Influence of Filler Type and Rheological Properties of Asphalt Mastic on the Asphalt Mastic–Aggregate Interaction
Abstract
:1. Introduction
2. Materials and Experimental
2.1. Materials
2.1.1. Bitumen
2.1.2. Filler
2.1.3. Aggregates
2.2. Preparation of the Specimens
2.2.1. Design of Asphalt Mastics
2.2.2. Design of the Asphalt Mastic–Aggregate Interface Specimens
2.3. Experimental Tests
2.3.1. Rheological Testing of Asphalt Mastics
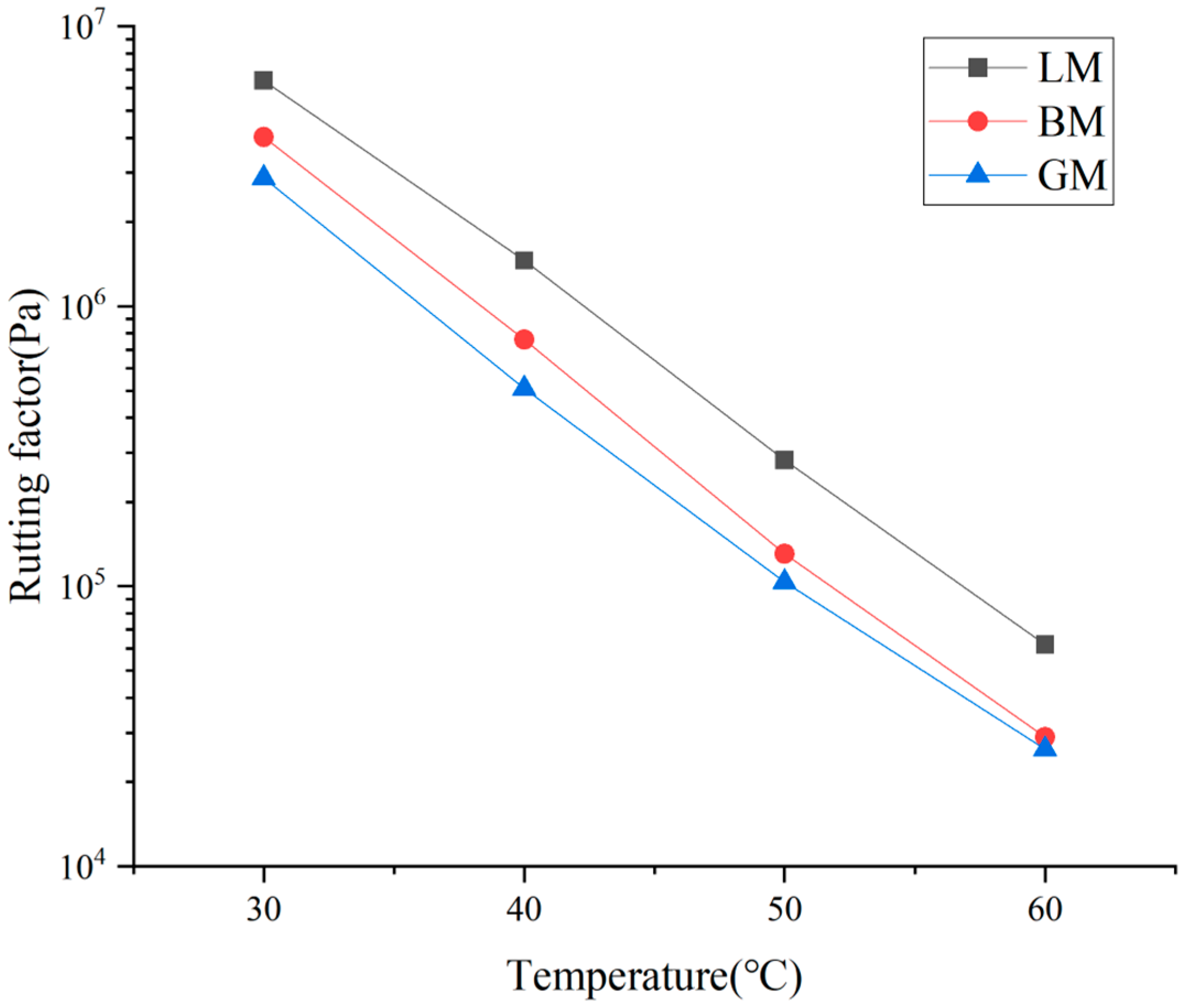
- A dynamic shear rheometer (DSR) was employed in this study to evaluate the rheological properties of the asphalt mastics, focusing on the influence of fillers. In this test, mastic specimens with a 2 mm thickness were prepared and sandwiched between two flat plates with a diameter of 8 mm. One of the two plates was fixed and the other one was oscillated back and forth around the central axis at a certain angular velocity. The mastic specimens were tested at a