Development of Mechanical Properties of Stainless Steel 316LN-IG after Cryo-Plastic Deformation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
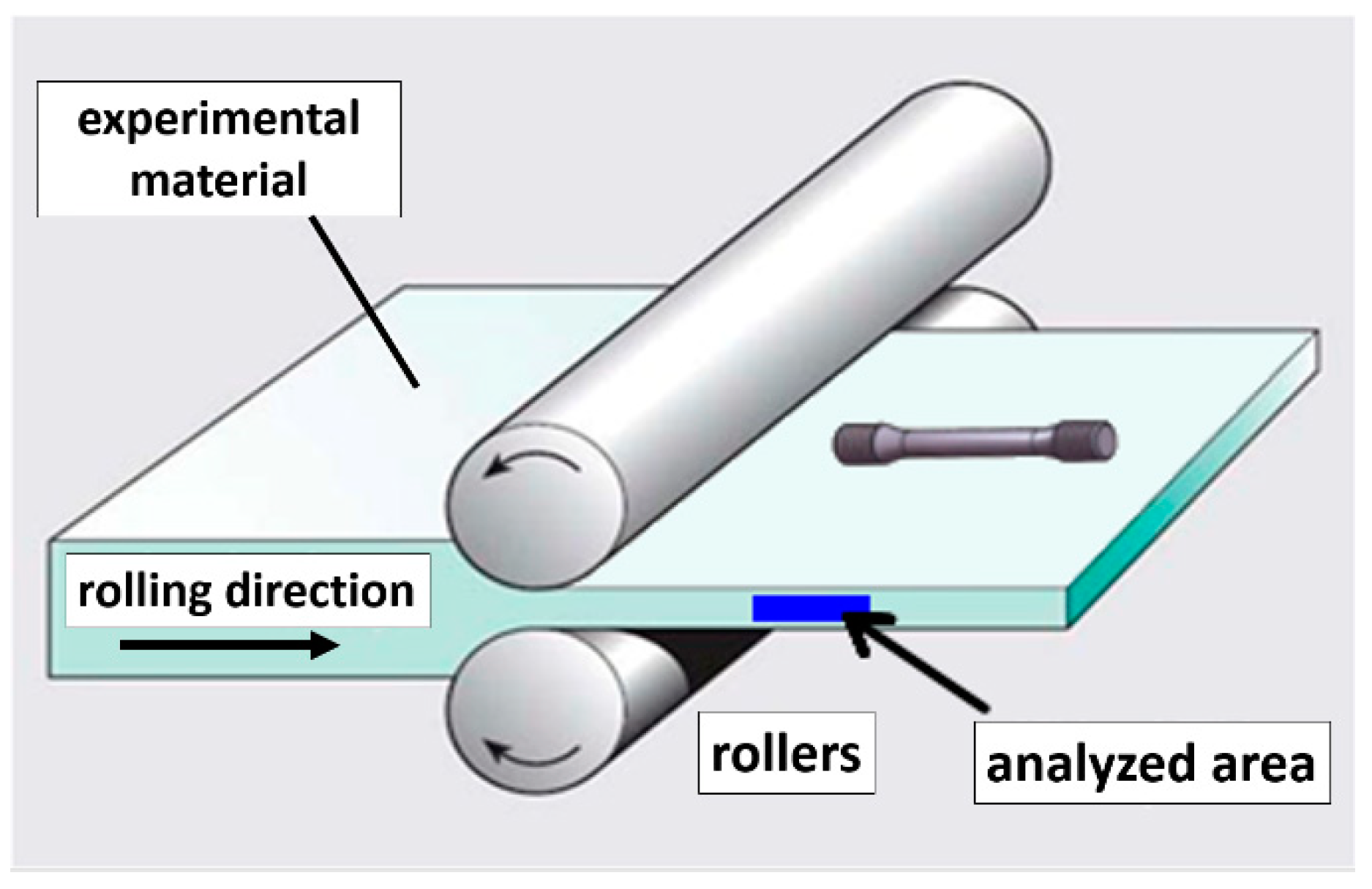
2.2. Physical Simulations—Experimental Rolling
2.3. Differential Scanning Calorimetry (DSC)
2.4. Heat Treatment
2.5. Mechanical Properties
2.6. Microstructure
3. Results and Discussion
3.1. Determination of Heat Treatment Using the DSC Analysis
3.2. Mechanical Properties after Plastic Deformation
3.2.1. Static Tensile Test
3.2.2. Structure Development after Plastic Deformations
3.3. Properties Stainles Steel 316 LN-IG after Heat Treatment
3.3.1. Static Tensile Test
Mechanical Properties after HT2 (1093 K/10 h)
Mechanical Properties after HT3 (1323 K/1.5 h)
3.3.2. Hardness
3.3.3. The Structure Development after Plastic Deformation and Heat Treatment
4. Conclusions
- Austenitic stainless steel 316 LN-IG, after solid solution annealing, shows excellent synergy between strength and plastic properties at cryogenic temperatures. The strength of the steel increases continuously with decreasing testing temperatures (UTS293K = 641 MPa, UTS77K = 1282 MPa, and UTS4.2K = 1543 MPa). Even at very low temperatures, the material shows high ductility TESA,77K = 57% and TESA,4.2K = 45%.
