Research Progress of Natural Products and Their Derivatives in Marine Antifouling
Abstract
:1. Introduction
2. Compounds from Marine Organisms
2.1. Compounds from Algae
2.2. Compounds from Marine Invertebrates
2.3. Bacterial and Fungal
3. Compounds from Terrestrial Plants
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Agostini, V.O.; Martinez, S.T.; Muxagata, E.; Macedo, A.J.; Pinho, G.L.L. Antifouling activity of isonitrosoacetanilides against microfouling and macrofouling. Environ. Sci. Pollut. Res. Int. 2023, 30, 26435–26444. [Google Scholar] [PubMed]
- Subbaiyan, R.; Ganesan, A.; Dhanuskodi, S. Scientific investigation of antifouling activity from biological agents and distribution of marine foulers—Coastal Areas of Tamil Nadu. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Cao, P.; Liu, D.; Liu, Y.; Wang, H.; Zhang, C.; Yuan, C.; Liu, X. Marine antifouling behavior of the surfaces modified by dopamine and antibacterial peptide. J. Oceanol. Limnol. 2022, 41, 174–188. [Google Scholar]
- Li, Y.; Liao, J.; Zhang, H.; Tang, X.; Zhong, S.; Li, Q. Acrylate boron silane polymer/carbon nitride–titanium dioxide composite coatings with enhancing photocatalytic antifouling performance under visible light. J. Coat. Technol. Res. 2023, 20, 1445–1458. [Google Scholar]
- Sarkar, P.K.; Pawar, S.S.; Rath, S.K.; Kandasubramanian, B. Anti-barnacle biofouling coatings for the protection of marine vessels: Synthesis and progress. Environ. Sci. Pollut. Res. Int. 2022, 29, 26078–26112. [Google Scholar]
- Evans, S.M.; Leksono, T.; McKinnell, P.D. Tributyltin pollution: A diminishing problem following legislation limiting the use of TBT-based anti-fouling paints. Mar. Pollut. Bull. 1995, 30, 14–21. [Google Scholar]
- Al-Lihaibi, S.S.; Abdel-Lateff, A.; Alarif, W.M.; Alorfi, H.S.; Nogata, Y.; Okino, T. Environmentally friendly antifouling metabolites from Red Sea organisms. J. Chem. 2019, 2019, 3278394. [Google Scholar]
- Khanam, M.R.M.; Shimasaki, Y.; Hosain, M.Z.; Chairil, A.E.; Mukai, K.; Wang, P.; Tsuyama, M.; Qiu, X.; Oshima, Y. Effects of the antifouling agent tributyltin on the sinking behavior, photosynthetic rate and biochemical composition of the marine planktonic diatom Thalassiosira pseudonana. Ecotoxicology 2022, 31, 1158–1168. [Google Scholar]
- Zhao, W.; Wang, X. Antifouling based on biocides: From toxic to green. In Antifouling Surfaces and Materials; Zhou, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 105–134. [Google Scholar]
