Synthesis of Imidazole-Based Deep Eutectic Solvents as Solid Lubricants: Lubricated State Transition
Abstract
:1. Introduction
2. Experiment Details
2.1. Materials
2.2. DESs Preparation
2.3. Friction Test
2.4. Characterization
3. Results and Discussion
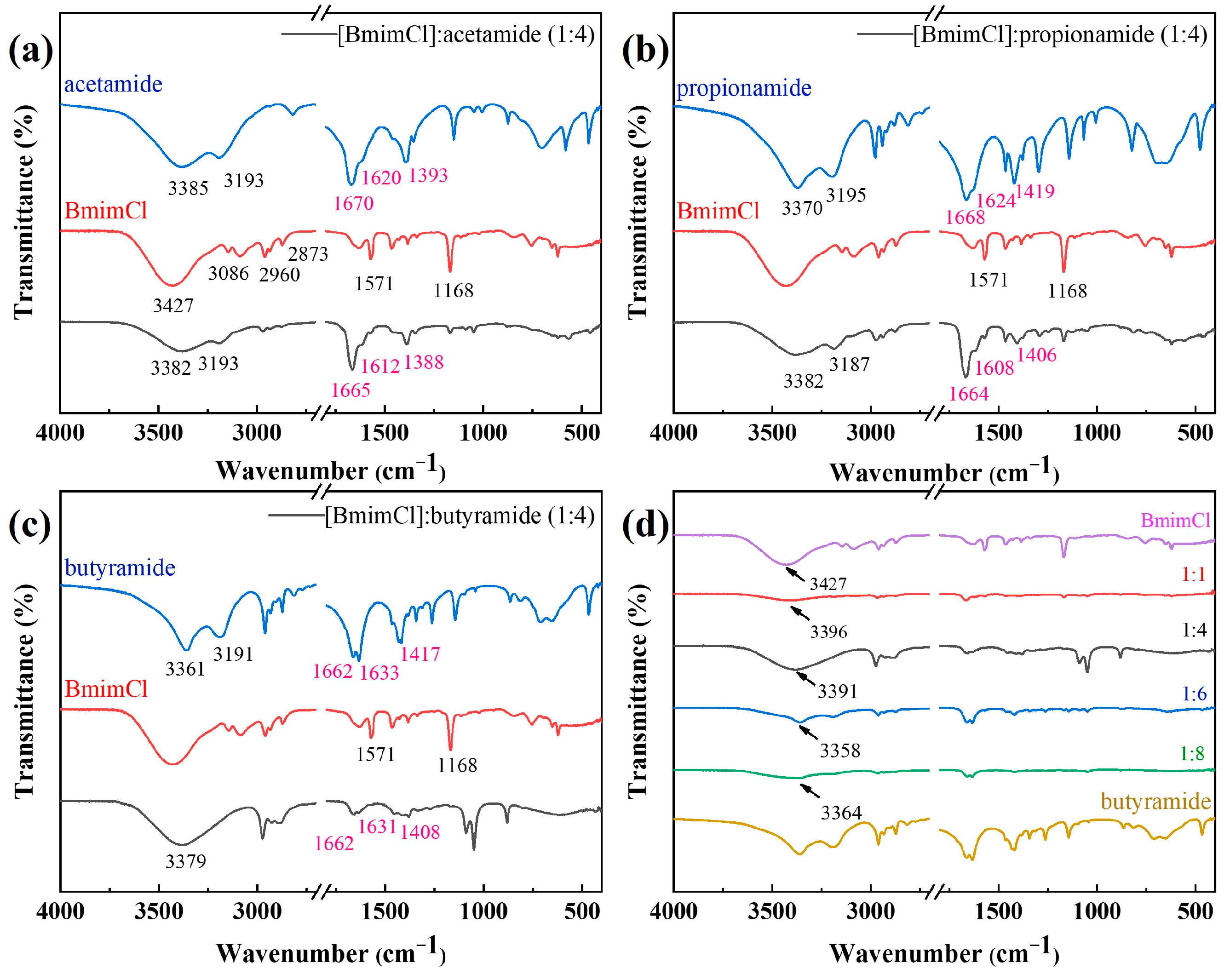
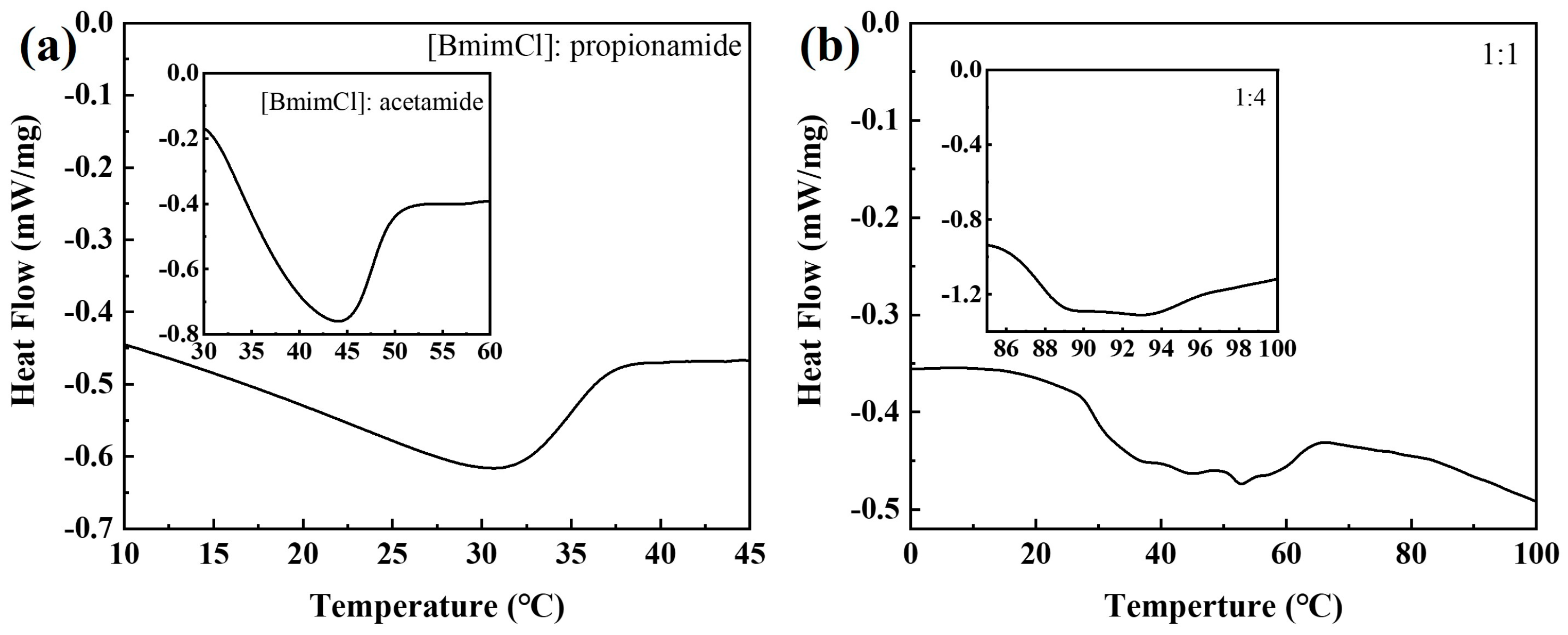
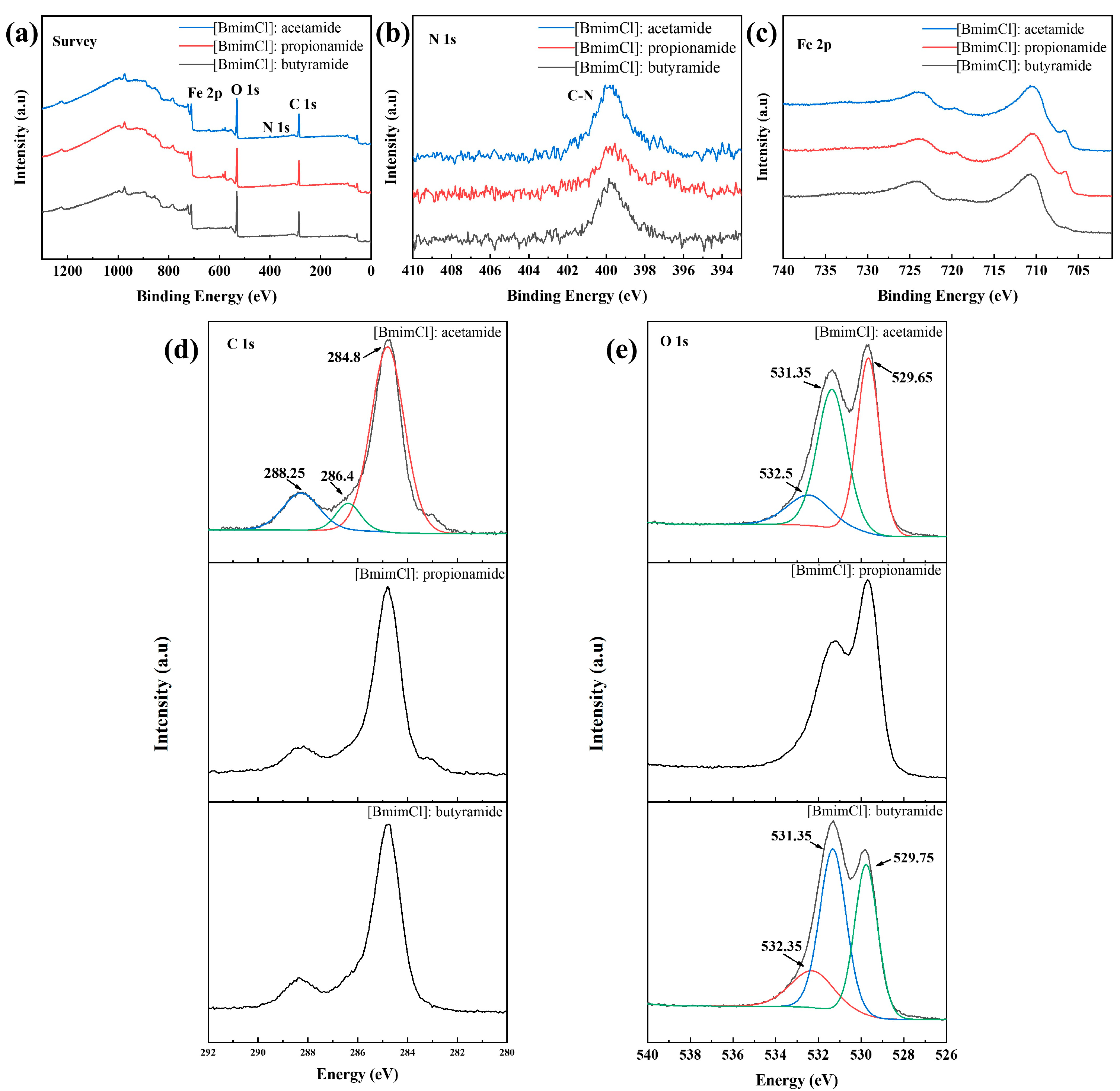
3.1. Characterization of DESs
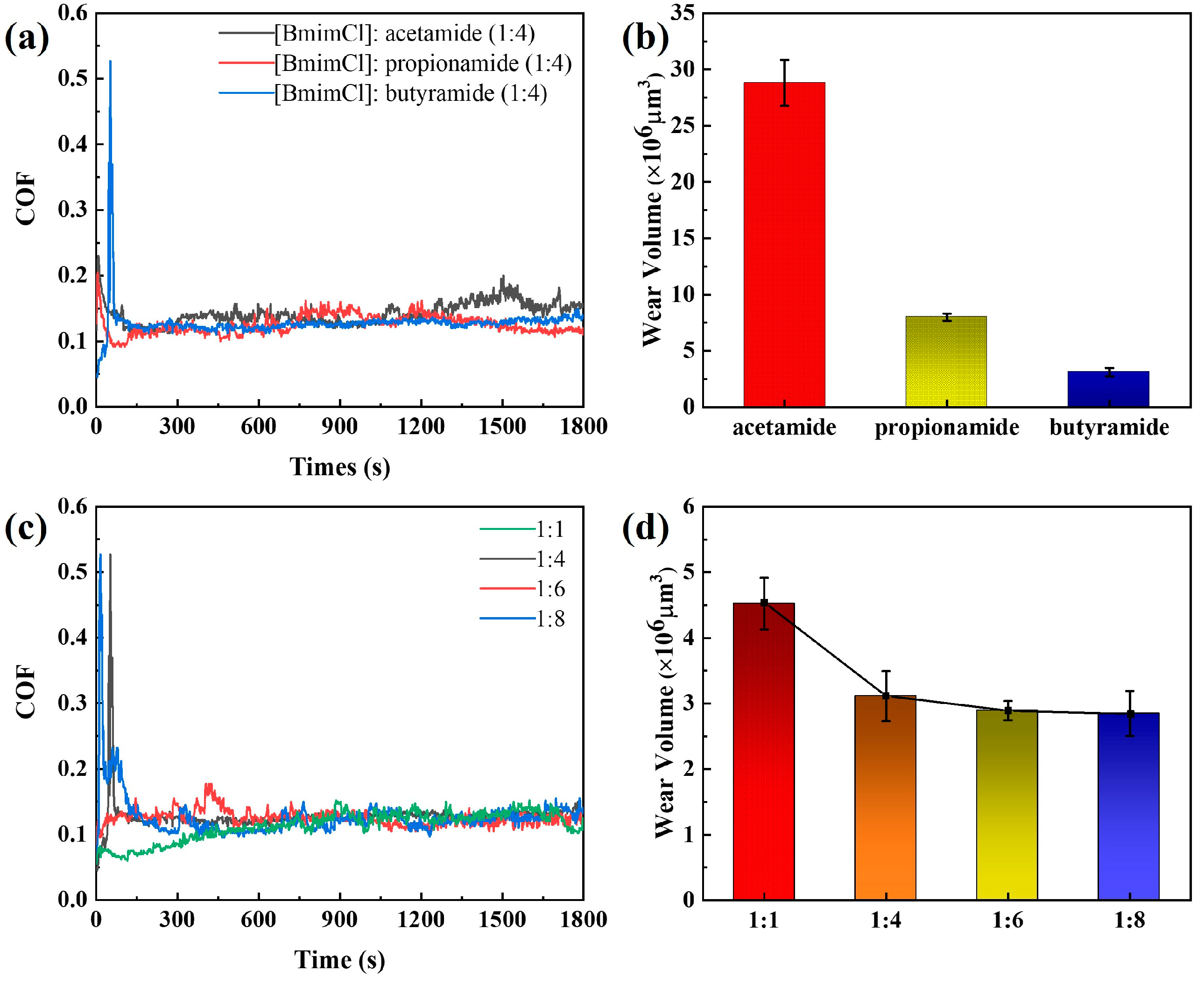
3.2. Frictional Wear
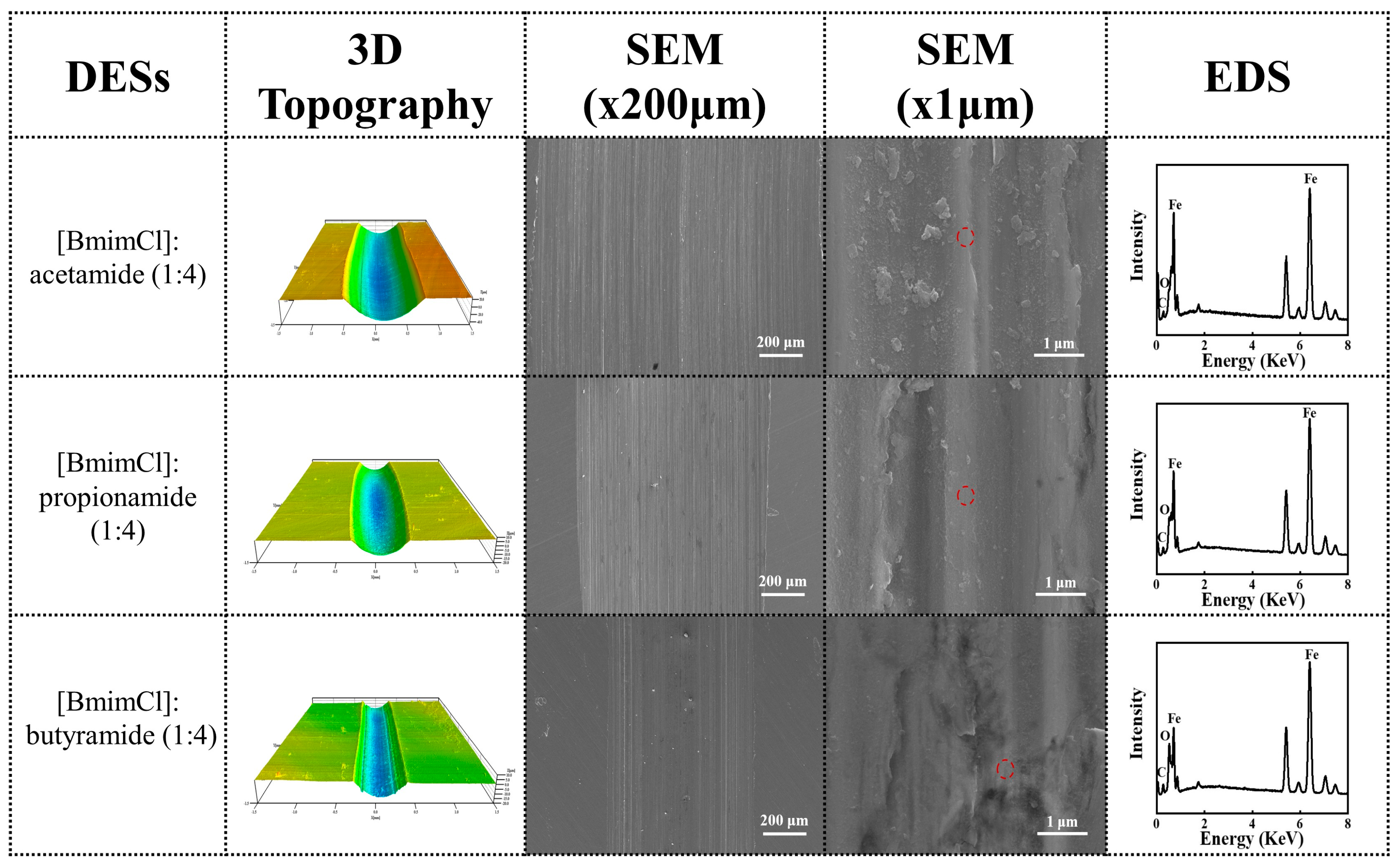
3.3. Surface and Compositional Analysis
3.4. Lubrication Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manikanta, J.E.; Raju, B.N.; Prasad, C.; Sankar, B.S.S.P. Machining performance on SS304 using nontoxic, biodegradable vegetable-based cutting fluids. Chem. Data Collect. 2022, 42, 100961. [Google Scholar] [CrossRef]
- Shen, Y.; Dong, Y.; Zhu, H.; Dong, L. Pseudomonas xiamenensis in the cutting fluids on corrosion behavior of aluminum alloy 2219. Bioelectrochemistry 2023, 150, 108350. [Google Scholar] [CrossRef]
- Li, B.-H.; Huang, J.-H.; Li, X.-L.; Ma, Q.; Zhao, P.-F.; Huang, W. The microbial degradation of AAOO based cutting fluid wastewater. J. Water Process Eng. 2023, 55, 104167. [Google Scholar] [CrossRef]
- Krolczyk, G.M.; Maruda, R.W.; Krolczyk, J.B.; Wojciechowski, S.; Mia, M.; Nieslony, P.; Budzik, G. Ecological trends in machining as a key factor in sustainable production—A review. J. Clean. Prod. 2019, 218, 601–615. [Google Scholar] [CrossRef]
