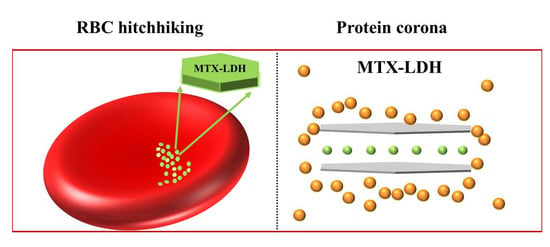
Blood Compatibility of Drug–Inorganic Hybrid in Human Blood: Red Blood Cell Hitchhiking and Soft Protein Corona
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MTX-LDH and LDH without Drug Moiety
2.3. In Vitro Biological Assay: Hemolysis Assay and Red Blood Cell Counting
2.4. Microscopy Studies
2.5. Colloidal Properties of MTX-LDH in Human Plasma
2.6. Human Plasma Fluorescence Quenching
2.7. Evaluation of Interaction between MTX-LDH and Protein by Quartz Crystal Microbalance (QCM)
3. Results and Discussion
3.1. In Vitro Blood Compatibility of MTX-LDH
3.2. Investigation of the RBC Morphology
3.3. Nanoparticle Attachment on the Surface of RBCs
3.4. Colloidal Stability of MTX-LDH in Human Plasma
3.5. Fluorescence Quenching
3.6. Reversible Adsorption Behavior of Proteins on the MTX-LDH Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nalawade, P.; Aware, B.; Kadam, V.; Hirlekar, R. Layered Double Hydroxides: A Review. 2009. Available online: http://nopr.niscpr.res.in/handle/123456789/3482 (accessed on 18 September 2023).
- Ambrogi, V.; Perioli, L.; Ciarnelli, V.; Nocchetti, M.; Rossi, C. Effect of gliclazide immobilization into layered double hydroxide on drug release. Eur. J. Pharm. Biopharm. 2009, 73, 285–291. [Google Scholar] [CrossRef]
- Guagliano, M.; Monteforte, F.; Bruni, G.; Friuli, V.; Maggi, L.; Quinzeni, I.; Bini, M. The peculiar dissolution behaviour of Piretanide hosted in layered double hydroxides. Appl. Clay Sci. 2020, 198, 105826. [Google Scholar] [CrossRef]
- Kang, H.; Kim, M.; Feng, Q.; Lin, S.; Wei, K.; Li, R.; Choi, C.J.; Kim, T.-H.; Li, G.; Oh, J.-M. Nanolayered hybrid mediates synergistic co-delivery of ligand and ligation activator for inducing stem cell differentiation and tissue healing. Biomaterials 2017, 149, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Choi, S.J.; Lee, G.E.; Kim, J.E.; Choy, J.H. Inorganic metal hydroxide nanoparticles for targeted cellular uptake through clathrin-mediated endocytosis. Chem. Asian J. 2009, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Vasti, C.; Bedoya, D.A.; Rojas, R.; Giacomelli, C.E. Effect of the protein corona on the colloidal stability and reactivity of LDH-based nanocarriers. J. Mater. Chem. B 2016, 4, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gu, W.; Chen, J.; Chen, W.; Xu, Z.P. Co-delivery of siRNAs and anti-cancer drugs using layered double hydroxide nanoparticles. Biomaterials 2014, 35, 3331–3339. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, L.; Xie, J.; Yin, Y.; Chang, T.; Duan, Y.; Jiang, N. In vitro controlled release of vitamin C from Ca/Al layered double hydroxide drug delivery system. Mater. Sci. Eng. C 2014, 39, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, E.; Namazi, H.; Pooresmaeil, M. Carboxymethyl starch encapsulated 5-FU and DOX co-loaded layered double hydroxide for evaluation of its in vitro performance as a drug delivery agent. Int. J. Biol. Macromol. 2022, 201, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Inorganic nanomaterials for bioimaging, targeted drug delivery and therapeutics. Chem. Commun. 2014, 50, 14071–14081. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Kim, H.