- The work-hardening rate of the steel increased continuously with a lowering of the temperature.
- After 50% deformation of thickness, a strongly deformed microstructure made up of tangles of deformation bands, shear bands, and twins was observed.
- The highest precipitation hardening of steel was achieved at a temperature of 823 K (HT1) after 50% deformation.
- At an annealing temperature of 1093 K, there was a loss of deformation and precipitation hardening in the steel.
- The completely recrystallized austenitic structure was observed after 50% deformation and following heat treatment at 1323 K for 1.5 h.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lesley, R.; Herrera, C.; Escriba, D.; Rios, P.; Padilha, A. A Short Review on Wrought Austenitic Stainless Steels at High Temperatures: Processing, Microstructure, Properties and Performance. Mater. Res. 2007, 10, 453–460. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Yuan, Y.; Zhang, P.; Yang, Z.; Zhou, Y.L.; Huang, J.Y.; Yin, H.F.; Dang, Y.Y.; Zhao, X.B.; Lu, J.T.; et al. A Modified HR3C Austenitic Heat-Resistant Steel for Ultra-Supercritical Power Plants Applications Beyond 650 °C. Met. Mater. Trans. A 2018, 49, 434–438. [Google Scholar] [CrossRef]
- Kvackaj, T.; Rozsypalova, A.; Kocisko, R.; Bidulska, J.; Petrousek, P.; Vlado, M.; Pokorny, I.; Sas, J.; Weiss, K.-P.; Duchek, M.; et al. Influence of Processing Conditions on Properties of AISI 316LN Steel Grade. J. Mater. Eng. Perform. 2020, 29, 1509–1514. [Google Scholar] [CrossRef]
- Foussat, A.; Weiyue, W.; Jing, W.; Shuangsong, D.; Sgobba, S.; Hongwei, L.; Libeyre, P.; Jong, C.; Klofac, K.; Mitchell, N. Mechanical Design and Construction Qualification Program on ITER Correction Coils Structures. Nucl. Eng. Des. 2014, 269, 116–124. [Google Scholar] [CrossRef]
- Sgobba, S.; Libeyre, P.; Marcinek, D.J.; Nyilas, A. A Comparative Assessment of Metallurgical and Mechanical Properties of Two Austenitic Stainless Steels for the Conductor Jacket of the ITER Central Solenoid. Fusion. Eng. Des. 2013, 88, 2484–2487. [Google Scholar] [CrossRef]
- Wawszczak, R.; Baczmański, A.; Marciszko, M.; Wróbel, M.; Czeppe, T.; Sztwiertnia, K.; Braham, C.; Berent, K. Evolution of Microstructure and Residual Stress during Annealing of Austenitic and Ferritic Steels. Mater. Charact. 2016, 112, 238–251. [Google Scholar] [CrossRef]
- Qin, J.; Dai, C.; Liao, G.; Wu, Y.; Zhu, X.; Huang, C.; Li, L.; Wang, K.; Shen, X.; Tu, Z.; et al. Tensile Test of SS 316LN Jacket with Different Conditions. Cryogenics 2014, 64, 16–20. [Google Scholar] [CrossRef]
- Krivykh, A.V.; Irodova, A.V.; Keilin, V.E. Magneto-Elastic Effect for 316LN-IG Stainless Steel at Low Temperature. Phys. Procedia 2015, 67, 976–981. [Google Scholar] [CrossRef]
- Lorenzetto, P.; Helie, M.; Molander, A. Stress Corrosion Cracking of AISI 316LN Stainless Steel in ITER Primary Water Conditions. J. Nucl. Mater. 1996, 233, 1387–1392. [Google Scholar] [CrossRef]
- Committee, A.H. Properties and Selection: Irons, Steels, and High-Performance Alloys; ASM International: Almere, The Netherlands, 1990; ISBN 978-1-62708-161-0. [Google Scholar]
- Byrnes, M.L.G.; Grujicic, M.; Owen, W.S. Nitrogen Strengthening of a Stable Austenitic Stainless Steel. Acta Metall. 1987, 35, 1853–1862. [Google Scholar] [CrossRef]
- Petrousek, P.; Kvackaj, T.; Kocisko, R.; Bidulska, J.; Luptak, M.; Manfredi, D.; Grande, M.A.; Bidulsky, R. Influence of Cryorolling on Properties of L-Pbf 316l Stainless Steel Tested at 298k and 77k. Acta Metall. Slovaca 2019, 25, 283–290. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, P.; Li, Y.; Wang, R.; Liu, G.; Xin, L.; Zhang, J.; Sun, J. Ratcheting-Induced Twinning/de-Twinning Behaviors in a 316LN Austenitic Stainless Steel. Mater. Sci. Eng. A 2022, 851, 143648. [Google Scholar] [CrossRef]