- Palacio, M.; Bhushan, B. A Review of Ionic Liquids for Green Molecular Lubrication in Nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- Hoghoughi, M.H.; Farahnakian, M.; Elhami, S. Environmental, economical, and machinability based sustainability assessment in hybrid machining process employing tool textures and solid lubricant. Sustain. Mater. Technol. 2022, 34, e00511. [Google Scholar] [CrossRef]
- Huang, Q.; Shi, X.; Xue, Y.; Zhang, K.; Wu, C. Recent progress on surface texturing and solid lubricants in tribology: Designs, properties, and mechanisms. Mater. Today Commun. 2023, 35, 105854. [Google Scholar] [CrossRef]
- Sarkar, M.; Mandal, N. Solid lubricant materials for high temperature application: A review. Mater. Today Proc. 2022, 66, 3762–3768. [Google Scholar] [CrossRef]
- Liu, S.; Gao, Q.; Hou, K.; Li, Z.; Wang, J.; Yang, S. Solvent-free covalent MXene nanofluid: A new lubricant combining the characteristics of solid and liquid lubricants. Chem. Eng. J. 2023, 462, 142238. [Google Scholar] [CrossRef]
- Marques, A.; Paipa Suarez, M.; Falco Sales, W.; Rocha Machado, Á. Turning of Inconel 718 with whisker-reinforced ceramic tools applying vegetable-based cutting fluid mixed with solid lubricants by MQL. J. Mater. Process. Technol. 2019, 266, 530–543. [Google Scholar] [CrossRef]
