Effect of Filler Types on Cellulose-Acetate-Based Composite Used as Coatings for Biodegradable Magnesium Implants for Trauma
Abstract
:1. Introduction
2. Materials and Methods
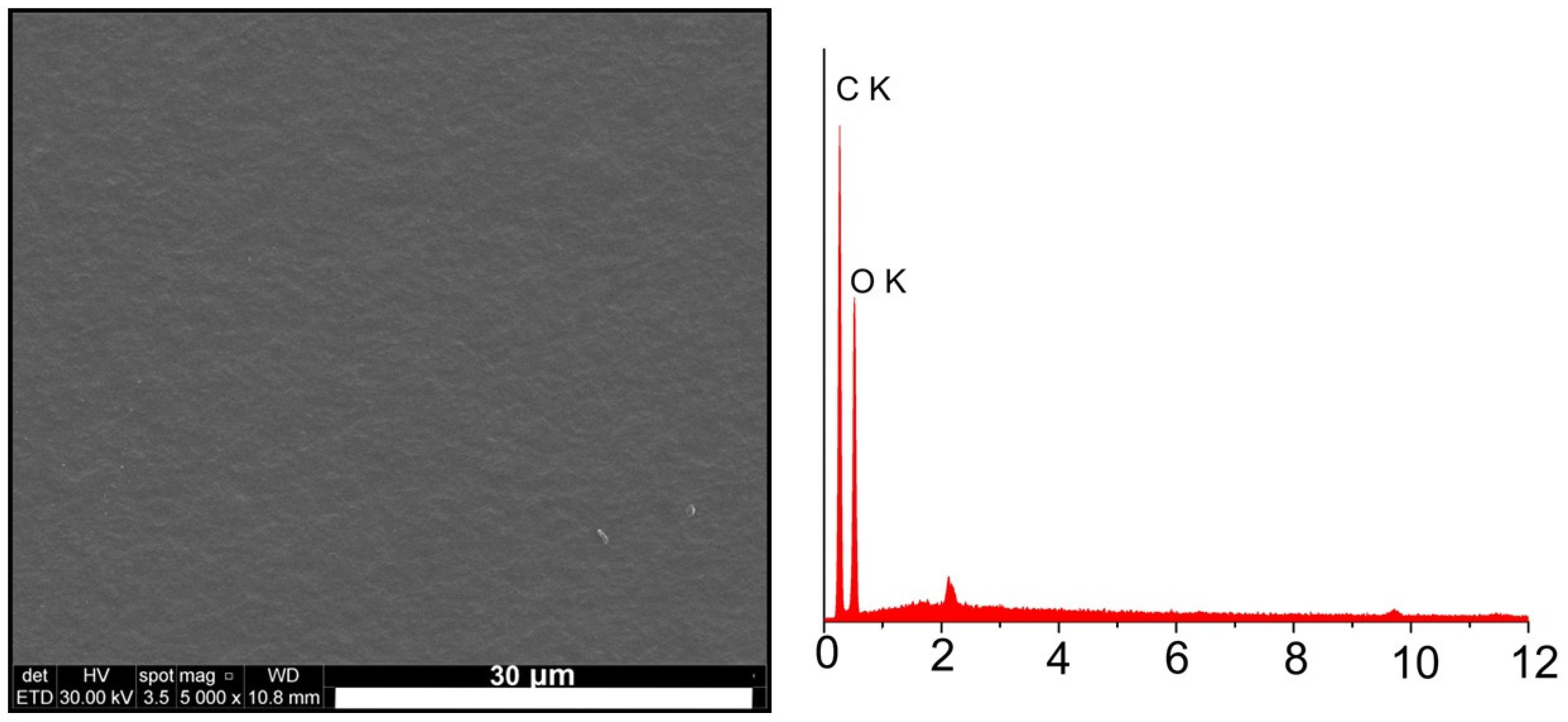
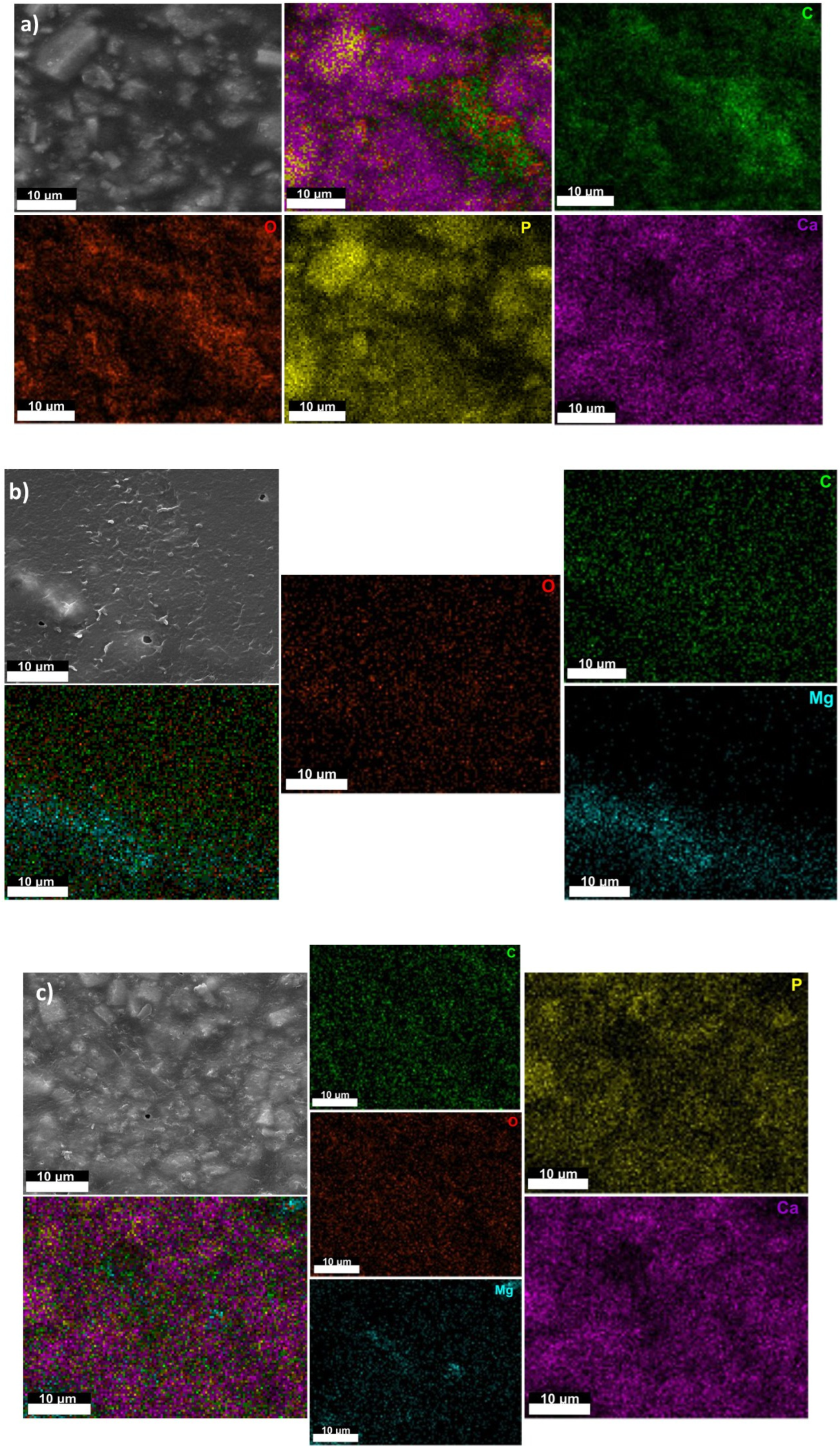
2.1. The Samples’ Surface Morphology and Elemental Composition
2.2. Infrared Spectroscopy
2.3. Raman Spectroscopy
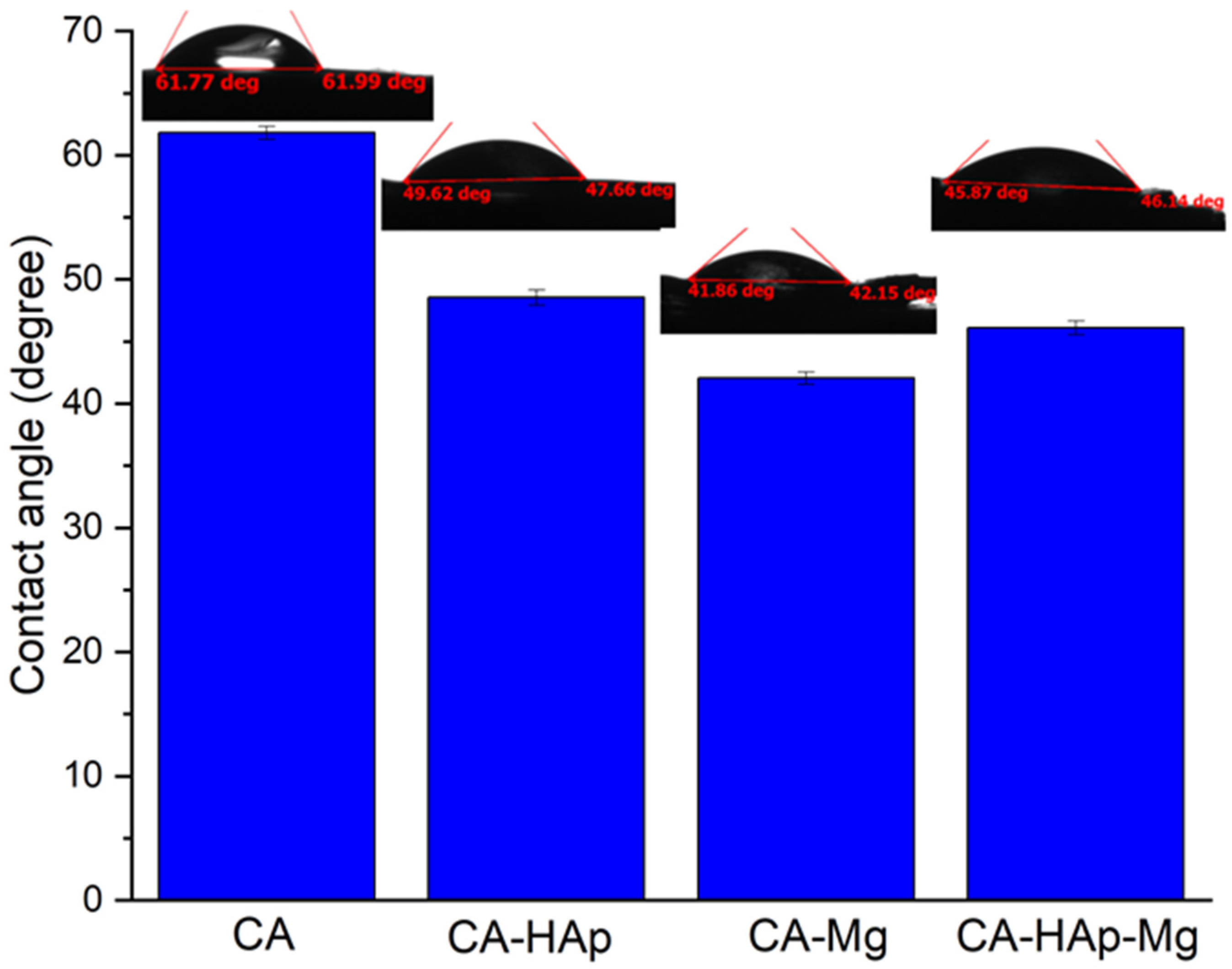
2.4. The Contact Angle Measurements
2.5. Thermogravimetric (TGA) Curves
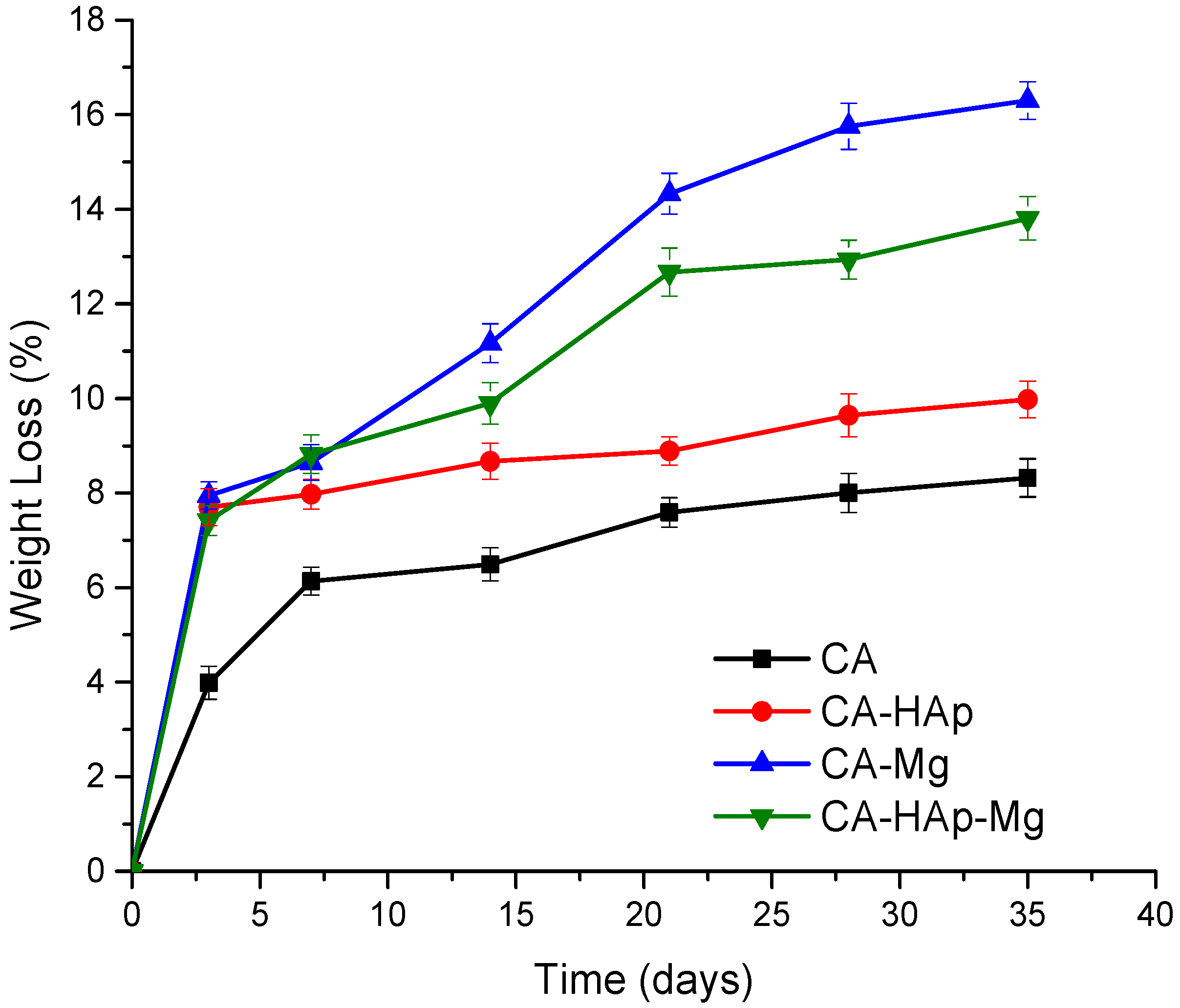
2.6. Degradation and Swelling Studies
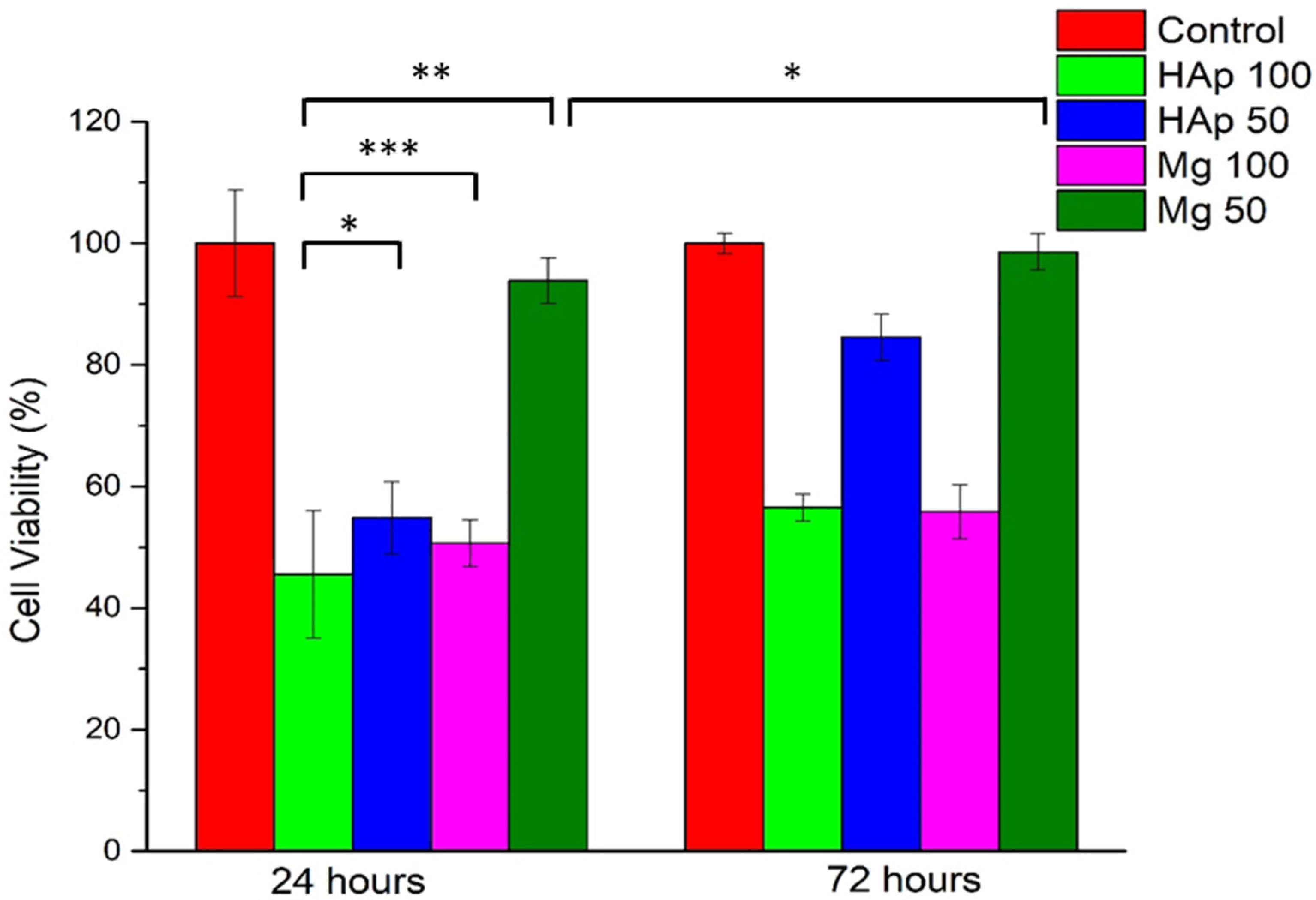
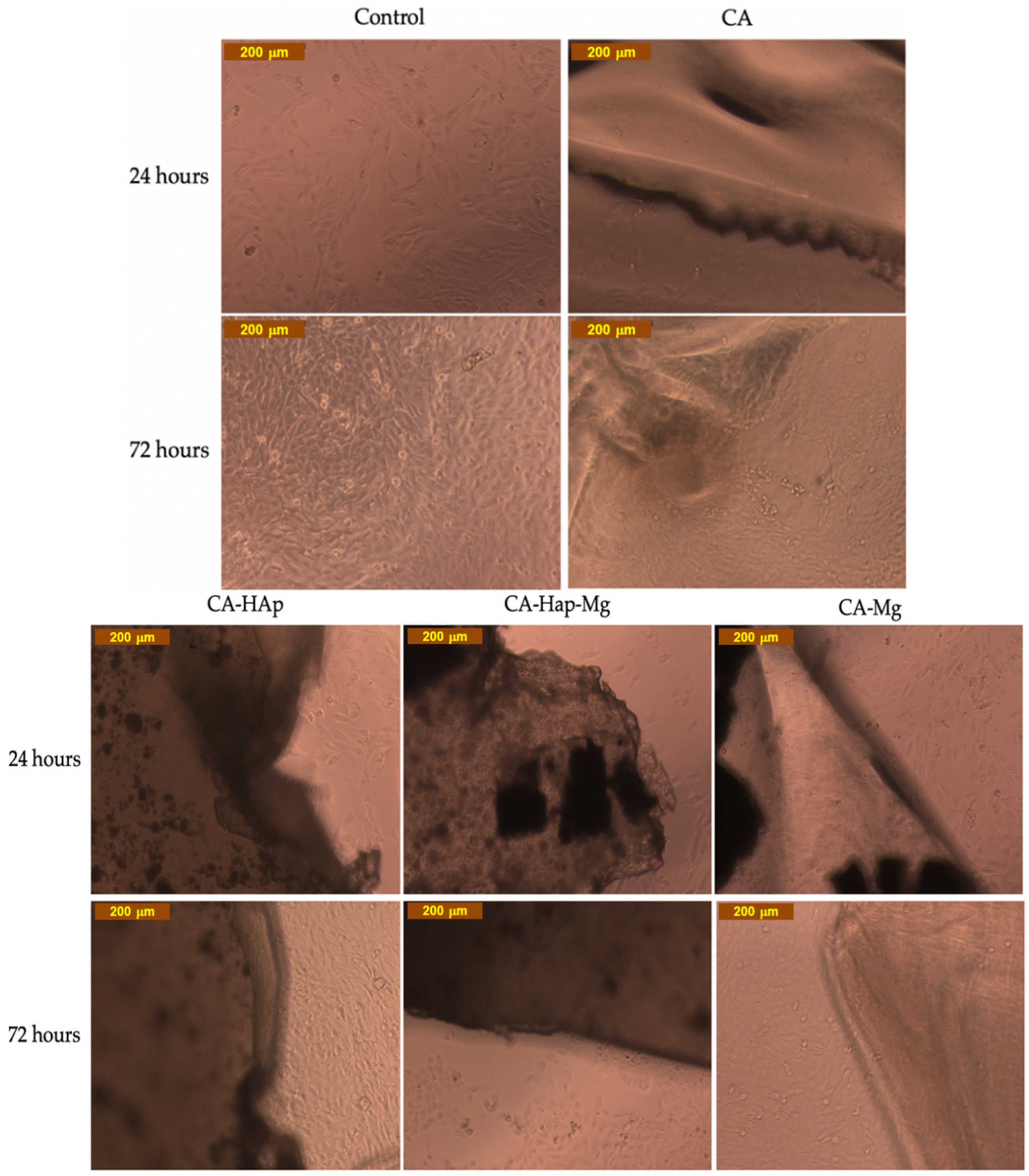
2.7. In Vitro Cytotoxicity Assays
- -
- Indirect contact method for particle materials (hydroxyapatite—HAp and magnesium particles—Mg) used to obtain the composite materials. The extracts were prepared by immersion of 2 mg/mL of each particle material in Dulbecco’s modified Eagle’s medium (DMEM) with 1% P/S/N, under stirring (200 rpm, 37 °C) for 24 h and filtering (70 μm), and finlaly mixed with 10% FBS.
- -
- Direct contact method for CA, CA-HAp, CA-Mg, and CA-HAp-Mg samples, after materials’ incubation with DMEM, suplemented with 10% FBS and 1% P/S/N.
2.8. MTT Assay
3. Results and Discussions
3.1. Scanning Electron Microscopy Analysis
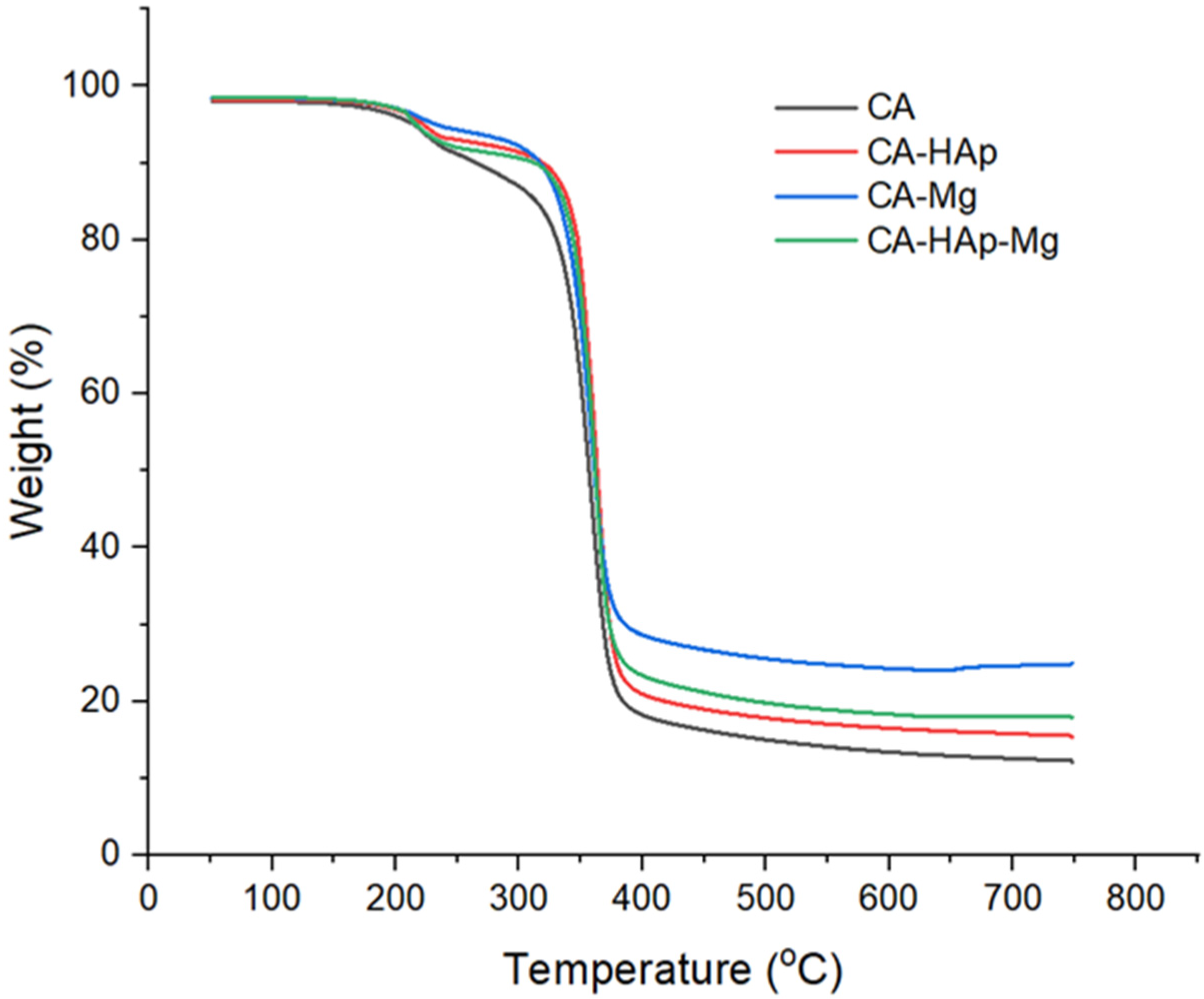
3.2. Thermogravimetric Analysis
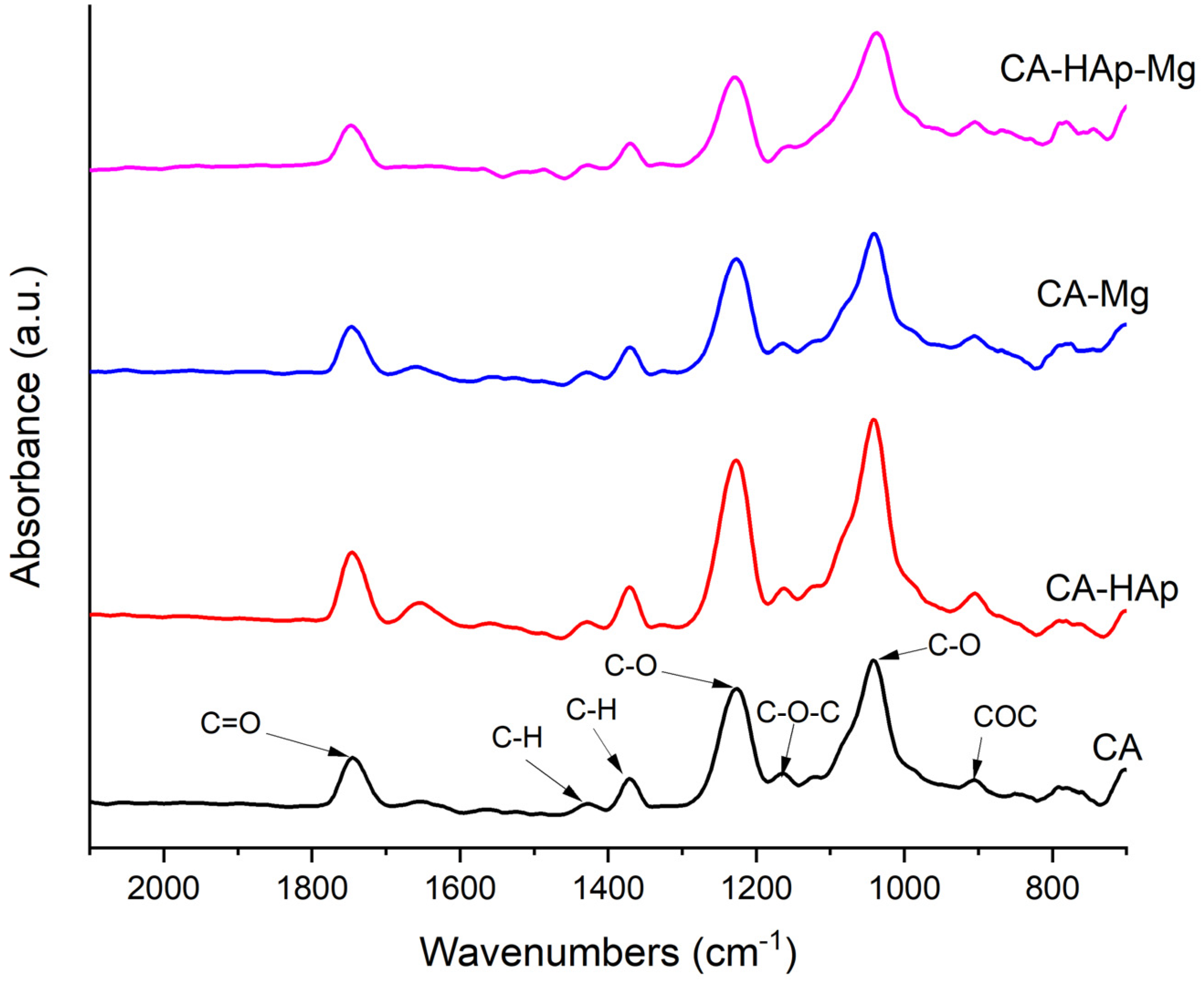
3.3. Fourier Transform Infrared Spectroscopy Analysis
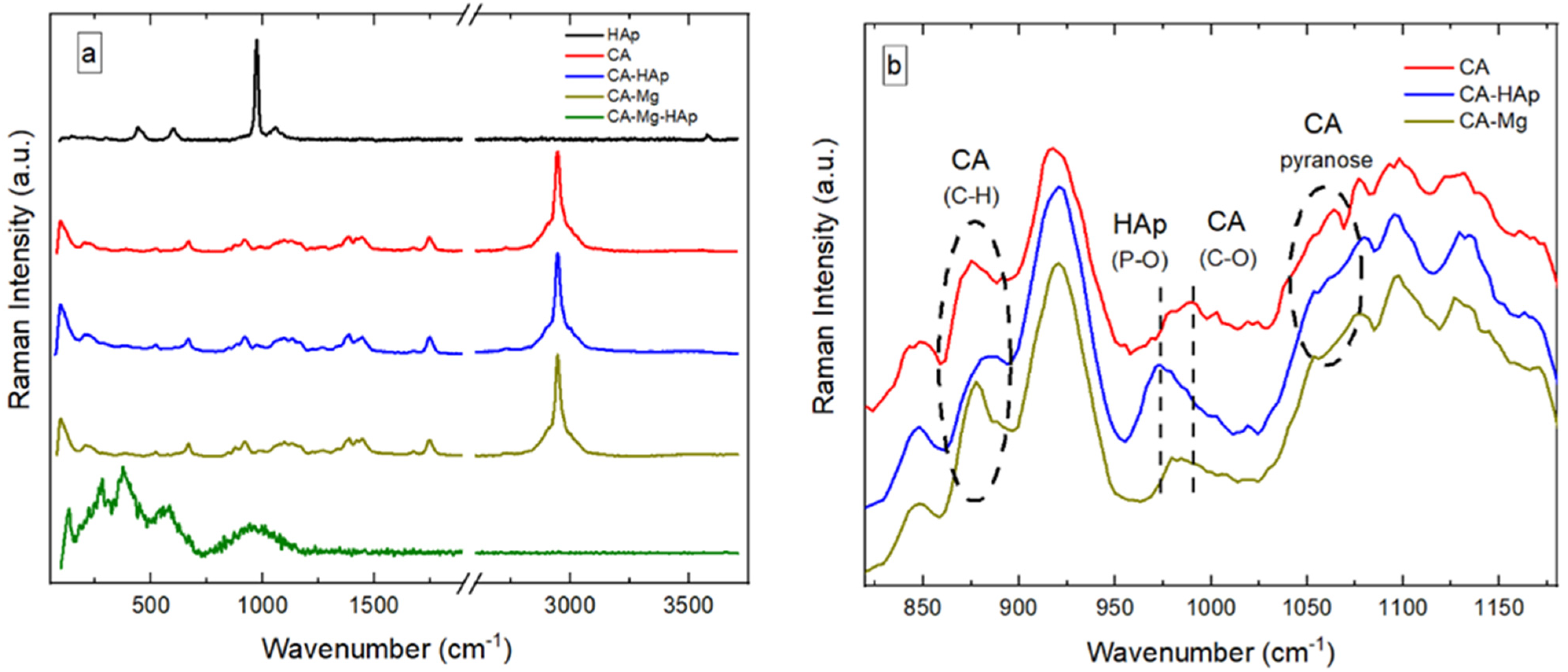
3.4. RAMAN Spectroscopy
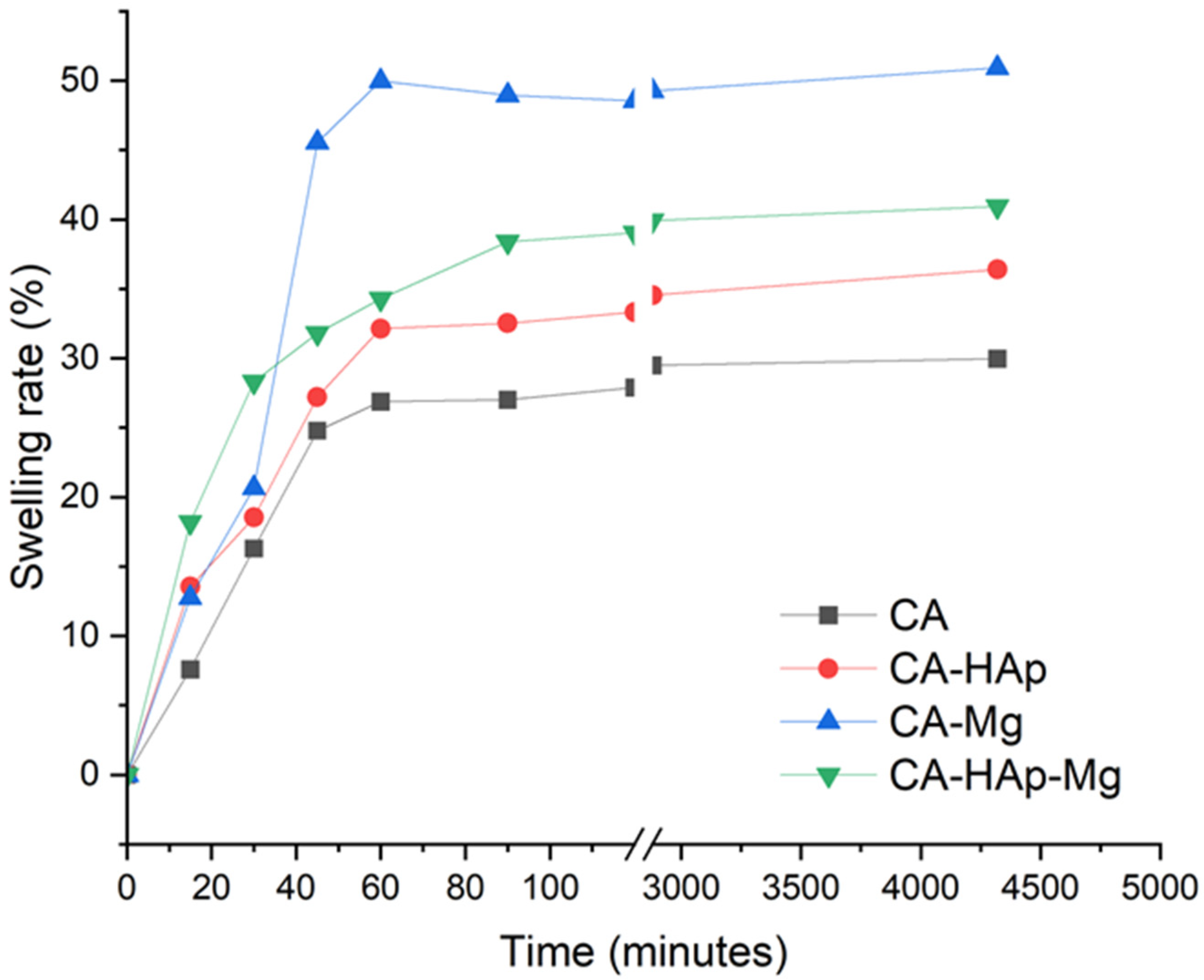
3.5. Degradation and Swelling Studies
3.6. Degradation Behavior
3.7. MTT Assay
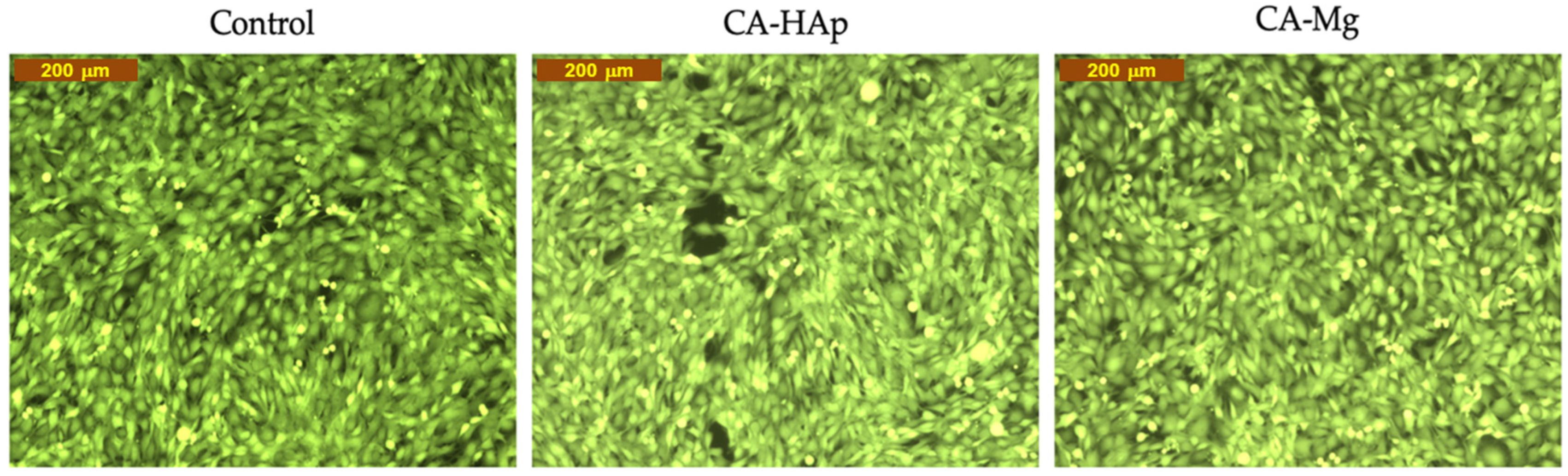
3.8. Calcein-AM Cell Viability Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quan, P.H.; Antoniac, I.; Miculescu, F.; Antoniac, A.; Manescu, V.; Robu, A.; Bița, A.-I.; Miculescu, M.; Saceleanu, A.; Bodog, A.D.; et al. Fluoride Treatment and In Vitro Corrosion Behavior of Mg-Nd-Y-Zn-Zr Alloys Type. Materials 2022, 15, 566. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Antoniac, I.; Miculescu, M.; Mănescu, V.; Stere, A.; Quan, P.H.; Păltânea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nissan, B.; Choi, A.H.; Macha, I.J.; Cazalbou, S. Sol-Gel Nanocoatings of Bioceramics. In Handbook of Bioceramics and Biocomposites; Springer International Publishing: Cham, Switzerland, 2016; pp. 735–756. [Google Scholar]
- Guo, Y.; Su, Y.; Gu, R.; Zhang, Z.; Li, G.; Lian, J.; Ren, L. Enhanced Corrosion Resistance and Biocompatibility of Biodegradable Magnesium Alloy Modified by Calcium Phosphate/Collagen Coating. Surf. Coat. Technol. 2020, 401, 126318. [Google Scholar] [CrossRef]
- Domocos, D.; Popovici, R.; Bei, M.; Anchidin, O.; Todor, L.; Bodog, F.D.; Marcu, O.A.; Bodog, A.; Ciavop, G.; Pogan, M.D. The effect of antioxidants on the evolution of precancerous oral lesions. Int. J. Med. Dent. 2021, 15, 154–158. [Google Scholar]
