- Xiang, J.; Lin, Q.; Yao, X.; Yin, G. Removal of Cd from aqueous solution by chitosan coated MgO-biochar and its in-situ remediation of Cd-contaminated soil. Environ. Res. 2021, 195, 110650. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Choppala, G.; Singh, R.S. Evaluation of modified chitosan for remediation of zinc contaminated soils. J. Geochem. Explor. 2017, 182, 180–184. [Google Scholar] [CrossRef]
- Wang, L.; Wei, J.; Yang, L.; Chen, Y.; Wang, M.; Xiao, L.; Yuan, G. Enhancing Soil Remediation of Cop-per-Contaminated Soil through Washing with a Soluble Humic Substance and Chemical Reductant. Agronomy 2023, 13, 1754. [Google Scholar] [CrossRef]


| Heavy Metals | Normal Value | Soil Type | |||
|---|---|---|---|---|---|
| Sensitive Soils | Less Sensitive Soils | ||||
| Alert Threshold | Intervention Threshold | Alert Threshold | Intervention Threshold | ||
| Pb | 20 | 50 | 100 | 250 | 1000 |
| Cu | 20 | 100 | 200 | 250 | 500 |
| Zn | 100 | 300 | 600 | 700 | 1500 |
| Cd | 1 | 3 | 5 | 5 | 10 |
| Cr | 30 | 100 | 300 | 300 | 600 |
| Extraction Solution | Extraction Solution Concentration | Solid/Liquid Ratio (S/L) | Stirring Time [h] | |||
|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | |||
| Water (A) | - | 1:4 | A, 1:4, 2 | A, 1:4, 4 | A, 1:4, 6 | A, 1:4, 8 |
| Chitosan (C) | 1% | 1:4 | C, 1%, 1:4, 2 | C, 1%, 1:4, 4 | C, 1%, 1:4, 6 | C, 1%, 1:4, 8 |
| 3% | 1:4 | C, 3%, 1:4, 2 | C, 3%, 1:4, 4 | C, 3%, 1:4, 6 | C, 3%, 1:4, 8 | |
| Humus (H) | 1% | 1:4 | H, 1%, 1:4, 2 | H, 1%, 1:4, 4 | H, 1%, 1:4, 6 | H, 1%, 1:4, 8 |
| 3% | 1:4 | H, 3%, 1:4, 2 | H, 3%, 1:4, 4 | H, 3%, 1:4, 6 | H, 3%, 1:4, 8 | |
| Malic acid (AM) | 1% | 1:4 | AM, 1%, 1:4, 2 | AM, 1%, 1:4, 4 | AM, 1%, 1:4, 6 | AM, 1%, 1:4, 8 |
| 3% | 1:4 | AM, 3%, 1:4, 2 | AM, 3%, 1:4, 4 | AM, 3%, 1:4, 6 | AM, 3%, 1:4, 8 | |
| Gluconic acid (AG) | 1% | 1:4 | AG, 1%, 1:4, 2 | AG, 1%, 1:4, 4 | AG, 1%, 1:4, 6 | AG, 1%, 1:4, 8 |
| 3% | 1:4 | AG, 3%, 1:4, 2 | AG, 3%, 1:4, 4 | AG, 3%, 1:4, 6 | AG, 3%, 1:4, 8 | |
| Soil Properties | Value | |
|---|---|---|
| pH | 4.67 | |
| Texture | coarse loamy-sand (UG) | |
| Hygroscopicity coefficient (CH) | 4.8 [% g/g] | |
| Content of hydrostable macrostructural elements (HA) | 9 [% g/g] | |
| Content of hydrostable microstructural elements (D) | 17 [% g/g] | |
| Structural Instability Index (SI) | 1.95 [-] | |
| Corg | 1.23 [%] | |
| Humus | 2.12 [%] | |
| Micronutrients | Nt | 0.107 [%] |
| PAL | 6 [mg kg−1] | |
| KAL | 26 [mg kg−1] | |
| Ca2+ | Mg2+ | K+ | Na+ | SB | H+ | T (SB + SH) | Ca2+ | Mg2+ | K+ | Na+ | H+ | V | Total Soluble Salt Content |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| me/100 g soil | wt% from T | mg/100 g soil | |||||||||||
| 1.29 | 0.20 | 0.13 | 0.01 | 1.63 | 9.07 | 10.70 | 12.1 | 1.8 | 1.2 | 0.1 | 84.8 | 15.2 | 10.70 |
| Heavy Metals | Pb | Cu | Zn | Cd | Cr |
|---|---|---|---|---|---|
| Measured value [mg kg−1] | 27,660 | 5590 | 2199 | 11.68 | 146 |
| How many times does the measured value exceed the normal value | 1383 | 279.5 | 21.99 | 11.68 | 5.33 |
| How many times does the measured value exceed the alert threshold for less sensitive soils | 110.6 | 22.36 | 3.14 | 2.33 | * |
| How many times does the measured value exceed the intervention threshold for less sensitive soils | 27.66 | 11.18 | 1.46 | 1.16 | * |
| Extraction Solution | Water | Chitosan 1% | Chitosan 3% | Humus 1% | Humus 3% | Malic Acid 1% | Malic Acid 3% | Gluconic Acid 1% | Gluconic Acid 3% |
|---|---|---|---|---|---|---|---|---|---|
| pH | 6.8 ± 0.1 | 4.9 ± 0.1 | 5.2 ± 0.1 | 5.3 ± 0.1 | 5.8 ± 0.1 | 3.8 ± 0.1 | 3.4 ± 0.1 | 4.8 ± 0.1 | 4.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sur, I.M.; Hegyi, A.; Micle, V.; Gabor, T.; Lăzărescu, A.-V. Influence of the Extraction Solution on the Removal of Heavy Metals from Polluted Soils. Materials 2023, 16, 6189. https://doi.org/10.3390/ma16186189
Sur IM, Hegyi A, Micle V, Gabor T, Lăzărescu A-V. Influence of the Extraction Solution on the Removal of Heavy Metals from Polluted Soils. Materials. 2023; 16(18):6189. https://doi.org/10.3390/ma16186189
Chicago/Turabian StyleSur, Ioana Monica, Andreea Hegyi, Valer Micle, Timea Gabor, and Adrian-Victor Lăzărescu. 2023. "Influence of the Extraction Solution on the Removal of Heavy Metals from Polluted Soils" Materials 16, no. 18: 6189. https://doi.org/10.3390/ma16186189
APA StyleSur, I. M., Hegyi, A., Micle, V., Gabor, T., & Lăzărescu, A.-V. (2023). Influence of the Extraction Solution on the Removal of Heavy Metals from Polluted Soils. Materials, 16(18), 6189. https://doi.org/10.3390/ma16186189