- Reed, R.; Walsh, R.P. Low-Temperature Creep of Austenitic Stainless Steels. J. Phys. Conf. Ser. 2017, 897, 012002. [Google Scholar] [CrossRef]
- Mcrae, D.; Balachandran, S.; Walsh, R. Fatigue and Fracture of Three Austenitic Stainless Steels at Cryogenic Temperatures. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 279, p. 012001. [Google Scholar] [CrossRef]
- Müllner, P.; Solenthaler, C.; Speidel, M.O. Second Order Twinning in Austenitic Steel. Acta Metall. Et. Mater. 1994, 42, 1727–1732. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Liao, X.Z.; Wu, X.L. Deformation Twinning in Nanocrystalline Materials. Prog. Mater. Sci. 2012, 57, 1–62. [Google Scholar] [CrossRef]
- Kim, J.-K.; Kwon, M.-H.; De Cooman, B.C. On the Deformation Twinning Mechanisms in Twinning-Induced Plasticity Steel. Acta Mater. 2017, 141, 444–455. [Google Scholar] [CrossRef]
- Üçok, İ.; Ando, T.; Grant, N.J. Property Enhancement in Type 316L Stainless Steel by Spray Forming. Mater. Sci. Eng. A 1991, 133, 284–287. [Google Scholar] [CrossRef]
- Xiong, Y.; He, T.; Wang, J.; Lu, Y.; Chen, L.; Ren, F.; Liu, Y.; Volinsky, A.A. Cryorolling Effect on Microstructure and Mechanical Properties of Fe–25Cr–20Ni Austenitic Stainless Steel. Mater. Des. 2015, 88, 398–405. [Google Scholar] [CrossRef]
- Roy, B.; Kumar, R.; Das, J. Effect of Cryorolling on the Microstructure and Tensile Properties of Bulk Nano-Austenitic Stainless Steel. Mater. Sci. Eng. A 2015, 631, 241–247. [Google Scholar] [CrossRef]
- Xiong, Y.; Yue, Y.; Fan, M.; Lu, Y.; Ren, F.; Cao, W. Cryorolling Impacts on Microstructure and Mechanical Properties of AISI 316 LN Austenitic Stainless Steel. Mater. Sci. Eng. A 2017, 709, 270–276. [Google Scholar] [CrossRef]
- Petrousek, P.; Kvackaj, T.; Bidulska, J.; Bidulsky, R.; Grande, M.A.; Manfredi, D.; Weiss, K.-P.; Kocisko, R.; Luptak, M.; Pokorny, I. Investigation of the Properties of 316L Stainless Steel after AM and Heat Treatment. Materials 2023, 16, 3935. [Google Scholar] [CrossRef] [PubMed]
- Gubicza, J.; El-Tahawy, M.; Huang, Y.; Choi, H.; Choe, H.; Lábár, J.L.; Langdon, T.G. Microstructure, Phase Composition and Hardness Evolution in 316L Stainless Steel Processed by High-Pressure Torsion. Mater. Sci. Eng. A 2016, 657, 215–223. [Google Scholar] [CrossRef]
- Yin, F.; Cheng, G.; Xu, R.; Zhao, K.; Li, Q.; Jian, J.; Hu, S.; Sun, S.; An, L.; Han, Q. Ultrastrong Nanocrystalline Stainless Steel and Its Hall-Petch Relationship in the Nanoscale. Scr. Mater. 2018, 155, 26–31. [Google Scholar] [CrossRef]
- Hu, G.; Wang, P.; Li, D.; Li, Y. Effects of Nitrogen on Precipitation and Tensile Behaviors of 25Cr 20Ni Austenitic Stainless Steels at Elevated Temperatures. Mater. Sci. Eng. A 2019, 752, 93–100. [Google Scholar] [CrossRef]
- Hong, H.; Rho, B.; Nam, S. Correlation of the M23C6 Precipitation Morphology with Grain Boundary Characteristics in Austenitic Stainless Steel. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. Mater. Sci. Eng. A-Struct. Mater. 2001, 318, 285–292. [Google Scholar] [CrossRef]
- Sourmail, T. Precipitation in Creep Resistant Austenitic Stainless Steels. Mater. Sci. Technol. 2001, 17, 1–14. [Google Scholar] [CrossRef]
- Padilha, A.F.; Rios, P.R. Decomposition of Austenite in Austenitic Stainless Steels. ISIJ Int. 2002, 42, 325–327. [Google Scholar] [CrossRef]
- ISO 6892-1 (420310); Metallic Materials. Tensile Testing. Part 1: Method of Test at Room Temperature. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 643; Steels—Micrographic Determination of the Apparent Grain Size. International Organization for Standardization: Geneva, Switzerland, 2019.
