Adhesion Performance of Rubber Modified Asphalt in Chip Seal: A Molecular Dynamic Study
Abstract
:1. Introduction
2. Methodology
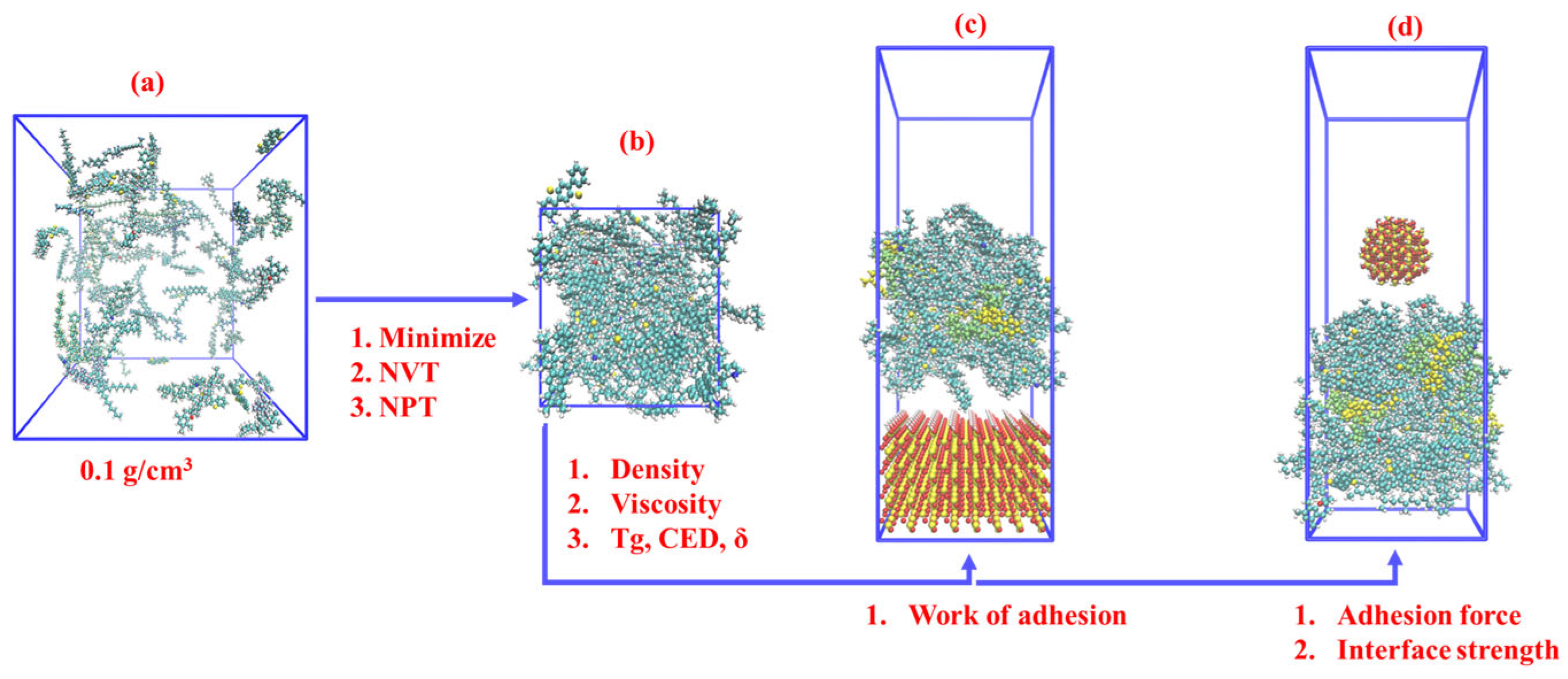
2.1. Molecular Model for Rubber-Modified Asphalt