- Vilas-Boas, C.; Silva, E.R.; Resende, D.; Pereira, B.; Sousa, G.; Pinto, M.; Almeida, J.R.; Correia-da-Silva, M.; Sousa, E. 3,4-Dioxygenated xanthones as antifouling additives for marine coatings: In silico studies, seawater solubility, degradability, leaching, and antifouling performance. Environ. Sci. Pollut. Res. Int. 2023, 30, 68987–68997. [Google Scholar]
- Unver, B.; Evingur, G.A.; Cavas, L. Effects of currently used marine antifouling paint biocides on green fluorescent proteins in Anemonia viridis. J. Fluoresc. 2022, 32, 2087–2096. [Google Scholar]
- Tadros, H.R.Z.; Elkady, E.M.; Saleh, S.M. Comparative study for marine antifouling agents based on natural sarcophine product and ZnO nanomaterials. Egypt. J. Aquat. Res. 2021, 47, 191–197. [Google Scholar]
- Omae, I. General aspects of tin-free antifouling paints. Chem. Rev. 2003, 103, 3431–3448. [Google Scholar] [PubMed]
- Vignesh, V.; Stafslien, S.; Evans, M.; Wise, K.; Marmo, A.; Tonks, M.; Brennan, A. Comparative analysis of two isocyanate-free urethane-based gels for antifouling applications. Biofouling 2021, 37, 131–144. [Google Scholar] [PubMed]
- Fusetani, N. Antifouling marine natural products. Nat. Prod. Rep. 2011, 28, 400–410. [Google Scholar] [PubMed]
- Ajay Krishna, M.S.; Mohan, S.; Ashitha, K.T.; Chandramouli, M.; Kumaran, A.; Ningaiah, S.; Babu, K.S.; Somappa, S.B. Marine based natural products: Exploring the recent developments in the identification of antimicrobial agents. Chem. Biodivers. 2022, 19, e202200513. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Martinelli, E.; Guazzelli, E.; Cuccaro, A.; De Marchi, L.; Fumagalli, G.; Monni, G.; Vasarri, M.; Degl’Innocenti, D.; Pretti, C. Posidonia oceanica (L.) (Delile, 1813) extracts as a potential booster biocide in fouling-release coatings. Environ. Sci. Pollut. Res. Int. 2023, 30, 18480–18490. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Jiang, X.; Yu, L. Preparation and properties of environmentally friendly marine antifouling coatings based on a collaborative strategy. Langmuir 2022, 38, 6676–6689. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, X.; Yu, L. Performance assessment of capsaicin derivatives containing amide groups used as active substances for antifouling coatings. Prog. Org. Coat. 2021, 160, 106515. [Google Scholar]
- Liu, H.; Yang, W.; Zhao, W.; Zhang, J.; Cai, M.; Pei, X.; Zhou, F. Natural product inspired environmentally friendly strategy based on dopamine chemistry toward sustainable marine antifouling. ACS Omega 2020, 5, 21524–21530. [Google Scholar] [CrossRef]