-M.; Hwang, S.; Jeung, D.-G.; Rhee, K.-J.; Oh, J.-M. Physicochemical properties and hematocompatibility of layered double hydroxide-based anticancer drug methotrexate delivery system. Pharmaceutics 2020, 12, 1210. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.Y.; Kim, T.-H.; Gwak, G.-H.; Park, J.H.; Oh, J.-M. Radioisotope and anticancer agent incorporated layered double hydroxide for tumor targeting theranostic nanomedicine. Appl. Clay Sci. 2020, 186, 105454. [Google Scholar] [CrossRef]
- Wang, X.; Yang, B.; Xu, X.; Su, M.; Xi, M.; Yin, Z. Dextran sulfate–modified pH-sensitive layered double hydroxide nanocomposites for treatment of rheumatoid arthritis. Drug Deliv. Transl. Res. 2021, 11, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Bajaj, G.; Yeo, Y. Drug delivery systems for intraperitoneal therapy. Pharm. Res. 2010, 27, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Holback, H.; Yeo, Y. Intratumoral drug delivery with nanoparticulate carriers. Pharm. Res. 2011, 28, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Wang, L.; Zhang, Z.; Lu, D.; Lu, M.; Chopp, M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001, 32, 1005–1011. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kim, H.-M.; Jung, B.C.; Kim, Y.S.; Oh, J.-M. Size and surface charge effect of layered double hydroxide particles upon blood cells. Appl. Clay Sci. 2022, 225, 106549. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kim, H.-M.; Oh, J.-M. Photochemical Consideration in the Interactions between Blood Proteins and Layered Inorganic Materials. Int. J. Mol. Sci. 2022, 23, 11367. [Google Scholar] [CrossRef]
- Hu, H.; Wang, X.; Xu, S.; Yang, W.; Xu, F.; Shen, J.; Mao, C. Preparation and evaluation of well-defined hemocompatible layered double hydroxide-poly (sulfobetaine) nanohybrids. J. Mater. Chem. 2012, 22, 15362–15369. [Google Scholar] [CrossRef]
- Singh, M.; Singh, R.K.; Singh, S.K.; Mahto, S.K.; Misra, N. In vitro biocompatibility analysis of functionalized poly (vinyl chloride)/layered double hydroxide nanocomposites. RSC Adv. 2018, 8, 40611–40620. [Google Scholar] [CrossRef]
- Chen, D.; Ganesh, S.; Wang, W.; Amiji, M. Plasma protein adsorption and biological identity of systemically administered nanoparticles. Nanomedicine 2017, 12, 2113–2135. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- Kopp, M.; Kollenda, S.; Epple, M. Nanoparticle–protein interactions: Therapeutic approaches and supramolecular chemistry. Acc. Chem. Res. 2017, 50, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Laurent, S.; Tawil, N.; Yahia, L.; Mahmoudi, M. Protein-nanoparticle interactions. Biophysics 2013, 15, 45–63. [Google Scholar]
- Mohammadi, M.R.; Corbo, C.; Molinaro, R.; Lakey, J.R. Biohybrid nanoparticles to negotiate with biological barriers. Small 2019, 15, 1902333. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef]
- Kah, J.C.Y.; Chen, J.; Zubieta, A.; Hamad-Schifferli, K. Exploiting the protein corona around gold nanorods for loading and triggered release. ACS Nano 2012, 6, 6730–6740. [Google Scholar] [CrossRef]
