Tuning Shear Thinning Factors of 3D Bio-Printable Hydrogels Using Short Fiber
Abstract
:1. Introduction
2. Materials and Methods
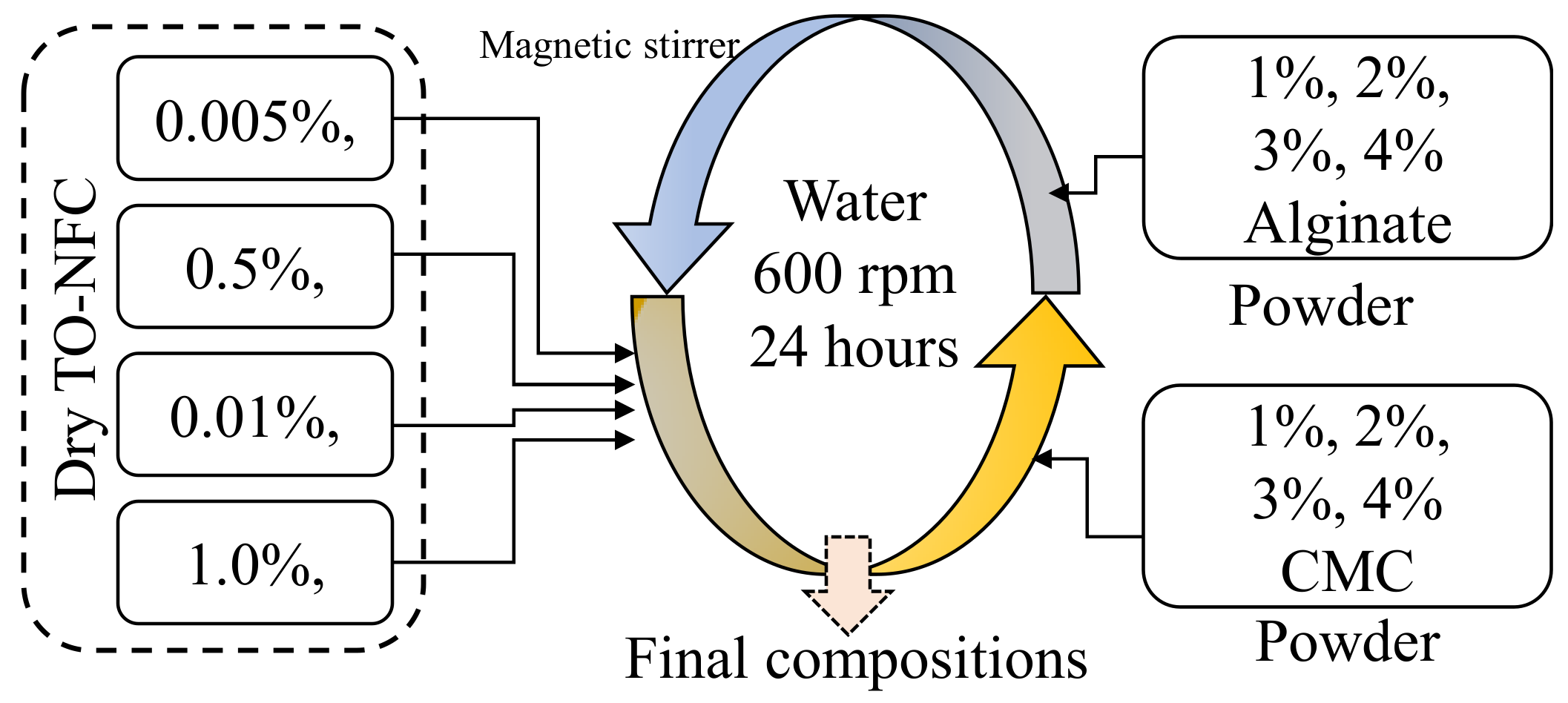
2.1. Processing of TO-NFC and Hydrogel Preparation
2.2. Rheological Analysis
2.3. 3D Printing and Shape Fidelity Analysis
2.4. Investigation on Microstructure and Biocompatibity
2.5. Statistical Analysis
3. Results
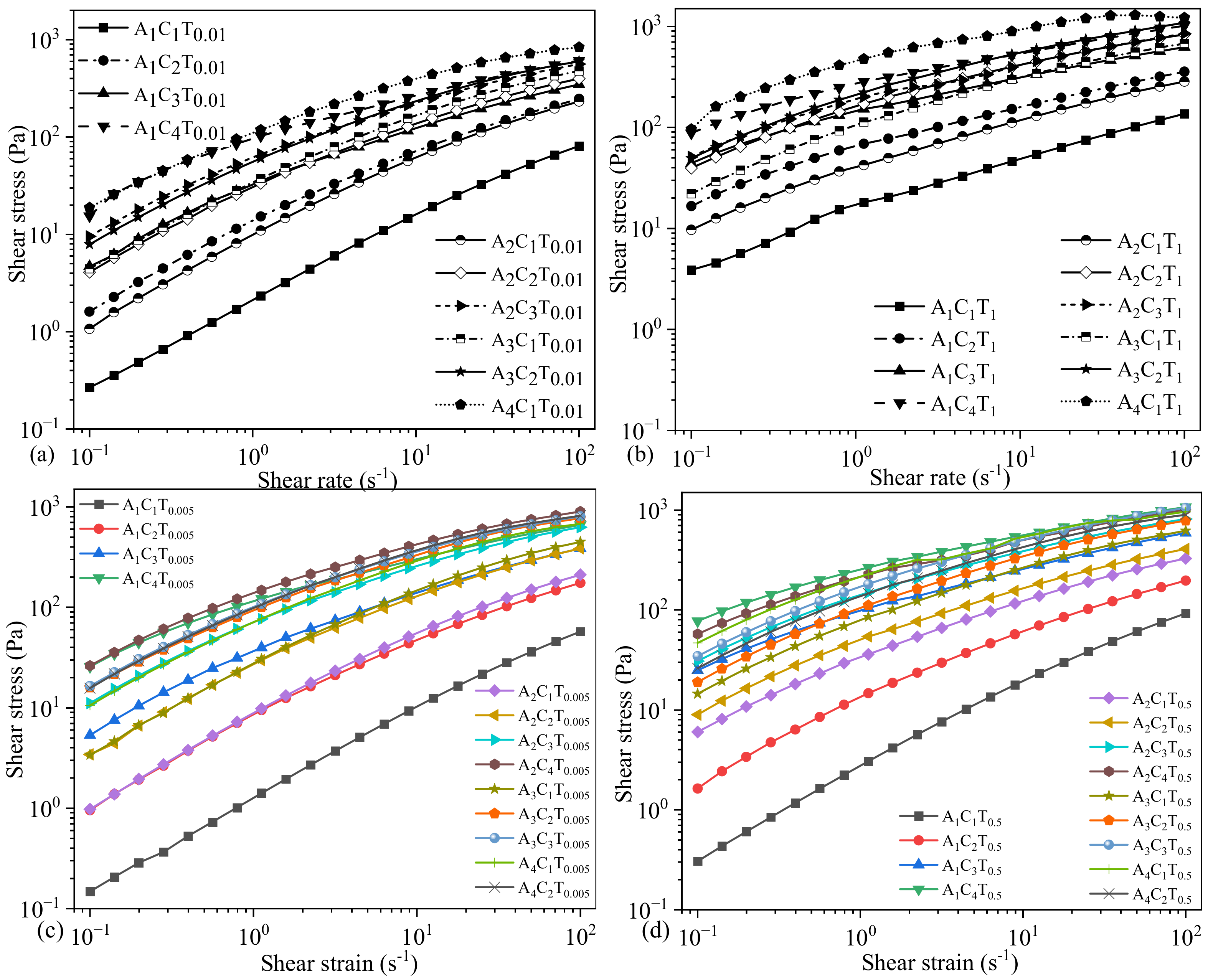
3.1. Flow Behavior of Hydrogels Prepared by 0.005% and 0.5% TO-NFC
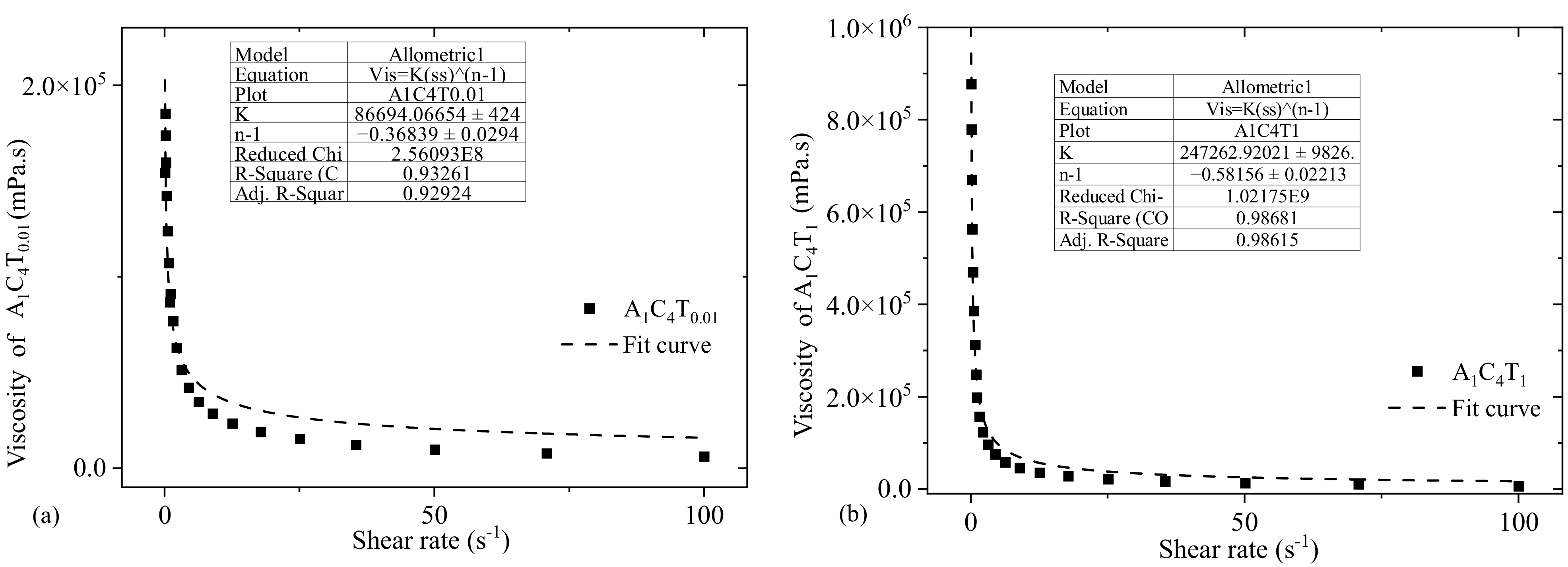
3.2. Flow Behavior of Hydrogels Prepared by 0.01% and 1.0% TO-NFC
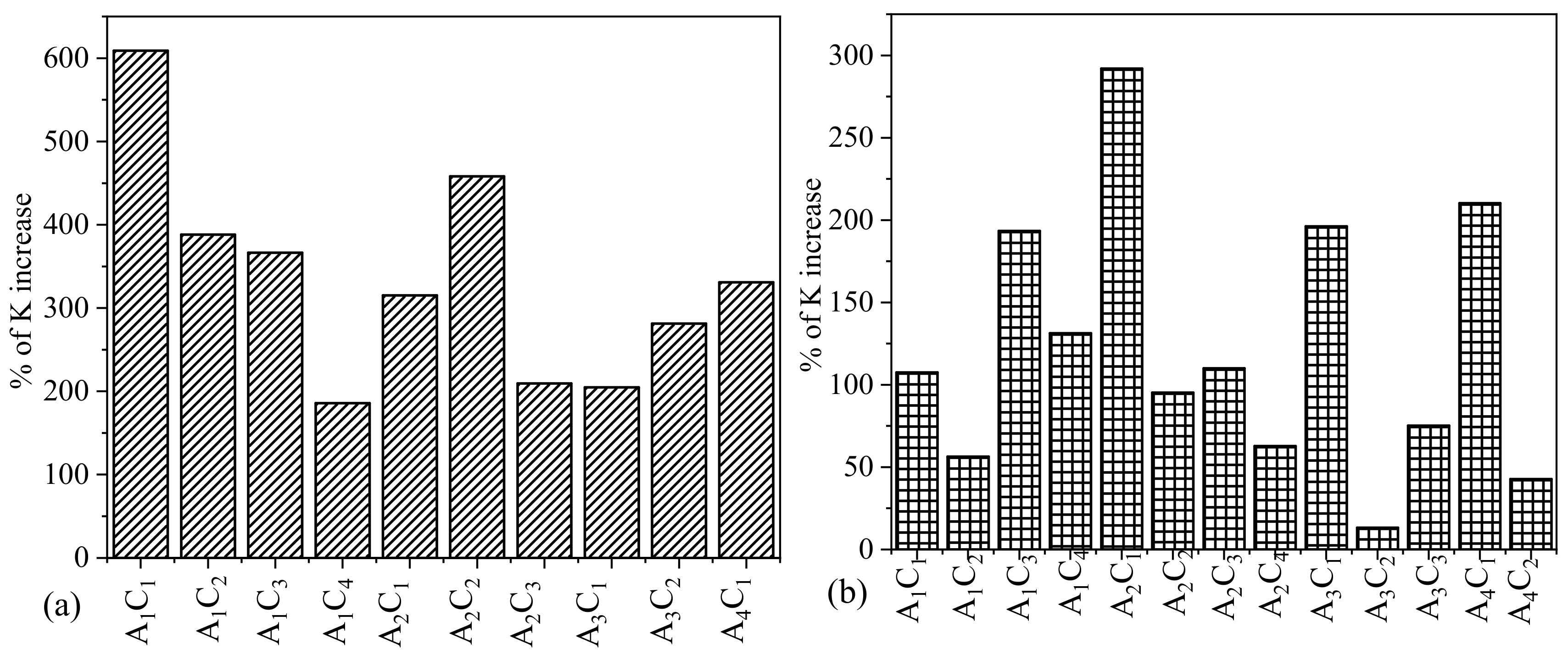
3.3. Impact of Higher Percentages of TO-NFC over Lower Percentages of TO-NFC
3.4. Dynamic Shear Stress and Yield Stress of Compositions Prepared with 0.005%, 0.01%, 0.5%, and 1.0% TO-NFC
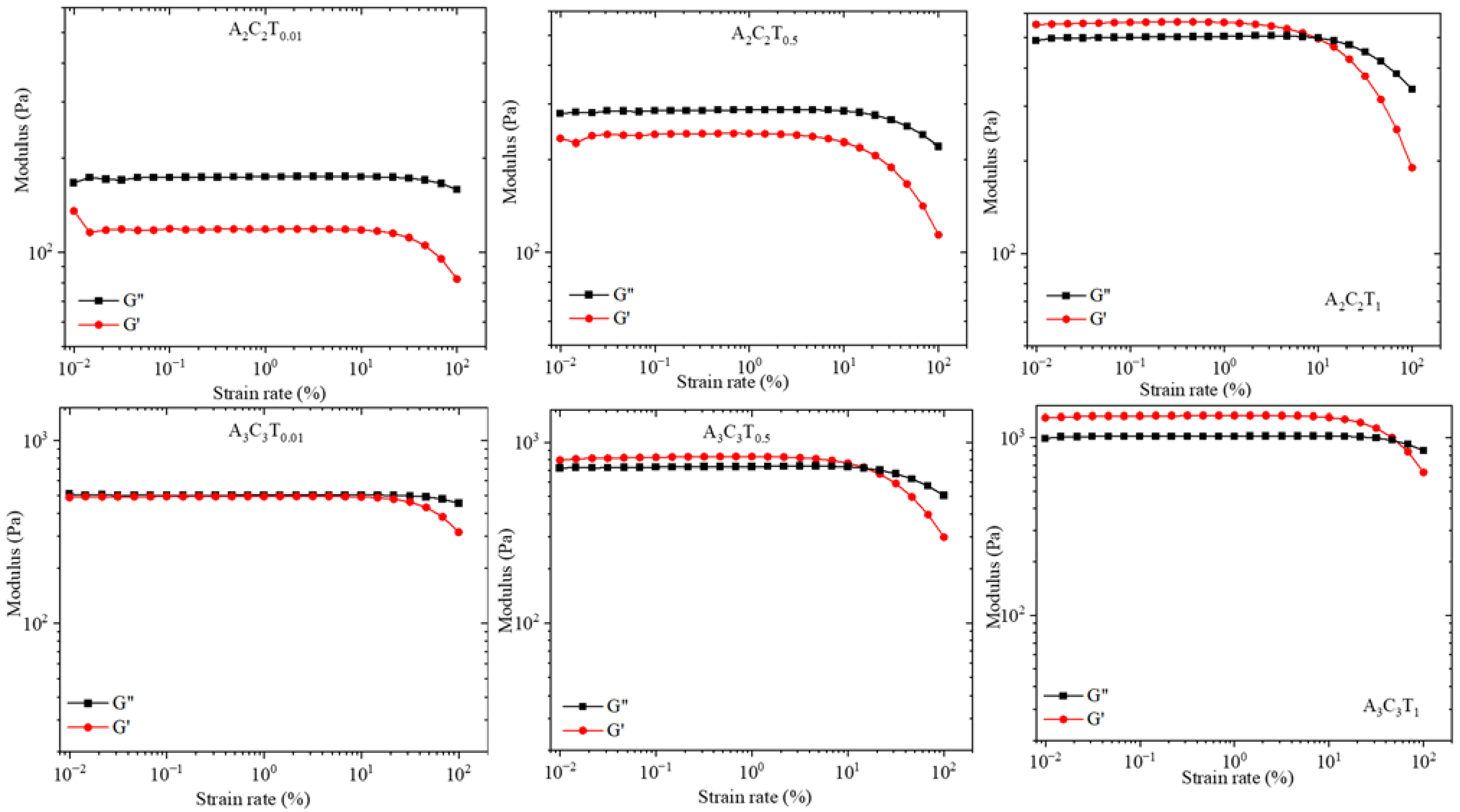
3.5. Amplitude Test: Storage and Loss Modulus
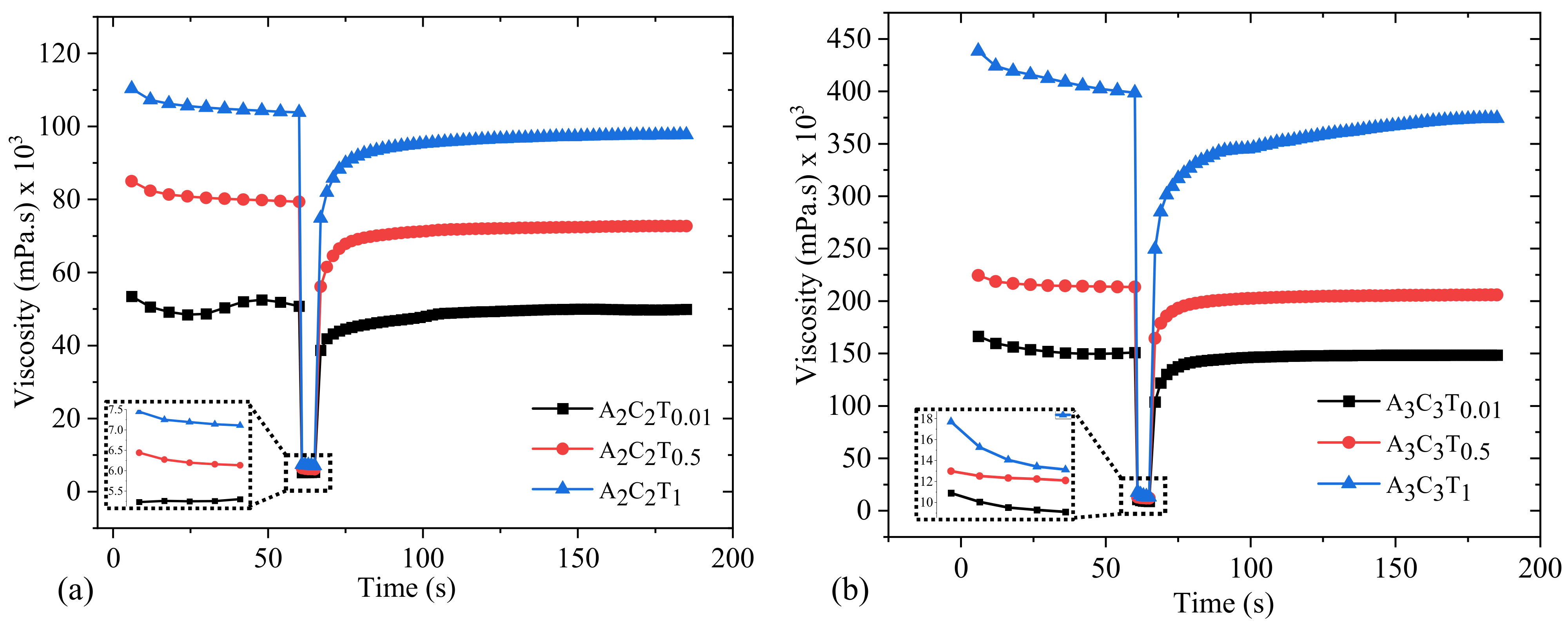
3.6. Three Point Thixotropic Test (3iTT)
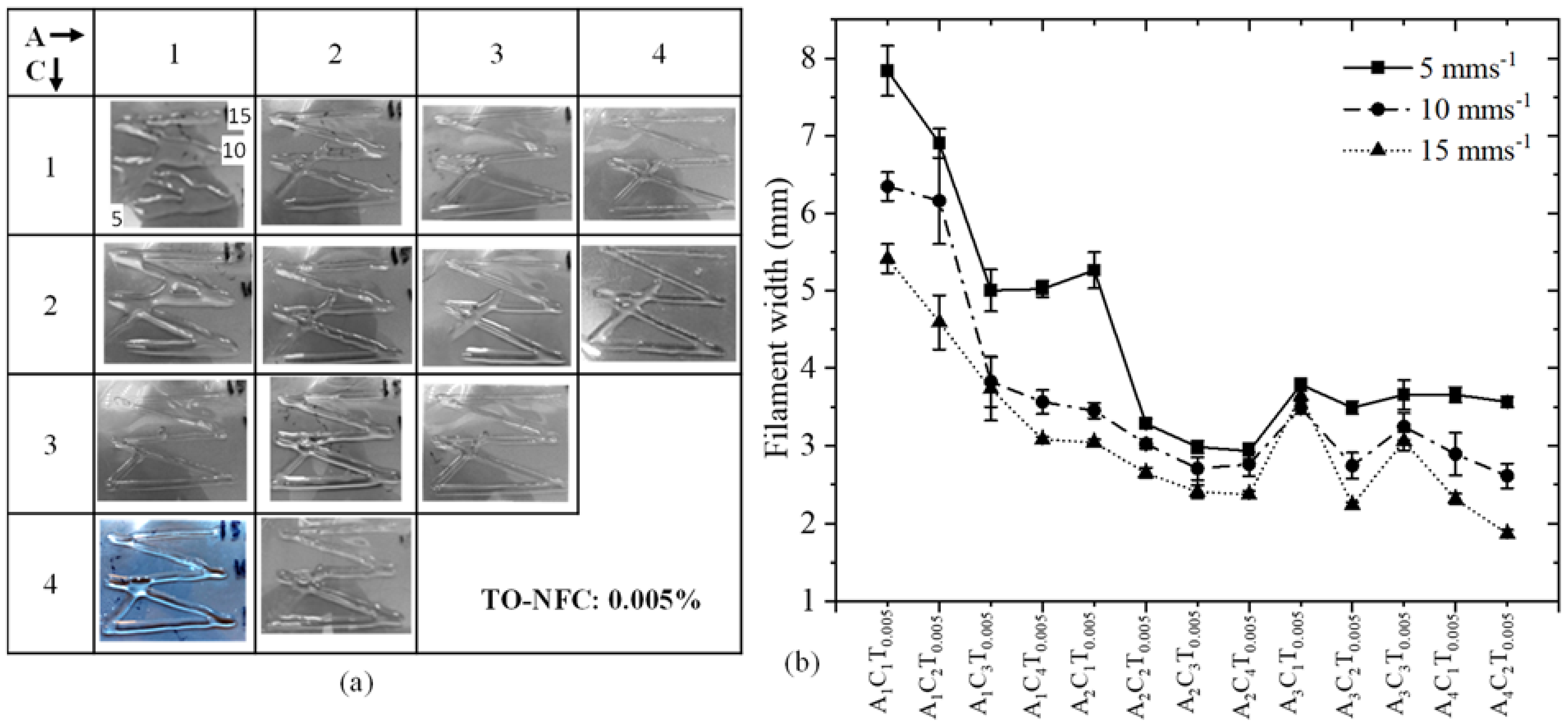
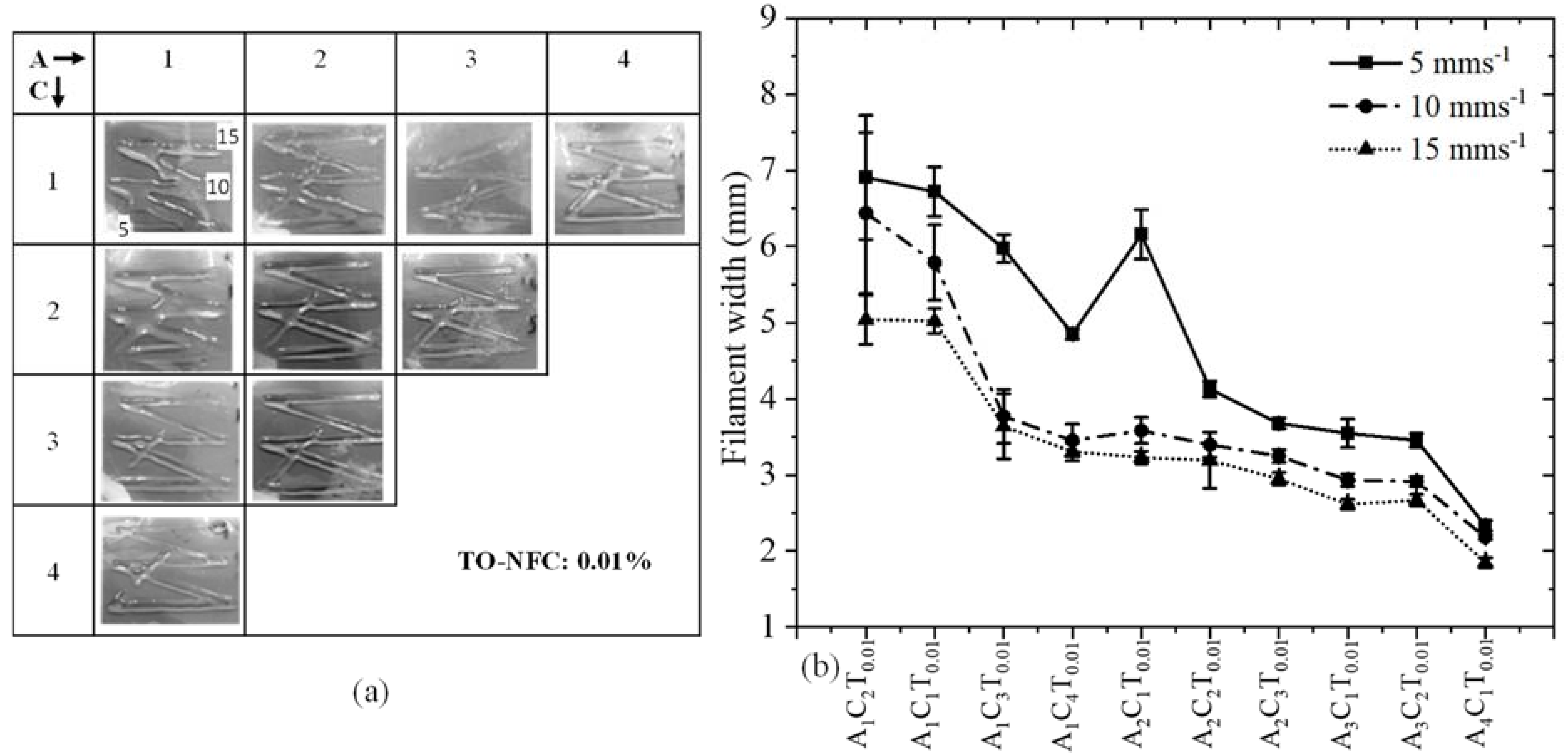
3.7. Analysis of Filament Width after 3D Printing
4. Discussion of Microstructure and Biocompatibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, W.L.; Chua, C.K.; Shen, Y.-F. Print me an organ! Why we are not there yet. Prog. Polym. Sci. 2019, 97, 101145. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Khademhosseini, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio 2019, 1, 100008. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D printing of shear-thinning hyaluronic acid hydrogels with secondary cross-linking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Kong, H.