- Tian, L.; Wang, J.; Yin, Y.; Bing, W.; Du, W.; Jin, H. Coffee/polydimethylsiloxane composite coating for preventing marine biofouling. J. Coat. Technol. Res. 2022, 20, 949–955. [Google Scholar] [CrossRef]
- Kamada, T.; Fukada, R.; Yamagishi, Y.; Nagasaka, M.; Osada, D.; Nimura, K.; Oshima, I.; Tsujimoto, K.; Kirihara, M.; Takizawa, S.; et al. Antifouling brominated diterpenoids from Japanese Marine Red alga Laurencia venusta Yamada. Chem. Biodivers. 2023, 20, e202300888. [Google Scholar]
- Chualáin, F.N.; Maggs, C.A.; Saunders, G.W.; Guiry, M.D. The invasive genus Asparagopsis (Bonnemaisoniaceae, Rhodophyta): Molecular systematics, morphology, and ecophysiology of Falkenbergia isolates. J. Phycol. 2004, 40, 1112–1126. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Silva, J.; Pedrosa, R. The marine invasive seaweeds Asparagopsis armata and Sargassum muticum as targets for greener antifouling solutions. Sci. Total Environ. 2021, 750, 141372. [Google Scholar] [CrossRef] [PubMed]
- Vasarri, M.; De Biasi, A.M.; Barletta, E.; Pretti, C.; Degl’Innocenti, D. An overview of new insights into the benefits of the seagrass Posidonia oceanica for human Health. Mar. Drugs 2021, 19, 476. [Google Scholar] [CrossRef]
- Muhring-Salamone, S.; Wanka, R.; Rosenhahn, A. Low fouling marine coatings based on nitric oxide-releasing polysaccharide-based hybrid materials. ACS Sustain. Chem. Eng. 2023, 11, 8858–8869. [Google Scholar] [CrossRef]
- Abdelsalam, K.M.; Shaltout, N.A.; Ibrahim, H.A.; Tadros, H.R.Z.; Aly-Eldeen, M.A.-E.; Beltagy, E.A. A comparative study of biosynthesized marine natural-product nanoparticles as antifouling biocides. Oceanologia 2022, 64, 35–49. [Google Scholar] [CrossRef]
- Prieto, I.M.; Paola, A.; Perez, M.; Garcia, M.; Blustein, G.; Schejter, L.; Palermo, J.A. Antifouling diterpenoids from the sponge Dendrilla antarctica. Chem. Biodivers. 2022, 19, e202100618. [Google Scholar] [CrossRef]
- Perez, M.; Sanchez, M.; Garcia, M.; Patino, C.L.; Blustein, G.; Palermo, J.A. Antifouling activity of peracetylated cholic acid, a natural bile acid derivative. Steroids 2019, 149, 108414. [Google Scholar] [CrossRef]
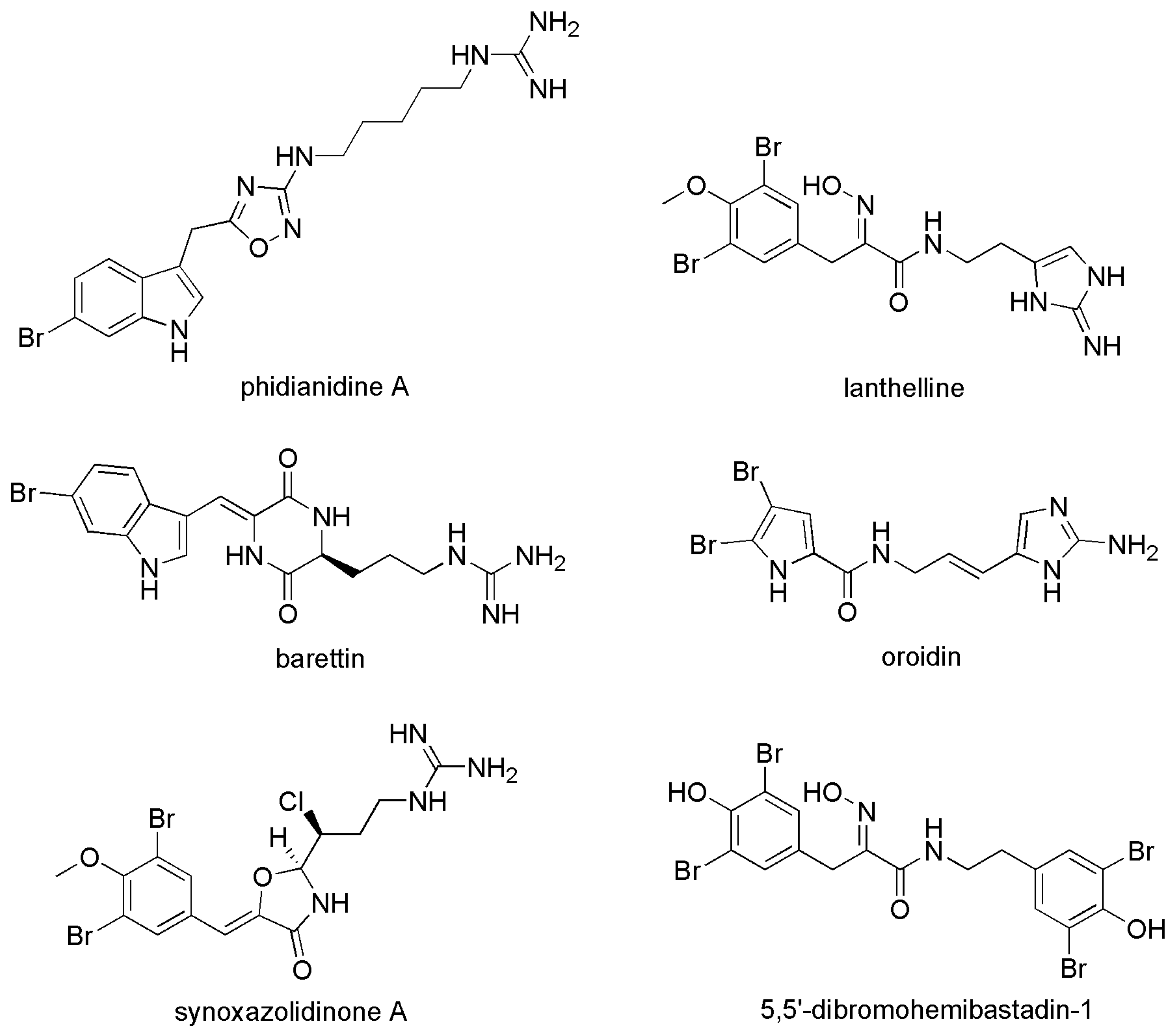
- Lidgren, G.; Bohlin, L.; Bergman, J. Studies of swedish marine organisms VII. A novel biologically active indole alkaloid from the sponge Geodia baretti. Tetrahedron Lett. 1986, 27, 3283–3284. [Google Scholar] [CrossRef]
