Marginal Adaptation of In Vitro Class II Restorations Made Out of Bulk or Conventional Composite Using Single- or Multi-Layered Techniques
Abstract
:1. Introduction
2. Materials and Method
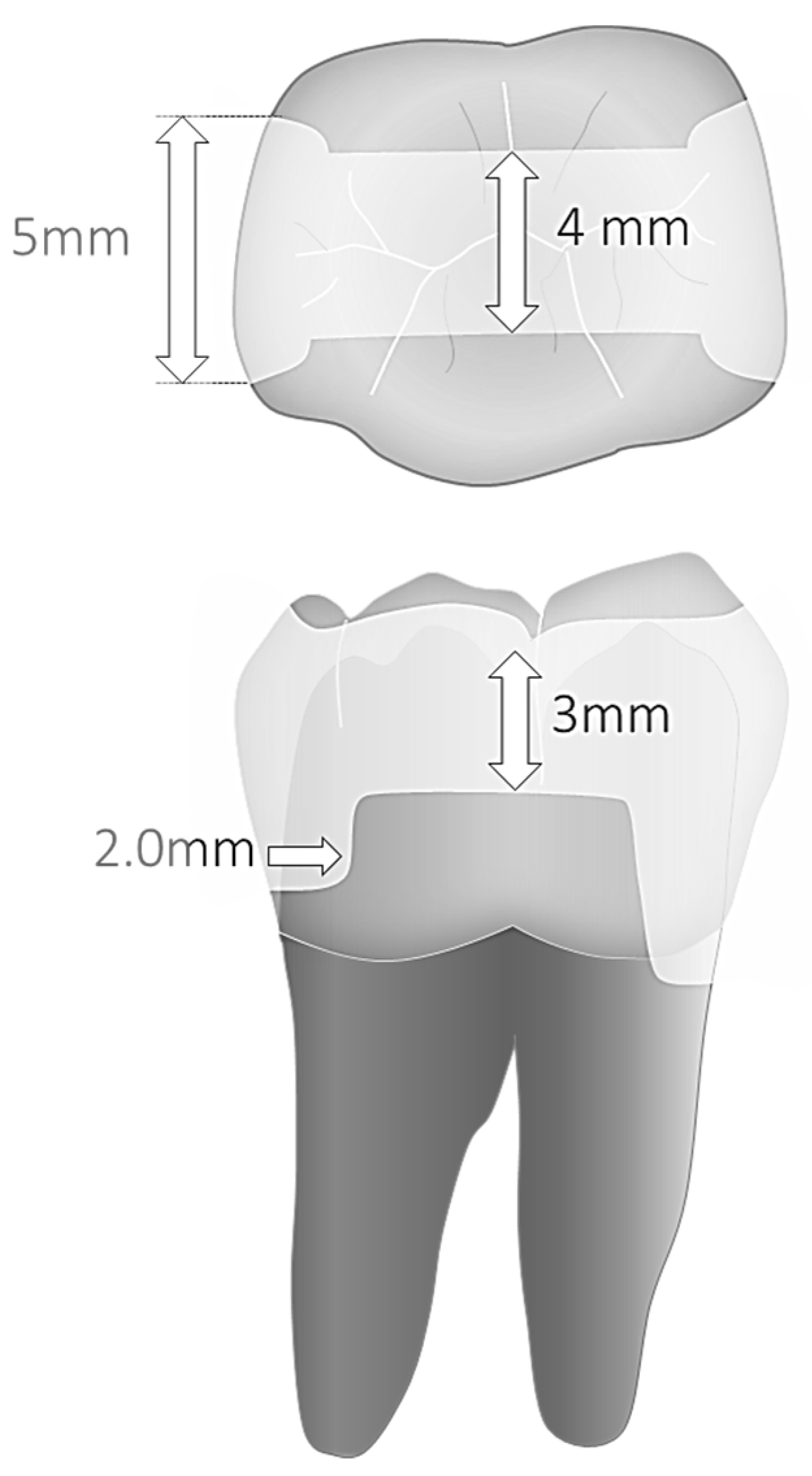
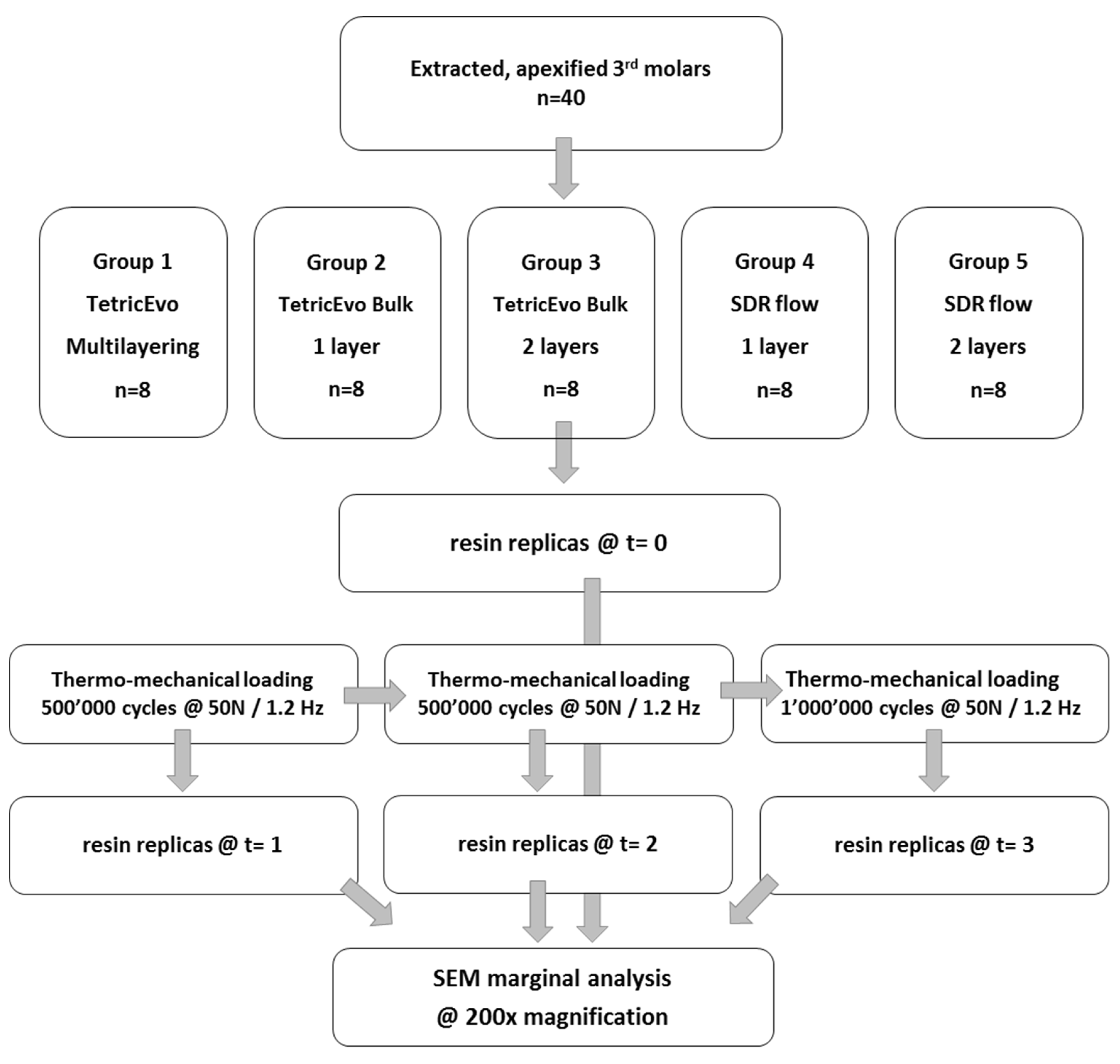
2.1. Basic Specimen Preparation and Group Distribution (New)
- Group 1: Multilayered restorations made of conventional nano-hybrid composite—Tetric EvoCeram (Ivoclar, Schaan, Liechtensetein) (control group);
- Group 2: Single layer restorations made of high-viscosity bulkfill composite—Tetric EvoCeram Bulk Fill (Ivoclar);
- Group 3: Trilaminated restorations made of high-viscosity bulkfill composite—Tetric EvoCeram Bulk Fill;
- Group 4: Bilaminated restorations made of one single increment of flowable bulkfill composite—Surefil SDR flow bulkfill (Sirona-Dentsply, Charlotte, NC, USA)—and one occlusal layer of conventional nano-hybrid composite Ceram-X (Sirona-Dentsply);
- Group 5: Trilaminated restorations made of two increments of flowable bulkfill composite—Surefil SDR flow bulkfill—and one occlusal layer of conventional nano-hybrid composite Ceram-X.