-J.; Lee, K.Y.; Mooney, D.J. Decoupling the dependence of rheological/mechanical properties of hydrogels from solids concentration. Polymers 2002, 43, 6239–6246. [Google Scholar] [CrossRef]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting technology: A current state-of-the-art review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Alexander, A.E.; Wake, N.; Chepelev, L.; Brantner, P.; Ryan, J.; Wang, K.C. A guideline for 3D printing terminology in biomedical research utilizing ISO/ASTM standards. 3D Print. Med. 2021, 7, 1–6. [Google Scholar] [CrossRef]
- Chung, J.H.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Kyle, S.; Thomas, B.; Badiei, N.; Whitaker, I.S. Printability of pulp derived crystal, fibril and blend nanocellulose-alginate bioinks for extrusion 3D bioprinting. Biofabrication 2019, 11, 045006. [Google Scholar] [CrossRef]
- Zhuang, P.; Ng, W.L.; An, J.; Chua, C.K.; Tan, L.P. Layer-by-layer ultraviolet assisted extrusion-based (UAE) bioprinting of hydrogel constructs with high aspect ratio for soft tissue engineering applications. PLoS ONE 2019, 14, e0216776. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Kort-Mascort, J.; Kinsella, J.M. Extrusion bioprinting of soft materials: An emerging technique for biological model fabrication. Appl. Phys. Rev. 2019, 6, 011310. [Google Scholar] [CrossRef]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, C. Predictive modeling of droplet formation processes in inkjet-based bioprinting. J. Manuf. Sci. Eng. 2018, 140, 101007. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Xu, T. Inkjet bioprinting of biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef]