- Takamura, H.; Kinoshita, Y.; Yorisue, T.; Kadota, I. Chemical synthesis and antifouling activity of monoterpene-furan hybrid molecules. Org. Biomol. Chem. 2023, 21, 632–638. [Google Scholar]
- Carbone, M.; Li, Y.; Irace, C.; Mollo, E.; Castelluccio, F.; Di Pascale, A.; Cimino, G.; Santamaria, R.; Guo, Y.-W.; Gavagnin, M. Structure and cytotoxicity of phidianidines A and B: First finding of 1,2,4-oxadiazole system in a marine natural product. Org. Lett. 2011, 13, 2516–2519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, X.; Han, X.; Feng, D.; Luo, X.; van Ofwegen, L.; Li, P.; Li, G. Sarcoglaucins A-I, new antifouling cembrane-type diterpenes from the South China Sea soft coral Sarcophyton glaucum. Org. Chem. Front. 2019, 6, 2004–2013. [Google Scholar] [CrossRef]
- Song, Y.; Cai, Z.H.; Lao, Y.M.; Jin, H.; Ying, K.Z.; Lin, G.H.; Zhou, J. Antibiofilm activity substances derived from coral symbiotic bacterial extract inhibit biofouling by the model strain Pseudomonas aeruginosa PAO1. Microb. Biotechnol. 2018, 11, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.W.; Wang, Z.X.; Guo, Y.Q.; Tang, S.A.; Feng, D.Q. Antifouling activity of terpenoids from the corals Sinularia flexibilis and Muricella sp. against the bryozoan Bugula neritina. J. Asian Nat. Prod. Res. 2023, 25, 85–94. [Google Scholar] [CrossRef]
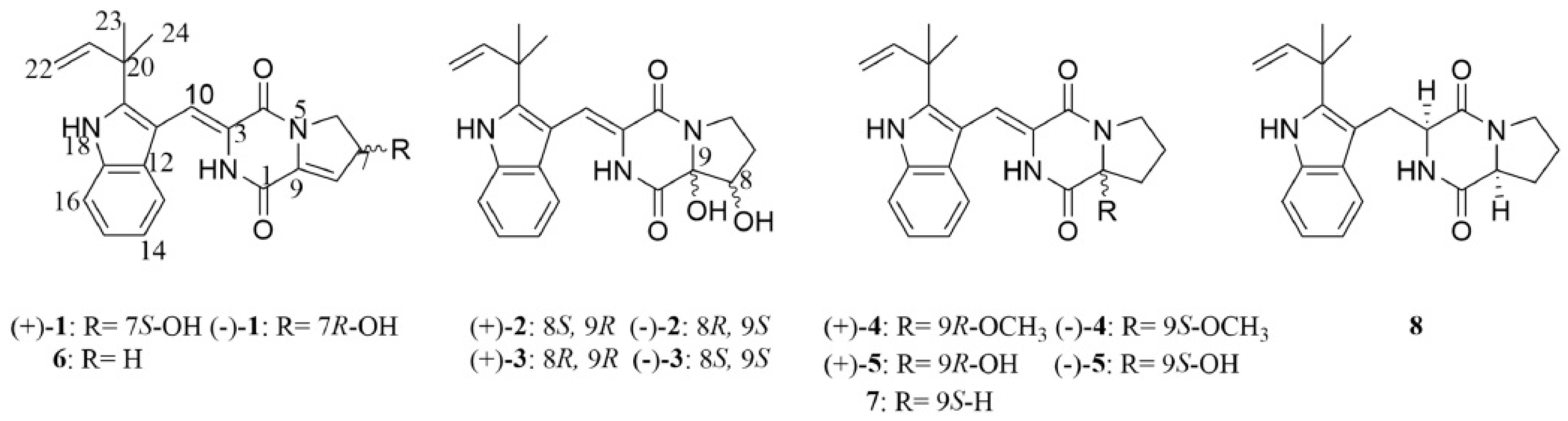
- Grant, T.M.; Rennison, D.; Cervin, G.; Pavia, H.; Hellio, C.; Foulon, V.; Brimble, M.A.; Cahill, P.; Svenson, J. Towards eco-friendly marine antifouling biocides—Nature inspired tetrasubstituted 2,5-diketopiperazines. Sci. Total Environ. 2022, 812, 152487. [Google Scholar] [CrossRef] [PubMed]
- Grant, T.M.; Rennison, D.; Arabshahi, H.J.; Brimble, M.A.; Cahill, P.; Svenson, J. Effect of regio- and stereoisomerism on antifouling 2,5-diketopiperazines. Org. Biomol. Chem. 2022, 20, 9431–9446. [Google Scholar] [CrossRef]
- Labriere, C.; Elumalai, V.; Staffansson, J.; Cervin, G.; Le Norcy, T.; Denardou, H.; Rehel, K.; Moodie, L.W.K.; Hellio, C.; Pavia, H.; et al. Phidianidine A and Synthetic Analogues as Naturally Inspired Marine Antifoulants. J. Nat. Prod. 2020, 83, 3413–3423. [Google Scholar] [CrossRef]
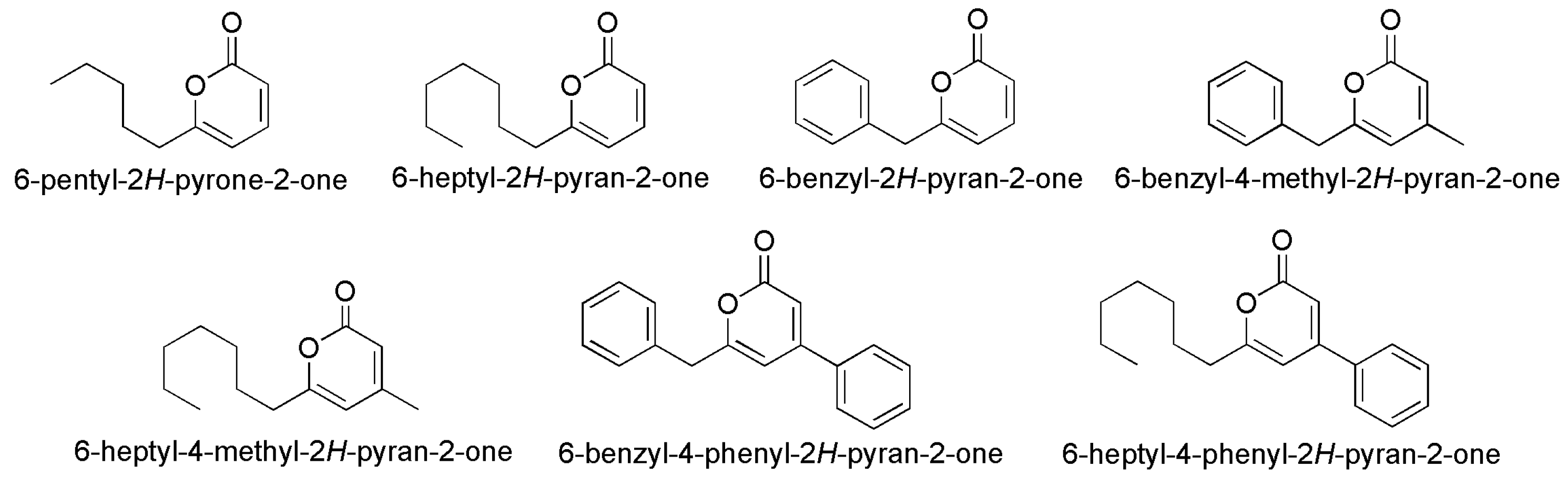
- Khan, M.A.R.; Wang, B.W.; Chen, Y.Y.; Lin, T.H.; Lin, H.C.; Yang, Y.L.; Pang, K.L.; Liaw, C.C. Natural polyketide 6-pentyl-2H-pyrone-2-one and its synthetic analogues efficiently prevent marine biofouling. Biofouling 2021, 37, 257–266. [Google Scholar] [CrossRef]
