Polypropylene Color Masterbatches Containing Layered Double Hydroxide Modified with Quinacridone and Phthalocyanine Pigments—Rheological, Thermal and Application Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
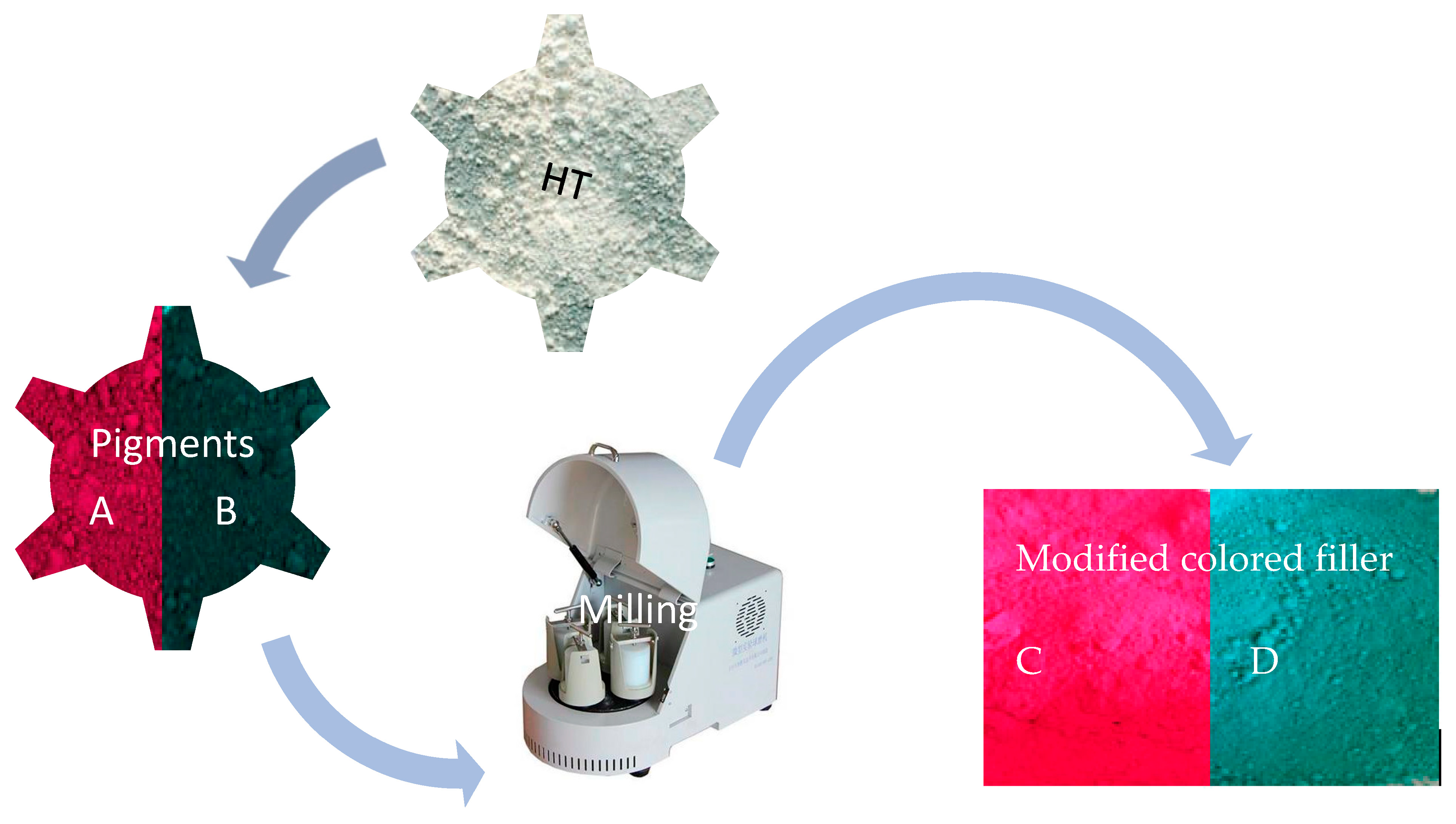
2.2. Preparation of Modified Colored Filler
2.3. Preparation of Masterbatches
2.4. The Characterization of Modified Colored LDH Fillers
2.5. Viscoelastic Properties at Processing Temperature 200 °C
2.6. The DSC and TGA Analysis
2.7. CIELab Measurements
3. Results
3.1. Surface Energy, the Tendency toward Aggregation and the Thermal Stability of Colored Layered Double Hydroxide Fillers
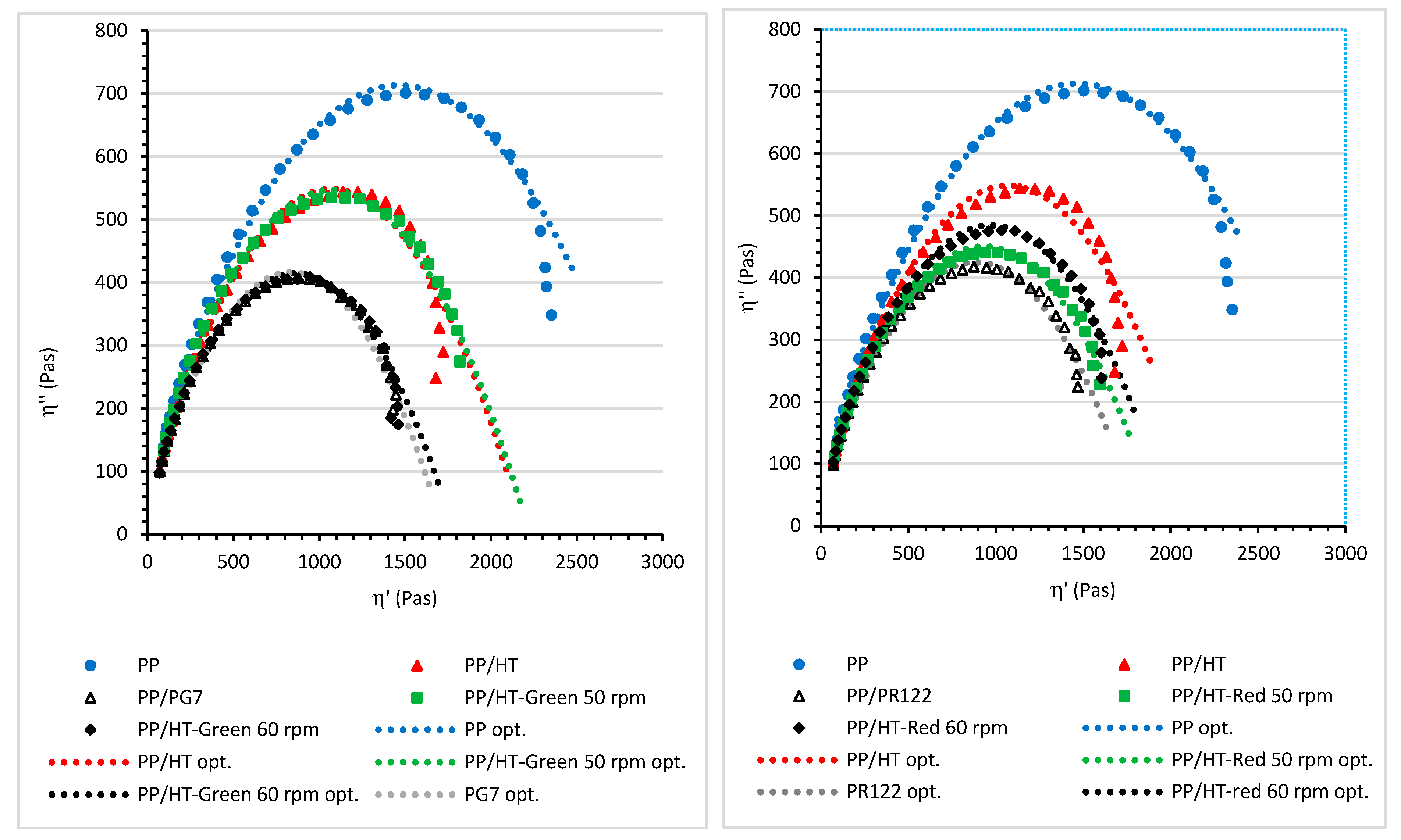
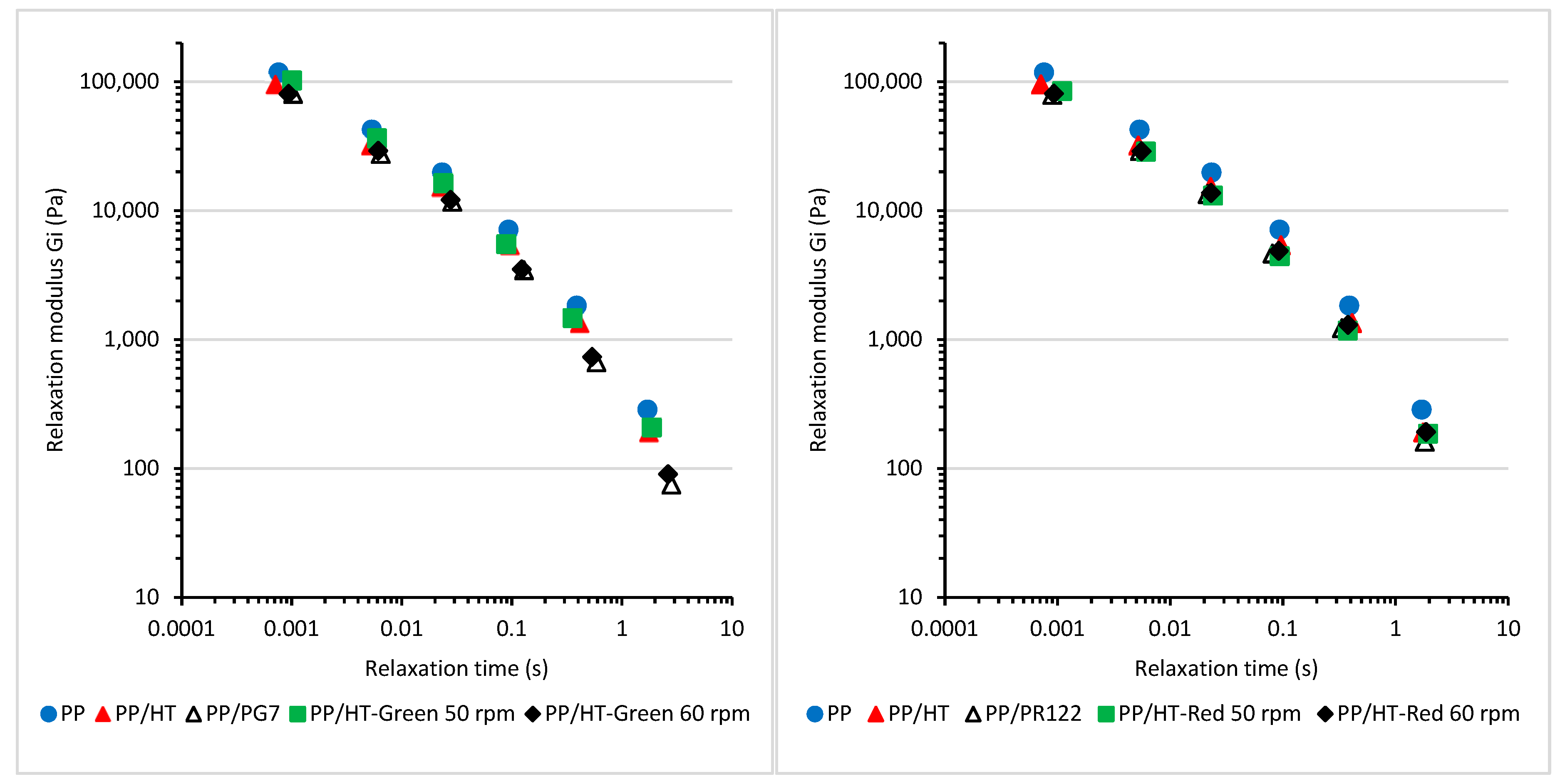
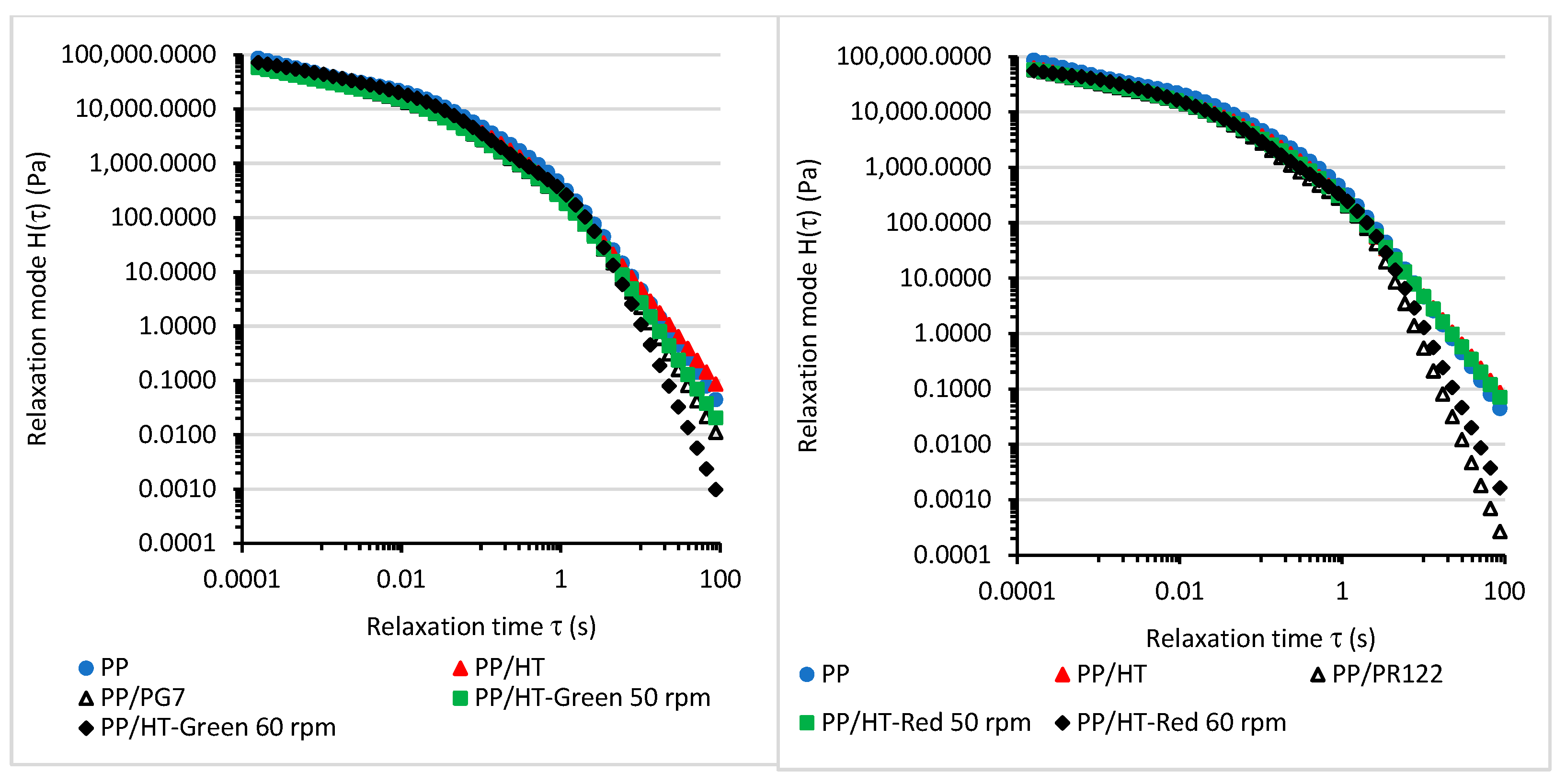
3.2. The Viscoelastic Properties and Relaxation Behavior of the Melted Masterbatches at a Processing Temperature of 200 °C
3.3. Thermal Properties and Crystallization of Masterbatches
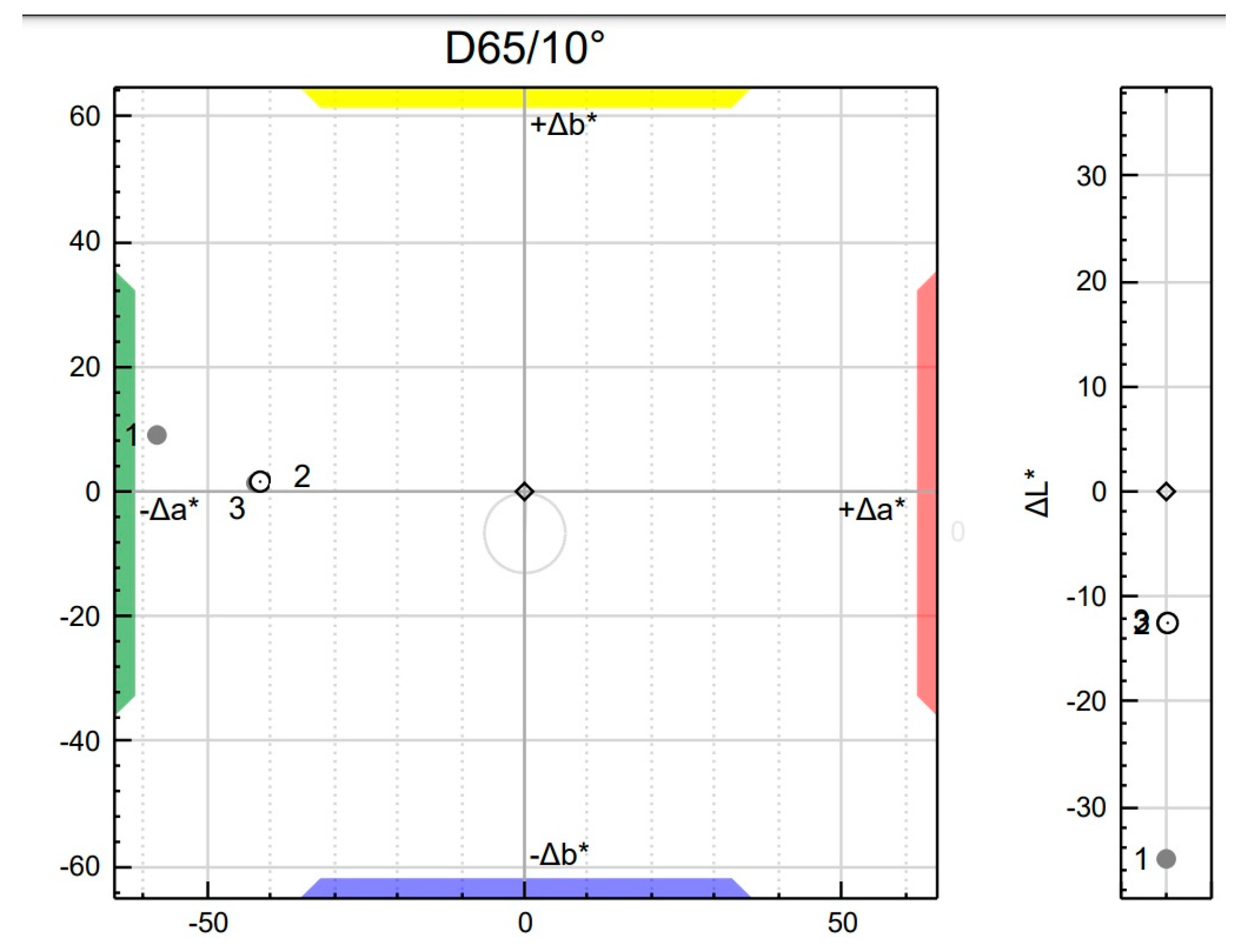
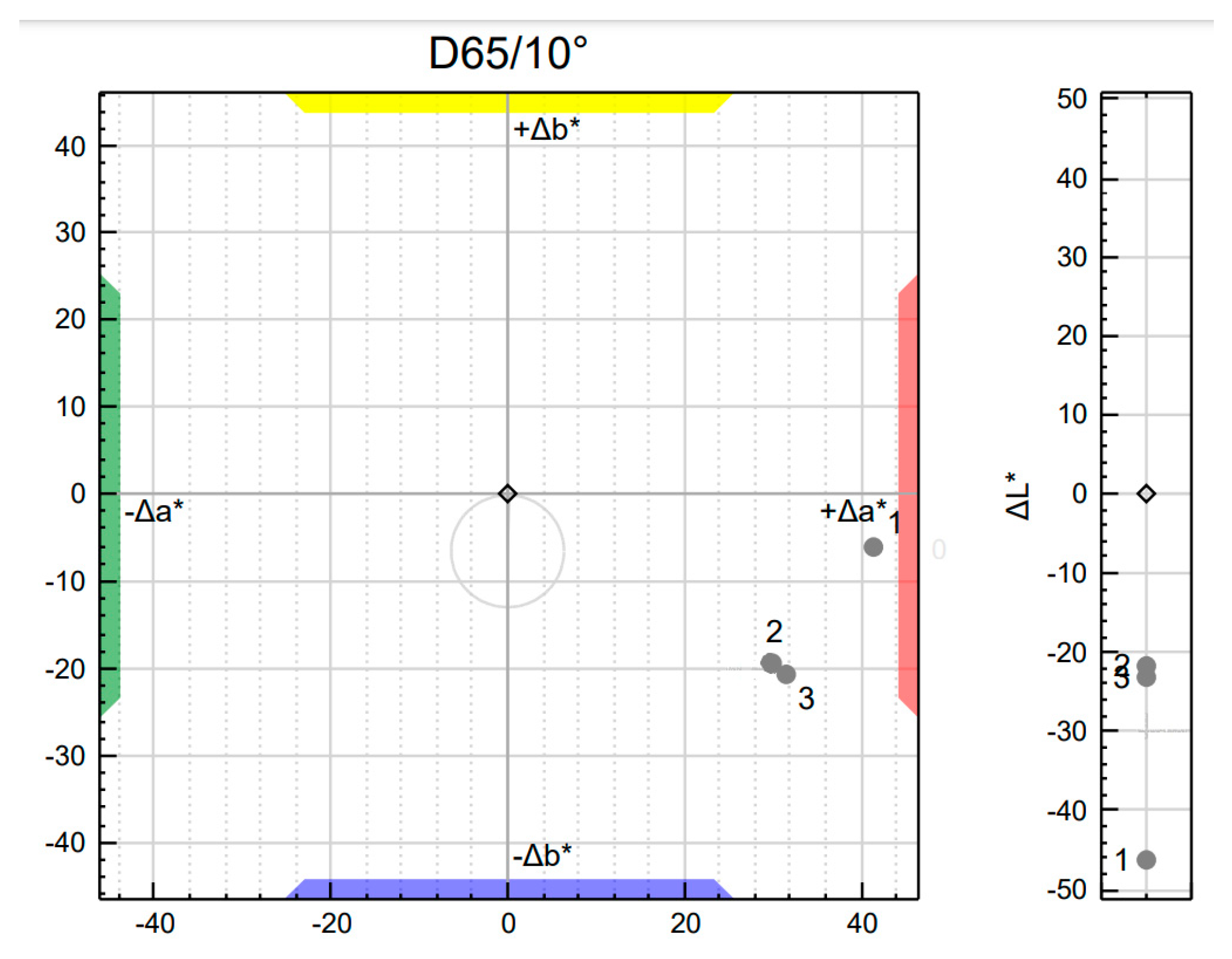
3.4. The Influence of Hybrid Pigment on the Color Profile of Masterbatch
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broda, J.; Gawlowski, A.; Slusarczyk, C.; Wlochowicz, A.; Fabia, J. The influence of additives on the structure of polypropylene fibres. Dye. Pigment. 2007, 74, 508–511. [Google Scholar] [CrossRef]
- Paleo, A.J.; Krause, B.; Mendes, A.R.; Tavares, C.J.; Cerqueira, M.F.; Muňoz, E.; Pötschke, P. Comparative thermoelectric properties of polypropylene composites melt-processed using Pytograf® III carbon nanofibers. J. Compos. Sci. 2023, 7, 173. [Google Scholar] [CrossRef]
- Harekrushna, S.; Mishra, B.; Senapti, P.; Murmu, R.; Sahu, D. Mechanical, thermal, and morphological properties of graphene nanoplatelet-reinforced polypropylene nanocomposites: Effects of nanofiller thickness. J. Compos. Sci. 2021, 5, 24. [Google Scholar] [CrossRef]
- Tsagdi, A.; Drossos, I.; Georgiou, D.; Exarhopoulos, S.; Karasiotas, G.; Kallitsis, J.K.; Kalogianni, E.P. Injection molded PP foams using food ingredients for food packaging applications. Polymers 2021, 13, 288. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Wang, C.J.; Zhu, J.; Guo, Z.; O’Hare, D. Polypropylene/layered double hydroxide nanocomposites. J. Mater. Chem. 2012, 22, 19113. [Google Scholar] [CrossRef]