2.2. Restorative Procedures
2.2.1. Adhesive Procedures
2.2.2. Restorative Protocols
- Group 1 (control):
- Groups 2 and 3:
- Groups 4 and 5:
2.3. Thermomechanical Cycling
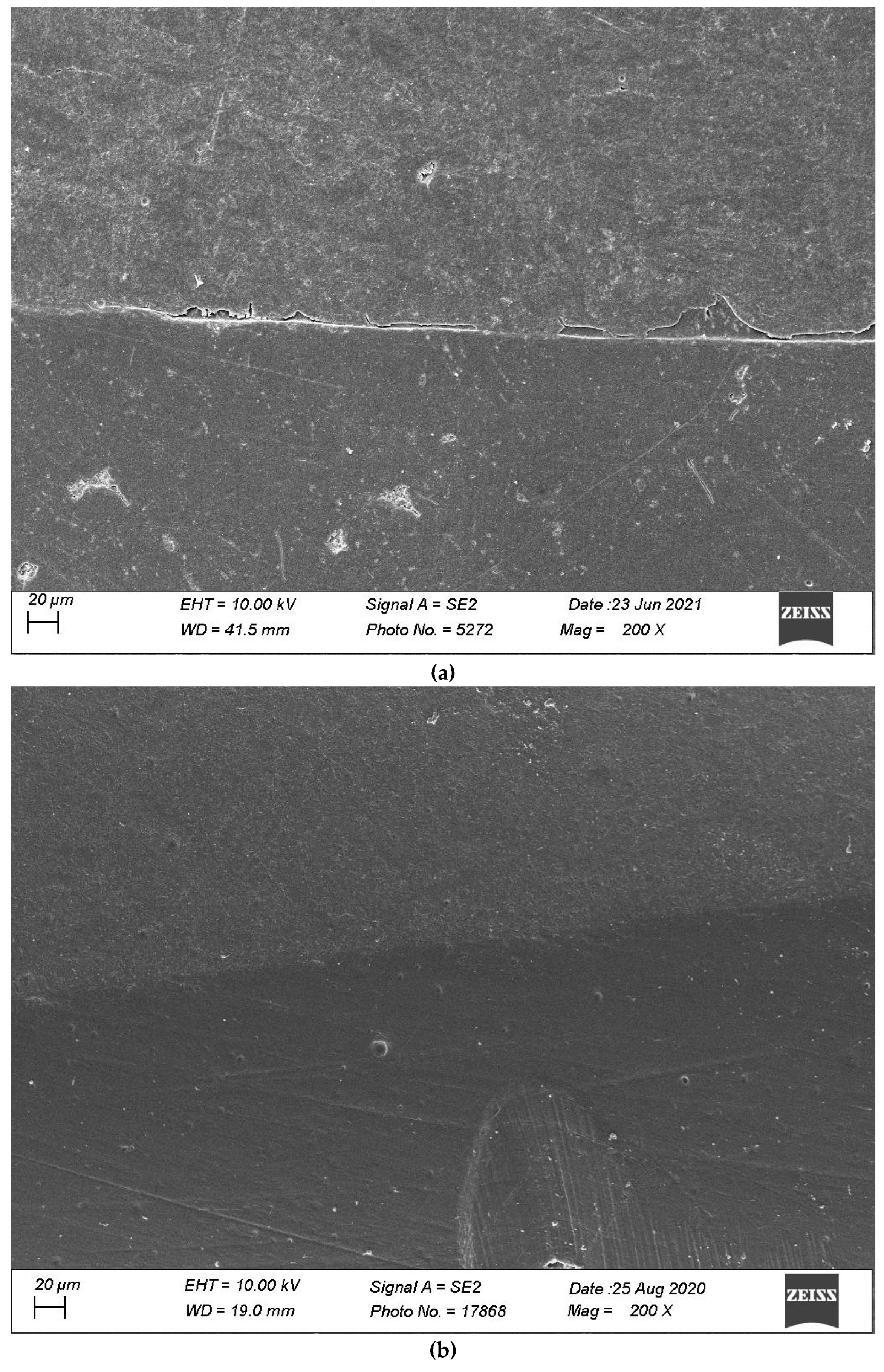
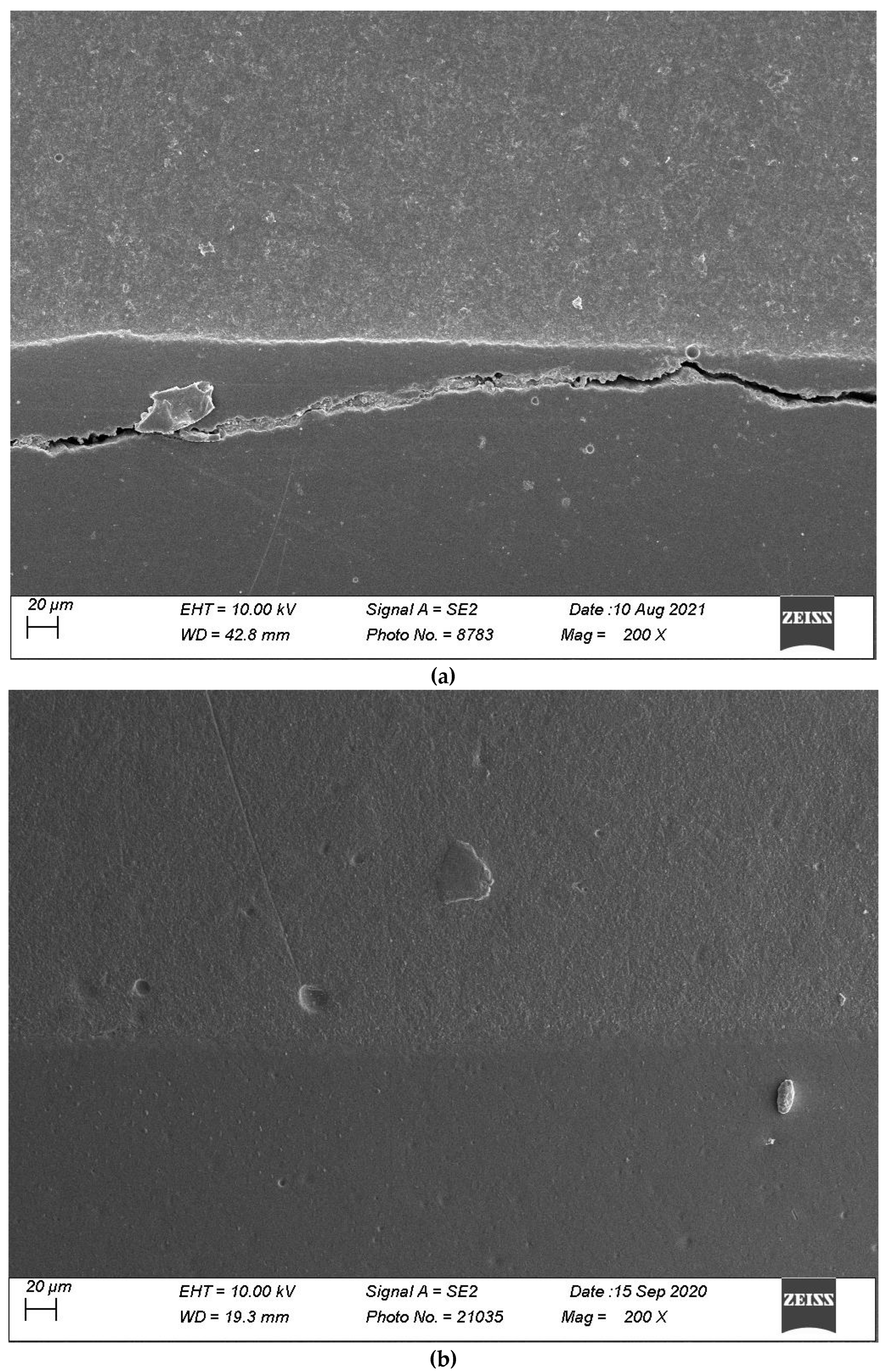
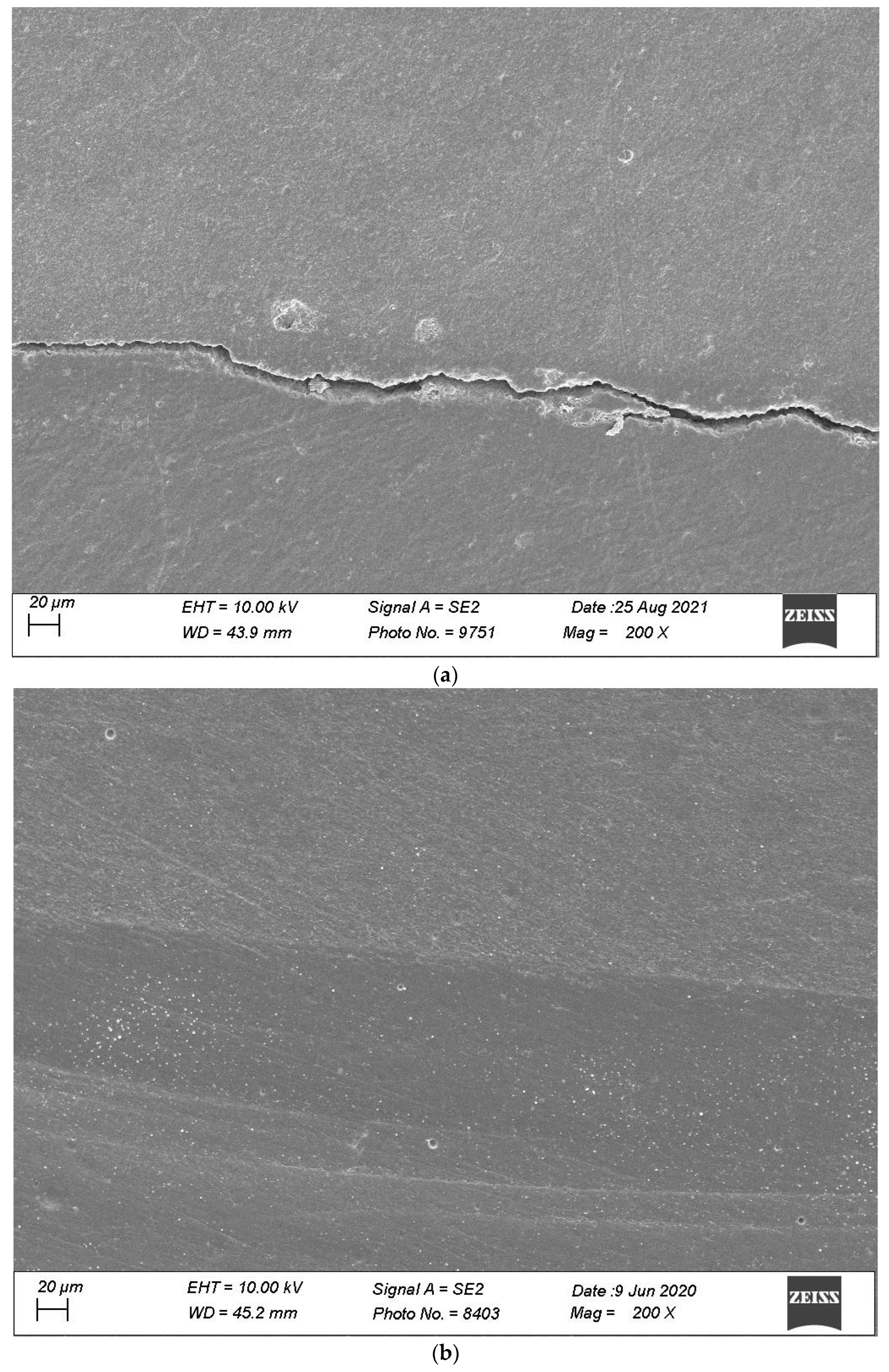
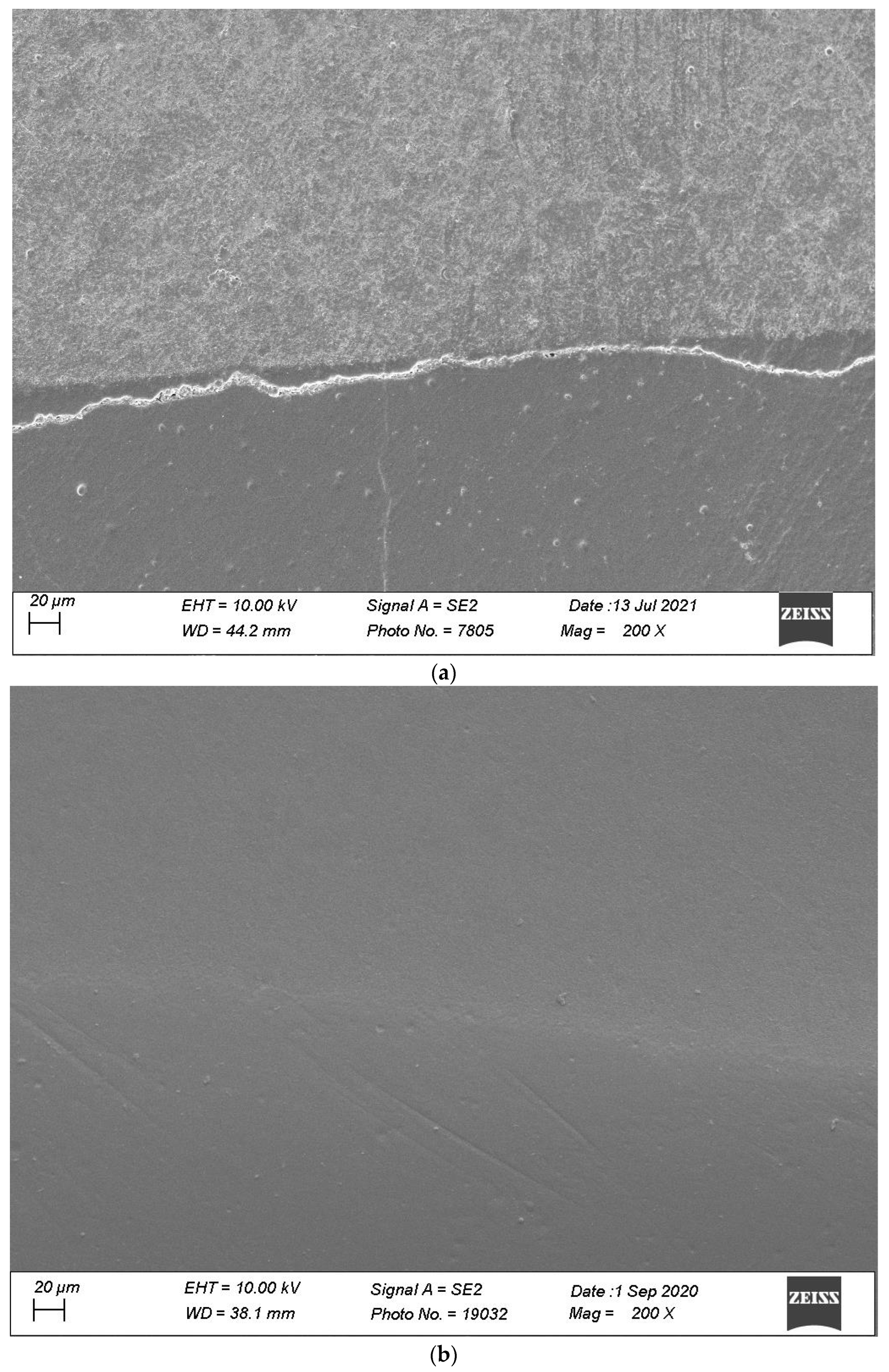
2.4. Specimen Evaluation
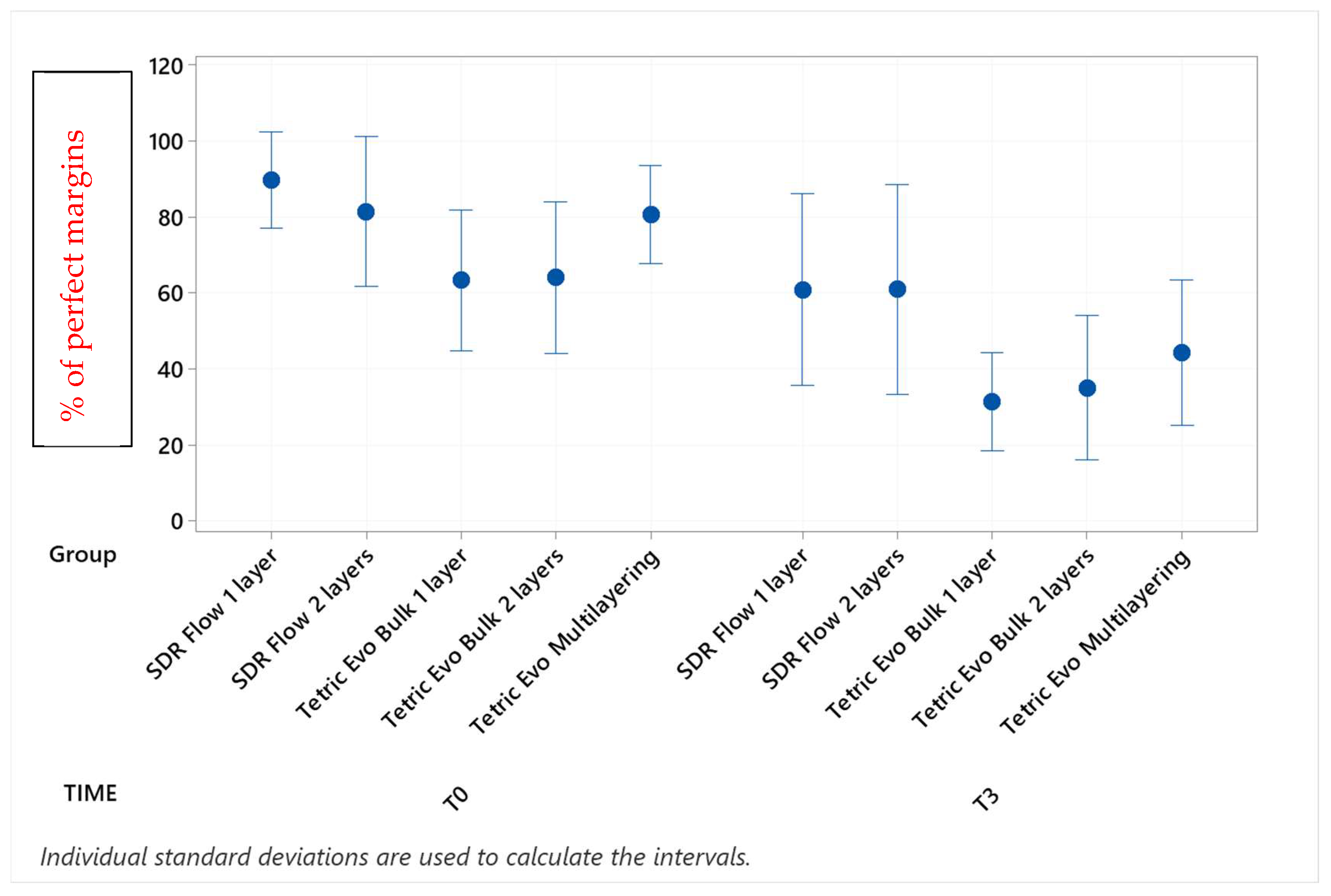
3. Results
4. Discussion
4.1. Impact of Thermomechanical Loading
4.2. Layering Protocols
4.3. Products and Inter-Group Comparisons
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellinaso, M.D.; Soares, F.Z.M.; Rocha, R.O. Do bulk-fill resins decrease the restorative time in posterior teeth? A systematic review and meta-analysis of in vitro studies. J. Investig. Clin. Dent. 2019, 10, e12463. [Google Scholar] [CrossRef]
- Chesterman, J.; Jowett, A.; Gallacher, A.; Nixon, P. Bulk-fill resin-based composite restorative materials: A review. Br. Dent. J. 2017, 222, 337–344. [Google Scholar] [CrossRef]
- Van Ende, A.; De Munck, J.; Lise, D.P.; Van Meerbeek, B. Bulk-Fill Composites: A Review of the Current Literature. J. Adhes. Dent. 2017, 19, 95–109. [Google Scholar]
- Reis, A.F.; Vestphal, M.; Amaral, R.C.D.; Rodrigues, J.A.; Roulet, J.F.; Roscoe, M.G. Efficiency of polymerization of bulk-fill composite resins: A systematic review. Braz. Oral Res. 2017, 31 (Suppl. S1), e59. [Google Scholar] [CrossRef]
- Lima, R.B.W.; Troconis, C.C.M.; Moreno, M.B.P.; Murillo-Gómez, F.; De Goes, M.F. Depth of cure of bulk fill resin composites: A systematic review. J. Esthet. Restor. Dent. 2018, 30, 492–501. [Google Scholar] [CrossRef]
- Rizzante, F.A.P.; Mondelli, R.F.L.; Furuse, A.Y.; Borges, A.F.S.; Mendonça, G.; Ishikiriama, S.K. Shrinkage stress and elastic modulus assessment of bulk-fill composites. J. Appl. Oral Sci. 2019, 27, e20180132. [Google Scholar] [CrossRef]
- Meereis, C.T.W.; Münchow, E.A.; de Oliveira da Rosa, W.L.; da Silva, A.F.; Piva, E. Polymerization shrinkage stress of resin-based dental materials: A systematic review and meta-analyses of composition strategies. J. Mech. Behav. Biomed. Mater. 2018, 82, 268–281. [Google Scholar] [CrossRef]
- Kim, R.J.; Kim, Y.J.; Choi, N.S.; Lee, I.B. Polymerization shrinkage, modulus, and shrinkage stress related to tooth-restoration interfacial debonding in bulk-fill composites. J. Dent. 2015, 43, 430–439. [Google Scholar] [CrossRef]
- Leprince, J.G.; Palin, W.M.; Vanacker, J.; Sabbagh, J.; Devaux, J.; Leloup, G. Physico-mechanical characteristics of commercially available bulk-fill composites. J. Dent. 2014, 42, 993–1000. [Google Scholar] [CrossRef]
- Czasch, P.; Ilie, N. In vitro comparison of mechanical properties and degree of cure of bulk fill composites. Clin. Oral Investig. 2013, 17, 227–235. [Google Scholar] [CrossRef]