- Li, L.; Chang, Q.-H.; Zhang, S.-S.; Yang, K.; Chen, F.-L.; Zhu, H.-J.; Cao, F.; Liu, Y.-F. (±)-Brevianamides Z and Z1, New Diketopiperazine alkaloids from the marine-derived fungus Aspergillus versicolor. J. Mol. Struct. 2022, 1261, 132904. [Google Scholar] [CrossRef]
- Lou, T.; Bai, X.; He, X.; Yuan, C. Antifouling performance analysis of peptide-modified glass microstructural surfaces. Appl. Surf. Sci. 2021, 541, 148384. [Google Scholar] [CrossRef]
- Amer, M.S.; Ibrahim, H.A.H. Chitosan from marine-derived Penicillum spinulosum MH2 cell wall with special emphasis on its antimicrobial and antifouling properties. Egypt. J. Aquat. Res. 2019, 45, 359–365. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Xiao, K.; Chandramouli, K.H.; Xu, Y.; Pan, K.; Wang, W.-X.; Qian, P.-Y. Acute toxicity of the antifouling compound butenolide in non-target organisms. PLoS ONE 2011, 6, e23803. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Pis Diez, C.M.; Belen Valdez, M.; Garcia, M.; Paola, A.; Avigliano, E.; Palermo, J.A.; Blustein, G. Isolation and Antimacrofouling Activity of Indole and Furoquinoline Alkaloids from ‘Guatambu’ Trees (Aspidosperma australe and Balfourodendron riedelianum). Chem. Biodivers. 2019, 16, e1900349. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, W.F.; Li, M.; Zhang, J.; Chai, T.; Cao, W.; Yan, T.; Zhao, X.; Yang, W.; Yu, B.; et al. Natural Product Zanthoxylum bungeanum Based Multi-Functionalized Self-Polishing Interface for Sustainable Marine Antifouling. Adv. Mater. Interfaces 2022, 9, 2201195. [Google Scholar] [CrossRef]
- Tanikawa, A.; Fujihara, T.; Nakajima, N.; Maeda, Y.; Nogata, Y.; Yoshimura, E.; Okada, Y.; Chiba, K.; Kitano, Y. Anti-barnacle activities of isothiocyanates derived from beta-citronellol and their structure-activity relationships. Chem. Biodivers. 2023, 20, e202200953. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D.; Jiang, Y.; Gao, C.; Liu, L.; Liu, Y. A silicone coating containing natural borneol fluorinated side chains with excellent static antifouling properties. Eur. Polym. J. 2023, 193, 112064. [Google Scholar] [CrossRef]
- Valente, S.; Oliveira, F.; Ferreira, I.J.; Paiva, A.; Sobral, R.G.; Diniz, M.S.; Gaudencio, S.P.; Duarte, A.R.C. Hydrophobic DES based on menthol and natural organic acids for use in antifouling marine coatings. ACS Sustain. Chem. Eng. 2023, 11, 9989–10000. [Google Scholar] [CrossRef]