2.2. Molecular Model Verification Parameter
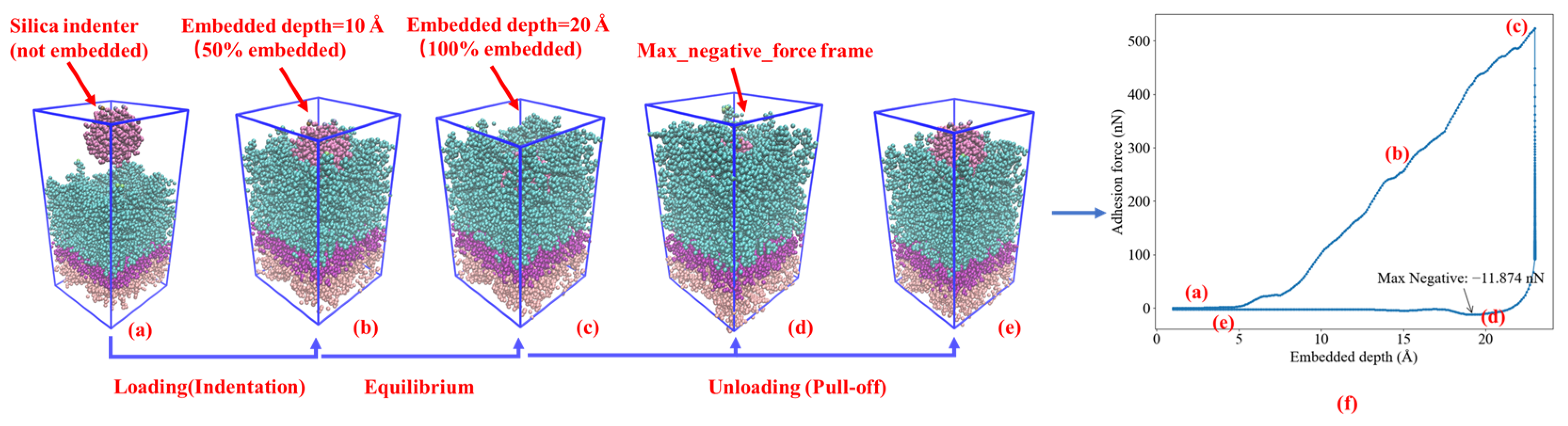
2.3. Nanoindentation Simulation
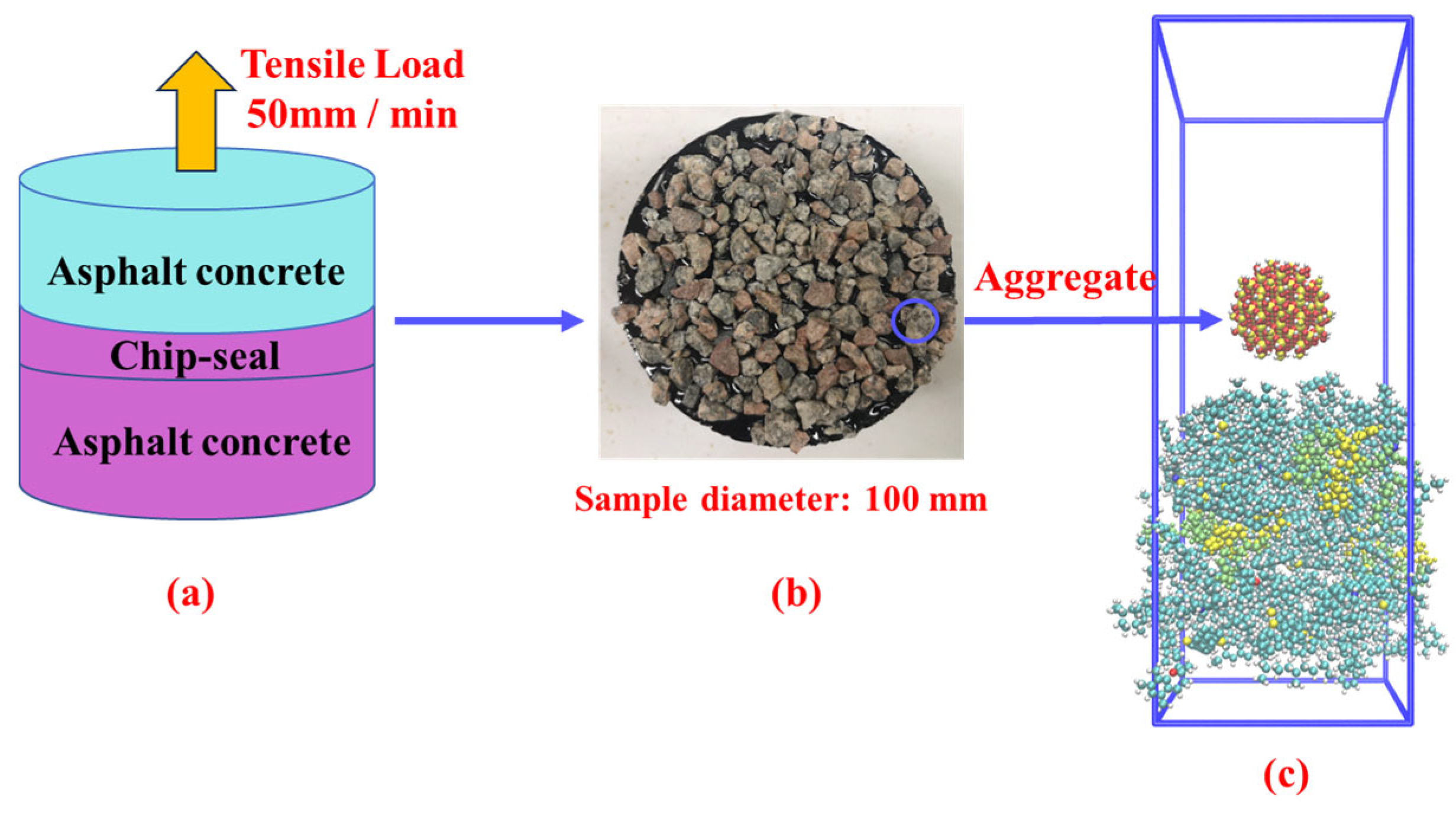
2.4. Experiment Validation
3. Results
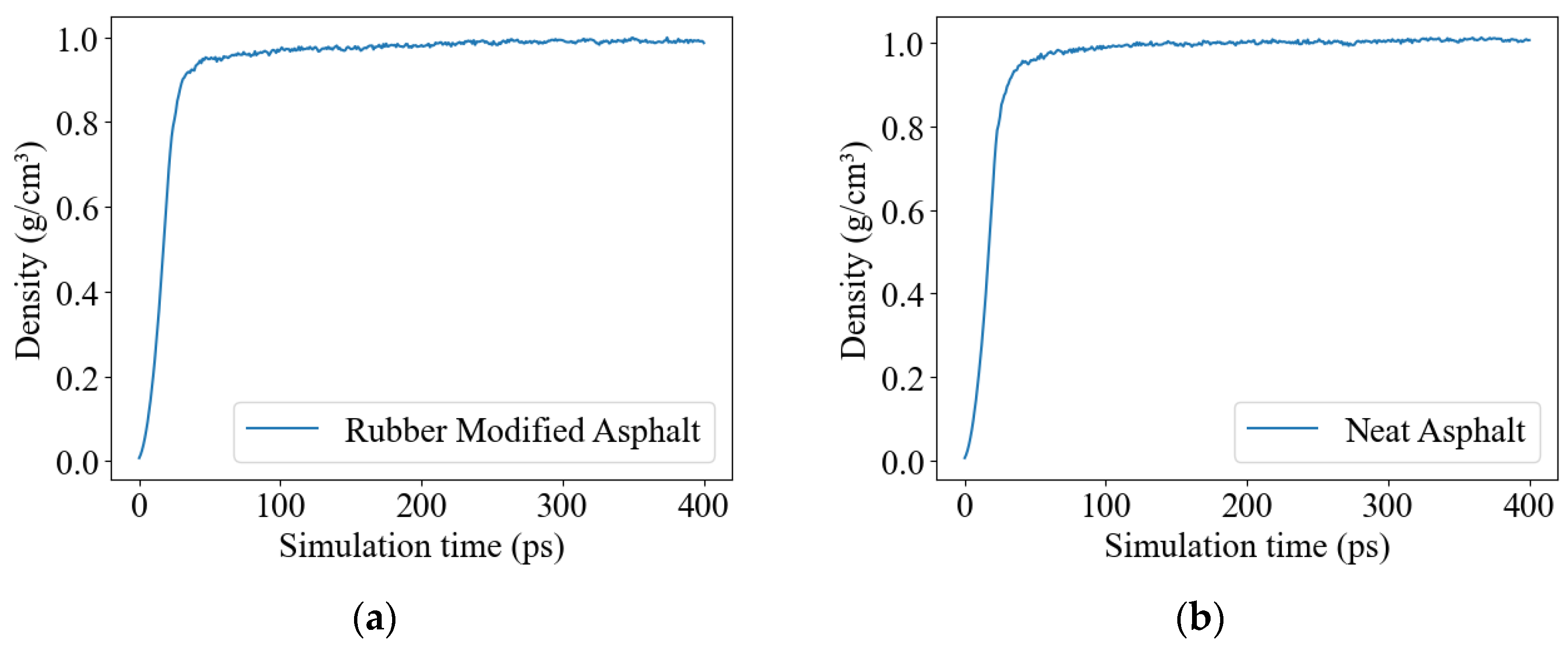
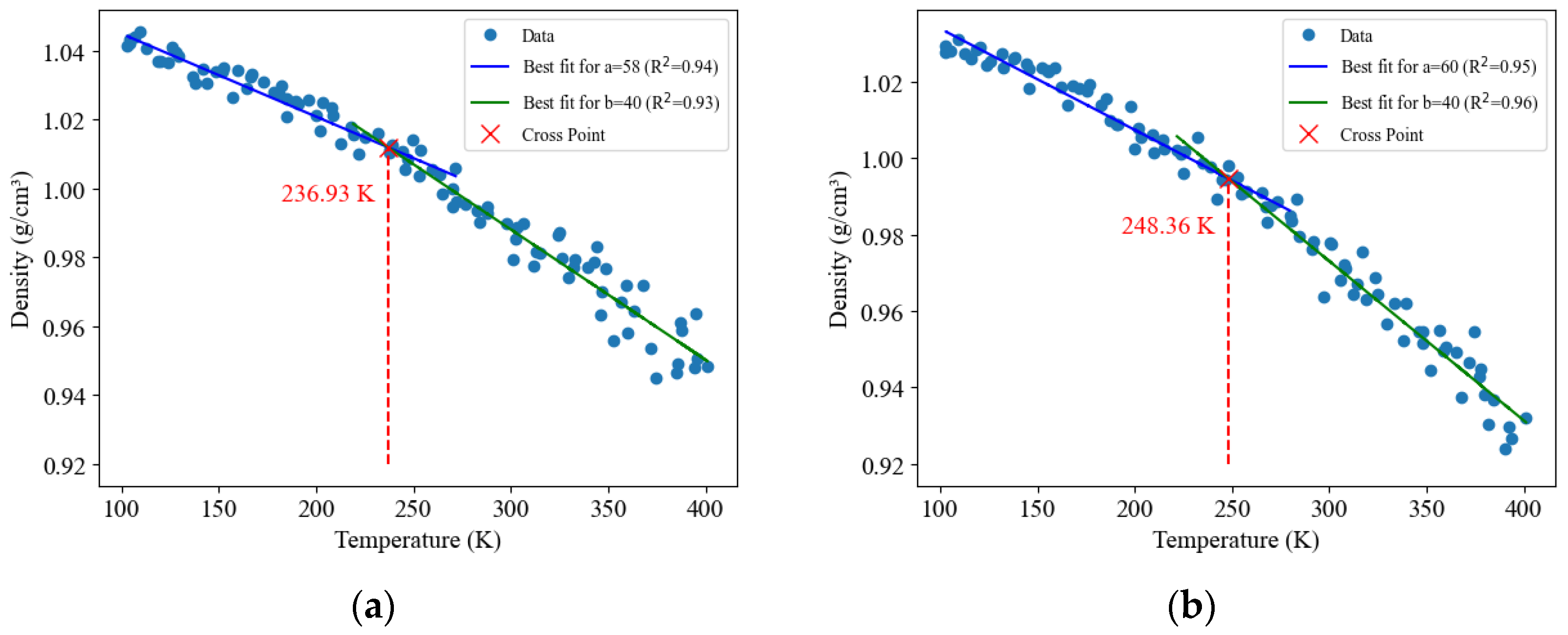