- Ng, W.L.; Huang, X.; Shkolnikov, V.; Goh, G.L.; Suntornnond, R.; Yeong, W.Y. Controlling droplet impact velocity and droplet volume: Key factors to achieving high cell viability in sub-nanoliter droplet-based bioprinting. Int. J. Bioprint. 2022, 8, 424. [Google Scholar] [CrossRef]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A state-of-the-art review of laser-assisted bioprinting and its future research trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Devillard, R.; Pagès, E.; Correa, M.M.; Kériquel, V.; Rémy, M.; Kalisky, J.; Guillemot, F. Cell patterning by laser-assisted bioprinting. In Methods in Cell Biology; Piel, M., Théry, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 159–174. [Google Scholar]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Guillemot, F. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.W.; Lee, K.X.A.; Yeong, W.Y.; Shen, Y.F. Vat polymerization-based bioprinting—Process, materials, applications and regulatory challenges. Biofabrication 2020, 12, 022001. [Google Scholar] [CrossRef]
- Li, W.; Mille, L.S.; Robledo, J.A.; Uribe, T.; Huerta, V.; Zhang, Y.S. Recent advances in formulating and processing biomaterial inks for vat polymerization-based 3D printing. Adv. Healthc. Mater. 2020, 9, 2000156. [Google Scholar] [CrossRef]
- Habib, M.; Khoda, B. Fiber Filled Hybrid Hydrogel for Bio-Manufacturing. J. Manuf. Sci. Eng. 2020, 143, 1–38. [Google Scholar]
- Di Giuseppe, M.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Doyle, B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Narayana, S.G.H.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [CrossRef]