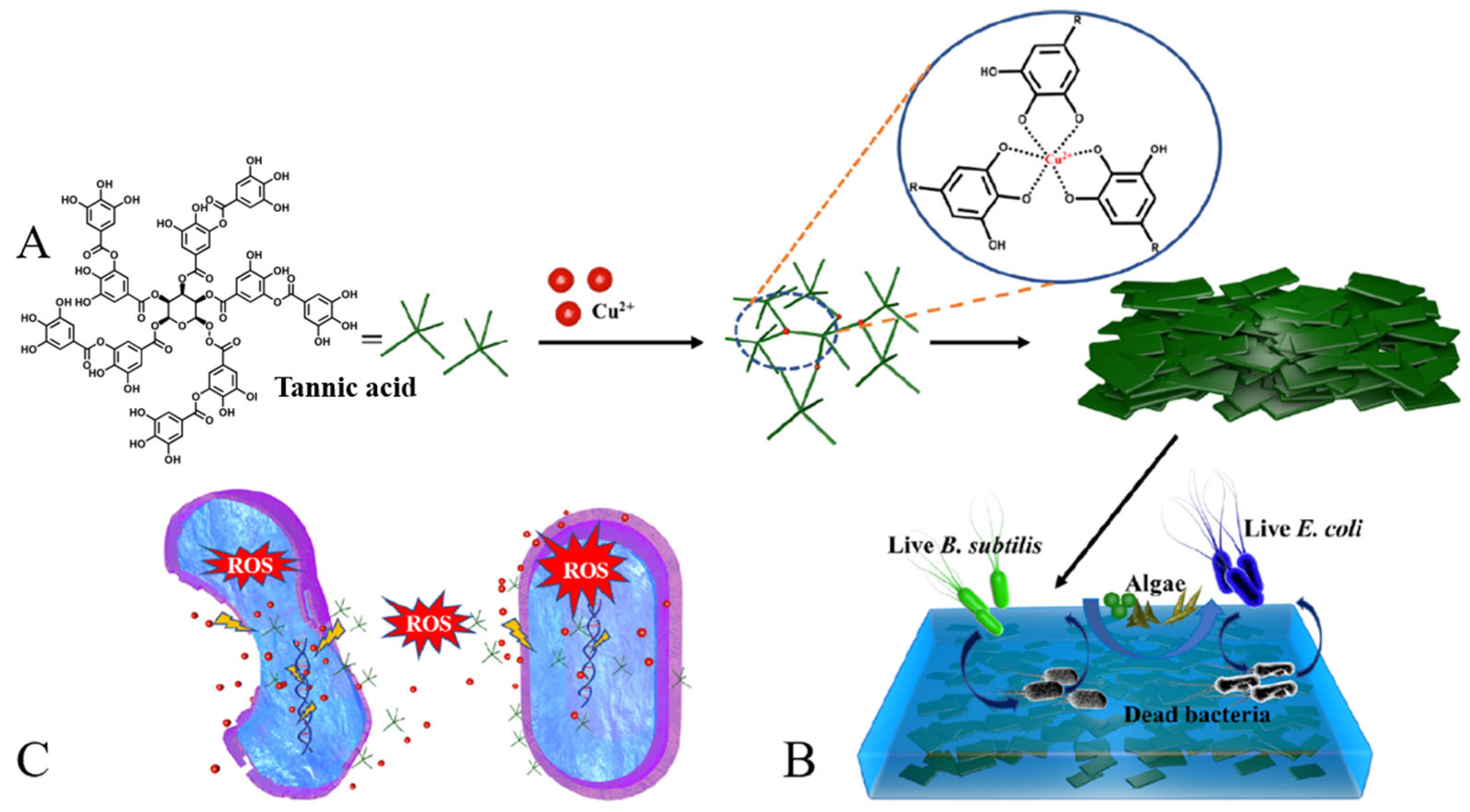
- Xu, L.; Pranantyo, D.; Neoh, K.-G.; Kang, E.-T. Tea stains-inspired antifouling coatings based on tannic acid-functionalized agarose. ACS Sustain. Chem. Eng. 2017, 5, 3055–3062. [Google Scholar] [CrossRef]
- Kim, S.; Gim, T.; Kang, S.M. Versatile, Tannic acid-mediated surface PEGylation for marine antifouling applications. ACS Appl. Mater. Interfaces 2015, 7, 6412–6416. [Google Scholar] [CrossRef]
- Weber, F.; Barrantes, A.; Tiainen, H. Silicic acid-mediated formation of tannic acid nanocoatings. Langmuir 2019, 35, 3327–3336. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Wang, H.; Jiang, R.; Zhou, X. Research progress of environmentally friendly marine antifouling coatings. Polym. Chem. 2021, 12, 3702–3720. [Google Scholar] [CrossRef]
- Lochab, B.; Shukla, S.; Varma, I.K. Naturally occurring phenolic sources: Monomers and polymers. RSC Adv. 2014, 4, 21712–21752. [Google Scholar] [CrossRef]
- Xu, G.; Neoh, K.G.; Kang, E.-T.; Teo, S.L.-M. Switchable antimicrobial and antifouling coatings from tannic acid-scaffolded binary polymer brushes. ACS Sustain. Chem. Eng. 2020, 8, 2586–2595. [Google Scholar] [CrossRef]
- He, X.; Gopinath, K.; Sathishkumar, G.; Guo, L.; Zhang, K.; Lu, Z.; Li, C.; Kang, E.-T.; Xu, L. UV-assisted deposition of antibacterial Ag–tannic acid nanocomposite coating. ACS Appl. Mater. Interfaces 2021, 13, 20708–20717. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Wei, J.; Zhu, X.; Qiu, S.; Zhao, H. Copper tannic acid-coordinated metal-organic nanosheets for synergistic antimicrobial and antifouling coatings. ACS Appl. Mater. Interfaces 2021, 13, 10446–10456. [Google Scholar] [CrossRef]
- Zhao, A.; Zhang, N.; Li, Q.; Zhou, L.; Deng, H.; Li, Z.; Wang, Y.; Lv, E.; Li, Z.; Qiao, M.; et al. Incorporation of silver-embedded carbon nanotubes coated with tannic acid into polyamide reverse osmosis membranes toward high permeability, antifouling, and antibacterial properties. ACS Sustain. Chem. Eng. 2021, 9, 11388–11402. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, J.; Yang, W.; Ma, Y.; Wang, R.; Ma, S.; Cai, M.; Yu, B.; Zhou, F. Transparent Janus hydrogel wet adhesive for underwater self-cleaning. ACS Appl. Mater. Interfaces 2021, 13, 50505–50515. [Google Scholar] [CrossRef]
- Pan, J.; Xie, Q.; Chiang, H.; Peng, Q.; Qian, P.-Y.; Ma, C.; Zhang, G. “From the Nature for the Nature”: An eco-friendly antifouling coating consisting of poly(lactic acid)-based polyurethane and natural antifoulant. ACS Sustain. Chem. Eng. 2019, 8, 1671–1678. [Google Scholar] [CrossRef]