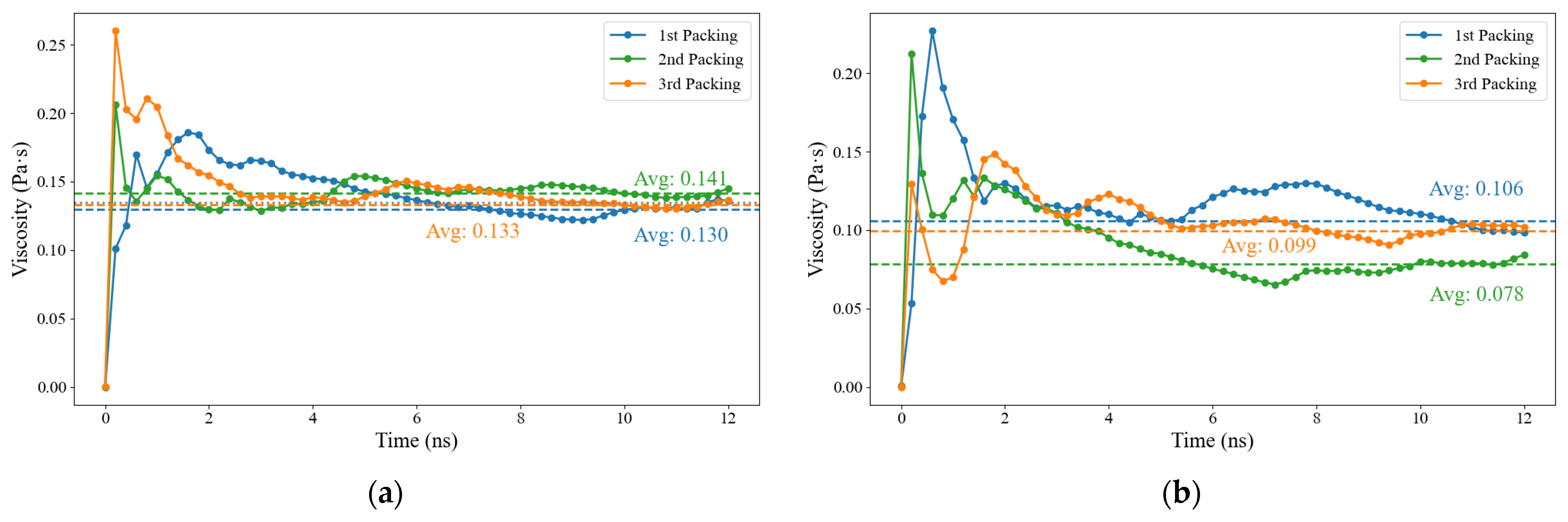
3.1. Molecular Model Verification
3.2. Silica-Asphalt Adhesion from Nanoindentation Simulation
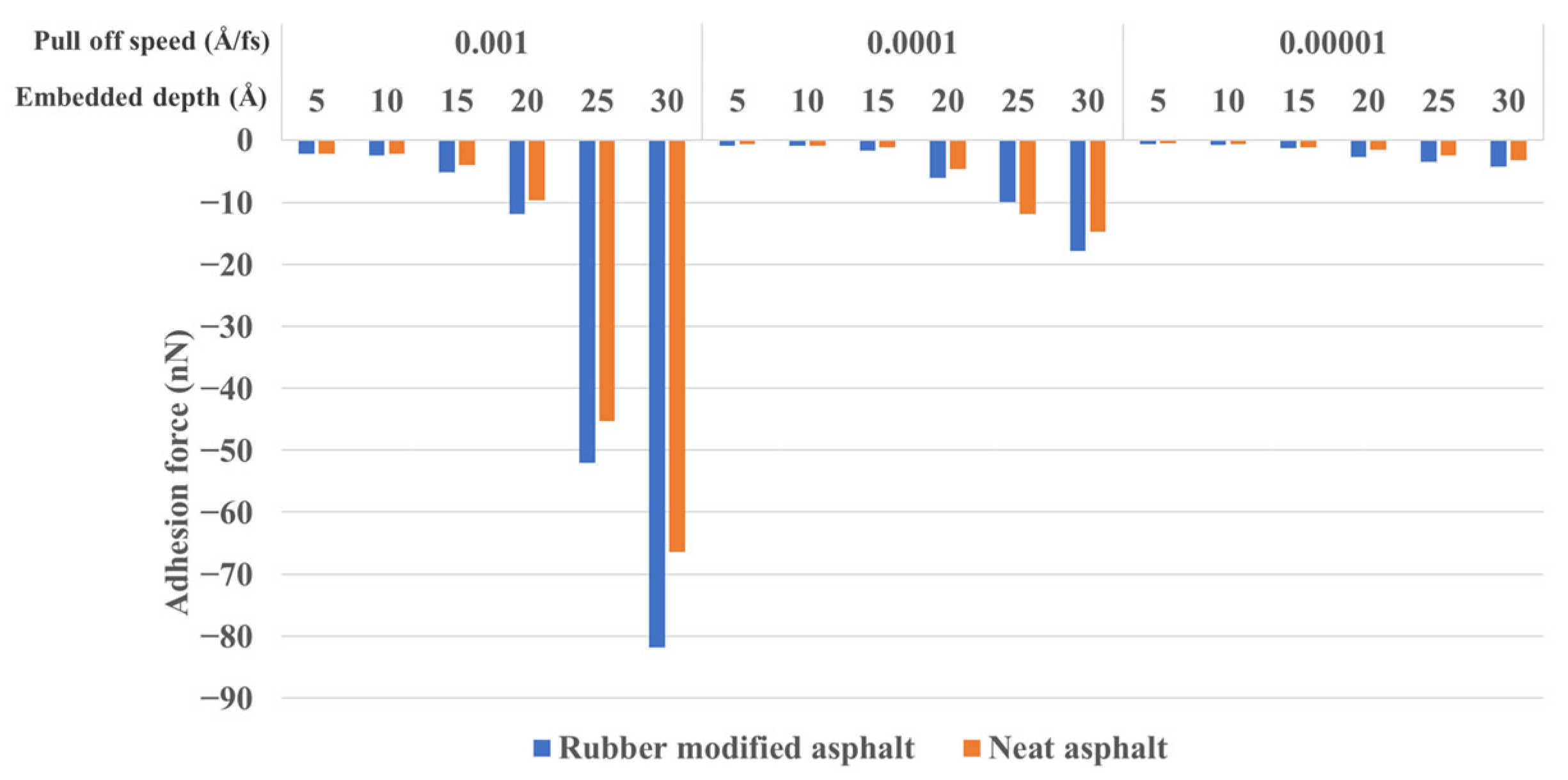
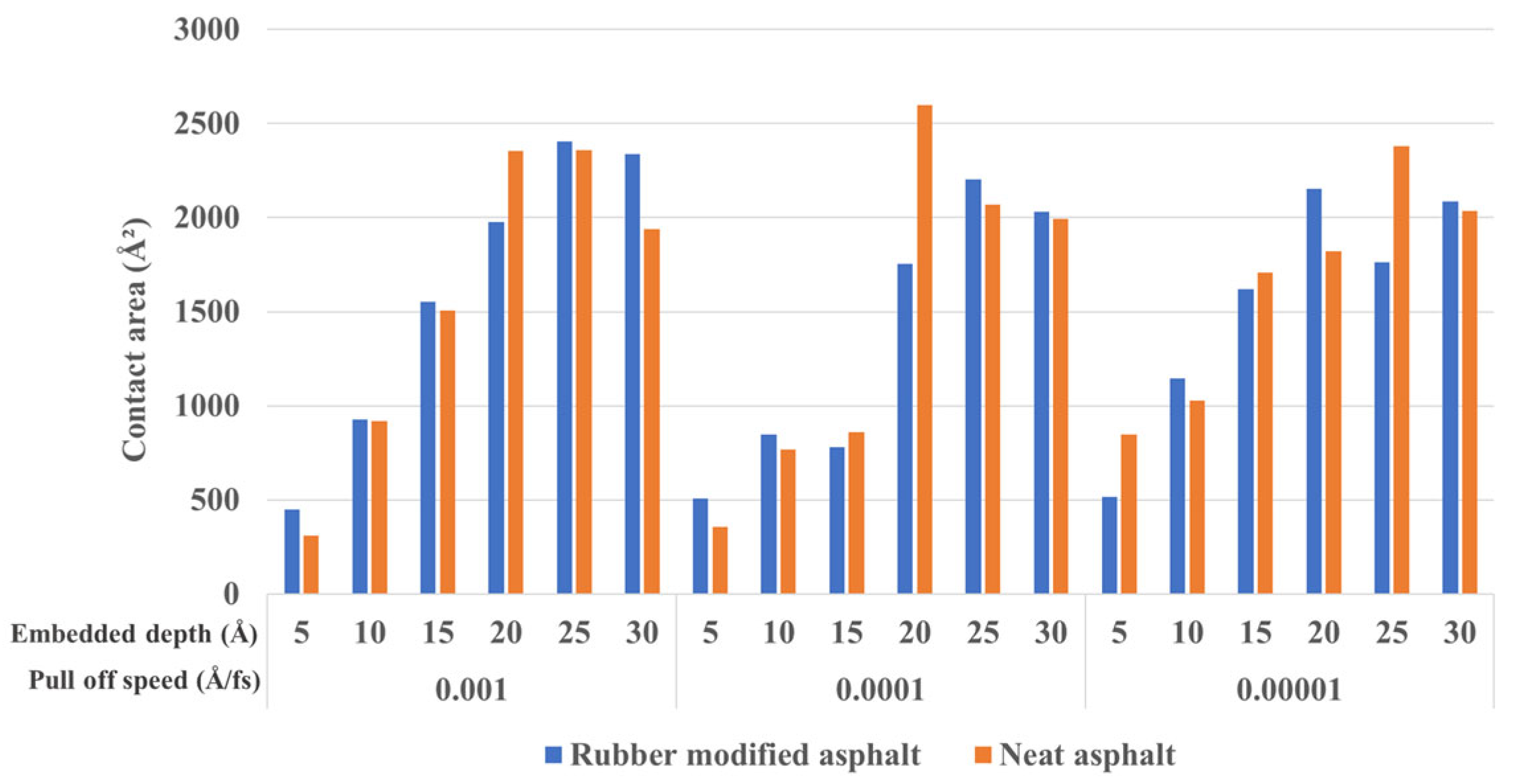