- Antoniac, I.; Adam, R.; Biță, A.; Miculescu, M.; Trante, O.; Petrescu, I.M.; Pogărășteanu, M. Comparative Assessment of In Vitro and In Vivo Biodegradation of Mg-1Ca Magnesium Alloys for Orthopedic Applications. Materials 2020, 14, 84. [Google Scholar] [CrossRef]
- Yin, Z.-Z.; Qi, W.-C.; Zeng, R.-C.; Chen, X.-B.; Gu, C.-D.; Guan, S.-K.; Zheng, Y.-F. Advances in Coatings on Biodegradable Magnesium Alloys. J. Magnes. Alloy. 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Tong, P.; Sheng, Y.; Hou, R.; Iqbal, M.; Chen, L.; Li, J. Recent Progress on Coatings of Biomedical Magnesium Alloy. Smart Mater. Med. 2022, 3, 104–116. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, K.; Satsangi, P.S.; Sharma, N. Surface Modification of Biodegradable Mg-4Zn Alloy Using PMEDM: An Experimental Investigation, Optimization and Corrosion Analysis. IRBM 2022, 43, 456–469. [Google Scholar] [CrossRef]
- Ahuja, N.; Batra, U.; Kumar, K. Experimental Investigation and Optimization of Wire Electrical Discharge Machining for Surface Characteristics and Corrosion Rate of Biodegradable Mg Alloy. J. Mater. Eng. Perform. 2020, 29, 4117–4129. [Google Scholar] [CrossRef]
- Pan, Y.K.; Chen, C.Z.; Wang, D.G.; Yu, X. Microstructure and Biological Properties of Micro-Arc Oxidation Coatings on ZK60 Magnesium Alloy. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1574–1586. [Google Scholar] [CrossRef]
- Yang, Q.; Yuan, W.; Liu, X.; Zheng, Y.; Cui, Z.; Yang, X.; Pan, H.; Wu, S. Atomic Layer Deposited ZrO2 Nanofilm on Mg-Sr Alloy for Enhanced Corrosion Resistance and Biocompatibility. Acta Biomater. 2017, 58, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Yu, W.; Yang, Z.; Li, Y.; Yang, L.; Lang, S. Properties of Titanium Oxide Coating on MgZn Alloy by Magnetron Sputtering for Stent Application. Coatings 2020, 10, 999. [Google Scholar] [CrossRef]
- Peron, M.; bin Afif, A.; Dadlani, A.; Berto, F.; Torgersen, J. Comparing Physiologically Relevant Corrosion Performances of Mg AZ31 Alloy Protected by ALD and Sputter Coated TiO2. Surf. Coat. Technol. 2020, 395, 125922. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Y.; Liu, X.; Liu, T.; Zou, L.; Wang, Y.; Ren, Y.; Fan, Z.; Lv, Y.; Zhang, M. In-Situ Hydrothermal Crystallization Mg(OH)2 Films on Magnesium Alloy AZ91 and Their Corrosion Resistance Properties. Mater. Chem. Phys. 2013, 143, 322–329. [Google Scholar] [CrossRef]
- Xu, R.; Shen, Y.; Zheng, J.; Wen, Q.; Li, Z.; Yang, X.; Chu, P.K. Effects of One-Step Hydrothermal Treatment on the Surface Morphology and Corrosion Resistance of ZK60 Magnesium Alloy. Surf. Coat. Technol. 2017, 309, 490–496. [Google Scholar] [CrossRef]
- Drynda, A.; Seibt, J.; Hassel, T.; Bach, F.W.; Peuster, M. Biocompatibility of Fluoride-Coated Magnesium-Calcium Alloys with Optimized Degradation Kinetics in a Subcutaneous Mouse Model. J. Biomed. Mater. Res. A 2013, 101, 33–43. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Wong, M.H.; Cheng, F.T.; Man, H.C. Characterization and Corrosion Studies of Fluoride Conversion Coating on Degradable Mg Implants. Surf. Coat. Technol. 2007, 202, 590–598. [Google Scholar] [CrossRef]