- Abbá, E.; Speidel, A.; Liao, Z.; Axinte, D.; Novovic, D. A bar of cutting fluid: Deep Eutectic Fluids with a novel flavour. Mater. Today Adv. 2022, 16, 100291. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.T.; Colaço, R.; Branco, L.C.; Saramago, B. A review on alternative lubricants: Ionic liquids as additives and deep eutectic solvents. J. Mol. Liq. 2021, 333, 116004. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Wazeer, I.; AlNashef, I.M.; Al-Zahrani, A.A.; Hadj-Kali, M.K. The subtle but substantial distinction between ammonium- and phosphonium-based deep eutectic solvents. J. Mol. Liq. 2021, 332, 115838. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Kovacs, A.; Neyts, E.C.; Cornet, I.; Wijnants, M.; Billen, P. Modeling the Physicochemical Properties of Natural Deep Eutectic Solvents. ChemSusChem 2020, 13, 3789–3804. [Google Scholar] [CrossRef]
- Lawes, S.D.A.; Hainsworth, S.V.; Blake, P.; Ryder, K.S.; Abbott, A.P. Lubrication of Steel/Steel Contacts by Choline Chloride Ionic Liquids. Tribol. Lett. 2009, 37, 103–110. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ahmed, E.I.; Harris, R.C.; Ryder, K.S. Evaluating water miscible deep eutectic solvents (DESs) and ionic liquids as potential lubricants. Green Chem. 2014, 16, 4156–4161. [Google Scholar] [CrossRef]
- Antunes, M.; Campinhas, A.-S.; de Sá Freire, M.; Caetano, F.; Diogo, H.P.; Colaço, R.; Branco, L.C.; Saramago, B. Deep eutectic solvents (DES) based on sulfur as alternative lubricants for silicon surfaces. J. Mol. Liq. 2019, 295, 111728. [Google Scholar] [CrossRef]