- Garrett, Q.; Simmons, P.A.; Xu, S.; Vehige, J.; Zhao, Z.; Ehrmann, K.; Willcox, M. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1559–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, L.K.; Huebner, P.; Fisher, M.B.; Spang, J.T.; Starly, B.; Shirwaiker, R.A. 3D-bioprinting of polylactic acid (PLA) nanofiber–alginate hydrogel bioink containing human adipose-derived stem cells. ACS Biomater. Sci. Eng. 2016, 2, 1732–1742. [Google Scholar] [CrossRef]
- Nguyen, D.; Hägg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Simonsson, S. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef] [Green Version]
- Li, V.C.; Mulyadi, A.; Dunn, C.K.; Deng, Y.; Qi, H.J. Direct Ink Write 3D Printed Cellulose Nanofiber Aerogel Structures with Highly Deformable, Shape Recoverable, and Functionalizable Properties. ACS Sustain. Chem. Eng. 2018, 6, 2011–2022. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Rodríguez-González, D.; Fernández, M.A.; Kucko, N.W.; Barrère-de Groot, F.; Aguilar, E. Effect on Rheological Properties and 3D Printability of Biphasic Calcium Phosphate Microporous Particles in Hydrocolloid-Based Hydrogels. Gels 2022, 8, 28. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, X.; Liu, X.; Cui, R.; Wang, C.; Zhao, G.; Ge, J. 3D Bioprinting of shear-thinning hybrid bioinks with excellent bioactivity derived from gellan/alginate and thixotropic magnesium phosphate-based gels. J. Mater. Chem. B 2020, 8, 5500–5514. [Google Scholar] [CrossRef]
- Therriault, D.; White, S.R.; Lewis, J.A. Rheological behavior of fugitive organic inks for direct-write assembly. Appl. Rheol. 2007, 17, 10112-1–10112-8. [Google Scholar] [CrossRef]