- Patra, S.C.; Swain, S.; Senapti, P.; Sahu, H.; Murmu, R.; Sutar, H. Polypropylene and graphene nanocomposites: Effects of selected 2D-Nanofiller’s Plate Sizes on Fundamental Physicochemical Properties. Inventions 2023, 8, 8. [Google Scholar] [CrossRef]
- Janostik, V.; Senkerik, V.; Manas, L.; Stanek, M.; Cvek, M. Injection-molded isotactic polypropylene colored with green transparent and opaque pigments. Int. J. Mol. Sci. 2023, 24, 9924. [Google Scholar] [CrossRef] [PubMed]
- Broda, J. Structure of polypropylene fibres coloured with a mixture of pigments with different nucleating ability. Polymer 2003, 44, 6943–6949. [Google Scholar] [CrossRef]
- Broda, J.; Włochowicz, A. Influence of pigments on supermolecular structure of polypropylene fibers. Eur. Polym. J. 2000, 36, 1283–1297. [Google Scholar] [CrossRef]
- Paulus, E.F.; Leusen, F.J.J.; Schmidt, M.U. Crystal structures of quinacridones. Cryst. Eng. Comm. 2007, 9, 131–143. [Google Scholar] [CrossRef]
- Kanbur, Y.; Coskun, H.; Głowacki, E.D.; Irimia-Vladu, M.; Sariciftci, N.S.; Yumusak, C. High temperature-stability of organic thin-film transitors based on quinacridone pigments. Org. Electron. 2019, 66, 53–57. [Google Scholar] [CrossRef]
- Chen, P.; Liu, G.-J.; Wang, Y.; Zhang, S.X.-A. A stable aggregated system of silyl ether substituted quinacridone and its aggregation-state changes induced by fluoride-ions: Inspiration for a dual guaranteed strategy for probe design. RSC Adv. 2016, 6, 25986. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Calvete, M.J.F. Phthalocyanines: An Old Dog Can Still Have New (Photo)Tricks! Molecules 2023, 26, 2823. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.; Nural, Y.; Seferoǧlu, Z. Recent advances in the nonlinear optical (NLO) properties of phthalocyanines: A review. Dye. Pigment. 2022, 196, 109960. [Google Scholar] [CrossRef]
- Zeinidenov, A.K.; Aimukanov, A.K.; Kambar, D.S.; Ilyassov, B.R.; Zavgorodniy, A.V. Effects of phthalocyanine nanostructure on photovoltaic performance of its polymer composite thin films. Mat. Chem. Phys. 2021, 267, 124680. [Google Scholar] [CrossRef]
- Pająk, A.; Rybiński, P.; Janowska, G.; Kucharska-Jastrząbek, A. The thermal properties and the flammability of pigmented elastomeric materials. Part I. Phthalocyanine pigments. Therm. Anal. Calorim. 2014, 117, 789–798. [Google Scholar] [CrossRef]
- Tawiah, B.; Asinyo, B.K.; Badoe, W.; Zhang, L. Phthalocyanine green aluminium pigment prepared by inorganic acid radical/radical polymerization for waterborne textile applications. Int. J. Ind. Chem. 2017, 8, 17–28. [Google Scholar] [CrossRef]