- Fernández, J.; el Ouardi, Y.; Bonastre, J.; Molina, J.M.; Cases, F. Modification of the Magnesium Corrosion Rate in Physiological Saline 0.9 Wt % NaCl via Chemical and Electrochemical Coating of Reduced Graphene Oxide. Corros. Sci. 2019, 152, 75–81. [Google Scholar] [CrossRef]
- Mahapatro, A.; Arshanapalli, S.A. Bioceramic Coatings on Magnesium Alloys. J. Bio Tribocorros. 2017, 3, 37. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Li, J.; Zhao, C.; Zhang, X. Electrodeposition of Ca–P Coatings on Biodegradable Mg Alloy: In Vitro Biomineralization Behavior. Acta Biomater. 2010, 6, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Seyfoori, A.; Mirdamadi, S.; Mehrjoo, M.; Khavandi, A. In-Vitro Assessments of Micro Arc Oxidized Ceramic Films on AZ31 Magnesium Implant: Degradation and Cell-Surface Response. Prog. Nat. Sci. Mater. Int. 2013, 23, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yamamoto, A. In Vitro Degradation of Biodegradable Polymer-Coated Magnesium under Cell Culture Condition. Appl. Surf. Sci. 2012, 258, 6353–6358. [Google Scholar] [CrossRef]
- Huniadi, A.; Sorian, A.; Iuhas, C.; Bodog, A.; Sandor, M.I. The Effect of Cannabis in the Treatment of Hodgkin’s Lymphoma in a Pregnant Patient-Extensive Case Report and Literature Review. J. BUON Off. J. Balk. Union Oncol. 2021, 26, 11–16. [Google Scholar]
- Yliniemi, K.; Wilson, B.P.; Singer, F.; Höhn, S.; Kontturi, E.; Virtanen, S. Dissolution Control of Mg by Cellulose Acetate–Polyelectrolyte Membranes. ACS Appl. Mater. Interfaces 2014, 6, 22393–22399. [Google Scholar] [CrossRef] [Green Version]
- Mohan, A.G.; Ciurea, A.V.; Antoniac, I.; Manescu (Paltanea), V.; Bodog, A.; Maghiar, O.; Marcut, L.; Ghiurau, A.; Bodog, F. Cranioplasty after Two Giant Intraosseous Angiolipomas of the Cranium: Case Report and Literature Review. Healthcare 2022, 10, 655. [Google Scholar] [CrossRef]
- Singh, N.; Batra, U.; Kumar, K.; Mahapatro, A. Investigating TiO2–HA–PCL Hybrid Coating as an Efficient Corrosion Resistant Barrier of ZM21 Mg Alloy. J. Magnes. Alloy. 2021, 9, 627–646. [Google Scholar] [CrossRef]
- Alabbasi, A.; Liyanaarachchi, S.; Kannan, M.B. Polylactic Acid Coating on a Biodegradable Magnesium Alloy: An in Vitro Degradation Study by Electrochemical Impedance Spectroscopy. Thin Solid Films 2012, 520, 6841–6844. [Google Scholar] [CrossRef]
- Sheng, Y.; Tian, L.; Wu, C.; Qin, L.; Ngai, T. Biodegradable Poly(l-Lactic Acid) (PLLA) Coatings Fabricated from Nonsolvent Induced Phase Separation for Improving Corrosion Resistance of Magnesium Rods in Biological Fluids. Langmuir 2018, 34, 10684–10693. [Google Scholar] [CrossRef]
- Li, J.N.; Cao, P.; Zhang, X.N.; Zhang, S.X.; He, Y.H. In Vitro Degradation and Cell Attachment of a PLGA Coated Biodegradable Mg–6Zn Based Alloy. J. Mater. Sci. 2010, 45, 6038–6045. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, Y.; Li, D.; Yu, K.; Jiang, D.; Yan, Y.; Chen, L.; Xiao, T. Effects of Polycaprolactone Coating on the Biodegradable Behavior and Cytotoxicity of Mg-6%Zn-10%Ca3(PO4) 2 Composite in Simulated Body Fluid. Mater. Lett. 2017, 198, 118–120. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Tayebi, L. Development and Degradation Behavior of Magnesium Scaffolds Coated with Polycaprolactone for Bone Tissue Engineering. Mater. Lett. 2014, 132, 106–110. [Google Scholar] [CrossRef]