- Barnes, H.A. A review of the slip (wall depletion) of polymer solutions, emulsions and particle suspensions in viscometers: Its cause, character, and cure. J. Non-Newton. Fluid Mech. 1995, 56, 221–251. [Google Scholar] [CrossRef]
- Brahme, A. Comprehensive Biomedical Physics; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Ng, H.M.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Hui, D.; Low, C.Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B: Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Moud, A.A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H. Suspensions and hydrogels of cellulose nanocrystals (CNCs): Characterization using microscopy and rheology. Cellulose 2022, 29, 3621–3623. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Pignon, F. Current progress in rheology of cellulose nanofibril suspensions. Biomacromolecules 2016, 17, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Pawcenis, D.; Leśniak, M.; Szumera, M.; Sitarz, M.; Profic-Paczkowska, J. Effect of hydrolysis time, pH and surfactant type on stability of hydrochloric acid hydrolyzed nanocellulose. Int. J. Biol. Macromol. 2022, 222, 1996–2005. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef] [PubMed]
- Emmermacher, J.; Spura, D.; Cziommer, J.; Kilian, D.; Wollborn, T.; Fritsching, U.; Lode, A. Engineering considerations on extrusion-based bioprinting: Interactions of material behavior, mechanical forces and cells in the printing needle. Biofabrication 2020, 12, 025022. [Google Scholar] [CrossRef]