- Chorobiński, M.; Skowroński, Ł.; Bieliński, M. Methodology for determining selected characteristics of polyethylene dyeing using CIELab system. Polimery 2019, 64, 690–696. [Google Scholar] [CrossRef]
- Chen, C.; Gu, J.; Weng, Y.; Huang, Z.; Qiu, D.; Shao, S. Optimization of the preparation process of biodegradable masterbatches and characterization of their rheological and application properties. Polym. Test. 2018, 70, 526–532. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.J.; Quiñones-Jurado, Z.V.; Cruz-Delgado, V.J.; Avila-Orta, C.A. Pigmentation and degradative activity of TiO2 on polyethylene films using masterbatches fabricated using variable-frequency ultrasound-assisted melt-extrusion. Materials 2020, 13, 3855. [Google Scholar] [CrossRef]
- Olivier-Ortega, H.; Tresserras, J.; Julian, F.; Alcalà, M.; Bala, A.; Espinach, F.X.; Méndez, J.A. Nanocomposites Materials of PLA reinforced with nanoclays using a masterbatch technology: A study of the mechanical performance and its sustainability. Polymers 2021, 13, 2133. [Google Scholar] [CrossRef] [PubMed]
- Ullah, J.; Harkin-Jones, E.; Mcllhagger, A.; Magee, C.; Tormey, D.; Dave, F.; Sherlock, R.; Dixon, D. The effect of masterbatch pigments on the crystallization, morphology, and shrinkage behaviour of isotactic polypropylene. J. Polym. Res. 2022, 29, 183. [Google Scholar] [CrossRef]
- Wu, S.; Brzozowski, K.J. Surface free energy and polarity of organic pigments. J. Coll. Interface Sci. 1971, 37, 686–690. [Google Scholar] [CrossRef]
- Agbo, C.; Acheampong, C.; Liping, Z.; Li, M.; Wang, D.; Fu, S. Synthesis and application of novel dispersant using a dichlorotriazine azo moiety and dodecan-1-ol. Prog. Org. Coat. 2019, 127, 1–7. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, K.; Liu, L.; Wen, S.; Gui, T. The design and preparation of antibacterial polymer brushes with phthalocyanine pigments. Coatings 2023, 13, 1114. [Google Scholar] [CrossRef]
- Saito, Y.; Iwamoto, S.; Tanaka, Y.; Hontama, N.; Endo, T. Suppresing aggregation of quinacridone pigment and improving its color strength by using chitosan nanofibres. Carbohydr. Polym. 2021, 255, 117365. [Google Scholar] [CrossRef]