- Neacsu, P.; Staras, A.; Voicu, S.; Ionascu, I.; Soare, T.; Uzun, S.; Cojocaru, V.; Pandele, A.; Croitoru, S.; Miculescu, F.; et al. Characterization and In Vitro and In Vivo Assessment of a Novel Cellulose Acetate-Coated Mg-Based Alloy for Orthopedic Applications. Materials 2017, 10, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liangjian, C.; Jun, Z.; Kun, Y.; Chang, C.; Yilong, D.; Xueyan, Q.; Zhiming, Y. Improving of in Vitro Biodegradation Resistance in a Chitosan Coated Magnesium Bio-Composite. Rare Met. Mater. Eng. 2015, 44, 1862–1865. [Google Scholar] [CrossRef]
- Hee Park, C.; Raj Pant, H.; Sang Kim, C. Effect on corrosion behavior of collagen film/fiber coated az31 magnesium alloy. Dig. J. Nanomater. Biostruct. (DJNB) 2013, 8, 1227–1234. [Google Scholar]
- Wang, C.; Fang, H.; Hang, C.; Sun, Y.; Peng, Z.; Wei, W.; Wang, Y. Fabrication and Characterization of Silk Fibroin Coating on APTES Pretreated Mg-Zn-Ca Alloy. Mater. Sci. Eng. C 2020, 110, 110742. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yagoshi, K.; Asakura, T.; Sasaki, M.; Niidome, T. Silk Fibroin as a Coating Polymer for Sirolimus-Eluting Magnesium Alloy Stents. ACS Appl. Bio. Mater. 2020, 3, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Fang, H.; Qi, X.; Hang, C.; Sun, Y.; Peng, Z.; Wei, W.; Wang, Y. Silk Fibroin Film-Coated MgZnCa Alloy with Enhanced in Vitro and in Vivo Performance Prepared Using Surface Activation. Acta Biomater. 2019, 91, 99–111. [Google Scholar] [CrossRef]
- Dang, N.; Wei, Y.H.; Hou, L.F.; Li, Y.G.; Guo, C.L. Investigation of the Inhibition Effect of the Environmentally Friendly Inhibitor Sodium Alginate on Magnesium Alloy in Sodium Chloride Solution. Mater. Corros. 2015, 66, 1354–1362. [Google Scholar] [CrossRef]
- Agarwal, S.; Riffault, M.; Hoey, D.; Duffy, B.; Curtin, J.; Jaiswal, S. Biomimetic Hyaluronic Acid-Lysozyme Composite Coating on AZ31 Mg Alloy with Combined Antibacterial and Osteoinductive Activities. ACS Biomater. Sci. Eng. 2017, 3, 3244–3253. [Google Scholar] [CrossRef]
- Jothi, V.; Adesina, A.Y.; Kumar, A.M.; Rahman, M.M.; Ram, J.S.N. Enhancing the Biodegradability and Surface Protective Performance of AZ31 Mg Alloy Using Polypyrrole/Gelatin Composite Coatings with Anodized Mg Surface. Surf. Coat. Technol. 2020, 381, 125139. [Google Scholar] [CrossRef]
- Akram, M.; Arshad, N.; Aktan, M.K.; Braem, A. Alternating Current Electrophoretic Deposition of Chitosan–Gelatin–Bioactive Glass on Mg–Si–Sr Alloy for Corrosion Protection. ACS Appl. Bio. Mater. 2020, 3, 7052–7060. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Ahmadi, T.; Dehaghani, M.T.; Mohemi, K. Synthesis, Corrosion and Bioactivity Evaluation of Gelatin/Silicon and Magnesium Co-Doped Fluorapatite Nanocomposite Coating Applied on AZ31 Mg Alloy. Russ. J. Non-Ferr. Met. 2018, 59, 458–464. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Y.; Gong, Z.; Liu, T.; Gong, T.; Liu, S.; Zhang, C.; Quan, L.; Kaveendran, B.; Pan, C. Fabrication of Chitosan/Heparinized Graphene Oxide Multilayer Coating to Improve Corrosion Resistance and Biocompatibility of Magnesium Alloys. Mater. Sci. Eng. C 2019, 104, 109947. [Google Scholar] [CrossRef]