- Xu, F.; Loh, H.T.; Wong, Y.S. Considerations and selection of optimalorientation for different rapid prototyping systems. Rapid Prototyp. J. 1999, 5, 54–60. [Google Scholar] [CrossRef]
- Kumar, V.; Rajagopalan, S.; Cutkosky, M.; Dutta, D. Representation and processing heterogeneous objects for solid freeform fabrication. In Proceedings of the Sixth IFIP WG 5.2 International Workshop on Geometric Modelling: Fundamentals and Applications, Tokyo, Japan, 7–9 December 1998. [Google Scholar]
- Liu, Z.; Zhang, M.; Bhandari, B.; Yang, C. Impact of rheological properties of mashed potatoes on 3D printing. J. Food Eng. 2018, 220, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Costakis Jr, W.J.; Rueschhoff, L.M.; Diaz-Cano, A.I.; Youngblood, J.P.; Trice, R.W. Additive manufacturing of boron carbide via continuous filament direct ink writing of aqueous ceramic suspensions. J. Eur. Ceram. Soc. 2016, 36, 3249–3256. [Google Scholar] [CrossRef]
- Zhang, M.; Vora, A.; Han, W.; Wojtecki, R.J.; Maune, H.; Le, A.B.; Nelson, A. Dual-responsive hydrogels for direct-write 3D printing. Macromolecules 2015, 48, 6482–6488. [Google Scholar] [CrossRef]
- Mouser, V.H.; Melchels, F.P.; Visser, J.; Dhert, W.J.; Gawlitta, D.; Malda, J. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication 2016, 8, 035003. [Google Scholar] [CrossRef] [PubMed]





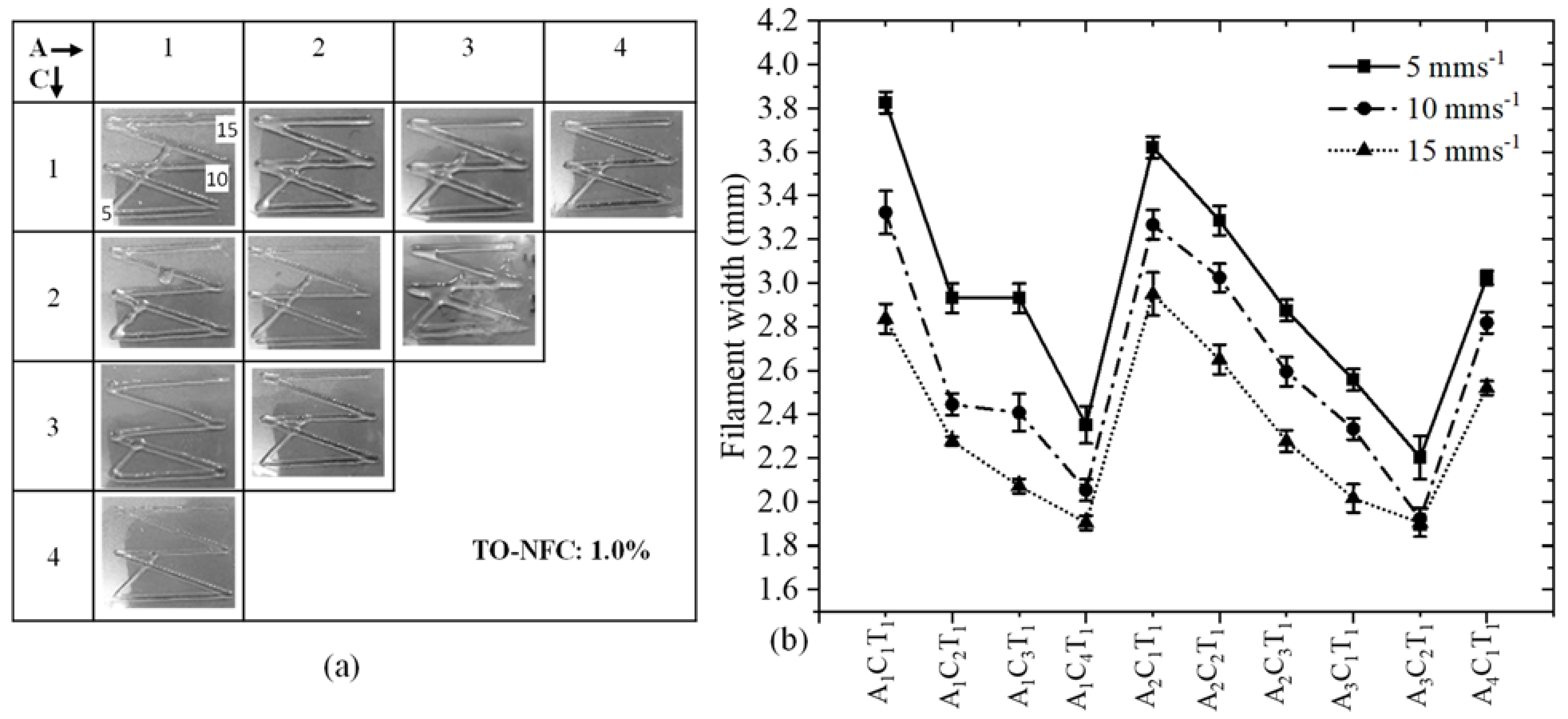
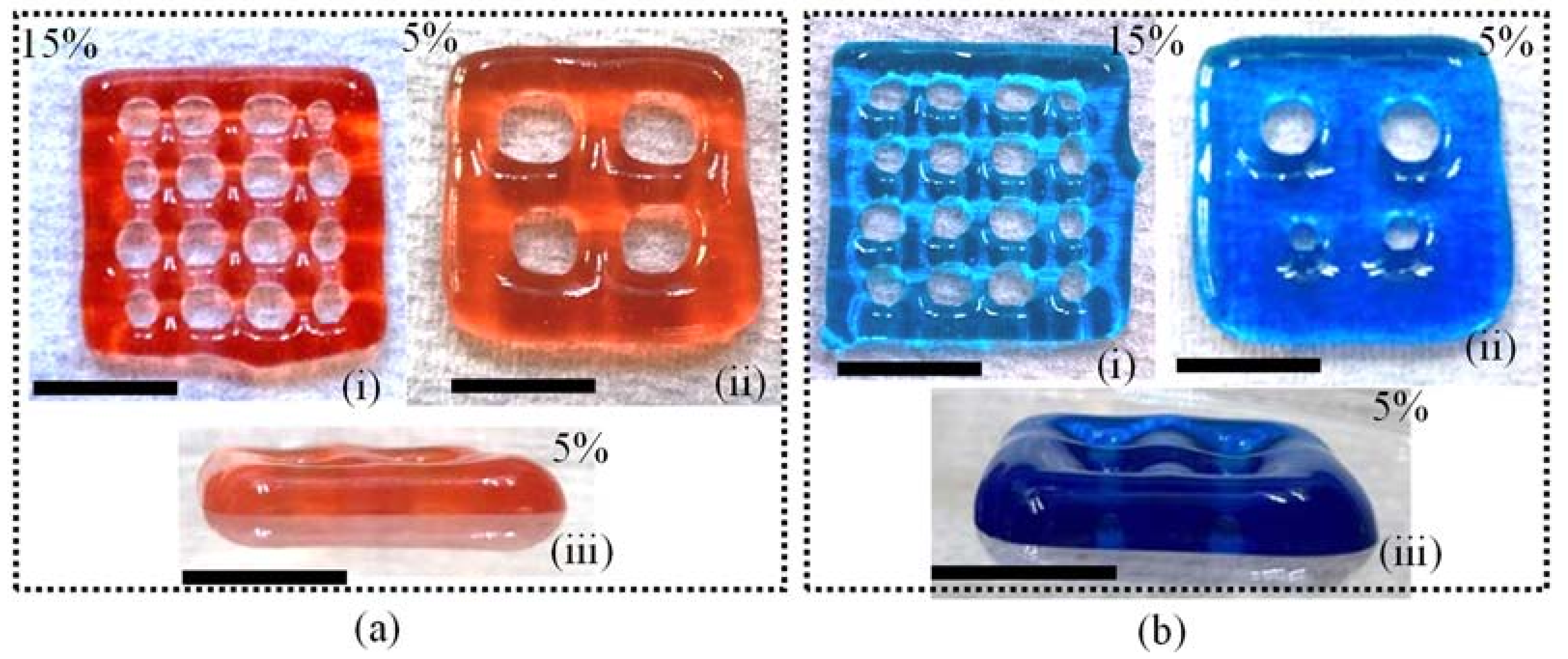

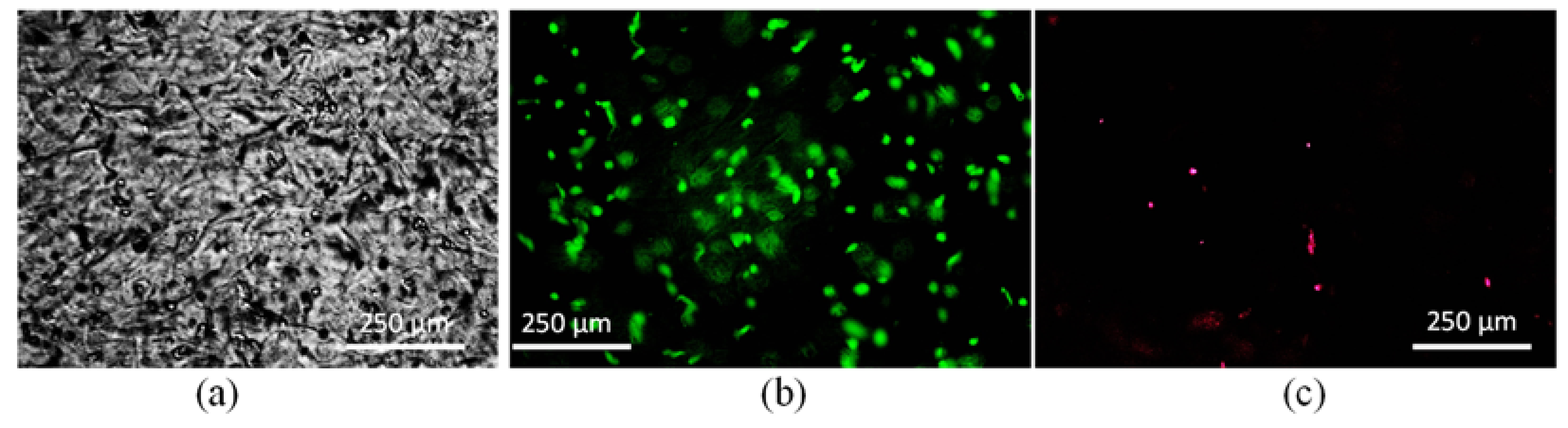
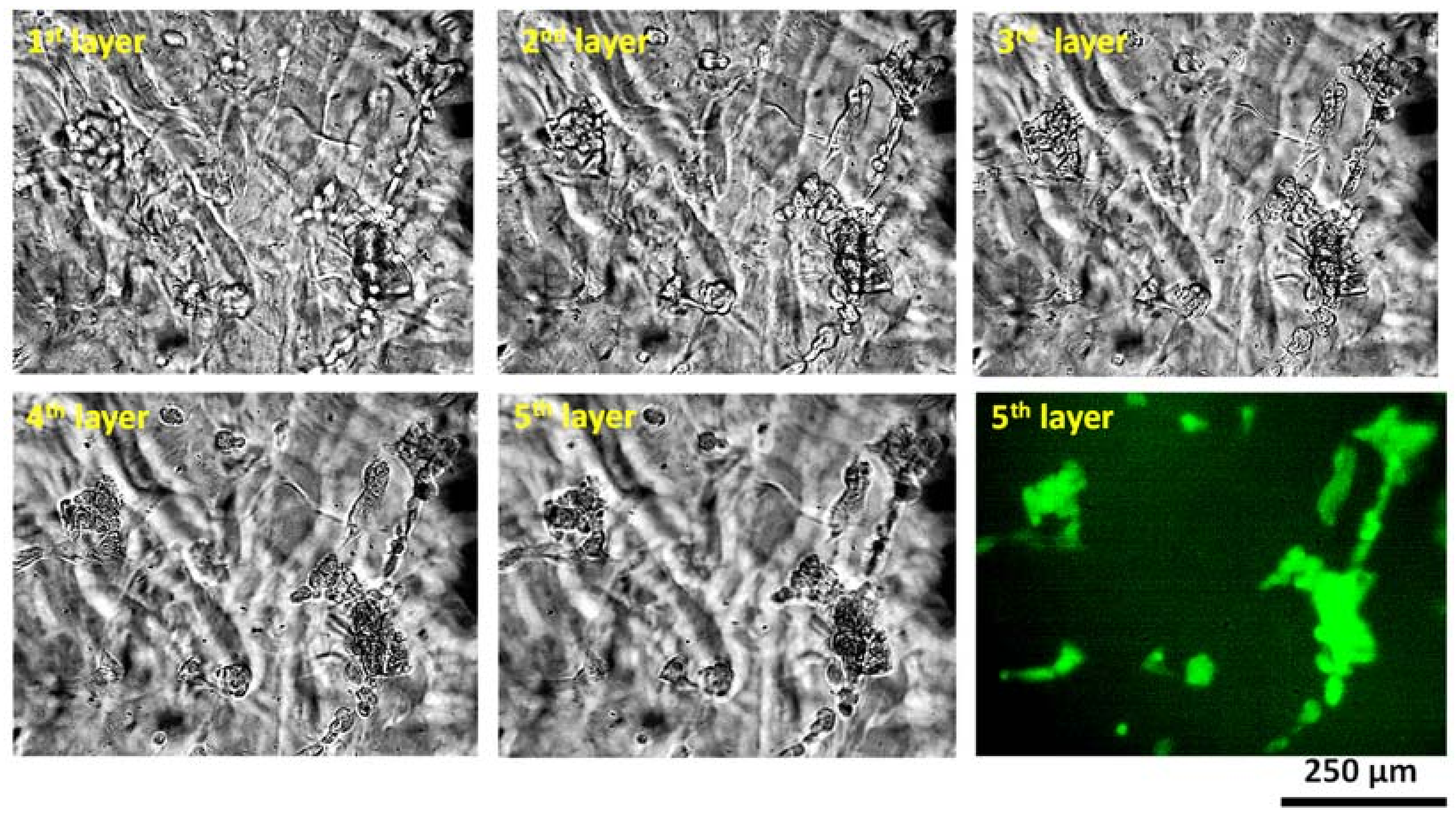
| (a) | |||
|---|---|---|---|
| Alginate (A) | CMC (C) | TO-NFC (T) | Symbol |
| 1 | 1 | 0.005 | A1C1T0.005 |
| 2 | A1C2T0.005 | ||
| 3 | A1C3T0.005 | ||
| 4 | A1C4T0.005 | ||
| 2 | 1 | A2C1T0.005 | |
| 2 | A2C2T0.005 | ||