- Marangoni, R.; Tavlot-Guého, C.; Illaik, A.; Wypych, F.; Leroux, F. Organic inorganic dye filler for polymer: Blue coloured layered double hydroxides into polystyrene. J. Coll. Interface Sci. 2008, 326, 366–373. [Google Scholar] [CrossRef]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Sadiku, E.R.; Mokhena, T.C. Morphology, thermal stability, and flammability properties of polymer-layered double hydroxide (LDH) nanocomposites: A Review. Crystals 2020, 10, 612. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, C.; Wang, Y.; Wang, Y.; Han, Z. Effects of modified layered double hydroxides on the thermal degradation and combustion behaviors of intumescent flame retardant polyethylene nanocomposites. Polymers 2022, 14, 1616. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Jung, S.-C.; Yoon, S.-M.; Kim, D.-K. Preparation and characterization of dye-intercalated Zn-Al-layered double hydroxide and its surface modification by silica coating. J. Phys. Chem Solids 2008, 69, 1061–1065. [Google Scholar] [CrossRef]
- Marangoni, R.; Mikowski, A.; Wypych, F. Effect of adsorbed/intercalated anionic dyes into the mechanical properties of PVA: Layered zinc hydroxide nitrate nanocomposites. J. Coll. Interface Sci. 2010, 351, 384–391. [Google Scholar] [CrossRef]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Kozanecki, M.; Moszyński, D.; Zaborski, M. Characterization and properties of new color-tunable hybrid pigments based on layered double hydroxides (LDH) and 1,2-dihydroxyantraquinone dye. J. Ind. Eng. Chem. 2019, 70, 427–438. [Google Scholar] [CrossRef]
- Coiai, S.; Javarone, S.; Cicogna, F.; Oberhauser, W.; Onor, M.; Pucci, A.; Minei, P.; Iasilli, G.; Passaglia, E. Fluoroscent LDPE and PLA nanocomposites containing fluorescein-modified layered double hydroxides and their ON/OFF responsive behavior towards humidity. Eur. Polym. J. 2018, 99, 189–201. [Google Scholar] [CrossRef]
- Kutlu, B.; Leuteritz, A.; Häuβler, L.; Oertel, U.; Heinrich, G. Stabilization of polypropylene using dye modified layered double hydroxides. Polym. Degrad. Stab. 2014, 102, 9–14. [Google Scholar] [CrossRef]
- Ran, B.; Chen, F.; Li, J.; Li, W.; Yang, F. Adsorption capability for anionic dyes on 2-hydroxyethylammonium acetate-intercalated layered double hydroxide. Colloids Surfaces A: Physicochem. Eng. Asp. 2016, 511, 312–319. [Google Scholar] [CrossRef]
- Marek, A.A.; Verney, V.; Totaro, G.; Sisti, L.; Celli, A.; Cionci, N.B.; Di Gioia, D.; Massacrier, L.; Leroux, F. Organo-modified LDH fillers endowing multi-functionality to bio-based poly (butylene succinate): An extended study from the laboratory to possible market. Appl. Clay Sci. 2020, 188, 105502. [Google Scholar] [CrossRef]