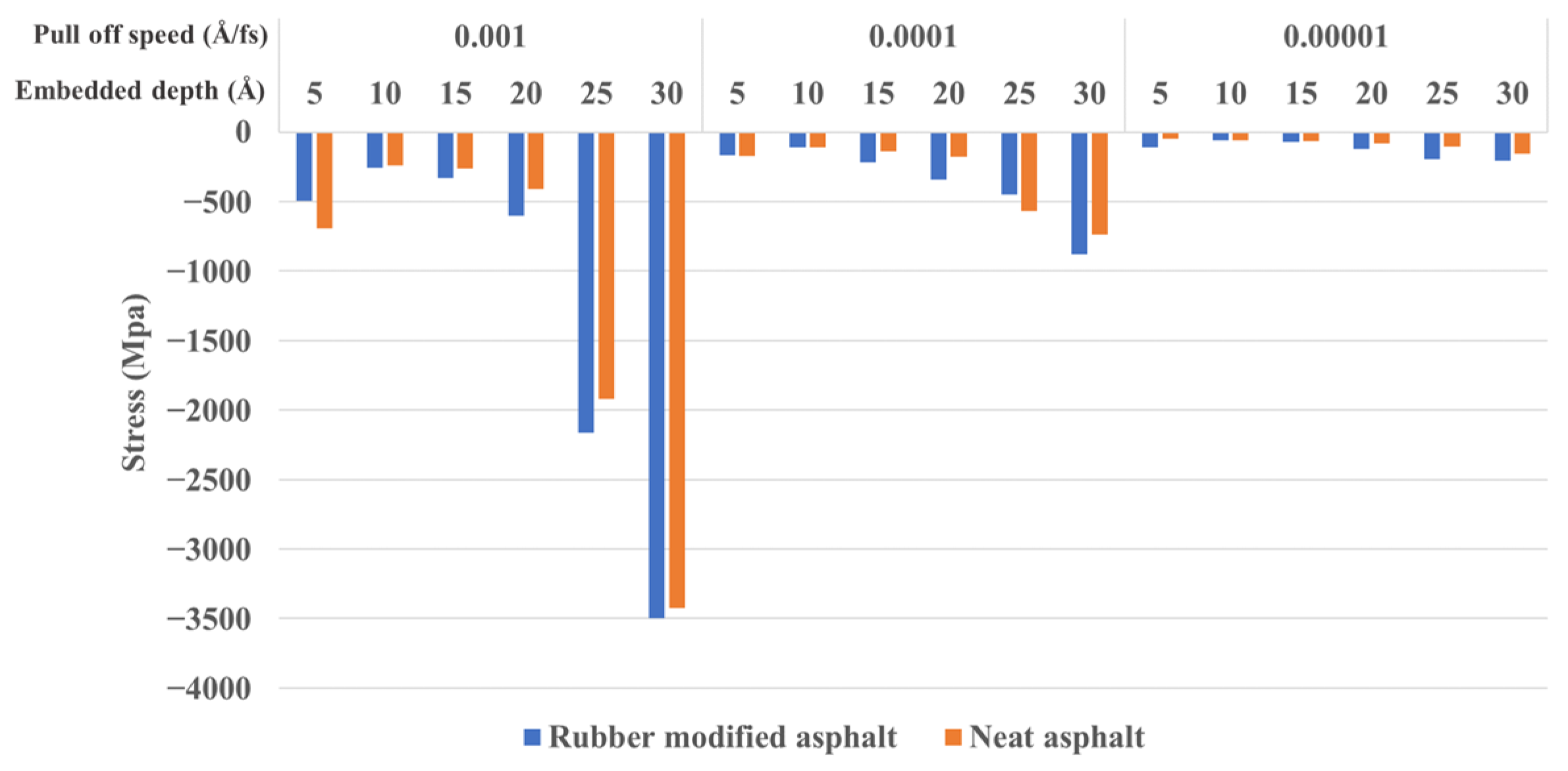
3.2.1. Embedded Depth and Pull-Off Speed Effect on Silica-Asphalt Adhesion
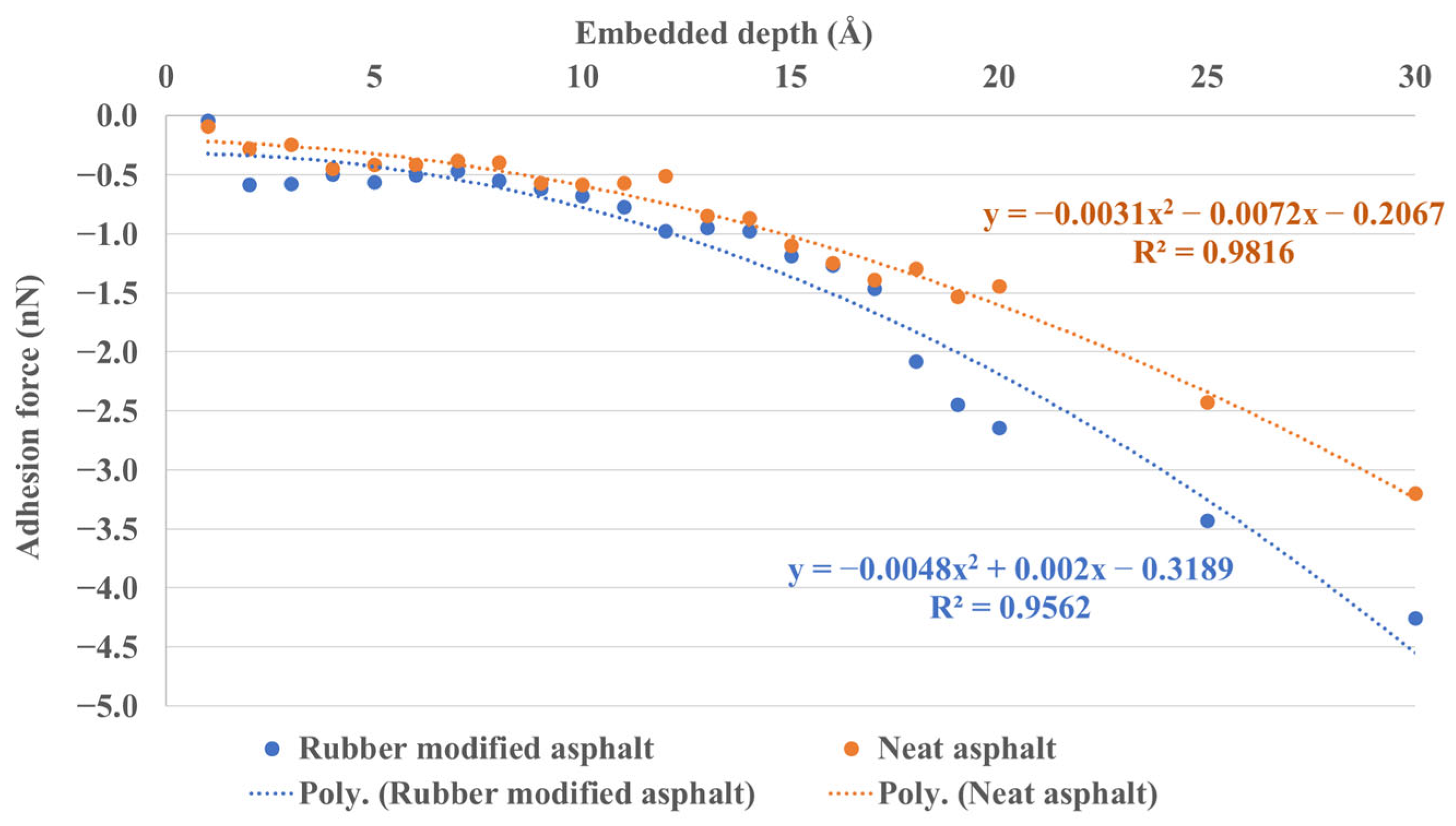
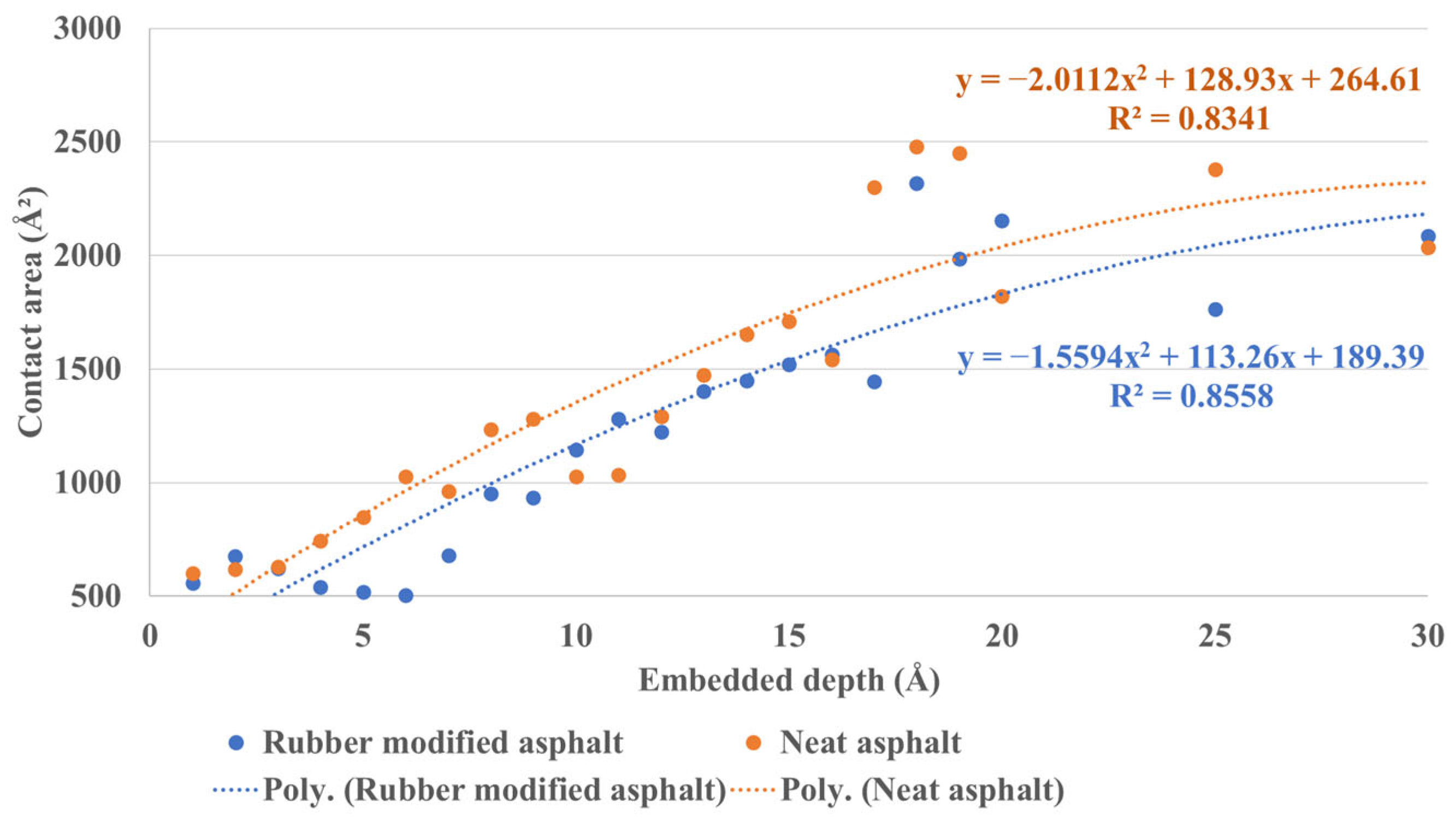
3.2.2. Silica–Asphalt Adhesion Simulated under Continuous Embedded Depth