- Hahn, B.-D.; Park, D.-S.; Choi, J.-J.; Ryu, J.; Yoon, W.-H.; Choi, J.-H.; Kim, H.-E.; Kim, S.-G. Aerosol Deposition of Hydroxyapatite–Chitosan Composite Coatings on Biodegradable Magnesium Alloy. Surf. Coat. Technol. 2011, 205, 3112–3118. [Google Scholar] [CrossRef]
- Córdoba, L.C.; Marques, A.; Taryba, M.; Coradin, T.; Montemor, F. Hybrid Coatings with Collagen and Chitosan for Improved Bioactivity of Mg Alloys. Surf. Coat. Technol. 2018, 341, 103–113. [Google Scholar] [CrossRef]
- Zhang, L.; Pei, J.; Wang, H.; Shi, Y.; Niu, J.; Yuan, F.; Huang, H.; Zhang, H.; Yuan, G. Facile Preparation of Poly(Lactic Acid)/Brushite Bilayer Coating on Biodegradable Magnesium Alloys with Multiple Functionalities for Orthopedic Application. ACS Appl. Mater. Interfaces 2017, 9, 9437–9448. [Google Scholar] [CrossRef]
- Chen, L.; Sheng, Y.; Zhou, H.; Li, Z.; Wang, X.; Li, W. Influence of a MAO + PLGA Coating on Biocorrosion and Stress Corrosion Cracking Behavior of a Magnesium Alloy in a Physiological Environment. Corros. Sci. 2019, 148, 134–143. [Google Scholar] [CrossRef]
- Lim, S.-W.; Loh, H.-S.; Ting, K.-N.; Bradshaw, T.D.; Allaudin, Z.N. Reduction of MTT to purple formazan by vitamin E isomers in the absence of cells. Trop. Life Sci. Res. 2015, 26, 111. [Google Scholar]
- Miao, X.; Lin, J.; Bian, F. Utilization of Discarded Crop Straw to Produce Cellulose Nanofibrils and Their Assemblies. J. Bioresour. Bioprod. 2020, 5, 26–36. [Google Scholar] [CrossRef]
- Fischer, S.; Thümmler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and Applications of Cellulose Acetate. Macromol. Symp. 2008, 262, 89–96. [Google Scholar] [CrossRef]
- Douglass, E.F.; Avci, H.; Boy, R.; Rojas, O.J.; Kotek, R. A Review of Cellulose and Cellulose Blends for Preparation of Bio-Derived and Conventional Membranes, Nanostructured Thin Films, and Composites. Polym. Rev. 2018, 58, 102–163. [Google Scholar] [CrossRef]
- Aoki, D.; Teramoto, Y.; Nishio, Y. SH-Containing Cellulose Acetate Derivatives: Preparation and Characterization as a Shape Memory-Recovery Material. Biomacromolecules 2007, 8, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Madaeni, S.S.; Derakhshandeh, K.; Ahmadi, S.; Vatanpour, V.; Zinadini, S. Effect of Modified Multi-Walled Carbon Nanotubes on Release Characteristics of Indomethacin from Symmetric Membrane Coated Tablets. J. Memb. Sci. 2012, 389, 110–116. [Google Scholar] [CrossRef]
- Vatankhah, E.; Prabhakaran, M.P.; Jin, G.; Mobarakeh, L.G.; Ramakrishna, S. Development of Nanofibrous Cellulose Acetate/Gelatin Skin Substitutes for Variety Wound Treatment Applications. J. Biomater. Appl. 2014, 28, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, W.; Shi, X.; Li, B.; Duan, S.; Guo, H.; Guo, J. Influence of Laser Process Parameters on the Densification, Microstructure, and Mechanical Properties of a Selective Laser Melted AZ61 Magnesium Alloy. J. Alloys Compd. 2019, 808, 151160. [Google Scholar] [CrossRef]