- Bragg, W.H.; Bragg, W.L. The reflection of X-rays by crystals. Proc. R. Soc. Lond. 1913, 88, 428–438. [Google Scholar] [CrossRef]
- Asma, B.; Hammoui, Y.; Adjeroud, N.; Djerrada, N.; Madani, K. Effect of filler load and high-energy ball milling process on properties of plasticized wheat gluten/olive pomace biocomposites. Adv. Powder Technol. 2018, 29, 1230–1238. [Google Scholar] [CrossRef]
- Loh, Z.H.; Samantha, A.K.; Heng, P.W.S. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J. Pharmaceut. Sci. 2015, 10, 255274. [Google Scholar] [CrossRef]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Ball milling: A green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale Adv. 2019, 1, 937. [Google Scholar] [CrossRef]
- Zhang, C.; Tominaga, Y.; Soto, K.; Imai, Y. Simultaneous attainment of particle dispersion and surface modification of Al2O3 nanoparticles via wet-jet milling. J. Compos. Mater. 2020, 55, 521–530. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Hejna, A.; Biskup, R.; Szulc, J.; Michałowski, S.; Piasecki, A.; Kloziński, A. The effect of surface treatment with isocyanate and aromatic carbodiimide of thermally expanded vermiculite used as a functional filler for polylactide-based composites. Polymers 2021, 13, 890. [Google Scholar] [CrossRef] [PubMed]
- ASTM D 1238; Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer. ASTM: West Conshohocken, PA, USA, 2013.
- ASTM D792; Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement. ASTM: West Conshohocken, PA, USA, 2020.
- Kaelble, D.H. Dispersion-polar surface tension properties of organic solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Malvern Instruments. A Basic Introduction to Rheology; Malvern Instruments White Paper; Malvern Instruments worldwide: Malvern, UK, 2016; pp. 1–20. Available online: https://cdn.technologynetworks.com/TN/Resources/PDF/WP160620BasicIntroRheology.pdf (accessed on 11 August 2023).
- Cuadri, A.A.; Martin-Alfonso, J.E. Thermal, thermo-oxidative and thermomechanical degradation of PLA: A comparative study based on rheological, chemical and thermal properties. Polym. Degrad. Stab. 2018, 150, 37–45. [Google Scholar] [CrossRef]
- Malkin, A.Y. Continuous relaxation spectrum–Its advantages and methods of calculation. Int. J. Appl. Mech. Eng. 2006, 11, 235–243. [Google Scholar]