- Cifuentes-Rius, A.; De Puig, H.; Kah, J.C.Y.; Borros, S.; Hamad-Schifferli, K. Optimizing the properties of the protein corona surrounding nanoparticles for tuning payload release. ACS Nano 2013, 7, 10066–10074. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, L.; Christner, C.; Storck, W.; Schick, I.; Krumbein, I.; Dähring, H.; Haedicke, K.; Heinz-Herrmann, K.; Teichgräber, U.; Reichenbach, J.R. A plasma protein corona enhances the biocompatibility of Au@ Fe3O4 Janus particles. Biomaterials 2015, 68, 77–88. [Google Scholar] [CrossRef]
- Hitchings, G.H.; Smith, S.L. Dihydrofolate reductases as targets for inhibitors. Adv. Enzym. Regul. 1980, 18, 349–371. [Google Scholar] [CrossRef]
- Göker, E.; Lin, J.T.; Trippett, T.; Elisseyeff, Y.; Tong, W.P.; Niedzwiecki, D.; Tan, C.; Steinherz, P.; Schweitzer, B.I.; Bertino, J.R. Decreased polyglutamylation of methotrexate in acute lymphoblastic leukemia blasts in adults compared to children with this disease. Leukemia 1993, 7, 1000–1004. [Google Scholar] [PubMed]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-M.; Park, M.; Kim, S.-T.; Jung, J.-Y.; Kang, Y.-G.; Choy, J.-H. Efficient delivery of anticancer drug MTX through MTX-LDH nanohybrid system. J. Phys. Chem. Solids 2006, 67, 1024–1027. [Google Scholar] [CrossRef]
- Ray, S.; Saha, S.; Sa, B.; Chakraborty, J. In vivo pharmacological evaluation and efficacy study of methotrexate-encapsulated polymer-coated layered double hydroxide nanoparticles for possible application in the treatment of osteosarcoma. Drug Deliv. Transl. Res. 2017, 7, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhou, M.; Zhang, X.; Huang, L.; Chen, W.; Roy, V.A.; Zhang, W.; Chen, X. A novel type of aqueous dispersible ultrathin-layered double hydroxide nanosheets for in vivo bioimaging and drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 34185–34193. [Google Scholar] [CrossRef]
- Chakraborty, J.; Roychowdhury, S.; Sengupta, S.; Ghosh, S. Mg–Al layered double hydroxide–methotrexate nanohybrid drug delivery system: Evaluation of efficacy. Mater. Sci. Eng. C 2013, 33, 2168–2174. [Google Scholar] [CrossRef]
- Chakraborty, M.; Dasgupta, S.; Sengupta, S.; Chakraborty, J.; Ghosh, S.; Ghosh, J.; Mitra, M.K.; Mishra, A.; Mandal, T.K.; Basu, D. A facile synthetic strategy for Mg–Al layered double hydroxide material as nanocarrier for methotrexate. Ceram. Int. 2012, 38, 941–949. [Google Scholar] [CrossRef]
- Choy, J.-H.; Jung, J.-S.; Oh, J.-M.; Park, M.; Jeong, J.; Kang, Y.-K.; Han, O.-J. Layered double hydroxide as an efficient drug reservoir for folate derivatives. Biomaterials 2004, 25, 3059–3064. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Seron, A.; Delorme, F. Synthesis of layered double hydroxides (LDHs) with varying pH: A valuable contribution to the study of Mg/Al LDH formation mechanism. J. Phys. Chem. Solids 2008, 69, 1088–1090. [Google Scholar] [CrossRef]
- Choimet, M.; Hyoung-Mi, K.; Jae-Min, O.; Tourrette, A.; Drouet, C. Nanomedicine: Interaction of biomimetic apatite colloidal nanoparticles with human blood components. Colloids Surf. B 2016, 145, 87–94. [Google Scholar] [CrossRef][Green Version]
- (ASTM) F756-17; Standard Practice for Assessment of Hemolytic Properties of Materials. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA., 2017.