- Ilie, N.; Bucuta, S.; Draenert, M. Bulk-fill Resin-based Composites: An In Vitro Assessment of Their Mechanical Performance. Oper. Dent. 2013, 38, 618–625. [Google Scholar] [CrossRef]
- Papadogiannis, D.; Tolidis, K.; Gerasimou, P.; Lakes, R.; Papadogiannis, Y. Viscoelastic properties, creep behavior and degree of conversion of bulk fill composite resins. Dent. Mater. 2015, 31, 1533–1541. [Google Scholar] [CrossRef]
- Arbildo-Vega, H.I.; Lapinska, B.; Panda, S.; Lamas-Lara, C.; Khan, A.S.; Lukomska-Szymanska, M. Clinical Effectiveness of Bulk-Fill and Conventional Resin Composite Restorations: Systematic Review and Meta-Analysis. Polymers 2020, 12, 1786. [Google Scholar] [CrossRef]
- Kunz, P.V.M.; Wambier, L.M.; Kaizer, M.D.R.; Correr, G.M.; Reis, A.; Gonzaga, C.C. Is the clinical performance of composite resin restorations in posterior teeth similar if restored with incremental or bulk-filling techniques? A systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 2281–2297. [Google Scholar] [CrossRef]
- Cidreira Boaro, L.C.; Pereira Lopes, D.; de Souza, A.S.C.; Lie Nakano, E.; Ayala Perez, M.D.; Pfeifer, C.S.; Gonçalves, F. Clinical performance and chemical-physical properties of bulk fill composites resin—A systematic review and meta-analysis. Dent. Mater. 2019, 35, e249–e264. [Google Scholar] [CrossRef]
- Veloso, S.R.M.; Lemos, C.A.A.; de Moraes, S.L.D.; do Egito Vasconcelos, B.C.; Pellizzer, E.P.; de Melo Monteiro, G.Q. Clinical performance of bulk-fill and conventional resin composite restorations in posterior teeth: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 221–233. [Google Scholar] [CrossRef]
- Heintze, S.D.; Loguercio, A.D.; Hanzen, T.A.; Reis, A.; Rousson, V. Clinical efficacy of resin-based direct posterior restorations and glass-ionomer restorations—An updated meta-analysis of clinical outcome parameters. Dent. Mater. 2022, 38, e109–e135. [Google Scholar] [CrossRef]
- Borgia, E.; Baron, R.; Borgia, J.L. Quality and Survival of Direct Light-Activated Composite Resin Restorations in Posterior Teeth: A 5- to 20-Year Retrospective Longitudinal Study. J. Prosthodont. 2019, 28, e195–e203. [Google Scholar] [CrossRef]
- Beck, F.; Lettner, S.; Graf, A.; Bitriol, B.; Dumitrescu, N.; Bauer, P.; Moritz, A.; Schedle, A. Survival of direct resin restorations in posterior teeth within a 19-year period (1996–2015): A meta-analysis of prospective studies. Dent. Mater. 2015, 31, 958–985. [Google Scholar] [CrossRef]
- Demarco, F.F.; Corrêa, M.B.; Cenci, M.S.; Moraes, R.R.; Opdam, N.J. Longevity of posterior composite restorations: Not only a matter of materials. Dent. Mater. 2012, 28, 87–101. [Google Scholar] [CrossRef]