- Malkin, A.Y.; Vasilyev, G.B.; Adrianov, A.V. On continuous relaxation spectrum. Method of calculation. Polym. Sci. Ser. A. 2010, 52, 1137–1141. [Google Scholar] [CrossRef]
- Bartenev, G.M.; Valishin, A.A.; Panchuk, I.I. Relaxation spectrometry of elastomers. Vysokomol. Soyed. 1977, 19, 187–193. [Google Scholar] [CrossRef]
- López-Barrón, C.R.; Macosko, C.W. Rheology of compatibilized immiscible blends with droplet-matrix and cocontinous morphologies during coarsening. J. Rheol. 2014, 58, 1935–1953. [Google Scholar] [CrossRef]
- Verney, V.; Michael, A. Influence de la polydispersité sur le comportement rhéologique á l’état fondu du polypropylene. Rheol. Acta 1985, 24, 627–631. [Google Scholar] [CrossRef]
- Martin, G.; Labaig, J.J.; Monge, P. Dynamic viscosity of entangled polymers. Polymer 1975, 16, 223–226. [Google Scholar] [CrossRef]
- Garcia-Franco, C.A.; Mead, D.W. Rheological and molecular characterization of linear backbone flexible polymers with the Cole-Cole relaxation spectrum. Rheol. Acta 1999, 38, 34–47. [Google Scholar] [CrossRef]
- Dobrzynska-Mizera, M.; Dutkiewicz, M.; Sterzynski, T.; Di Lorenzo, M.L. Isotactic polypropylene modified with sorbitol-based derivative and siloxane-silsesquioxane resin. Eur. Polym. J. 2016, 85, 62–71. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Andrzejewski, J.; Piasecki, A.; Strzemięcka, B.; Adamek, G. The inhibiting effect of basalt powder on crystallization behavior and the structure-property relationship of α-nucleated polypropylene composites. Polym. Test. 2021, 103, 107372. [Google Scholar] [CrossRef]
- Alghyamah, A.A.; Elnour, A.Y.; Shaikh, H.; Haider, S.; Poulose, A.M.; Al-Zahrani, S.M.; Almasry, W.A.; Park, S.Y. Biochar/polypropylene composites: A study on the effect of pyrolysis temperature on crystallization kinetics, crystalline structure, and thermal stability. J. King Saud Univ.–Sci. 2021, 33, 101409. [Google Scholar] [CrossRef]
- Bernard, E.; Zucha, W.J.; Lothenbach, B.; Mäder, U. Stability of hydrotalcite (Mg-Al layered double hydroxide) in presence of different anions. Cement Concrete Res. 2022, 152, 106674. [Google Scholar] [CrossRef]

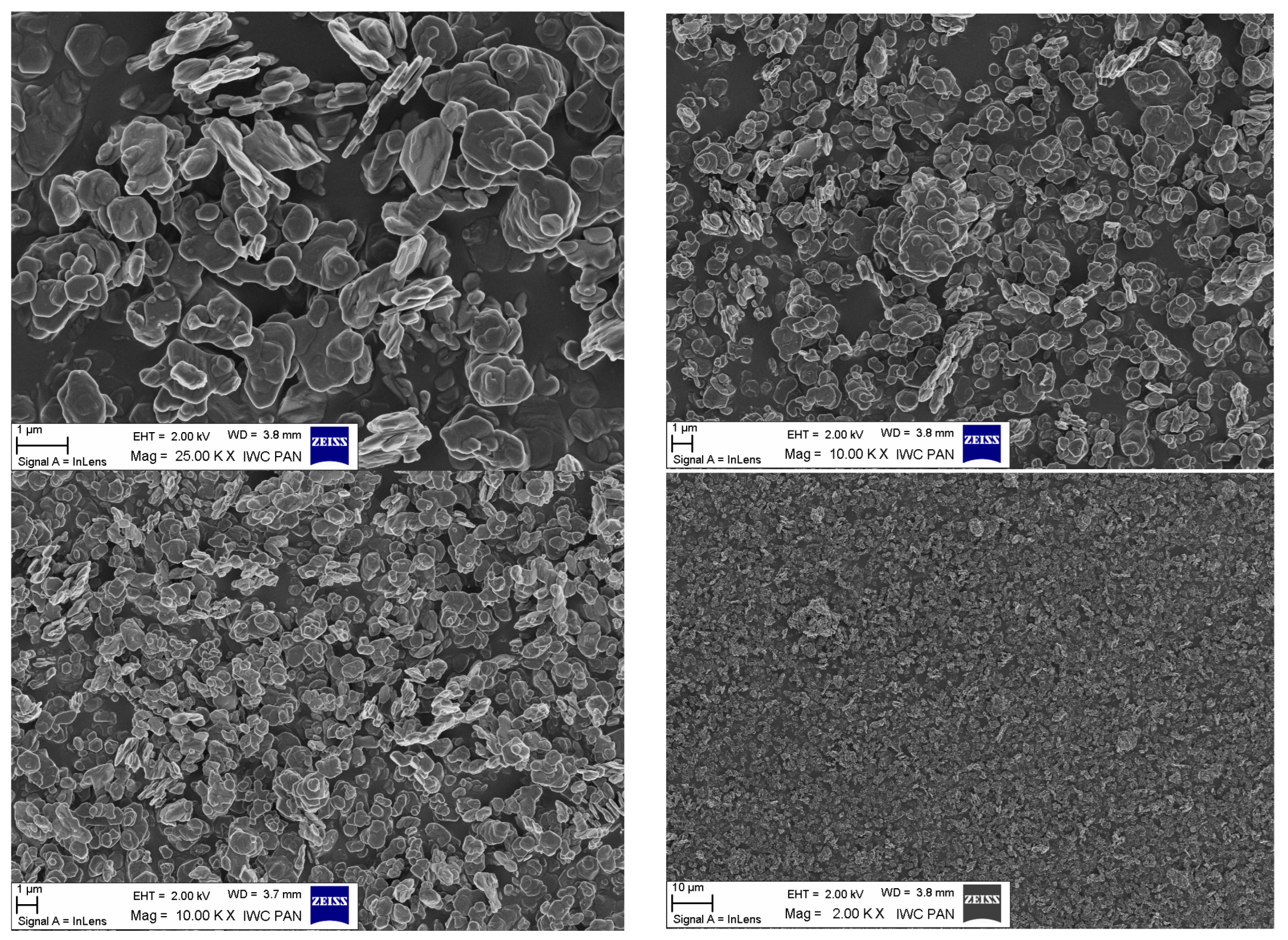





| Sample | Range of Aggregates Size (nm) * | Size of the Main Fraction (nm) * | Percentage as Number of Main Fraction (%) * |
|---|---|---|---|
| Unmodified HT | 940–2118 | 1332 | 38.0 |