- Abdelhamid, A.E.; Khalil, A.M. Polymeric Membranes Based on Cellulose Acetate Loaded with Candle Soot Nanoparticles for Water Desalination. J. Macromol. Sci. Part A 2019, 56, 153–161. [Google Scholar] [CrossRef]
- de Guzman, M.R.; Andra, C.K.A.; Ang, M.B.M.Y.; Dizon, G.V.C.; Caparanga, A.R.; Huang, S.-H.; Lee, K.-R. Increased Performance and Antifouling of Mixed-Matrix Membranes of Cellulose Acetate with Hydrophilic Nanoparticles of Polydopamine-Sulfobetaine Methacrylate for Oil-Water Separation. J. Memb. Sci. 2021, 620, 118881. [Google Scholar] [CrossRef]
- Demir, A.G.; Taketa, T.B.; Tolouei, R.; Furlan, V.; Paternoster, C.; Beppu, M.M.; Mantovani, D.; Previtali, B. Laser Surface Structuring Affects Polymer Deposition, Coating Homogeneity, and Degradation Rate of Mg Alloys. Mater. Lett. 2015, 160, 359–362. [Google Scholar] [CrossRef]
- Voicu, S.I.; Condruz, R.M.; Mitran, V.; Cimpean, A.; Miculescu, F.; Andronescu, C.; Miculescu, M.; Thakur, V.K. Sericin Covalent Immobilization onto Cellulose Acetate Membrane for Biomedical Applications. ACS Sustain. Chem. Eng. 2016, 4, 1765–1774. [Google Scholar] [CrossRef]
- Bikiaris, D. Can Nanoparticles Really Enhance Thermal Stability of Polymers? Part II: An Overview on Thermal Decomposition of Polycondensation Polymers. Thermochim. Acta 2011, 523, 25–45. [Google Scholar] [CrossRef]
- Kumar, M.; Isloor, A.M.; Todeti, S.R.; Nagaraja, H.S.; Ismail, A.F.; Susanti, R. Effect of Binary Zinc-Magnesium Oxides on Polyphenylsulfone/Cellulose Acetate Derivatives Hollow Fiber Membranes for the Decontamination of Arsenic from Drinking Water. Chem. Eng. J. 2021, 405, 126809. [Google Scholar] [CrossRef]
- Al-Kubaisi, O.; Nasseri, R.; Moresoli, C.; Yu, A. Thermal Behavior and Kinetic Study of Plasticized Cellulose Acetate Magnesium Hydroxide Polypropylene Materials. Mater. Today Proc. 2021, 42, 2410–2421. [Google Scholar] [CrossRef]
- Nosenko, V.V.; Yaremko, A.M.; Dzhagan, V.M.; Vorona, I.P.; Romanyuk, Y.A.; Zatovsky, I.V. Nature of Some Features in Raman Spectra of Hydroxyapatite-Containing Materials. J. Raman Spectrosc. 2016, 47, 726–730. [Google Scholar] [CrossRef]
- Sánchez-Márquez, J.A.; Fuentes-Ramírez, R.; Cano-Rodríguez, I.; Gamiño-Arroyo, Z.; Rubio-Rosas, E.; Kenny, J.M.; Rescignano, N. Membrane Made of Cellulose Acetate with Polyacrylic Acid Reinforced with Carbon Nanotubes and Its Applicability for Chromium Removal. Int. J. Polym. Sci. 2015, 2015, 320631. [Google Scholar] [CrossRef] [Green Version]
- Mundozah, A.L.; Tridon, C.C.; Cartwright, J.J.; Salman, A.D.; Hounslow, M.J. Wetting of Binary Powder Mixtures. Int. J. Pharm. 2019, 572, 118770. [Google Scholar] [CrossRef]