- Opdam, N.J.M.; Collares, K.; Hickel, R.; Bayne, S.C.; Loomans, B.A.; Cenci, M.S.; Lynch, C.D.; Correa, M.B.; Demarco, F.; Schwendicke, F.; et al. Clinical studies in restorative dentistry: New directions and new demands. Dent. Mater. 2018, 34, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Flecha, O.D.; Douglas de Oliveira, D.W.; Marques, L.S.; Gonçalves, P.F. A commentary on randomized clinical trials: How to produce them with a good level of evidence. Perspect. Clin. Res. 2016, 7, 75–80. [Google Scholar] [CrossRef]
- Ernest, P.; Jandrain, B.; Scheen, A.J. Strengths and weaknesses of randomized clinical trials: Evolving changes according to personalized medicine. Rev. Med. Liege 2015, 70, 232–236. [Google Scholar]
- Luescher, B.; Lutz, F.; Oschenbein, H.; Muehlemann, H.R. Microleakage and marginal adaptation in conventional and adhesive Class II restorations. J. Prosthet. Dent. 1977, 37, 300–309. [Google Scholar] [CrossRef]
- Krejci, I.; Heinzmann, J.L.; Lutz, F. Verschleiss von Schmelz, Amalgam und ihrer Schmelz-Antagonisten im Computer gesteuerte Kausimulator. Schweiz. Monatschr. Zahmed. 1990, 100, 1285–1291. [Google Scholar]
- Krejci, I.; Lutz, F. In-vitro test results of the evaluation of dental restoration systems. Correlation with in-vivo results. Schweiz. Monatsschr. Zahnmed. 1990, 100, 1445–1449. [Google Scholar] [PubMed]
- DeLong, R.; Douglas, W.H. An artificial oral environment for testing dental materials. IEEE Trans. Biomed. Eng. 1991, 38, 339–345. [Google Scholar] [CrossRef]
- Dietschi, D.; Argente, A.; Krejci, I.; Mandikos, M. In vitro performance of Class I and II composite restorations: A literature review on nondestructive laboratory trials—Part I. Oper. Dent. 2013, 38, E166–E181. [Google Scholar] [CrossRef]
- Dietschi, D.; Argente, A.; Krejci, I.; Mandikos, M. In vitro performance of Class I and II composite restorations: A literature review on nondestructive laboratory trials—Part II. Oper. Dent. 2013, 38, E182–E200. [Google Scholar] [CrossRef]
- Kumagai, H.; Suzuki, T.; Hamada, T.; Sondang, P.; Fujitani, M.; Nikawa, H. Occlusal force distribution on the dental arch during various levels of clenching. J. Oral Rehabil. 1999, 26, 932–935. [Google Scholar] [CrossRef]
- Hattori, Y.; Satoh, C.; Kunieda, T.; Endoh, R.; Hisamatsu, H.; Watanabe, M. Bite forces and their resultants during forceful intercuspal clenching in humans. J. Biomech. 2009, 22, 1533–1538. [Google Scholar] [CrossRef]
- Hidaka, O.; Iwasaki, M.; Saito, M.; Morimoto, T. Influence of clenching intensity on bite force balance, occlusal contact area, and average bite pressure. J. Dent. Res. 1999, 78, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Winocur, E.; Guarda-Nardini, L.; Paesani, D.; Lobbezoo, F. Epidemiology of bruxism in adults: A systematic review of the literature. J. Orofac. Pain 2013, 27, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Restrepo, C.; Diaz-Serrano, K.; Winocur, E.; Lobbezoo, F. Prevalence of sleep bruxism in children: A systematic review of the literature. J. Oral Rehabil. 2013, 40, 631–642. [Google Scholar] [CrossRef]