| HT-Green 50 rpm | 1484–7456 | 3091 | 27.0 |
| HT-Green 60 rpm | 1718–7456 | 4145 | 26.7 |
| HT-Red 50 rpm | 1246–1589 | 1471 | 24.1 |
| HT-Red 60 rpm | 1351–1893 | 1763 | 23.4 |
| Sample | Range of Aggregates Size (nm) * | Size of the Main Fraction (nm) * | Percentage by Number of Main Fraction (%) * |
|---|---|---|---|
| Unmodified HT | 995–1545 | 1270 | 35.6 |
| HT-Green 50 rpm | 1152–2780 | 2376 | 27.8 |
| HT-Green 60 rpm | 2073–2780 | 2586 | 54.9 |
| HT-Red 50 rpm | 1152–1790 | 1438 | 34.3 |
| HT-Red 60 rpm | 1152–2073 | 1561 | 33.1 |
| Sample | γp dynes·cm−1 | γd dynes·cm−1 | γ dynes·cm−1 | γp/γ |
|---|---|---|---|---|
| Unmodified HT | 3.0 | 16.1 | 19.2 | 0.16 |
| PG7 | 5.8 | 17.2 | 23.0 | 0.25 |
| HT-Green 50 rpm | 2.7 | 21.8 | 24.5 | 0.11 |
| HT-Green 60 rpm | 8.1 | 10.5 | 18.6 | 0.44 |
| PR122 | 1.0 | 11.9 | 12.8 | 0.08 |
| HT-Red 50 rpm | 0.5 | 10.2 | 10.7 | 0.05 |
| HT-Red 60 rpm | 8.9 | 18.9 | 27.8 | 0.32 |
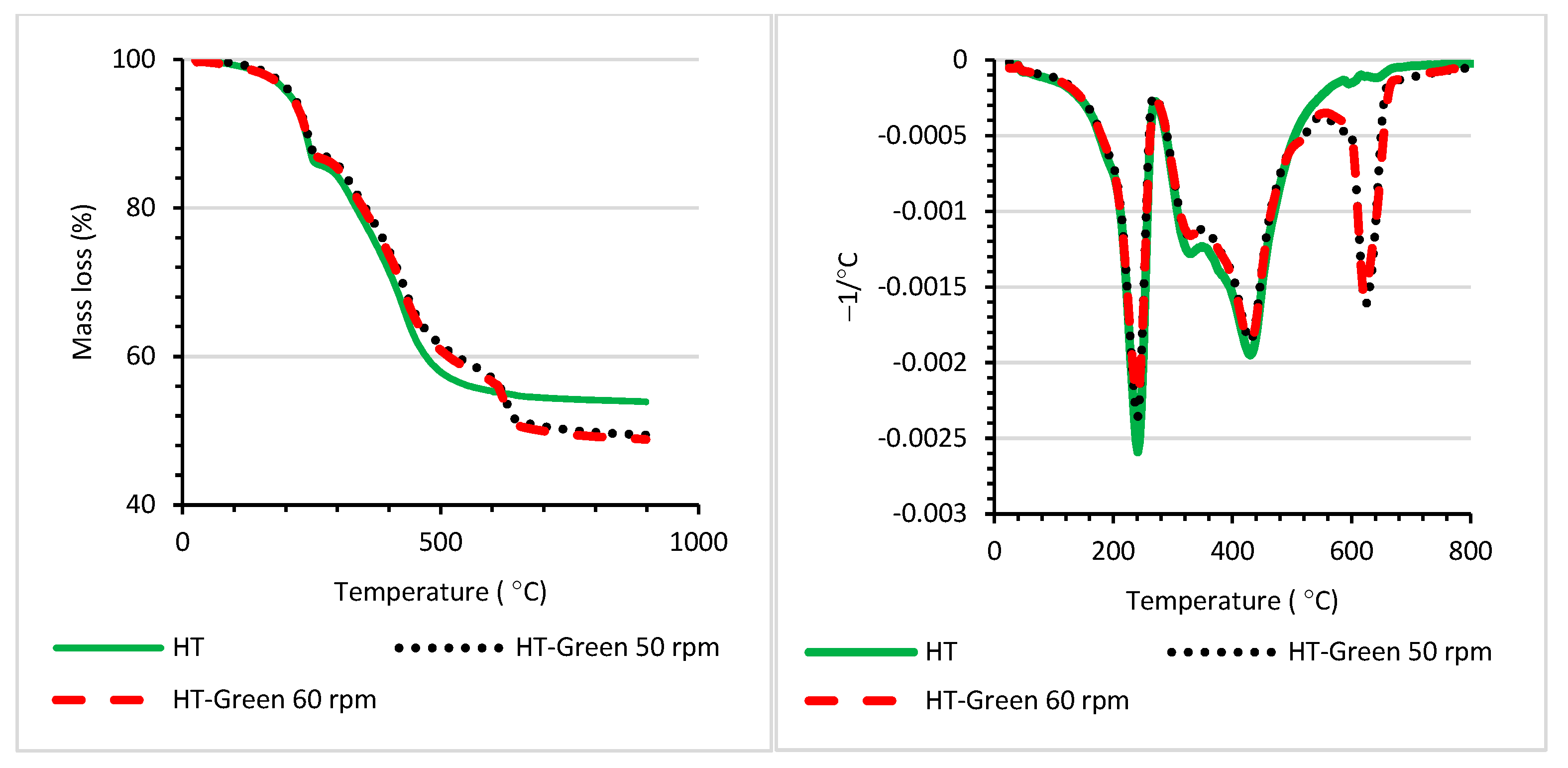
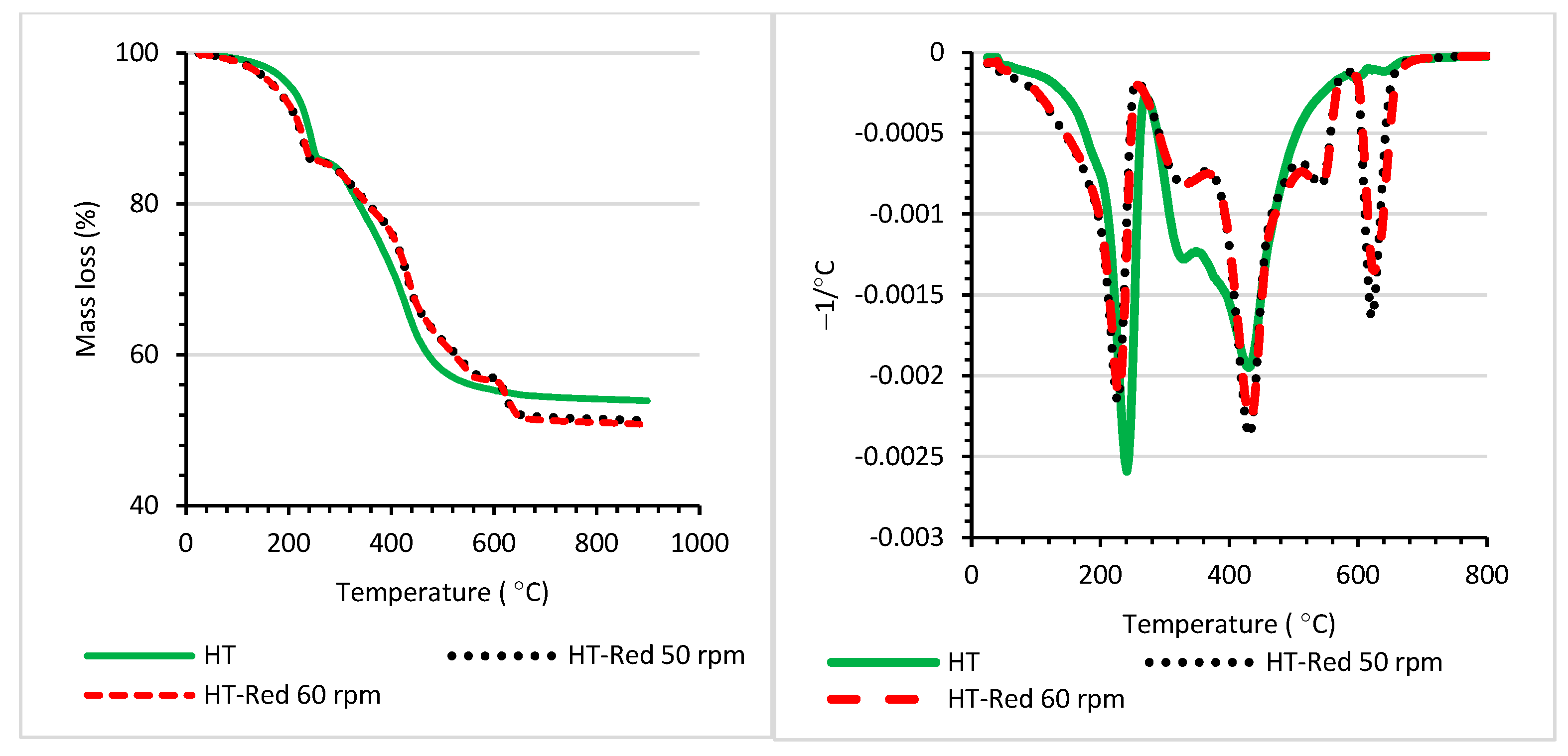
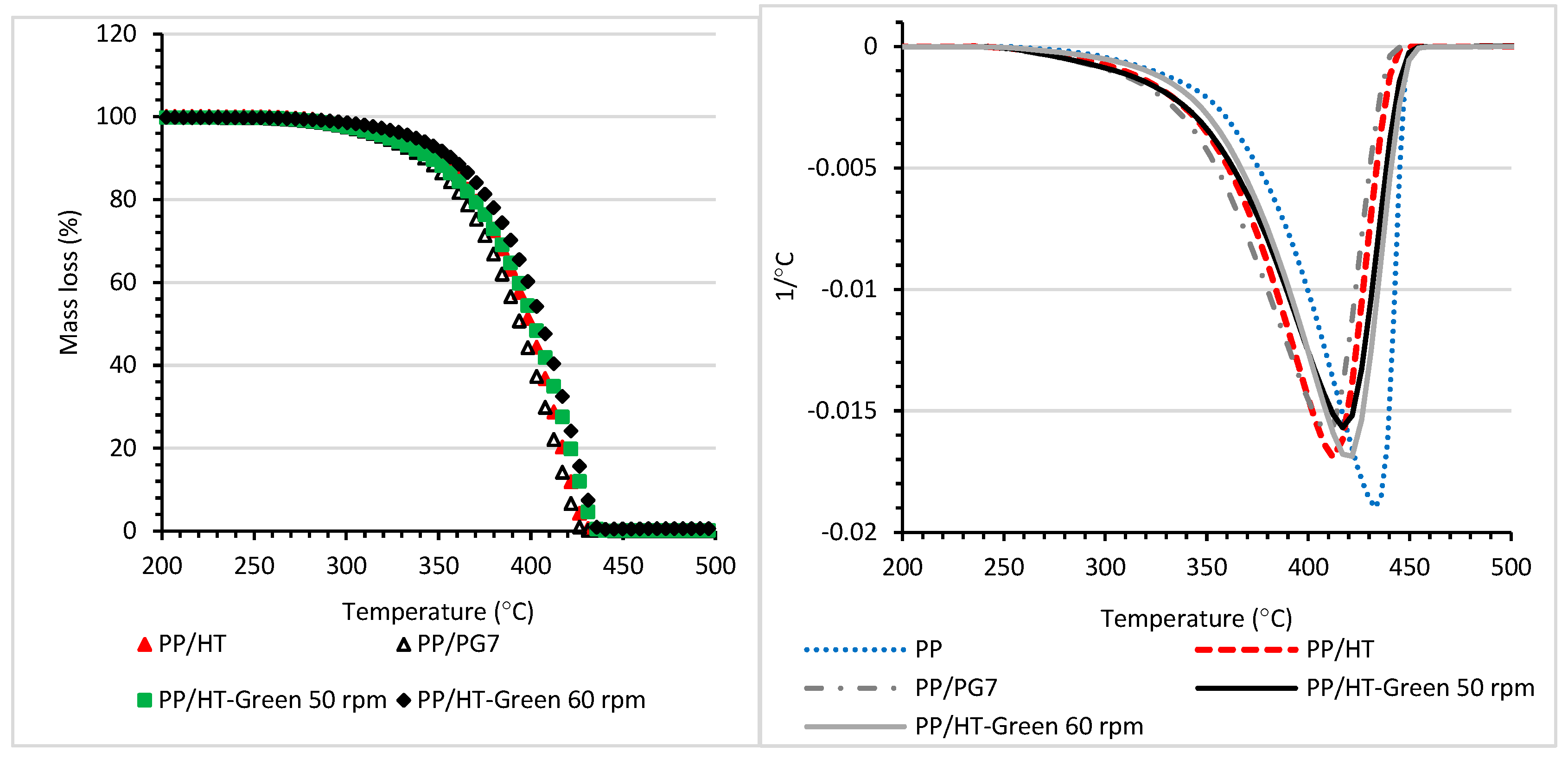
| T5% (°C) | T10% (°C) | T20% (°C) | Tmax (°C) | |
|---|---|---|---|---|
| PG7 | 598 | 622 | 649 | 697 |
| PR122 | 532 | 559 | 583 | 601; 733 |
| HT | 211 | 238 | 337 | 241; 430 |
| HT−Green 50 rpm | 217 | 244 | 355 | 241; 431; 625 |
| HT−Green 60 rpm | 214 | 241 | 352 | 241; 430; 622 |
| HT−Red 50 rpm | 181 | 223 | 355 | 226; 430; 622 |
| HT−Red 60 rpm | 181 | 223 | 352 | 229; 433; 625 |
| Composition | G′LVR (Pa) | G″LVR (Pa) | Tan δ (−) |
|---|---|---|---|
| PP | 4614 ± 49 | 8371 ± 57 | 1.81 ± 0.01 |
| PP/HT | 3292 ± 28 | 6056 ± 44 | 1.84 ± 0.01 |
| PP/PG7 | 3476 ± 85 | 6478 ± 101 | 1.86 ± 0.02 |
| PP/HT-Green 50 rpm | 4360 ± 55 | 8125 ± 93 | 1.86 ± 0.01 |
| PP/HT-Green 60 rpm | 4154 ± 59 | 7724 ± 113 | 1.86 ± 0.04 |
| PP/PR122 | 3665 ± 115 | 7207 ± 159 | 1.97 ± 0.02 |
| PP/HT-Red 50 rpm | 5413 ± 95 | 9264 ± 84 | 1.71 ± 0.02 |
| PP/HT-Red 60 rpm | 3895 ± 88 | 6938 ± 92 | 1.78 ± 0.03 |
| Composition | τm (s) | η0 (Pas) |
|---|---|---|
| PP | 1.123 | 3041 |
| PP/HT | 0.892 | 2120 |
| PP/PG7 | 1.001 | 1728 |
| PP/HT-Green 50 rpm | 0.708 | 2225 |
| PP/HT-Green 60 rpm | 0.795 | 1789 |
| PP/PR122 | 1.013 | 1829 |
| PP/HT-Red 50 rpm | 0.885 | 1942 |
| PP/HT-Red 60 rpm | 0.889 | 2014 |
| T2% °C | T5% °C | T10% °C | T20% °C | Tmax °C | |
|---|---|---|---|---|---|
| PP | 316 | 337 | 361 | 385 | 433 |
| PP/HT | 305 | 328 | 347 | 370 | 427 |