- Weiss, B.; Stickler, R. Phase Instabilities during High Temperature Exposure of 316 Austenitic Stainless Steel. Met. Trans. 1972, 3, 851–866. [Google Scholar] [CrossRef]
- Singhal, L.K.; Martin, J.W. The Formation of Ferrite and Sigma-Phase in Some Austenitic Stainless Steels. Acta Metall. 1968, 16, 1441–1451. [Google Scholar] [CrossRef]
- Wasnik, D.N.; Dey, G.K.; Kain, V.; Samajdar, I. Precipitation Stages in a 316L Austenitic Stainless Steel. Scr. Mater. 2003, 49, 135–141. [Google Scholar] [CrossRef]
- Spruiell, J.E.; Scott, J.A.; Ary, C.S.; Hardin, R.L. Microstructural Stability of Thermal-Mechanically Pretreated Type 316 Austenitic Stainless Steel. Met. Trans. 1973, 4, 1533–1544. [Google Scholar] [CrossRef]
- Han, W.; Liu, Y.; Wan, F.; Liu, P.; Yi, X.; Zhan, Q.; Morrall, D.; Ohnuki, S. Deformation Behavior of Austenitic Stainless Steel at Deep Cryogenic Temperatures. J. Nucl. Mater. 2018, 504, 29–32. [Google Scholar] [CrossRef]
- Pustovalov, V.V. Serrated Deformation of Metals and Alloys at Low Temperatures (Review). Low. Temp. Phys. 2008, 34, 683–723. [Google Scholar] [CrossRef]
- Tabin, J. Kinematic and Thermal Characteristic of Discontinuous Plastic Flow in Metastable Austenitic Stainless Steels. Mech. Mater. 2021, 163, 104090. [Google Scholar] [CrossRef]
- Herrera, C.; Plaut, R.L.; Padilha, A.F. Microstructural Refinement during Annealing of Plastically Deformed Austenitic Stainless Steels. Mater. Sci. Forum 2007, 550, 423–428. [Google Scholar] [CrossRef]

















| Heat Treatment | Temperature/K | Holding Time/h |
|---|---|---|
| SA | 1323 | 1 |
| HT0 | as rolled | - |
| HT1 | 823 | 10 |
| HT2 | 1093 | 10 |
| HT3 (for A50 and C50) | 1323 | 1.5 |
| Total Thickness Deformation ε/% | ||||||
|---|---|---|---|---|---|---|
| Heat Treatment | 10% Ambient | 10% Cryo | 30% Ambient | 30% Cryo | 50% Ambient | 50% Cryo |
| HT0 | A10 HT0 | C10 HT0 | A30 HT0 | C30 HT0 | A50 HT0 | C50 HT0 |
| HT1 | A10 HT1 | C10 HT1 | A30 HT1 | C30 HT1 | A50 HT1 | C50 HT1 |
| HT2 | A10 HT2 | C10 HT2 | A30 HT2 | C30 HT2 | A50 HT2 | C50 HT2 |
| HT3 | - | - | - | - | A50 HT3 | C50 HT3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedoriková, A.; Petroušek, P.; Kvačkaj, T.; Kočiško, R.; Zemko, M. Development of Mechanical Properties of Stainless Steel 316LN-IG after Cryo-Plastic Deformation. Materials 2023, 16, 6473. https://doi.org/10.3390/ma16196473
Fedoriková A, Petroušek P, Kvačkaj T, Kočiško R, Zemko M. Development of Mechanical Properties of Stainless Steel 316LN-IG after Cryo-Plastic Deformation. Materials. 2023; 16(19):6473. https://doi.org/10.3390/ma16196473
Chicago/Turabian StyleFedoriková, Alica, Patrik Petroušek, Tibor Kvačkaj, Róbert Kočiško, and Michal Zemko. 2023. "Development of Mechanical Properties of Stainless Steel 316LN-IG after Cryo-Plastic Deformation" Materials 16, no. 19: 6473. https://doi.org/10.3390/ma16196473
APA StyleFedoriková, A., Petroušek, P., Kvačkaj, T., Kočiško, R., & Zemko, M. (2023). Development of Mechanical Properties of Stainless Steel 316LN-IG after Cryo-Plastic Deformation. Materials, 16(19), 6473. https://doi.org/10.3390/ma16196473