- Melo, G.; Duarte, J.; Pauletto, P.; Porporatti, A.L.; Stuginski-Barbosa, J.; Winocur, E.; Flores-Mir, C.; De Luca Canto, G. Bruxism: An umbrella review of systematic reviews. J. Oral Rehabil. 2019, 46, 666–690. [Google Scholar] [CrossRef] [PubMed]
- DeLong, R.; Pintado, M.R.; Douglas, W.H.; Fok, A.S.; Wilder, A.D., Jr.; Swift, E.J., Jr.; Bayne, S.C. Wear of a dental composite in an artificial oral environment: A clinical correlation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2297–2306. [Google Scholar] [CrossRef]
- Heintze, S.D. Systematic reviews: I. The correlation between laboratory tests on marginal quality and bond strength. II. The correlation between marginal quality and clinical outcome. J. Adhes. Dent. 2007, 1, 77–106. [Google Scholar]
- Heintze, S.D.; Ilie, N.; Hickel, R.; Reis, A.; Loguercio, A.; Rousson, V. Laboratory mechanical parameters of composite resins and their relation to fractures and wear in clinical trials—A systematic review. Dent. Mater. 2017, 33, e101–e114. [Google Scholar] [CrossRef]
- Dietschi, D.; Del Curto, F.; Di Bella, E.; Krejci, I.; Ardu, S. In vitro evaluation of marginal adaptation in medium- and large size direct class II restorations using a bulk-fill or layering technique. J. Dent. 2021, 115, 103828. [Google Scholar] [CrossRef]
- Shahidi, S.; Krejci, I.; Dietschi, D. In Vitro Evaluation of Marginal Adaptation of Direct Class II Composite Restorations Made of Different “Low-Shrinkage” Systems. Oper. Dent. 2017, 42, 273–283. [Google Scholar] [CrossRef]
- Dietschi, D.; Olsburgh, S.; Krejci, I.; Davidson, C. In vitro evaluation of marginal and internal adaptation after occlusal stressing of indirect class II composite restorations with different resinous bases. Eur. J. Oral Sci. 2003, 111, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Kruzic, J.J.; Arsecularatne, J.A.; Tanaka, C.B.; Hoffman, M.J.; Cesar, P.F. Recent advances in understanding the fatigue and wear behavior of dental composites and ceramics. J. Mech. Behav. Biomed. Mater. 2018, 88, 504–533. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Blunck, U.; Göhring, T.N.; Rousson, V. Marginal adaptation in vitro and clinical outcome of Class V restorations. Dent. Mater. 2009, 25, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Zimmerli, B. Relevance of in vitro tests of adhesive and composite dental materials. A review in 3 parts. Part 3: In vitro tests of adhesive systems. Schweiz. Monatsschr. Zahnmed. 2011, 121, 1024–1040. [Google Scholar]
- Haak, R.; Brückner, A.; Häfer, M.; Scholz, M.; Schneider, H. Is There an Association Between Clinical and SEM Quantitative Marginal Analysis in a 90-month Trial? J. Adhes. Dent. 2021, 23, 37–46. [Google Scholar]