| 3 | A2C3T0.005 | ||
| 4 | A2C4T0.5 | ||
| 3 | 1 | A3C1T0.005 | |
| 2 | A3C2T0.005 | ||
| 3 | A3C3T0.005 | ||
| 4 | 1 | A4C1T0.005 | |
| 2 | A4C2T0.005 | ||
| (b) | |||
| Alginate (A) | CMC (C) | TO-NFC (T) | Symbol |
| 1 | 1 | 0.5 | A1C1T0.5 |
| 2 | A1C2T0.5 | ||
| 3 | A1C3T0.5 | ||
| 4 | A1C4T0.5 | ||
| 2 | 1 | A2C1T0.5 | |
| 2 | A2C2T0.5 | ||
| 3 | A2C3T0.5 | ||
| 4 | A2C4T0.5 | ||
| 3 | 1 | A3C1T0.5 | |
| 2 | A3C2T0.5 | ||
| 3 | A3C3T0.5 | ||
| 4 | 1 | A4C1T0.5 | |
| 2 | A4C2T0.5 | ||
| (a) | |||
|---|---|---|---|
| Alginate (A) | CMC (C) | TO-NFC (T) | Symbol |
| 1 | 1 | 0.01 | A1C1T0.01 |
| 2 | A1C2T0.01 | ||
| 3 | A1C3T0.01 | ||
| 4 | A1C4T0.01 | ||
| 2 | 1 | A2C1T0.01 | |
| 2 | A2C2T0.01 | ||
| 3 | A2C3T0.01 | ||
| 3 | 1 | A3C1T0.01 | |
| 2 | A3C2T0.01 | ||
| 4 | 1 | A4C1T0.01 | |
| (b) | |||
| Alginate (A) | CMC (C) | TO-NFC (T) | Symbol |
| 1 | 1 | 1 | A1C1T1 |
| 2 | A1C2T1 | ||
| 3 | A1C3T1 | ||
| 4 | A1C4T1 | ||
| 2 | 1 | A2C1T1 | |
| 2 | A2C2T1 | ||
| 3 | A2C3T1 | ||
| 3 | 1 | A3C1T1 | |
| 2 | A3C2T1 | ||
| 4 | 1 | A4C1T1 | |
| Symbol | SR | YS (Pa) | Symbol | SR | YS (Pa) | Symbol | SR | YS (Pa) | Symbol | SR | YS (Pa) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1C1T0.005 | 0.199 | 0.28 | A1C1T0.5 | 0.141 | 0.43 | A1C1T0.01 | 0.141 | 0.35 | A1C1T1 | 0.199 | 5.64 |
| A1C2T0.005 | 0.282 | 2.65 | A1C2T0.5 | 0.199 | 3.38 | A1C2T0.01 | 0.282 | 4.47 | A1C2T1 | 1.58 | 77.29 |
| A1C3T0.005 | 0.794 | 31.33 | A1C3T0.5 | 0.282 | 50.70 | A1C3T0.01 | 0.398 | 16.64 | A1C3T1 | 2.24 | 186.36 |
| A2C4T0.005 | 3.16 | 189.40 | A1C4T0.5 | 2.24 | 341.76 | A1C4T0.01 | 0.562 | 69.61 | A1C4T1 | 6.31 | 476.16 |