- Trager, W. The development of the malaria parasite Plasmodium lophurae in red blood cell suspensions in vitro. J. Parasitol. 1947, 33, 345–350. [Google Scholar] [CrossRef]
- Lu, N.; Sui, Y.; Tian, R.; Peng, Y.-Y. Adsorption of plasma proteins on single-walled carbon nanotubes reduced cytotoxicity and modulated neutrophil activation. Chem. Res. Toxicol. 2018, 31, 1061–1068. [Google Scholar] [CrossRef]
- Panico, S.; Capolla, S.; Bozzer, S.; Toffoli, G.; Dal Bo, M.; Macor, P. Biological Features of Nanoparticles: Protein Corona Formation and Interaction with the Immune System. Pharmaceutics 2022, 14, 2605. [Google Scholar] [CrossRef]
- Gehlen, M.H. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C 2020, 42, 100338. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, P.; Zhu, Z.; Wang, Y. Interaction of γ-Fe2O3 nanoparticles with fibrinogen. Spectrochim. Acta Part A 2015, 151, 40–47. [Google Scholar] [CrossRef]
- Keizer, J. Nonlinear fluorescence quenching and the origin of positive curvature in Stern-Volmer plots. J. Am. Chem. Soc. 1983, 105, 1494–1498. [Google Scholar] [CrossRef]
- Udenfriend, S. Fluorescence Assay in Biology and Medicine; Academic Press: Cambridge, MA, USA, 2014; Volume 2. [Google Scholar]
- Fraiji, L.K.; Hayes, D.M.; Werner, T. Static and dynamic fluorescence quenching experiments for the physical chemistry laboratory. J. Chem. Educ. 1992, 69, 424. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Lakowicz, J.R. Instrumentation for fluorescence spectroscopy. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 1999; pp. 25–61. [Google Scholar] [CrossRef]
- Kronenthal, R. Polymers in Medicine and Surgery; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 8. [Google Scholar]
- Rahman, M.; Khan, J.A.; Kanwal, U.; Awan, U.A.; Raza, A. Methotrexate-loaded PEGylated gold nanoparticles as hemocompatible and pH-responsive anticancer drug nanoconjugate. J. Nanopart. Res. 2021, 23, 195. [Google Scholar] [CrossRef]
- DeVita, V.T.; Lawrence, T.S.; Rosenberg, S.A. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; Volume 2. [Google Scholar]
- Woolley III, P.V.; Sacher, R.A.; Priego, V.M.; Schanfield, M.S.; Bonnem, E.M. Methotrexate-induced immune haemolytic anaemia. Br. J. Haematol. 1983, 54, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Ford, J. Red blood cell morphology. Int. J. Labor. Hematol. 2013, 35, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Avsievich, T.; Popov, A.; Bykov, A.; Meglinski, I. Mutual interaction of red blood cells influenced by nanoparticles. Sci. Rep. 2019, 9, 5147. [Google Scholar] [CrossRef]
- Jaworski, S.; Hinzmann, M.; Sawosz, E.; Grodzik, M.; Kutwin, M.; Wierzbicki, M.; Strojny, B.; Vadalasetty, K.P.; Lipińska, L.; Chwalibog, A. Interaction of different forms of graphene with chicken embryo red blood cells. Environ. Sci. Pollut. Res. 2017, 24, 21671–21679. [Google Scholar] [CrossRef]
- Brenner, J.S.; Mitragotri, S.; Muzykantov, V.R. Red blood cell hitchhiking: A novel approach for vascular delivery of nanocarriers. Annu. Rev. Biomed. Eng. 2021, 23, 225–248. [Google Scholar] [CrossRef]
- Kontos, S.; Hubbell, J.A. Improving protein pharmacokinetics by engineering erythrocyte affinity. Mol. Pharm. 2010, 7, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Brenner, J.S.; Pan, D.C.; Myerson, J.W.; Marcos-Contreras, O.A.; Villa, C.H.; Patel, P.; Hekierski, H.; Chatterjee, S.; Tao, J.-Q.; Parhiz, H. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 2018, 9, 2684. [Google Scholar] [CrossRef]