- Sakaguchi, R.L.; Douglas, W.H.; DeLong, R.; Pintado, M.R. The wear of a posterior composite in an artificial mouth: A clinical correlation. Dent. Mater. 1986, 2, 235–240. [Google Scholar] [CrossRef]
- Schimmel, M.; Leemann, B.; Herrmann, F.R.; Kiliaridis, S.; Schnider, A.; Müller, F. Masticatory function and bite force in stroke patients. J. Dent. Res. 2011, 90, 230–234. [Google Scholar] [CrossRef]
- Takahashi, T.; Hayakawa, F.; Kumagai, M.; Akiyama, Y.; Kohyam, K. Relations among mechanical properties, human bite parameters, and ease of chewing of solid foods with various textures. J. Food Eng. 2009, 95, 400–409. [Google Scholar] [CrossRef]
- Lepley, C.R.; Throckmorton, G.S.; Ceen, R.F.; Buschang, P.H. Relative contributions of occlusion, maximum bite force, and chewing cycle kinematics to masticatory performance. Am. J. Orthod. Dentofac. Orthop. 2011, 139, 606–613. [Google Scholar] [CrossRef]
- Aggarwal, V.; Logani, A.; Jain, V.; Shah, N. Effect of cyclic loading on marginal adaptation and bond strength in direct vs. indirect class II MO composite restorations. Oper. Dent. 2008, 33, 587–592. [Google Scholar] [CrossRef]
- Rocca, G.T.; Gregor, L.; Sandoval, M.J.; Krejci, I.; Dietschi, D. In vitro evaluation of marginal and internal adaptation after occlusal stressing of indirect class II composite restorations with different resinous bases and interface treatments. “Post-fatigue adaptation of indirect composite restorations”. Clin. Oral Investig. 2012, 16, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Furness, A.; Tadros, M.Y.; Looney, S.W.; Rueggeberg, F.A. Effect of bulk/incremental fill on internal gap formation of bulk-fill composites. J. Dent. 2014, 42, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Hamza, B.; Zimmerman, M.; Attin, T.; Tauböck, T.T. Marginal integrity of classical and bulk-fill composite restorations in permanent and primary molars. Sci. Rep. 2022, 11, 13670. [Google Scholar] [CrossRef] [PubMed]
- Benetti, A.R.; Peutzfeldt, A.; Lussi, A.; Flury, S. Resin composites: Modulus of elasticity and marginal quality. J. Dent. 2014, 42, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef]
- Soares, C.J.; Faria-E-Silva, A.L.; Rodrigues, M.P.; Vilela, A.B.F.; Pfeifer, C.S.; Tantbirojn, D.; Versluis, A. Polymerization shrinkage stress of composite resins and resin cements—What do we need to know? Braz. Oral Res. 2017, 28, e62. [Google Scholar] [CrossRef]
- Cadenaro, M.; Maravic, T.; Comba, A.; Mazzoni, A.; Fanfoni, L.; Hilton, T.; Ferracane, J.; Breschi, L. The role of polymerization in adhesive dentistry. Dent. Mater. 2019, 35, e1–e22. [Google Scholar] [CrossRef]
- Staninec, M.; Marshall, G.W.; Hilton, J.F.; Pashley, D.H.; Gansky, S.A.; Marshall, S.J.; Kinney, J.H. Ultimate tensile strength of dentin: Evidence for a damage mechanics approach to dentin failure. J. Biomed. Mater. Res. 2002, 63, 342–345. [Google Scholar] [CrossRef]