- Donato, M.T.; Santos, L.; Diogo, H.P.; Colaço, R.; Branco, L.C.; Saramago, B. Eutectic systems containing an ionic liquid and PEG200 as lubricants for silicon surfaces: Effect of the mixture’s molar ratio. J. Mol. Liq. 2022, 350, 118572. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Cao, C.; Li, H.; Fan, X.; Xu, X.; Zhu, M. Solid-liquid phase change of choline chloride type deep eutectic solvents towards lubrication regime. J. Mol. Liq. 2022, 365, 120162. [Google Scholar] [CrossRef]
- Khan, A.; Singh, R.; Gupta, P.; Gupta, K.; Khatri, O.P. Aminoguanidine-based deep eutectic solvents as environmentally-friendly and high-performance lubricant additives. J. Mol. Liq. 2021, 339, 116829. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Fan, X.; Xu, X.; Zhu, M. Oil-soluble deep eutectic solvent as high-performance green lubricant additives for PAO 40 and PEG 200. Tribol. Int. 2023, 186, 108602. [Google Scholar] [CrossRef]
- Singh, J.; Chatha, S.S.; Bhatia, R. Behaviour and applications of ionic liquids as lubricants in tribology: A review. Mater. Today Proc. 2022, 56, 2659–2665. [Google Scholar] [CrossRef]
- Al-Sallami, W.; Parsaeian, P.; Dorgham, A.; Neville, A. Effect of ionic liquids’ chemistry on their lubrication behaviour under various contact pressures. Tribol. Int. 2020, 151, 106465. [Google Scholar] [CrossRef]
- Huang, G.; Fan, S.; Ba, Z.; Cai, M.; Qiao, D. Insight into the lubricating mechanism for alkylimidazolium phosphate ionic liquids with different alkyl chain length. Tribol. Int. 2019, 140, 105886. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, T.; Xing, H. Butyl-3-methylimidazolium Chloride Preparation in Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2005, 45, 525–529. [Google Scholar] [CrossRef]