| A2C1T0.005 | 0.398 | 3.78 | A2C1T0.5 | 0.199 | 10.79 | A2C1T0.01 | 0.141 | 1.585 | A2C1T1 | 0.282 | 20.05 |
| A2C2T0.005 | 0.794 | 22.32 | A2C2T0.5 | 0.282 | 21.61 | A2C2T0.01 | 0.199 | 7.92 | A2C2T1 | 3.16 | 266.19 |
| A2C3T0.005 | 3.16 | 141.22 | A2C3T0.5 | 0.562 | 103.65 | A2C3T0.01 | 0.282 | 24.34 | A2C3T1 | 17.8 | 508.53 |
| A2C4T0.5 | 8.91 | 403.84 | A2C4T0.5 | 6.31 | 400.69 | A3C1T0.01 | 0.282 | 15.97 | A3C1T1 | 1.58 | 130.4 |
| A3C1T0.005 | 0.794 | 22.71 | A3C1T0.5 | 0.282 | 33.594 | A3C2T0.01 | 0.794 | 46.80 | A3C2T1 | 2.24 | 302.48 |
| A3C2T0.005 | 1.12 | 100.42 | A3C3T0.5 | 1.12 | 111.49 | A4C1T0.01 | 1.58 | 146.09 | A4C1T1 | 6.31 | 799.96 |
| A3C3T0.005 | 8.91 | 340.51 | A3C3T0.5 | 3.16 | 304.04 | ||||||
| A4C1T0.005 | 3.16 | 150.75 | A4C1T0.5 | 1.12 | 226.71 | ||||||
| A4C2T0.005 | 12.6 | 412.70 | A4C2T0.5 | 17.8 | 540.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuladhar, S.; Clark, S.; Habib, A. Tuning Shear Thinning Factors of 3D Bio-Printable Hydrogels Using Short Fiber. Materials 2023, 16, 572. https://doi.org/10.3390/ma16020572
Tuladhar S, Clark S, Habib A. Tuning Shear Thinning Factors of 3D Bio-Printable Hydrogels Using Short Fiber. Materials. 2023; 16(2):572. https://doi.org/10.3390/ma16020572
Chicago/Turabian StyleTuladhar, Slesha, Scott Clark, and Ahasan Habib. 2023. "Tuning Shear Thinning Factors of 3D Bio-Printable Hydrogels Using Short Fiber" Materials 16, no. 2: 572. https://doi.org/10.3390/ma16020572
APA StyleTuladhar, S., Clark, S., & Habib, A. (2023). Tuning Shear Thinning Factors of 3D Bio-Printable Hydrogels Using Short Fiber. Materials, 16(2), 572. https://doi.org/10.3390/ma16020572