- Giannini, M.; Soares, C.J.; de Carvalho, R.M. Ultimate tensile strength of tooth structures. Dent. Mater. 2004, 20, 322–329. [Google Scholar] [CrossRef]
- Yamada, Y.; Inoue, T.; Saito, M.; Nishimura, F.; Miyazaki, T. Anisotropic ultimate strength and microscopic fracture patterns during tensile testing in the dentin-enamel junction region. Dent. Mater. J. 2020, 31, 272–277. [Google Scholar] [CrossRef] [PubMed]


| Product/Shade (Abbreviation) | Batch Number | Composition | Manufacturer |
|---|---|---|---|
| OptiBond FL (adhesive) | 8626023 | Primer: HEMA, GPDM, MMEP, ethanol (20–25%), water, initiators Adhesive: uncured methacrylate Ester, TEGDMA, Ytterbium trifluoride, inert mineral fillers, photoinitiators, stabilizers | Kerr Corpopration, Orange, CA, USA |
| Tetric EvoCeram B2 (TEC) | W82536 | Matrix: Bis-GMA, UDMA, Ethoxylated Bis A Dimethacrylate Fillers: Barium glass filler, Ytterbium trifluoride, mixed oxide, pre-polymers, fumed silica Photoinitiator (CQ) | Ivoclar, Schaan, Liechtensetein |
| Tetric EvoCeram Bulkfill (TECB) | Y46174 | Matrix: Bis-GMA, UDMA, Ethoxylated Bis A Dimethacrylate Fillers: Barium glass filler, Ytterbium trifluoride, mixed oxide, pre-polymers, fumed silica Photoinitiators (CQ and Ivocerine) | Ivoclar, Schaan, Liechtensetein |
| SDR Flow Univ (SDR) | 00043289 | Matrix: modified urethane dimethacrylate resin, ethoxylated bisphenol-A dimethacrylate, triethyleneglycol dimethacrylate, camphorquinone, butylated hydroxyl toluene, UV stabilizer, titanium oxide, iron oxide pigments Filler: barium-alumino-fluoro-borosilicate glass, strontium alumino-fluoro-silicate glass | Dentsply DeTrey, Constance, Germany |
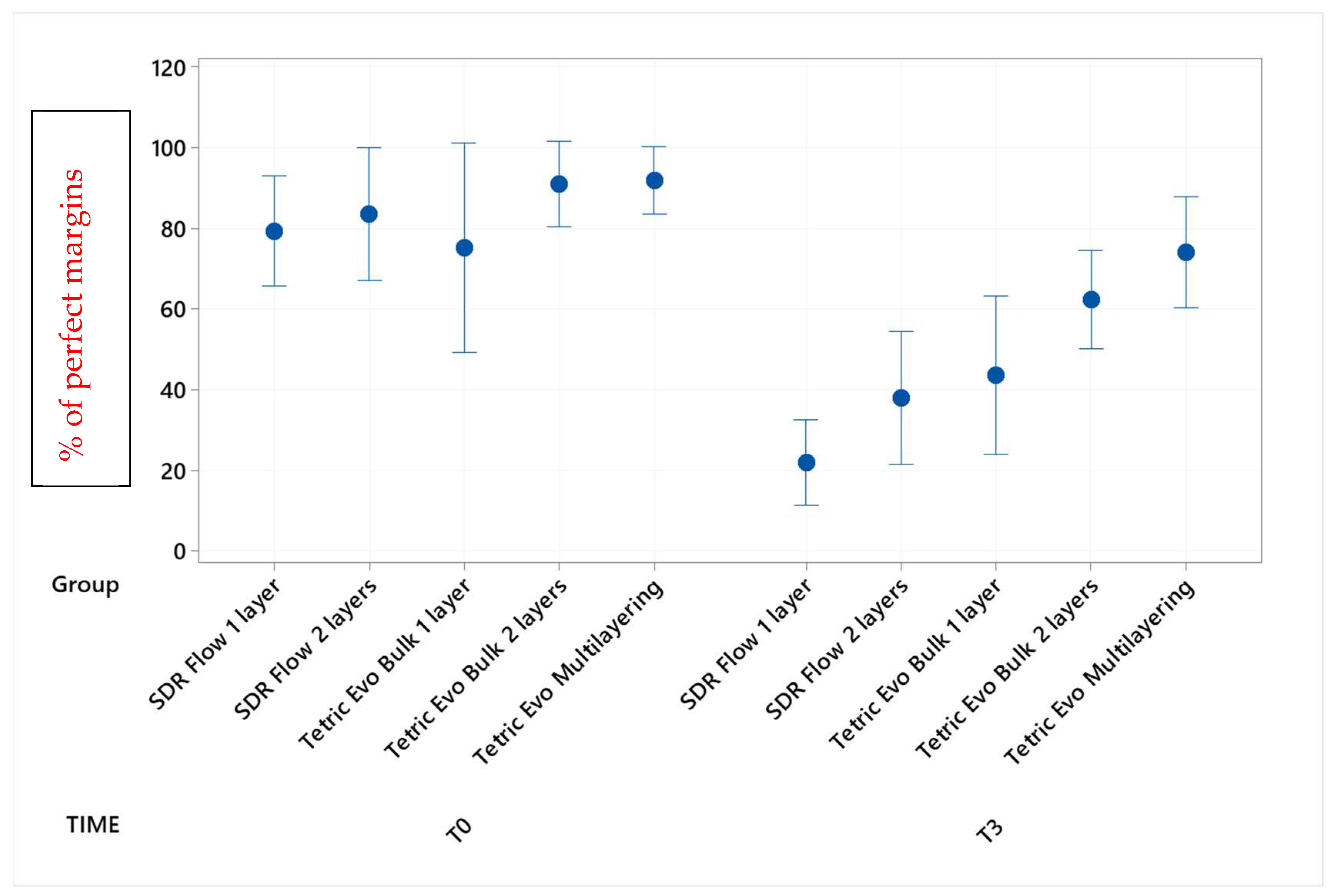
| Group × Timepoint | % Mean | SD | Grouping | ||
|---|---|---|---|---|---|
| Tetric Evo Multilayering T0 | 91.8 | 9.9 | A | ||
| Tetric Evo Bulk 2 layers T0 | 90.9 | 12.8 | A | ||
| SDR Flow 2 layers T0 | 83.4 | 19.7 | A | ||
| SDR Flow 1 layer T0 | 79.2 | 16.3 | A | ||
| Tetric Evo Bulk 1 layer T0 | 75.2 | 31.0 | A | ||
| Tetric Evo Multilayering T3 | 73.9 | 16.4 | A | ||
| Tetric Evo Bulk 2 layers T3 | 62.2 | 14.6 | A | B | |
| Tetric Evo Bulk 1 layer T3 | 43.6 | 23.4 | B | C | |
| SDR Flow 2 layers T3 | 37.9 | 19.7 | B | C | |
| SDR Flow 1 layer T3 | 21.9 | 12.6 | C | ||
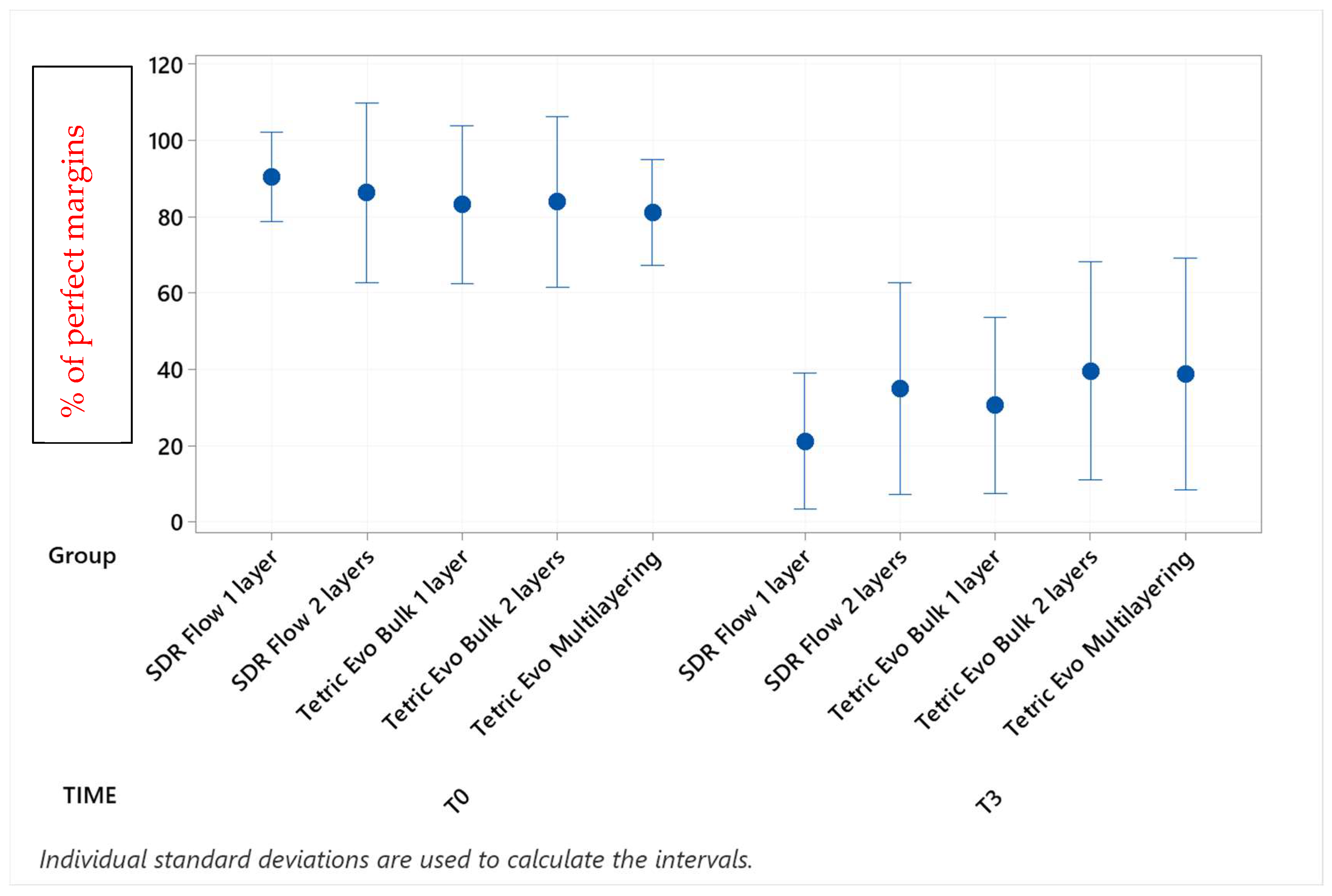
| Group × Timepoint | % Mean | SD | Grouping | |||
|---|---|---|---|---|---|---|
| Tetric Evo Bulk 1 layer T0 | 90.7 | 8.5 | A | |||
| Tetric Evo Bulk 2 layers T0 | 90.5 | 8.5 | A | |||
| Tetric Evo Multilayering T0 | 85.2 | 13.7 | A | |||
| SDR Flow 2 layers T0 | 85.1 | 18.5 | A | |||
| SDR Flow 1 layer T0 | 81.2 | 16.5 | A | B | ||