- Kozak, A.; Pindelska, E. Spectroscopic analysis of the influence of various external factors on ethenzamide-glutaric acid (1:1) cocrystal formation. Eur. J. Pharm. Sci. 2019, 133, 59–68. [Google Scholar] [CrossRef]
- Li, Y.; Cao, C.; Cai, M.; Li, H.; Fan, X.; Gao, Y.; Lu, Z.; Zhu, M. Green hydrophobic deep eutectic solvents as low-viscosity and efficient lubricants. Tribol. Int. 2023, 185, 108531. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Fan, X.; Cai, M.; Xu, X.; Zhu, M. Green and Economical Bet-Based Natural Deep Eutectic Solvents: A Novel High-Performance Lubricant. ACS Sustain. Chem. Eng. 2022, 10, 7253–7264. [Google Scholar] [CrossRef]
- Joseph, J.; Jemmis, E.D. Red-, blue-, or no-shift in hydrogen bonds: A unified explanation. J. Am. Chem. Soc. 2007, 129, 4620–4632. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, R.; Thakur, R.C.; Singh, L. An overview of deep eutectic solvents: Alternative for organic electrolytes, aqueous systems & ionic liquids for electrochemical energy storage. J. Energy Chem. 2023, 82, 592–626. [Google Scholar]
- Chandran, K.; Kait, C.F.; Wilfred, C.D.; Zaid, H.F.M. A review on deep eutectic solvents: Physiochemical properties and its application as an absorbent for sulfur dioxide. J. Mol. Liq. 2021, 338, 117021. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Database of deep eutectic solvents and their physical properties: A review. J. Mol. Liq. 2023, 384, 121899. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhu, F.; Dong, Q.; Wu, R.; Su, E. Insight into the physicochemical properties of deep eutectic solvents by systematically investigating the components. J. Mol. Liq. 2022, 346, 118315. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Xiong, W.; Liang, J.; Zhang, S.; Hu, X.; Wu, Y. Deep eutectic behavior in binary mixtures of protic ionic liquids. J. Mol. Liq. 2022, 366, 120251. [Google Scholar] [CrossRef]
- Cotroneo-Figueroa, V.P.; Gajardo-Parra, N.F.; López-Porfiri, P.; Leiva, Á.; Gonzalez-Miquel, M.; Garrido, J.M.; Canales, R.I. Hydrogen bond donor and alcohol chain length effect on the physicochemical properties of choline chloride based deep eutectic solvents mixed with alcohols. J. Mol. Liq. 2022, 345, 116986. [Google Scholar] [CrossRef]
- Li, Y.; Yang, F.; Li, Y.; Cai, M.; Li, H.; Fan, X.; Zhu, M. Choline amino acid ionic Liquids: A novel green potential lubricant. J. Mol. Liq. 2022, 360, 119539. [Google Scholar] [CrossRef]
- Nowosielski, B.; Jamrógiewicz, M.; Łuczak, J.; Śmiechowski, M.; Warmińska, D. Experimental and predicted physicochemical properties of monopropanolamine-based deep eutectic solvents. J. Mol. Liq. 2020, 309, 113110. [Google Scholar] [CrossRef]