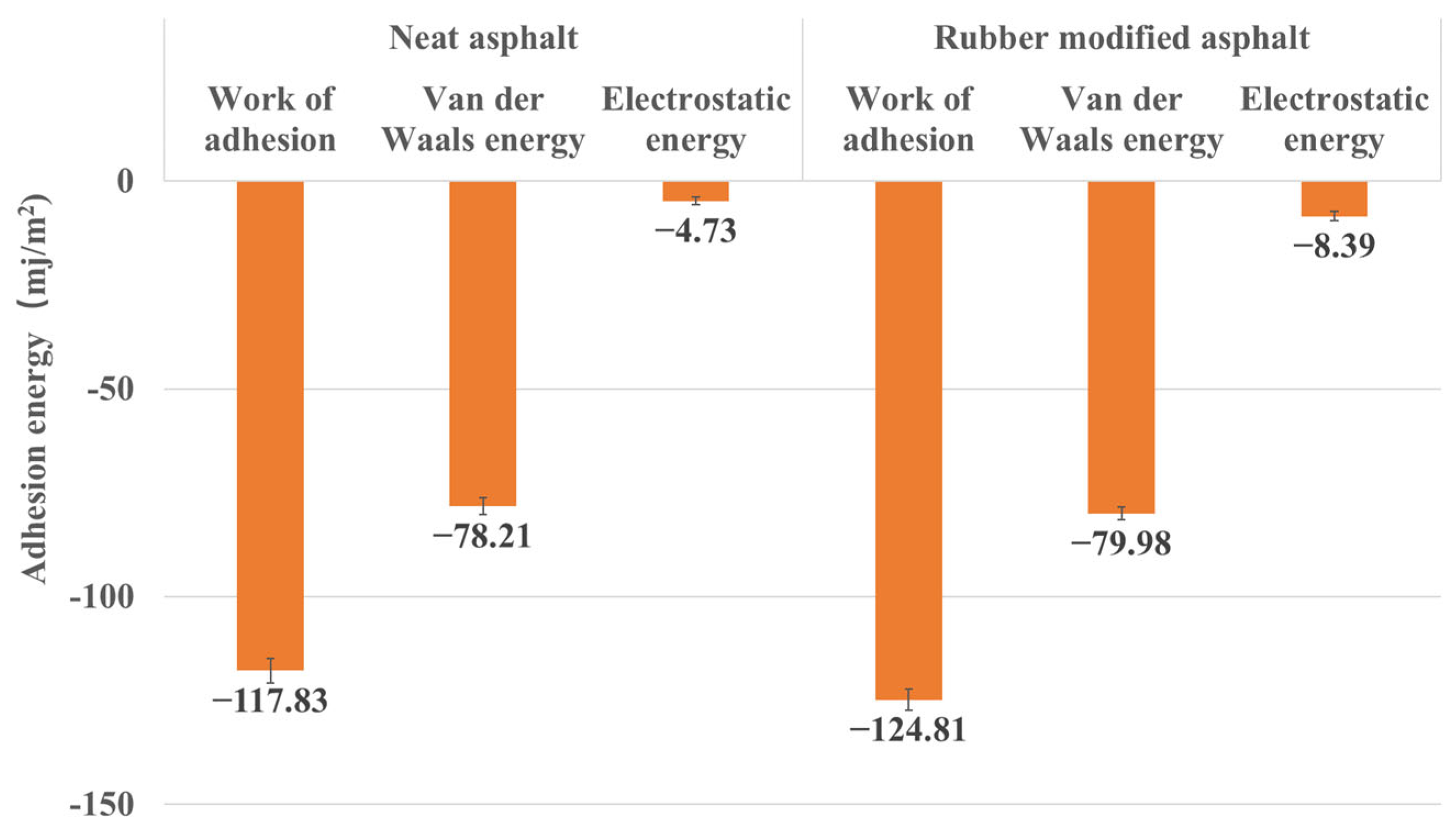
3.2.3. Work of Adhesion between Asphalt and Silica
4. Discussion
5. Conclusions
- Rubber-modified asphalt exhibits slightly lower density, cohesive energy density, and solubility parameters and obviously lower glass transition temperature compared to neat asphalt, which is attributed to the inclusion of rubber. Conversely, the viscosity of rubber-modified asphalt is slightly higher than that of neat asphalt.