- Ehnert, S.; Rinderknecht, H.; Aspera-Werz, R.H.; Häussling, V.; Nussler, A.K. Use of in Vitro Bone Models to Screen for Altered Bone Metabolism, Osteopathies, and Fracture Healing: Challenges of Complex Models. Arch. Toxicol. 2020, 94, 3937–3958. [Google Scholar] [CrossRef] [PubMed]
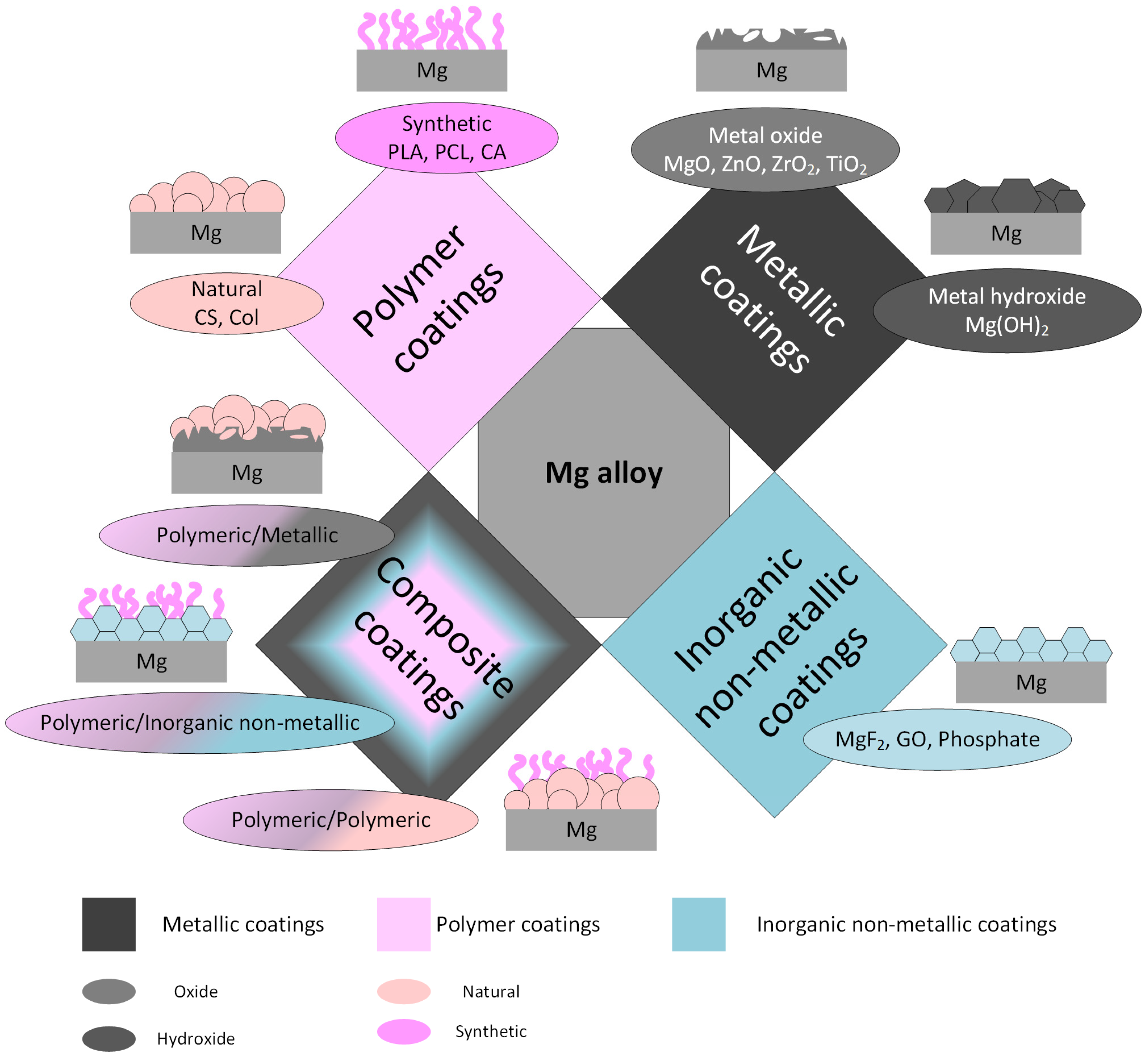
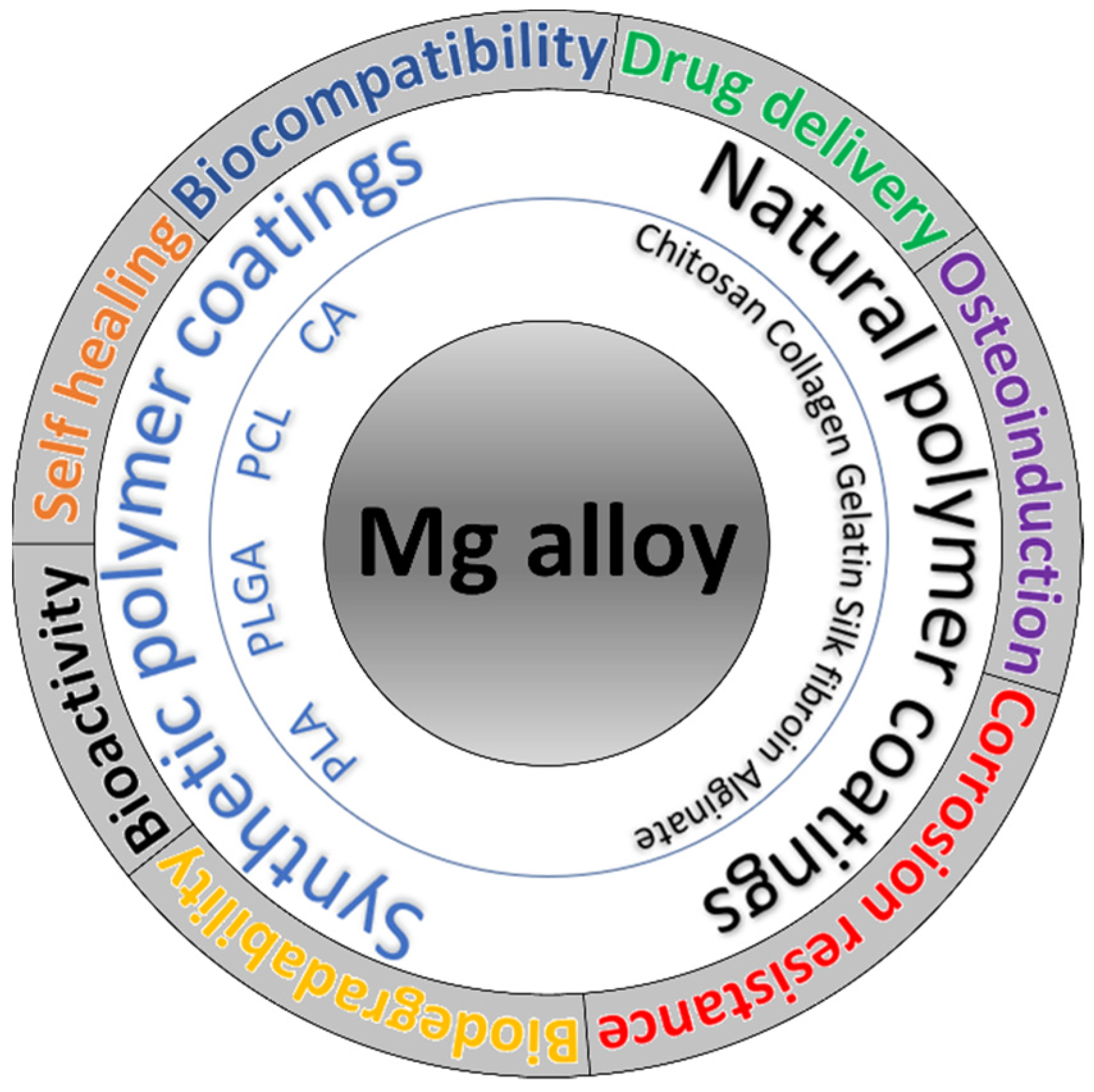


| Polymer | Polymer Properties | Coating | Mg-Based Substrate | Coating Technology | Remarks | Ref. |
|---|---|---|---|---|---|---|
| Polylactic Acid (PLA) | Biodegradable, Young’s modulus of about 3 GPa, ultimate tensile strength (UTS) of 50–70 MPa, fracture toughness of about 2.5 kJ/m2, biocompatible, degrades through hydrolysis | Poly(L-lactic acid) (PLLA) | Pure Mg rods | Dip-coating | PLLA coatings with porous structures and dense surfaces were obtained. The corrosion resistance was improved, and the lower pH of the coating sustained the emission of Mg2+ ions | [30] |
| PLA | Mg-9Al-1Zn (AZ91) | Spin-coating | PLA coating improved the corrosion behavior of the Mg-based alloy | [29] | ||
| Poly(Lactide-Co-Glycolic) Acid (PLGA) | High biocompatibility, already approved for use in human clinical trials, biodegradability, exhibits controllable degradation properties | PLGA | Mg-6Zn | Dip-coating | The degradation rate was reduced by a high amount | [31] |
| Polycapro lactone (PCL) | PCL is Food and Drug Administration (FDA)-approved. Biocompatibiliy, biodegradability, nontoxicity, high strain failure rate | PCL | Mg-based scaffolds produced through powder metallurgy route | Dip-coating | The degradation rate was reduced | [33] |
| PCL | Mg-6%Zn-10%Ca3(PO4)2 scaffolds | Surface sprayed and solidified at 50 °C for 5 min | Corrosion activity, surface morphology, and cytotoxicity were improved | [32] | ||
| Cellulose acetate (CA) | It increases corrosion resistance. CA is a thermoplastic material, and its main source is cellulose that is a natural polymer. CA is not manufactured by polymerization of any monomer | CA | Mg-Ca-Mn-Zr | Dip-coating | The coating increased the corrosion resistance. The material exhibited good cytocompatibility, promoting cell adhesion, viability, proliferation, and osteogenic differentiation | [34] |
| Composite Coating | Type of Composite Coating | Mg-Based Substrate | Coating Technology | Remarks | Ref. |
|---|---|---|---|---|---|
| Polylactic Acid (PLA)/Brushite | Synthetic polymeric/inorganic non-metallic ceramic based on phosphate | Mg-Nd-Zn-Zr | Composite coating with an inner layer of PLA and an outer layer of brushite prepared through chemical deposition | The biocorrosion resistance and biocompatibility were increased. The developed materials can be used as drug-delivery systems. | [48] |
| Micro-arc oxidation (MAO) + Poly(Lactide-Co-Glycolic) acid (PLGA) composite | Metallic/Synthetic polymeric | Mg-4Zn-0.6Zr-0.4Sr | Micro-arc oxidation | The MAO+PLGA coating increased the corrosion and stress corrosion cracking resistance of the Mg-based alloy. The high mechanical stability of the coated alloy was put in evidence. | [49] |