- Fajardo, O.Y.; Bresme, F.; Kornyshev, A.A.; Urbakh, M. Water in Ionic Liquid Lubricants: Friend and Foe. ACS Nano 2017, 11, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Gera, R.; Moll, C.J.; Bhattacherjee, A.; Bakker, H.J. Water-Induced Restructuring of the Surface of a Deep Eutectic Solvent. J. Phys. Chem. Lett. 2022, 13, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Palmelund, H.; Rantanen, J.; Löbmann, K. Deliquescence Behavior of Deep Eutectic Solvents. Appl. Sci. 2021, 11, 1601. [Google Scholar] [CrossRef]
- Esteves, P.J.; Seriacopi, V.; de Macêdo, M.C.S.; Souza, R.M.; Scandian, C. Combined effect of abrasive particle size distribution and ball material on the wear coefficient in micro-scale abrasive wear tests. Wear 2021, 476, 203639. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, T.; Zhang, S. Influence of oxides on the formation of self-lubricating layer and anti-wear performance during sliding. Tribol. Int. 2023, 179, 108188. [Google Scholar] [CrossRef]
- Fang, H.; Li, Y.; Zhang, S.; Ding, Q.; Hu, L. Novel binary oil-soluble ionic liquids with high lubricating performance. Tribol. Int. 2022, 174, 107724. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Zhang, S.; Che, Q.; Hu, L.; Zhang, J. Outstanding lubrication properties of carbon dot-based ionic liquids. J. Mol. Liq. 2023, 376, 121458. [Google Scholar] [CrossRef]


| DESs | Hydrogen Bond Acceptor | Hydrogen Bond Donors | Molar Ratio |
|---|---|---|---|
| [BmimCl]: acetamide | 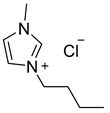 | 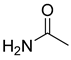 | 1:4 |
| [BmimCl]: propionamide |  | 1:4 | |
| [BmimCl]: butyramide | 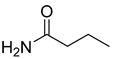 | 1:1 | |