- In the nanoindentation model, the adhesion force between silica and asphalt increases proportionally with the embedded depth and pull-off speed. The contact area of the silica-asphalt corresponding to the maximum adhesion force increases with the embedded depth while showing no significant correlation with the pull-off speed. Furthermore, the silica–asphalt interface stress displays an increment with both embedded depth and pull-off rate. This indicates that deeper embedded aggregates in the asphalt layer exhibit stronger adhesion at the molecular level in the chip seal application.
- The simulation results based on continuous embedded depth reveal a quadratic regression relationship between adhesion force, interface stress, and indentation depth.
- The work of adhesion results demonstrate that the adhesion between rubber-modified asphalt and silica is slightly stronger than that between neat asphalt and silica, thereby validating the concordance between energy (work of adhesion) and mechanical outcomes (adhesion force).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, B.A.; Willis, J.R.; Shacat, J. Asphalt Pavement Industry Survey on Recycled Materials and Warm-Mix Asphalt Usage: 2019; NAPA: Greenbelt, MD, USA, 2020. [Google Scholar]
- Johnson, A.M. Best Practices Handbook on Asphalt Pavement Maintenance; University of Minnesota, Center for Transportation Studies: Minneapolis, MN, USA, 2000. [Google Scholar]
- You, L.; You, Z.; Dai, Q.; Xie, X.; Washko, S.; Gao, J. Investigation of adhesion and interface bond strength for pavements underlying chip-seal: Effect of asphalt-aggregate combinations and freeze-thaw cycles on chip-seal. Constr. Build. Mater. 2019, 203, 322–330. [Google Scholar] [CrossRef]
- Gransberg, D.D.; James, D.M. Chip Seal Best Practices; Transportation Research Board: Washington, DC, USA, 2005; Volume 342. [Google Scholar]
- Jin, D.; Yin, L.; Xin, K.; You, Z. Comparison of Asphalt Emulsion-Based Chip Seal and Hot Rubber Asphalt-Based Chip Seal. Case Stud. Constr. Mater. 2023, 18, e02175. [Google Scholar] [CrossRef]
- Elseifi, M.A.; Rahman, N.; Sarkar, T.; Abohamer, H. Evaluation of Asphalt Rubber and Reclaimed Tire Rubber in Chip Seal Applications; Louisiana Department of Transportation and Development: Baton Rouge, LA, USA, 2022. [Google Scholar]
- Abedini, M.; Hassani, A.; Kaymanesh, M.R.; Yousefi, A.A. Low-temperature adhesion performance of polymer-modified Bitumen emulsion in chip seals using different SBR latexes. Pet. Sci. Technol. 2017, 35, 59–65. [Google Scholar] [CrossRef]
- Drelich, J.; Mittal, K.L. Atomic Force Microscopy in Adhesion Studies; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Landman, U.; Luedtke, W.; Burnham, N.A.; Colton, R.J. Atomistic mechanisms and dynamics of adhesion, nanoindentation, and fracture. Science 1990, 248, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Luan, Y.; Ma, T.; Wang, S.; Chen, J.; Li, J.; Wu, M. Multilevel analysis of the aging mechanisms and performance evolution of rubber-modified asphalt. J. Mater. Civ. Eng. 2021, 33, 04021365. [Google Scholar] [CrossRef]
- Wu, M.; Xu, G.; Luan, Y.; Zhu, Y.; Ma, T.; Zhang, W. Molecular dynamics simulation on cohesion and adhesion properties of the emulsified cold recycled mixtures. Constr. Build. Mater. 2022, 333, 127403. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Zhang, L.; Greenfield, M.L. Relaxation time, diffusion, and viscosity analysis of model asphalt systems using molecular simulation. J. Chem. Phys. 2007, 127, 194502. [Google Scholar] [CrossRef]
- Corbett, L.W. Composition of asphalt based on generic fractionation, using solvent deasphaltening, elution-adsorption chromatography, and densimetric characterization. Anal. Chem. 1969, 41, 576–579. [Google Scholar] [CrossRef]
- Drobny, J.G. Handbook of Thermoplastic Elastomers; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Tur Rasool, R.; Song, P.; Wang, S. Thermal analysis on the interactions among asphalt modified with SBS and different degraded tire rubber. Constr. Build. Mater. 2018, 182, 134–143. [Google Scholar] [CrossRef]
- Mondello, M.; Grest, G.S. Viscosity calculations of n-alkanes by equilibrium molecular dynamics. J. Chem. Phys. 1997, 106, 9327–9336. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D. LAMMPS-a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Jewett, A.I.; Stelter, D.; Lambert, J.; Saladi, S.M.; Roscioni, O.M.; Ricci, M.; Autin, L.; Maritan, M.; Bashusqeh, S.M.; Keyes, T. Moltemplate: A tool for coarse-grained modeling of complex biological matter and soft condensed matter physics. J. Mol. Biol. 2021, 433, 166841. [Google Scholar] [CrossRef] [PubMed]