- Liu, T.; Gao, C.; Gu, D.; Tang, H. Cell-based carrier for targeted hitchhiking delivery. Drug Deliv. Transl. Res. 2022, 12, 2634–2648. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Gao, Y.; Kim, J.; Mitragotri, S. Erythrocyte leveraged chemotherapy (ELeCt): Nanoparticle assembly on erythrocyte surface to combat lung metastasis. Sci. Adv. 2019, 5, eaax9250. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, X.; Zhang, G.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: Size and surface effects. ACS Nano 2011, 5, 1366–1375. [Google Scholar] [CrossRef]
- Sharma, H.S. Nanoneuroscience and Nanoneuropharmacology; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Kim, T.-H.; Lee, J.Y.; Xie, J.; Park, J.H.; Oh, J.-M. Topology dependent modification of layered double hydroxide for therapeutic and diagnostic platform. Adv. Drug Deliv. Rev. 2022, 188, 114459. [Google Scholar] [CrossRef]
- Xie, J.; Yamaguchi, T.; Oh, J.-M. Synthesis of a mesoporous Mg–Al–mixed metal oxide with P123 template for effective removal of Congo red via aggregation-driven adsorption. J. Solid State Chem. 2021, 293, 121758. [Google Scholar] [CrossRef]
- Xie, J.; Khalid, Z.; Oh, J.M. Recent advances in the synthesis of layered double hydroxides nanosheets. Bull. Korean Chem. Soc. 2023, 44, 100–111. [Google Scholar] [CrossRef]
- Vasti, C.; Bonnet, L.V.; Galiano, M.R.; Rojas, R.; Giacomelli, C.E. Relevance of protein–protein interactions on the biological identity of nanoparticles. Colloids Surf. B 2018, 166, 330–338. [Google Scholar] [CrossRef]
- Tantra, R.; Tompkins, J.; Quincey, P. Characterisation of the de-agglomeration effects of bovine serum albumin on nanoparticles in aqueous suspension. Colloids Surf. B 2010, 75, 275–281. [Google Scholar] [CrossRef]
- Cai, R.; Chen, C. The crown and the scepter: Roles of the protein corona in nanomedicine. Adv. Mater. 2019, 31, 1805740. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, G.; Liu, Q.S.; Liu, W.; Qu, G.; Hu, L.; Long, Y.; Cai, Z.; Zhao, X.; Jiang, G. Identification and interaction mechanism of protein corona on silver nanoparticles with different sizes and the cellular responses. J. Hazard. Mater. 2021, 414, 125582. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, S.H.D.P.; Park, J.J.; Meuse, C.; Pristinski, D.; Becker, M.L.; Karim, A.; Douglas, J.F. Interaction of gold nanoparticles with common human blood proteins. ACS Nano 2010, 4, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef]
- Norde, W.; Lyklema, J. Why proteins prefer interfaces. J. Biomater. Sci. Polym. Ed. 1991, 2, 183–202. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Zia, M.K.; Siddiqui, T.; Ali, S.S.; Ahsan, H.; Khan, F.H. Understanding the binding interaction between methotrexate and human alpha-2-macroglobulin: Multi-spectroscopic and computational investigation. Arch. Biochem. Biophys. 2019, 675, 108118. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska, A.; Maciążek, M.; Rownicka, J.; Bojko, B.; Pentak, D.; Sułkowski, W. Effect of temperature on the methotrexate–BSA interaction: Spectroscopic study. J. Mol. Struct. 2007, 834, 162–169. [Google Scholar] [CrossRef]
- Cheng, L.Y.; Fang, M.; Bai, A.M.; Ouyang, Y.; Hu, Y.J. Insights into the interaction of methotrexate and human serum albumin: A spectroscopic and molecular modeling approach. Luminescence 2017, 32, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Cisse, L.; Djande, A.; Capo-Chichi, M.; Delattre, F.; Saba, A.; Brochon, J.-C.; Sanouski, S.; Tine, A.; Aaron, J.-J. Fluorescence quenching of two coumarin-3-carboxylic acids by trivalent lanthanide ions. J. Fluoresc. 2017, 27, 619–628. [Google Scholar] [CrossRef]
- Khan, S.N.; Islam, B.; Yennamalli, R.; Sultan, A.; Subbarao, N.; Khan, A.U. Interaction of mitoxantrone with human serum albumin: Spectroscopic and molecular modeling studies. Eur. J. Pharm. Sci. 2008, 35, 371–382. [Google Scholar] [CrossRef]
- Eftink, M.R.; Ghiron, C.A. Fluorescence quenching studies with proteins. Anal. Biochem. 1981, 114, 199–227. [Google Scholar] [CrossRef]