| [BmimCl]: butyramide | 1:4 | ||
| [BmimCl]: butyramide | 1:6 | ||
| [BmimCl]: butyramide | 1:8 |
| DESs | Freezing Point (°C) | Melting Point (°C) |
|---|---|---|
| [BmimCl]: acetamide (1:4) | 7.45 | 30.24 |
| [BmimCl]: propionamide (1:4) | 9.78 | 44.10 |
| [BmimCl]: butyramide (1:1) | 24.56 | 44.19 |
| [BmimCl]: butyramide (1:4) | 40.72 | 93.01 |
| DESs | Molar Ratio | Fe Element (wt%) | C Element (wt%) | O Element (wt%) |
|---|---|---|---|---|
| [BmimCl]: acetamide | 1:4 | 68.11 | 3.75 | 0.59 |
| [BmimCl]: propionamide | 1:4 | 67.89 | 3.78 | 1.78 |
| [BmimCl]: butyramide | 1:1 | 69.06 | 2.67 | 1.32 |
| [BmimCl]: butyramide | 1:4 | 64.79 | 4.86 | 3.46 |
| [BmimCl]: butyramide | 1:6 | 65.93 | 3.48 | 2.00 |
| [BmimCl]: butyramide | 1:8 | 64.55 | 4.73 | 5.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Chen, Y.; Chu, A.; Hu, H.; Zhao, Y. Synthesis of Imidazole-Based Deep Eutectic Solvents as Solid Lubricants: Lubricated State Transition. Materials 2023, 16, 6579. https://doi.org/10.3390/ma16196579
Zhang H, Chen Y, Chu A, Hu H, Zhao Y. Synthesis of Imidazole-Based Deep Eutectic Solvents as Solid Lubricants: Lubricated State Transition. Materials. 2023; 16(19):6579. https://doi.org/10.3390/ma16196579
Chicago/Turabian StyleZhang, Houjie, Youming Chen, Aimin Chu, Hairong Hu, and Yuping Zhao. 2023. "Synthesis of Imidazole-Based Deep Eutectic Solvents as Solid Lubricants: Lubricated State Transition" Materials 16, no. 19: 6579. https://doi.org/10.3390/ma16196579