- Dodda, L.S.; Cabeza de Vaca, I.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen web server: An automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Bhasin, A.; Little, D.N.; Vasconcelos, K.L.; Masad, E. Surface free energy to identify moisture sensitivity of materials for asphalt mixes. Transp. Res. Rec. 2007, 2001, 37–45. [Google Scholar] [CrossRef]
- Xu, G.; Yao, Y.; Wu, M.; Zhao, Y. Molecular simulation and experimental analysis on co-aging behaviors of SBS modifier and asphalt in SBS-modified asphalt. Mol. Simul. 2023, 49, 629–642. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Z.-J.; Tan, Y.-Q.; Liu, Z.-Y. Investigating the Interactions of the Saturate, Aromatic, Resin, and Asphaltene Four Fractions in Asphalt Binders by Molecular Simulations. Energy Fuels 2015, 29, 112–121. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Apostolidis, P.; Wang, D.; Leng, Z.; Lu, G.; Erkens, S.; Skarpas, A. Investigating the high-and low-temperature performance of warm crumb rubber–modified bituminous binders using rheological tests. J. Transp. Eng. Part B Pavements 2021, 147, 04021067. [Google Scholar] [CrossRef]
- Li, P.; Jiang, X.; Ding, Z.; Zhao, J.; Shen, M. Analysis of viscosity and composition properties for crumb rubber modified asphalt. Constr. Build. Mater. 2018, 169, 638–647. [Google Scholar] [CrossRef]
- Jing, Y.; Cui, Z.; Zou, H.; Tu, J.; Jiang, X.; Shi, X.; Yong, Z.; Liu, S.; Liu, G. Three-dimensional solubility parameters of natural rubber and its predictive power in diffusion coefficients. J. Appl. Polym. Sci. 2022, 139, 51473. [Google Scholar] [CrossRef]
- Loadman, M. The glass transition temperature of natural rubber. J. Therm. Anal. Calorim. 1985, 30, 929–941. [Google Scholar] [CrossRef]
- Lindemann, N.; Schawe, J.E.; Lacayo-Pineda, J. Kinetics of the glass transition of styrene-butadiene-rubber: Dielectric spectroscopy and fast differential scanning calorimetry. J. Appl. Polym. Sci. 2021, 138, 49769. [Google Scholar] [CrossRef]
- Koh, S.; Lee, H.; Lu, C.; Cheng, Q. Molecular dynamics simulation of a solid platinum nanowire under uniaxial tensile strain: Temperature and strain-rate effects. Phys. Rev. B 2005, 72, 085414. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H. Molecular dynamics study of interfacial mechanical behavior between asphalt binder and mineral aggregate. Constr. Build. Mater. 2016, 121, 246–254. [Google Scholar] [CrossRef]

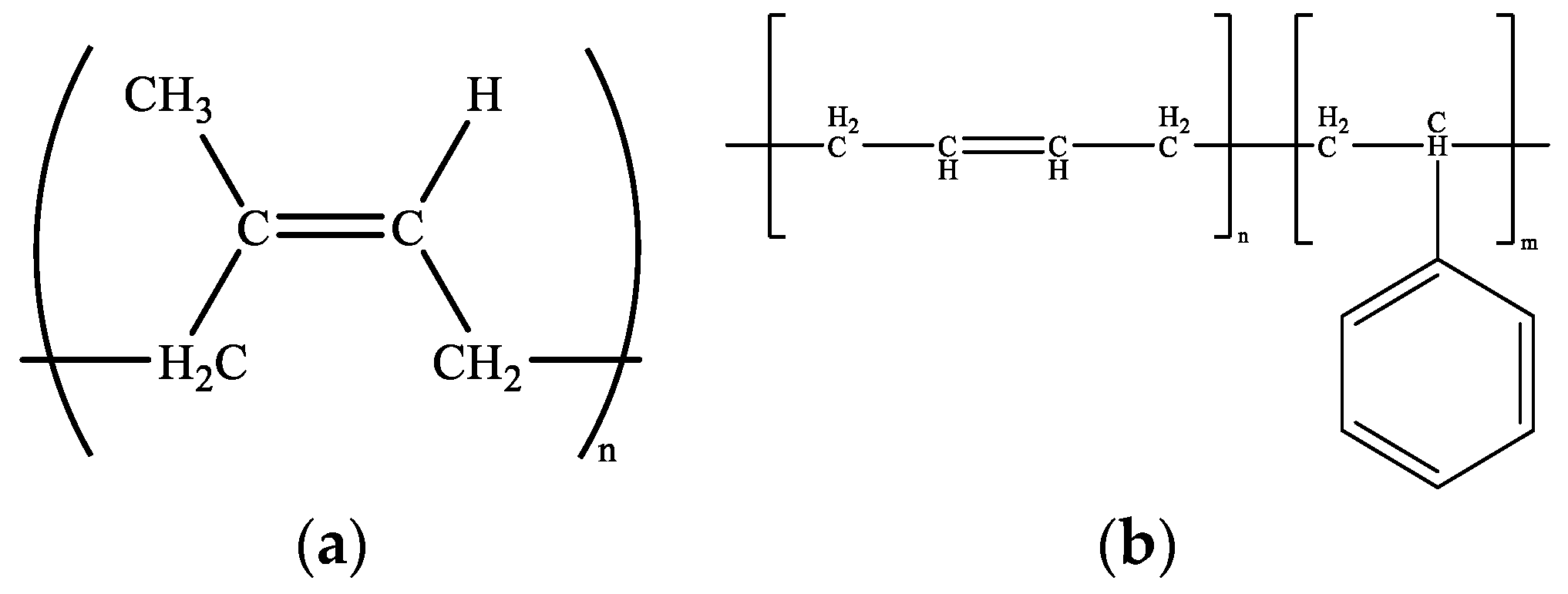



| Component | Number of Molecules | Weight % | |
|---|---|---|---|
| Saturate | Squalane | 4 | 9.83 |
| Hopane | 4 | ||
| Aromatic | Perhydrophenanthrene-naphthalene | 11 | 28.22 |
| Dioctyl-cyclohexane-naphthalene | 13 | ||
| Resin | Quinolinohopane | 4 | 35.15 |
| Thioisorenieratane | 4 | ||
| Trimethylbenzeneoxane | 5 | ||
| Pyridinohopane | 4 | ||
| Benzobisbenzothiophene | 15 | ||
| Asphaltene | Asphaltene-phenol | 3 | 15.19 |
| Asphaltene-pyrrole | 2 | ||
| Asphaltene-thiophene | 3 | ||
| Rubber | NR (natural rubber) | 6 | 11.61 |
| SBR (styrene butadiene rubber) | 3 |
| Rubber Modified Asphalt | Neat Asphalt | ||
|---|---|---|---|
| Embedded Depth (Å) | |||
| Pull-off speed (Å/fs) | 0.001 | 5, 10, 15, 20, 25, 30 | 5, 10, 15, 20, 25, 30 |
| 1 × 10−4 | 5, 10, 15, 20, 25, 30 | 5, 10, 15, 20, 25, 30 | |
| 1 × 10−5 | 1–20, 25, 30 | 1–20, 25, 30 | |
| 1 × 10−6 | 15 | / | |
| Rubber Modified Asphalt | References | Neat Asphalt | References | |
|---|---|---|---|---|
| Density (g/cm3) | 0.99 | / | 1.01 | 0.99–1.15 [11,28] |
| Cohesive energy density (J/m3) | 3.95 × 108 | / | 3.98 × 108 | 3.19–4.00 × 108 [11,29] |
| Solubility parameter (J/cm3)0.5 | 19.88 | / | 19.94 | 18–22.5 [11,29] |
| Viscosity (Pa·s, 408.15 K) | 0.135 | 0.1–3.6 [13,30] | 0.095 | 0.06–0.12 [13,30] |
| Glass transition temperature Tg (K) | Around 236.93 | 233–255 [17] | Around 248.36 | 255–282 [17,31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; You, Z.; Jin, D. Adhesion Performance of Rubber Modified Asphalt in Chip Seal: A Molecular Dynamic Study. Materials 2023, 16, 6324. https://doi.org/10.3390/ma16186324
Wu M, You Z, Jin D. Adhesion Performance of Rubber Modified Asphalt in Chip Seal: A Molecular Dynamic Study. Materials. 2023; 16(18):6324. https://doi.org/10.3390/ma16186324
Chicago/Turabian StyleWu, Meng, Zhanping You, and Dongzhao Jin. 2023. "Adhesion Performance of Rubber Modified Asphalt in Chip Seal: A Molecular Dynamic Study" Materials 16, no. 18: 6324. https://doi.org/10.3390/ma16186324
APA StyleWu, M., You, Z., & Jin, D. (2023). Adhesion Performance of Rubber Modified Asphalt in Chip Seal: A Molecular Dynamic Study. Materials, 16(18), 6324. https://doi.org/10.3390/ma16186324