| PP/PG7 | 291 | 319 | 342 | 361 | 428 |
| PP/HT-Green 50 rpm | 296 | 324 | 347 | 370 | 431 |
| PP/HT-Green 60 rpm | 310 | 338 | 356 | 379 | 431 |
| PP/PR122 | 300 | 323 | 342 | 366 | 429 |
| PP/HT-Red 50 rpm | 300 | 324 | 347 | 370 | 437 |
| PP/HT-Red 60 rpm | 300 | 324 | 342 | 366 | 427 |
| Tm (°C) * | ΔHm (J·g−1) * | χc (%) * | |
|---|---|---|---|
| PP | 170 | 86.70 | 41.9 |
| PP/HT | 169 | 84.58 | 40.9 |
| PP/PG7 | 169 | 92.86 | 44.9 |
| PP/HT-Green 50 rpm | 168 | 100.79 | 48.8 |
| PP/HT-Green 60 rpm | 168 | 95.32 | 46.1 |
| PP/PR122 | 167 | 101.68 | 49.2 |
| PP/HT-Red 50 rpm | 169 | 94.16 | 45.6 |
| PP/HT-Red 60 rpm | 168 | 102.92 | 49.8 |
| Tc (°C) * | Hc (J·g−1) * | Tm (°C) * | ΔHm (J·g−1) * | χc (%) * | |
|---|---|---|---|---|---|
| PP/HT | |||||
| PP/PG7 | 119.7 | 96.47 | 166.5 | 91.95 | 44.5 |
| PP/HT-Green 50 rpm | 127.2 | 92.95 | 170.8 | 81.7 | 39.6 |
| PP/HT-Green 60 rpm | 128.0 | 102.14 | 169.1 | 89.24 | 43.2 |
| PP/PR122 | 127.8 | 95.24 | 170.5 | 84.26 | 40.8 |
| PP/HT-Red 50 rpm | 129.6 | 100.94 | 170.3 | 87.74 | 42.5 |
| PP/HT-Red 60 rpm | 129.1 | 98.95 | 172.2 | 83.45 | 40.4 |
| 129.1 | 98.63 | 169.7 | 88.99 | 43.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, M.; Lipińska, M.; Okraska, M.; Pietrasik, J. Polypropylene Color Masterbatches Containing Layered Double Hydroxide Modified with Quinacridone and Phthalocyanine Pigments—Rheological, Thermal and Application Properties. Materials 2023, 16, 6243. https://doi.org/10.3390/ma16186243
Kozłowska M, Lipińska M, Okraska M, Pietrasik J. Polypropylene Color Masterbatches Containing Layered Double Hydroxide Modified with Quinacridone and Phthalocyanine Pigments—Rheological, Thermal and Application Properties. Materials. 2023; 16(18):6243. https://doi.org/10.3390/ma16186243
Chicago/Turabian StyleKozłowska, Magdalena, Magdalena Lipińska, Michał Okraska, and Joanna Pietrasik. 2023. "Polypropylene Color Masterbatches Containing Layered Double Hydroxide Modified with Quinacridone and Phthalocyanine Pigments—Rheological, Thermal and Application Properties" Materials 16, no. 18: 6243. https://doi.org/10.3390/ma16186243
APA StyleKozłowska, M., Lipińska, M., Okraska, M., & Pietrasik, J. (2023). Polypropylene Color Masterbatches Containing Layered Double Hydroxide Modified with Quinacridone and Phthalocyanine Pigments—Rheological, Thermal and Application Properties. Materials, 16(18), 6243. https://doi.org/10.3390/ma16186243