- Eftink, M.R. Fluorescence quenching reactions: Probing biological macromolecular structures. In Biophysical and Biochemical Aspects of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 1991; pp. 1–41. [Google Scholar]
- Kim, J.Y.; Choi, S.-J.; Oh, J.-M.; Park, T.; Choy, J.-H. Anticancer drug-inorganic nanohybrid and its cellular interaction. J. Nanosci. Nanotechnol. 2007, 7, 3700–3705. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.; Demeester, J.; De Smedt, S. Advanced nanogel engineering for drug delivery. Soft Matter 2009, 5, 707–715. [Google Scholar] [CrossRef]
- Afshari, M.J.; Sabzi, M.; Jiang, L.; Behshad, Y.; Zanjanijam, A.R.; Mahdavinia, G.R.; Ahmadi, M. Incorporation of dynamic boronate links and Ag nanoparticles into PVA hydrogels for pH-Regulated and prolonged release of methotrexate. J. Drug Deliv. Sci. Technol. 2021, 63, 102502. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Sakhtianchi, R.; Müller, B.; Landfester, K.; Crespy, D.; Mahmoudi, M. Protein corona change the drug release profile of nanocarriers: The “overlooked” factor at the nanobio interface. Colloids Surf. B 2014, 123, 143–149. [Google Scholar] [CrossRef]
- Obst, K.; Yealland, G.; Balzus, B.; Miceli, E.; Dimde, M.; Weise, C.; Eravci, M.; Bodmeier, R.; Haag, R.; Calderón, M. Protein corona formation on colloidal polymeric nanoparticles and polymeric nanogels: Impact on cellular uptake, toxicity, immunogenicity, and drug release properties. Biomacromolecules 2017, 18, 1762–1771. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Landry, M.P.; Moore, A.; Coreas, R. The protein corona from nanomedicine to environmental science. Nat. Rev. Mater. 2023, 8, 422–438. [Google Scholar] [CrossRef] [PubMed]
- Ashby, J.; Pan, S.; Zhong, W. Size and surface functionalization of iron oxide nanoparticles influence the composition and dynamic nature of their protein corona. ACS Appl. Mater. Interfaces 2014, 6, 15412–15419. [Google Scholar] [CrossRef] [PubMed]
- Molino, P.J.; Higgins, M.J.; Innis, P.C.; Kapsa, R.M.; Wallace, G.G. Fibronectin and bovine serum albumin adsorption and conformational dynamics on inherently conducting polymers: A QCM-D study. Langmuir 2012, 28, 8433–8445. [Google Scholar] [CrossRef]
- Delcroix, M.; Laurent, S.; Huet, G.; Dupont-Gillain, C.C. Protein adsorption can be reversibly switched on and off on mixed PEO/PAA brushes. Acta Biomater. 2015, 11, 68–79. [Google Scholar] [CrossRef] [PubMed]








| Quenchers | Type of Quenching | Regression Equation | Number | R2 | KSV | KS |
|---|---|---|---|---|---|---|
| MTX-LDH | Stern–Volmer | N.A. | N.A. | N.A. | N.A. | N.A. |
| LDH | Stern–Volmer | F0/F = 1.01 + 0.12[C] | 8 | 0.9043 | 0.116 | N.A. |
| Perrin | Log(F0/F) = 0.0064 + 0.049[C] | 8 | 0.8591 | N.A. | 0.049 | |
| Polynomial | F0/F = 1.00 + 0.20[C] − 0.054[C]2 | 8 | 0.9785 | N.A. | N.A. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Kim, H.-M.; Kamada, K.; Oh, J.-M. Blood Compatibility of Drug–Inorganic Hybrid in Human Blood: Red Blood Cell Hitchhiking and Soft Protein Corona. Materials 2023, 16, 6523. https://doi.org/10.3390/ma16196523
Xie J, Kim H-M, Kamada K, Oh J-M. Blood Compatibility of Drug–Inorganic Hybrid in Human Blood: Red Blood Cell Hitchhiking and Soft Protein Corona. Materials. 2023; 16(19):6523. https://doi.org/10.3390/ma16196523
Chicago/Turabian StyleXie, Jing, Hyoung-Mi Kim, Kai Kamada, and Jae-Min Oh. 2023. "Blood Compatibility of Drug–Inorganic Hybrid in Human Blood: Red Blood Cell Hitchhiking and Soft Protein Corona" Materials 16, no. 19: 6523. https://doi.org/10.3390/ma16196523
APA StyleXie, J., Kim, H.-M., Kamada, K., & Oh, J.-M. (2023). Blood Compatibility of Drug–Inorganic Hybrid in Human Blood: Red Blood Cell Hitchhiking and Soft Protein Corona. Materials, 16(19), 6523. https://doi.org/10.3390/ma16196523