| Tetric Evo Bulk 2 layers T3 | 70.5 | 15.6 | A | B | C | |
| Tetric Evo Multilayering T3 | 55.5 | 23.1 | B | C | D | |
| Tetric Evo Bulk 1 layer T3 | 55.3 | 22.5 | B | C | D | |
| SDR Flow 2 layers T3 | 49.2 | 18.5 | C | D | ||
| SDR Flow 1 layer T3 | 29.3 | 11.7 | D | |||
| Group × Timepoint | % Mean | SD | Grouping | |||
|---|---|---|---|---|---|---|
| SDR Flow 1 layer T0 | 90.5 | 14.1 | A | |||
| SDR Flow 2 layers T0 | 86.4 | 28.2 | A | |||
| Tetric Evo Bulk 2 layers T0 | 83.9 | 26.8 | A | B | ||
| Tetric Evo Bulk 1 layer T0 | 83.2 | 24.6 | A | B | C | |
| Tetric Evo Multilayering T0 | 81.2 | 16.5 | A | B | C | |
| Tetric Evo Bulk 2 layers T3 | 39.6 | 34.2 | B | C | D | |
| Tetric Evo Multilayering T3 | 38.9 | 36.2 | C | D | ||
| SDR Flow 2 layers T3 | 35.0 | 33.2 | D | |||
| Tetric Evo Bulk 1 layer T3 | 30.6 | 27.5 | D | |||
| SDR Flow 1 layer T3 | 21.3 | 21.2 | D | |||
| Group × Timepoint | % Mean | SD | Grouping | ||
|---|---|---|---|---|---|
| SDR Flow 1 layer T0 | 89.8 | 15.1 | A | ||
| SDR Flow 2 layers T0 | 81.4 | 23.5 | A | B | |
| Tetric Evo Multilayering T0 | 80.6 | 15.5 | A | B | |
| Tetric Evo Bulk 2 layers T0 | 64.1 | 23.8 | A | B | C |
| Tetric Evo Bulk 1 layer T0 | 63.4 | 22.1 | A | B | C |
| SDR Flow 2 layers T3 | 61.0 | 32.9 | A | B | C |
| SDR Flow 1 layer T3 | 61.0 | 30.0 | A | B | C |
| Tetric Evo Multilayering T3 | 44.4 | 22.9 | B | C | |
| Tetric Evo Bulk 2 layers T3 | 35.1 | 22.7 | C | ||
| Tetric Evo Bulk 1 layer T3 | 31.4 | 15.4 | C | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietschi, D.; Askari, M.; Rossier, I.; Caseiro, L.; Krejci, I.; Leprince, J.G.; Di Bella, E.; Ardu, S. Marginal Adaptation of In Vitro Class II Restorations Made Out of Bulk or Conventional Composite Using Single- or Multi-Layered Techniques. Materials 2023, 16, 6325. https://doi.org/10.3390/ma16186325
Dietschi D, Askari M, Rossier I, Caseiro L, Krejci I, Leprince JG, Di Bella E, Ardu S. Marginal Adaptation of In Vitro Class II Restorations Made Out of Bulk or Conventional Composite Using Single- or Multi-Layered Techniques. Materials. 2023; 16(18):6325. https://doi.org/10.3390/ma16186325
Chicago/Turabian StyleDietschi, Didier, Mustafa Askari, Isaline Rossier, Luciana Caseiro, Ivo Krejci, Julian Gregoire Leprince, Enrico Di Bella, and Stefano Ardu. 2023. "Marginal Adaptation of In Vitro Class II Restorations Made Out of Bulk or Conventional Composite Using Single- or Multi-Layered Techniques" Materials 16, no. 18: 6325. https://doi.org/10.3390/ma16186325
APA StyleDietschi, D., Askari, M., Rossier, I., Caseiro, L., Krejci, I., Leprince, J. G., Di Bella, E., & Ardu, S. (2023). Marginal Adaptation of In Vitro Class II Restorations Made Out of Bulk or Conventional Composite Using Single- or Multi-Layered Techniques. Materials, 16(18), 6325. https://doi.org/10.3390/ma16186325