- Chiang, H.Y.; Pan, J.; Ma, C.; Qian, P.Y. Combining a bio-based polymer and a natural antifoulant into an eco-friendly antifouling coating. Biofouling 2020, 36, 200–209. [Google Scholar] [CrossRef]
- Li, P.; Su, X.; Hao, D.; Yang, M.; Gui, T.; Cong, W.; Jiang, W.; Ge, X.; Guo, X. One-pot method for preparation of capsaicin-containing double-network hydrogels for marine antifouling. RSC Adv. 2022, 12, 15613–15622. [Google Scholar] [CrossRef]
- Hao, X.; Chen, S.; Qin, D.; Zhang, M.; Li, W.; Fan, J.; Wang, C.; Dong, M.; Zhang, J.; Cheng, F.; et al. Antifouling and antibacterial behaviors of capsaicin-based pH responsive smart coatings in marine environments. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110361. [Google Scholar] [CrossRef] [PubMed]
- Agostini, V.O.; Macedo, A.J.; Muxagata, E.; da Silva, M.V.; Pinho, G.L.L. Non-toxic antifouling potential of Caatinga plant extracts: Effective inhibition of marine initial biofouling. Hydrobiologia 2019, 847, 45–60. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Sofy, M.R.; Ghareeb, D.A.; Yacout, G.A.; Eldemellawy, M.A.; Ibrahim, B.M. Eco-friendly polyurethane acrylate (PUA)/natural filler-based composite as an antifouling product for marine coating. Appl. Microbiol. Biotechnol. 2021, 105, 7023–7034. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.C.K.; Qian, P.Y. Inhibitory effect of phenolic compounds and marine bacteria on larval settlement of the barnacle Balanus amphitrite amphitrite darwin. Biofouling 2000, 16, 47–58. [Google Scholar] [CrossRef]
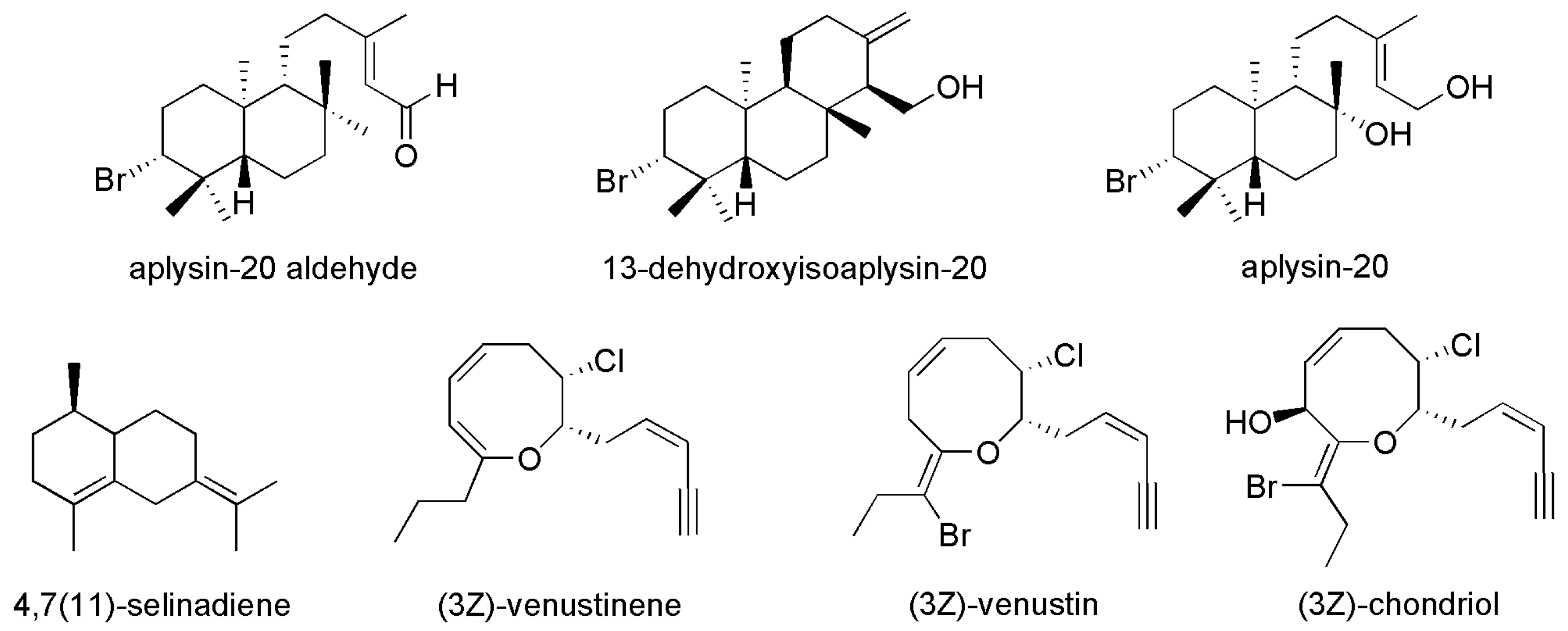
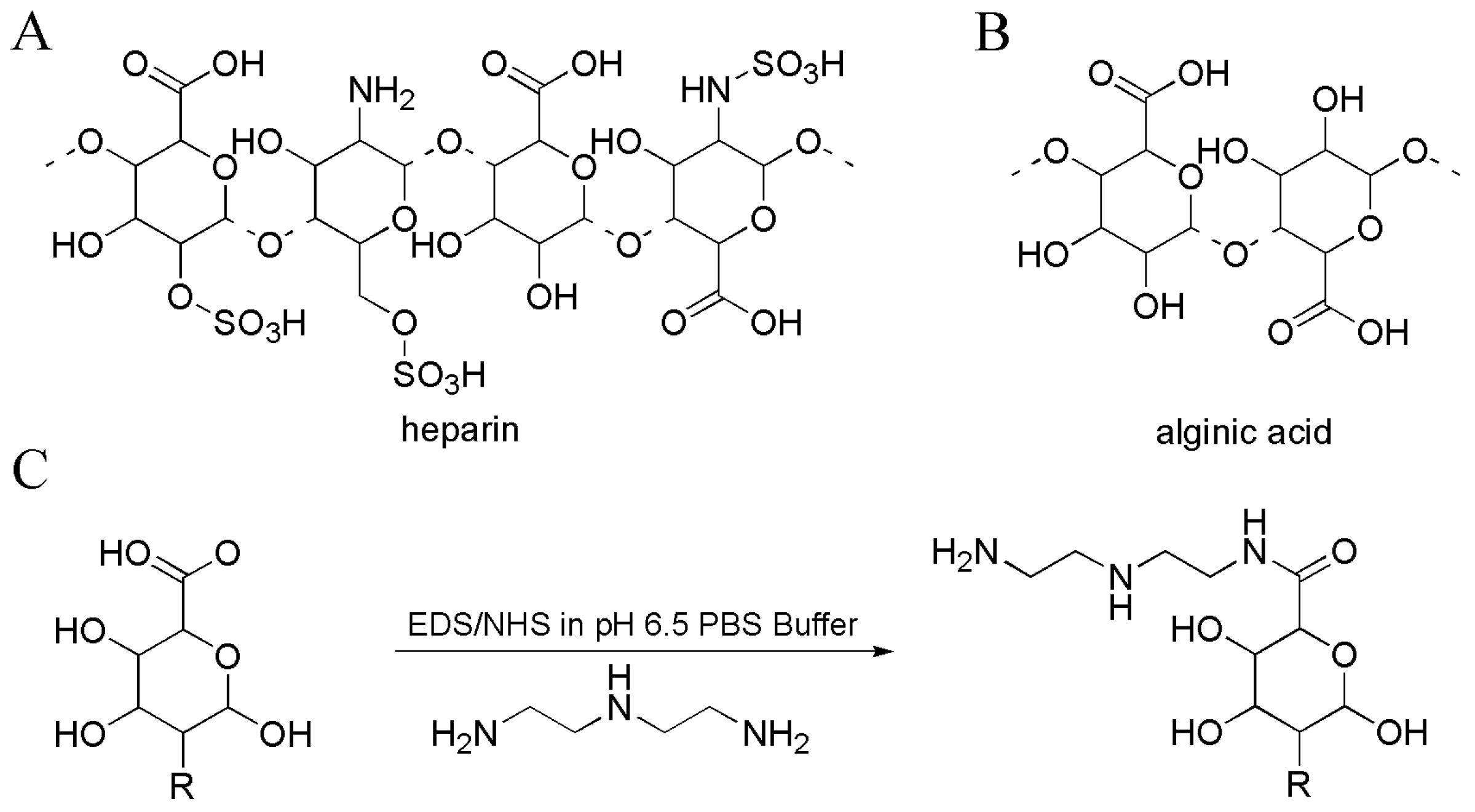
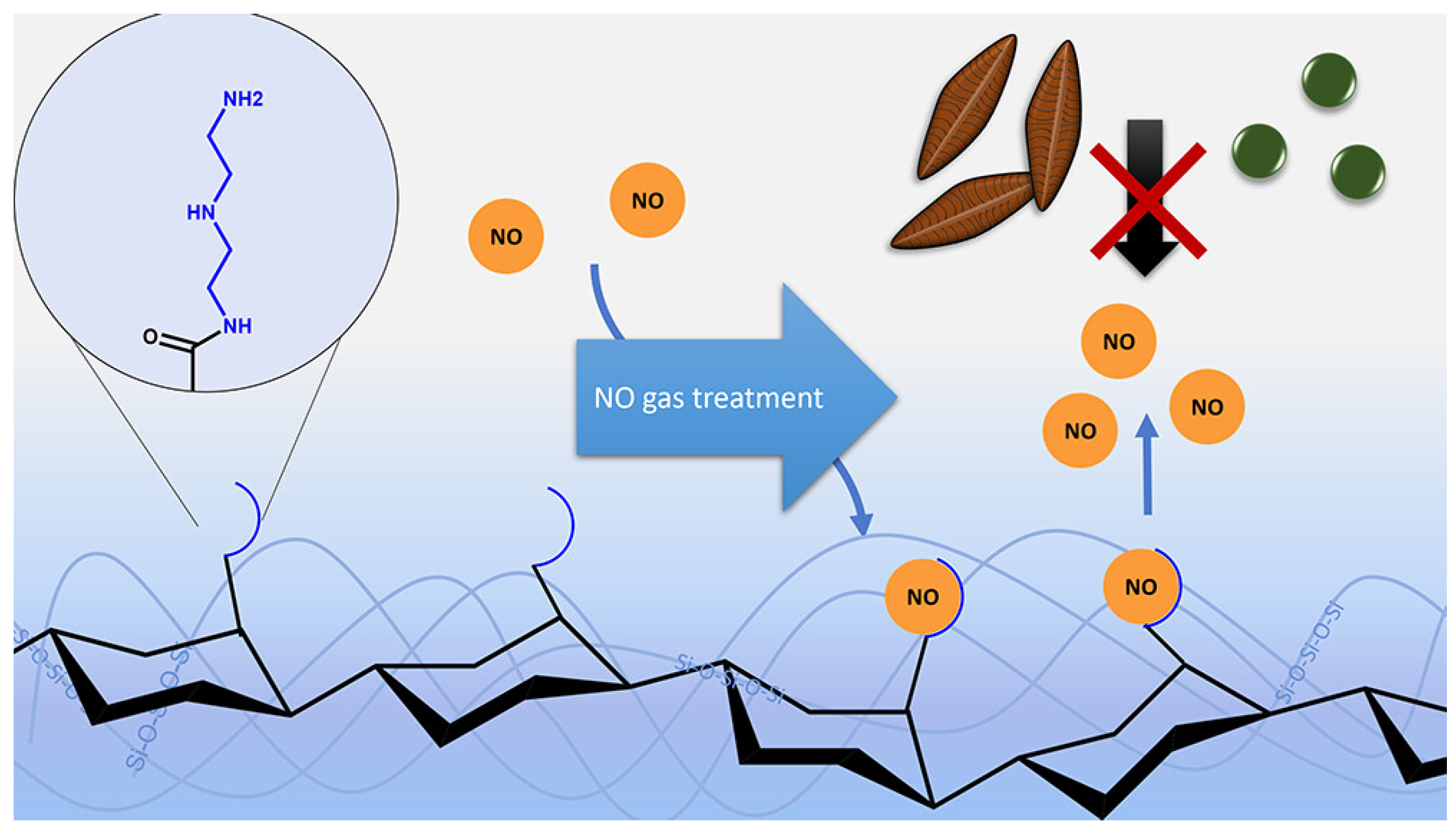
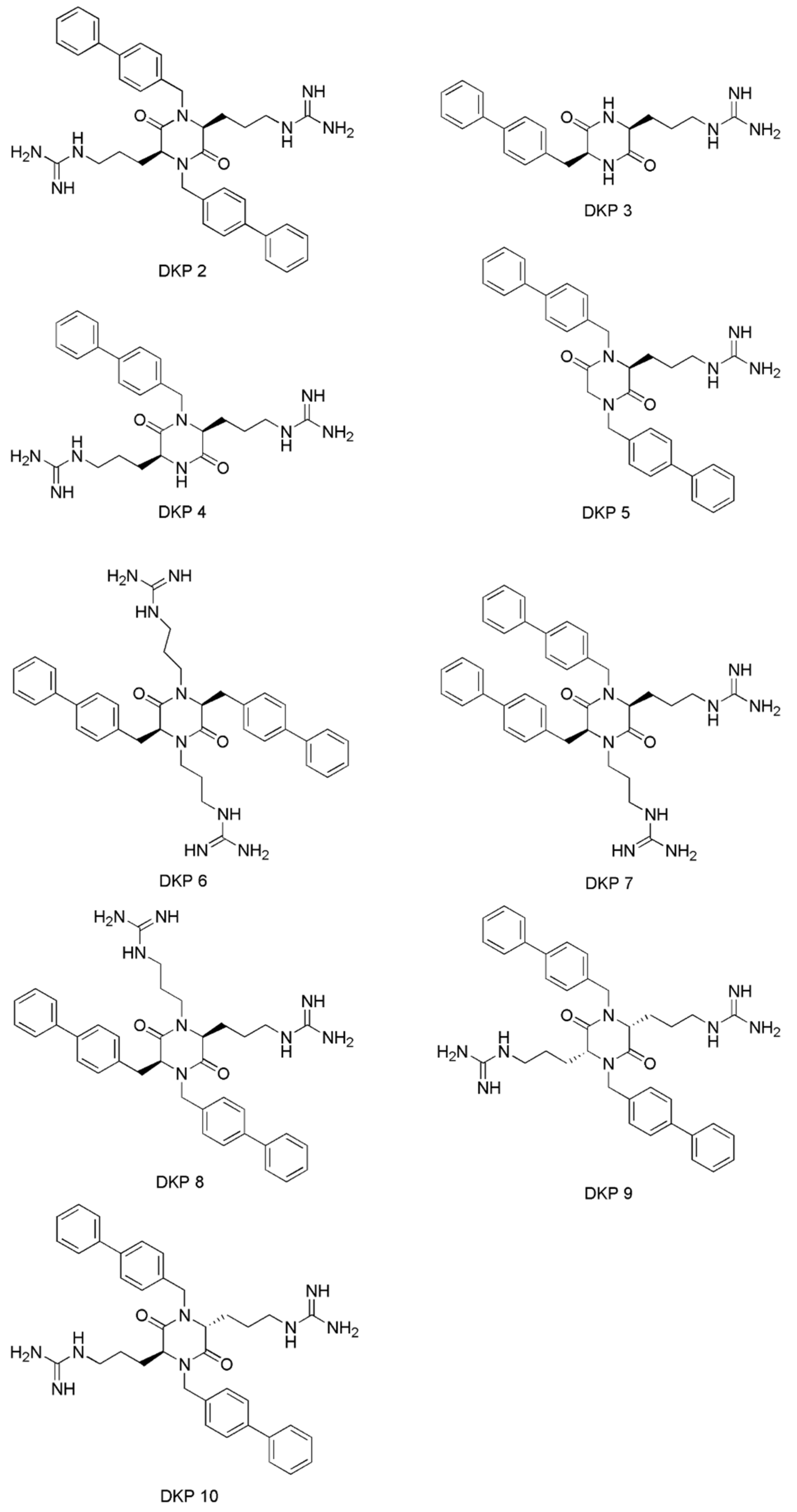





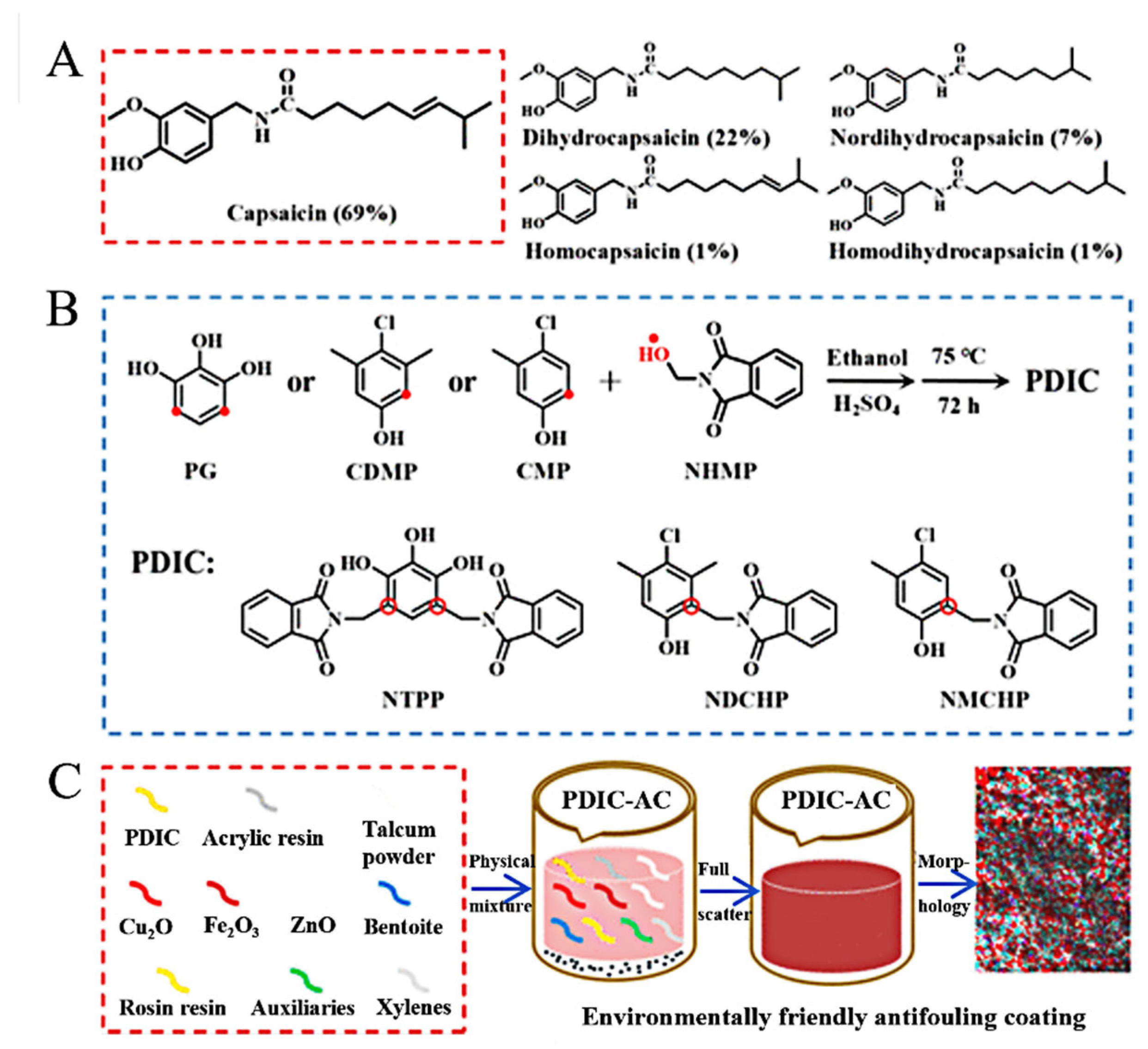
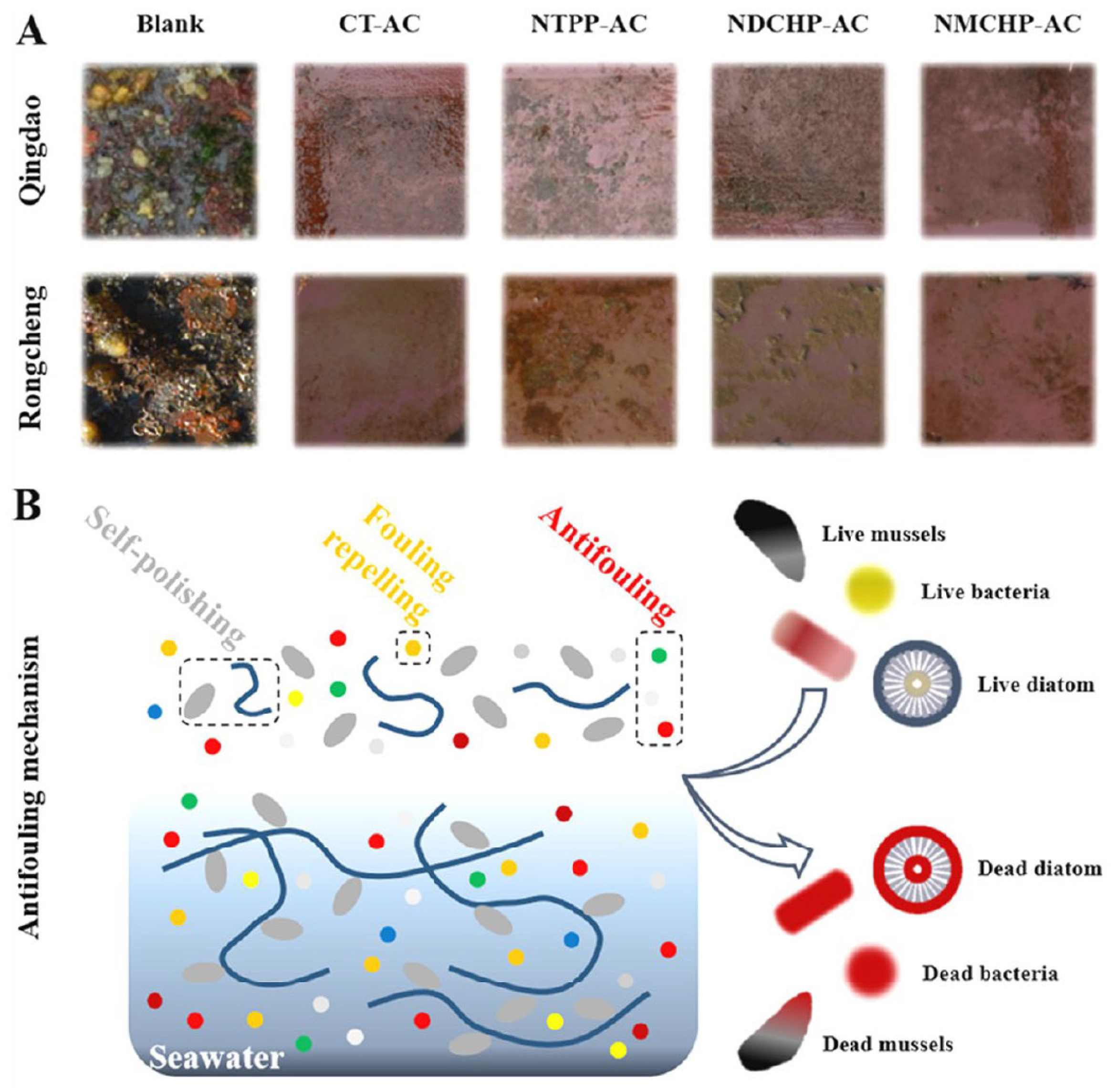

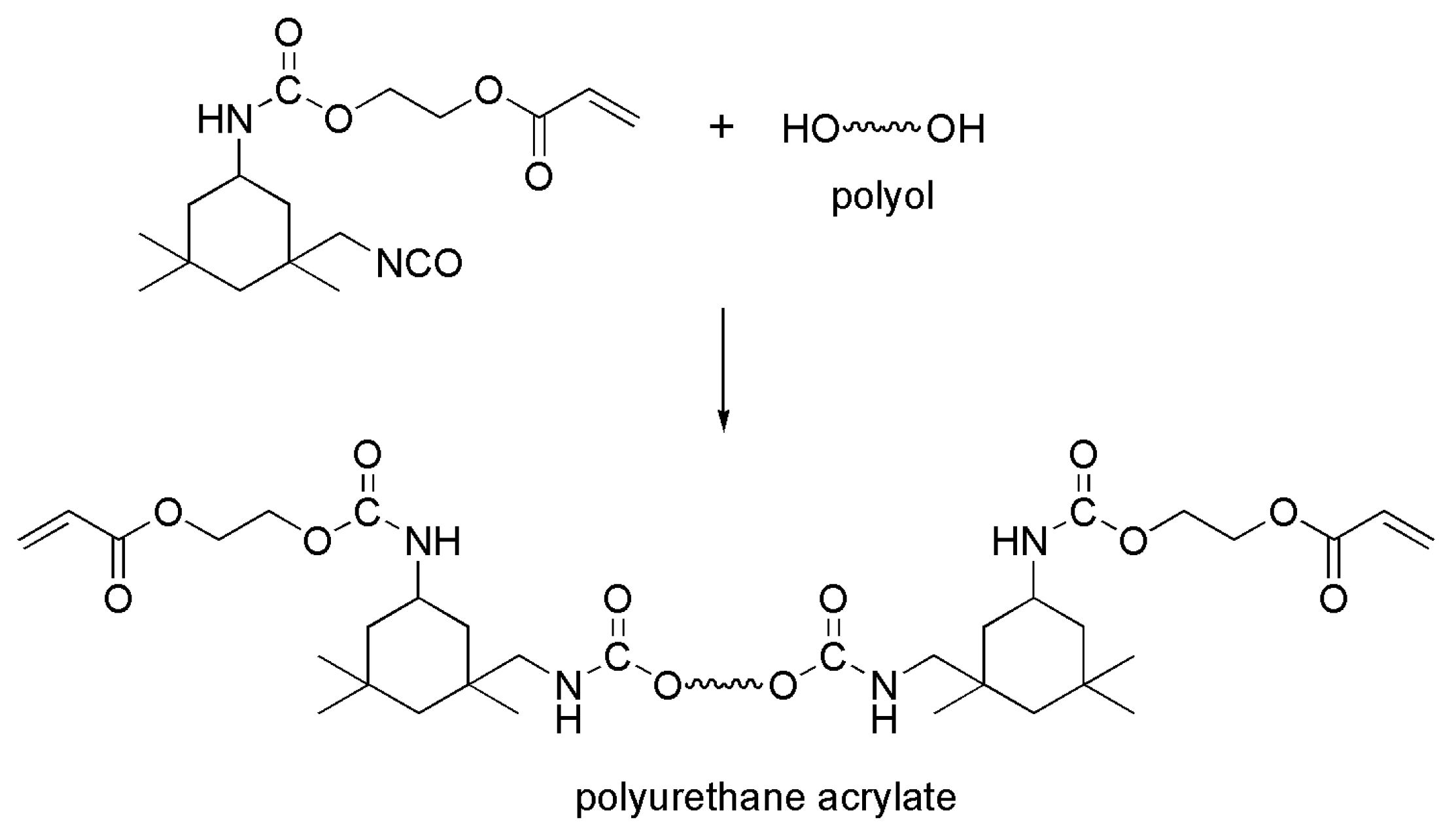
| No. | Compounds’ Name | Resources | Targets | Ref. |
|---|---|---|---|---|
| 1 | (+)-Catechin | Posidonia oceanica | Phaeodactylum tricornutum Aliivibrio fischeri Navicula salinicola Ficopomatus enigmaticus | [17] |
| 2 | Ferulic acid | |||
| 3 | Epicatechin | |||
| 4 | Chlorogenic acid | |||
| 5 | Gallic acid | |||
| 6 | Aplysin-20 aldehyde | Laurencia venusta | Mytilus galloprovincialis | [22] |
| 7 | 13-dehydroxyisoaplysin-20 |
| No. | Compounds’ Name | Resources | Targets | Ref. |
|---|---|---|---|---|