| Chitosan (CS)/heparinized graphene oxide (HGO) composite film | Natural polymeric/inorganic non-metallic based on graphene oxide | Mg-3Al-1Zn (AZ31B) | Layer-by-layer self-assembly method (LBL) | The CS/HGO composite coating degraded slowly in simulated body fluid (SBF) solution and offered the Mg-based alloy high corrosion protection. The multilayer film promoted the proliferation and adhesion of the endothelial cells and played an essential role in the reduction in the hemolysis rate. | [45] |
| Highly dense Hydroxyapatite (Hap)/Chitosan (CS) film | Inorganic non-metallic ceramic based on phosphate/natural polymeric | Mg-3Al-1Zn -Mn (AZ31) | Aerosol deposition | The aerosol deposition is a method used to manufacture high-density ceramic-polymer composite coatings. The coated samples exhibited a high adhesion phenomenon and a reduced corrosion rate. The Mg-based alloy biocompatibility was highly improved. | [46] |
| Bi-layered functional coatings consisting of an inner silane–TiO2 coating and an outer polymeric layer made from collagen (Col) or chitosan (CS) | Metallic/Natural polymeric | Mg-3Al-1Zn (AZ31), Mg-4Zn-2RE-0.7Zr (ZE41) | Sol–gel deposition and thermal conditioning | The coating has a beneficial effect against corrosion. High biocompatibility was put in evidence due to natural polymers’ existence. The bi-layered coating prevents the hydrogen from being released. | [47] |
| Polypyrrole (Ppy)/Gelatin (Gel) | Synthetic polymeric/natural polymeric | Mg-3Al-1Zn (AZ31) | Anodization and electrodeposition | A composite coating, which contains an anodization film and a Ppy/Gel layer, increases the corrosion resistance and reduces the H2 elimination | [42] |
| Sample | Composition |
|---|---|
| CA | Cellulose acetate |
| CA-HAp | Cellulose acetate + 5% hydroxyapatite |
| CA-Mg | Cellulose acetate + 5% Mg particles |
| CA-HAp-Mg | Cellulose acetate + 5% hydroxyapatite + 5% Mg particles |
| Samples | Contact Angle (Degree) |
|---|---|
| CA | 61.80 ± 0.53 |
| CA-HAp | 48.54 ± 0.62 |
| CA-Mg | 42.05 ± 0.49 |
| CA-HAp-Mg | 46.09 ± 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streza, A.; Antoniac, A.; Manescu, V.; Paltanea, G.; Robu, A.; Dura, H.; Verestiuc, L.; Stanica, E.; Voicu, S.I.; Antoniac, I.; et al. Effect of Filler Types on Cellulose-Acetate-Based Composite Used as Coatings for Biodegradable Magnesium Implants for Trauma. Materials 2023, 16, 554. https://doi.org/10.3390/ma16020554
Streza A, Antoniac A, Manescu V, Paltanea G, Robu A, Dura H, Verestiuc L, Stanica E, Voicu SI, Antoniac I, et al. Effect of Filler Types on Cellulose-Acetate-Based Composite Used as Coatings for Biodegradable Magnesium Implants for Trauma. Materials. 2023; 16(2):554. https://doi.org/10.3390/ma16020554
Chicago/Turabian StyleStreza, Alexandru, Aurora Antoniac, Veronica Manescu (Paltanea), Gheorghe Paltanea, Alina Robu, Horatiu Dura, Liliana Verestiuc, Enache Stanica, Stefan Ioan Voicu, Iulian Antoniac, and et al. 2023. "Effect of Filler Types on Cellulose-Acetate-Based Composite Used as Coatings for Biodegradable Magnesium Implants for Trauma" Materials 16, no. 2: 554. https://doi.org/10.3390/ma16020554




.png)