| 1 | 9,11-dihydrogracilin A | Dendrilla antarctica | Botrylloides sp. | [28] |
| 2 | 9,11-dihydrogracillinone A | |||
| 3 | Peracetylated cholic acid | Siphonochalina fortis | Mytilus edulis platensis | [29] |
| 4 | 2,5-diketopiperazine | Geodia barretti | - | [30] |
| 5 | dihydrofurospongin II (2) | Mediterranean sponges | Balanus (Amphibalanus) amphitrite | [31] |
| 6 | euryfuran | |||
| 7 | Phidianidine A | Phidiana militaris | - | [32] |
| 8 | Sarcoglaucin B | Sarcophyton glaucum | Balanus amphitrite | [33] |
| 9 | sarcoglaucin E | |||
| 10 | trochelioid | |||
| 11 | 7α-hydroxy-Δ8(19)-deepoxysarcophine | |||
| 12 | (−)-sartrochine | |||
| 13 | H12-Vibrio alginolyticus | Pocillopora damicornis | Pseudomonas aeruginosa | [34] |
| 14 | ent-sinuflexibilin D | Sinularia flexibilis | Bugula neritina | [35] |
| 15 | sinulaflexiolide O | |||
| 16 | sinulaflexiolide L | |||
| 17 | diepoxycembrene A | |||
| 18 | orphirin and sinensin |
| No. | Compounds’ Name | Resources | Targets | Ref. |
|---|---|---|---|---|
| 1 | Stellera chamaejasme extracts | Stellera chamaejasme | Porphyridium sp. | [20] |
| 2 | N-methyltetrahydroellipticine | Aspidosperma austral | Mytilus edulis platensis | [44] |
| 3 | furoquinoline alkaloids kokusaginine | Balfourodendron riedelianum | ||
| 4 | flindersiamine | |||
| 5 | CT-6(extracted from leaves) | Zanthoxylum bungeanum | Navicula sp. Amphora sp. Porphyridium sp. | [45] |
| 6 | TJ-2(extracted from fruit) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Wu, Z.; Liu, Y.; Dai, P.; Hai, G.; Liu, F.; Shang, Y.; Cao, Z.; Yang, W. Research Progress of Natural Products and Their Derivatives in Marine Antifouling. Materials 2023, 16, 6190. https://doi.org/10.3390/ma16186190
Zhao W, Wu Z, Liu Y, Dai P, Hai G, Liu F, Shang Y, Cao Z, Yang W. Research Progress of Natural Products and Their Derivatives in Marine Antifouling. Materials. 2023; 16(18):6190. https://doi.org/10.3390/ma16186190
Chicago/Turabian StyleZhao, Wenwen, Zhiqiang Wu, Yanming Liu, Pan Dai, Guojuan Hai, Feng Liu, Yu Shang, Zhongyue Cao, and Wufang Yang. 2023. "Research Progress of Natural Products and Their Derivatives in Marine Antifouling" Materials 16, no. 18: 6190. https://doi.org/10.3390/ma16186190
APA StyleZhao, W., Wu, Z., Liu, Y., Dai, P., Hai, G., Liu, F., Shang, Y., Cao, Z., & Yang, W. (2023). Research Progress of Natural Products and Their Derivatives in Marine Antifouling. Materials, 16(18), 6190. https://doi.org/10.3390/ma16186190