temperature interval of 10 °C in the range of 30–60 °C and the scanning rate was 10 rad/s. According to the curves, the complex shear modulus G*, phase angle δ, and rutting factor (G*/sin δ) of the asphalt mastics at different temperatures were obtained. The results were employed to evaluate high-temperature performance of the asphalt mastics at moderate temperatures;
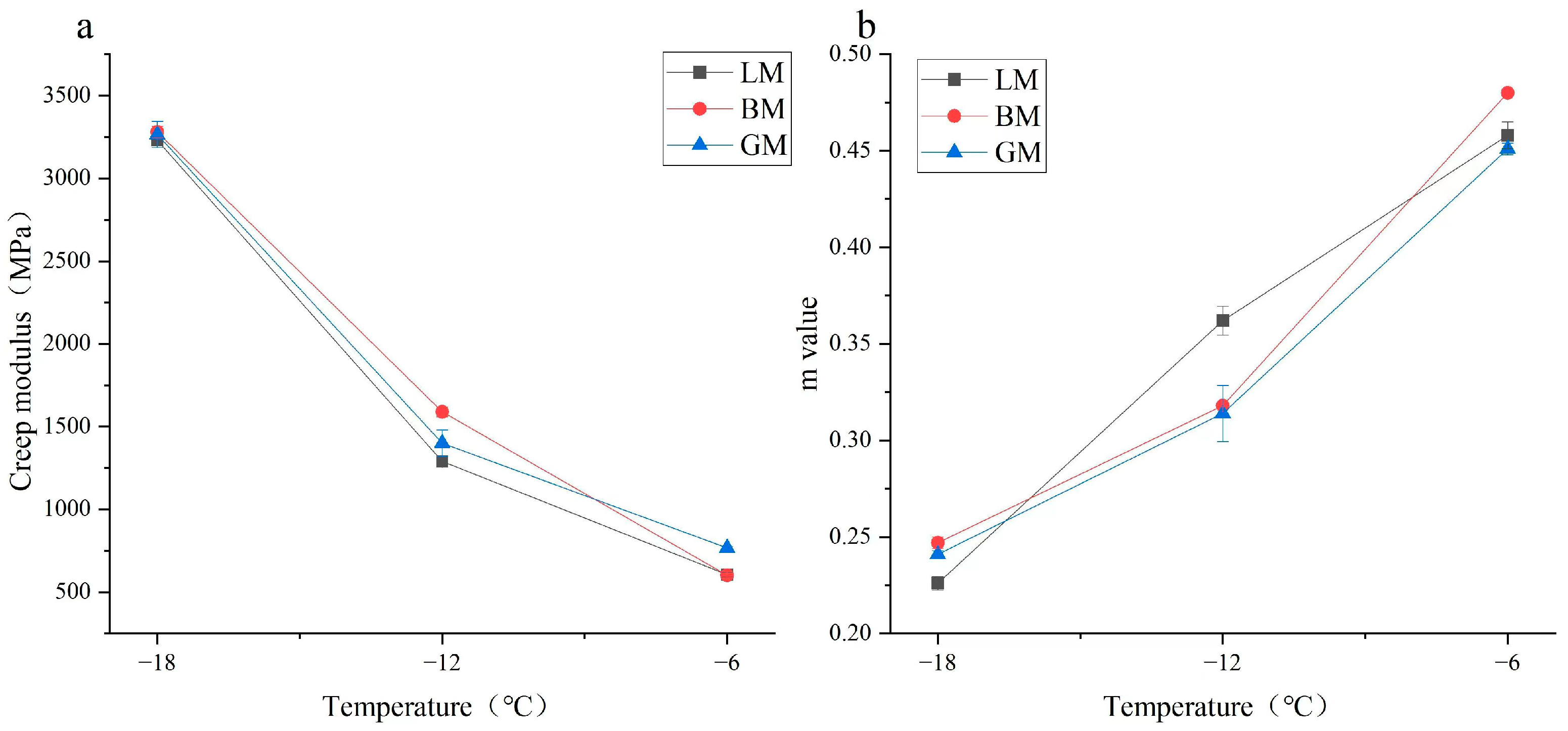
- The bending-beam rheometer (BBR) test was employed to characterize the low-temperature cracking resistance of the asphalt mastics. In this research, the BBR test was performed at −6 °C, −12 °C, and −18 °C. During testing, a constant load of 980 ± 50 mN was added in the middle of the mastic beam for 240 s. The deflection was automatically recorded in order to calculate the creep stiffness (S) and m-value (m). In order to resist thermal cracking at low temperatures, the creep stiffness (S) and the m-value must meet certain requirements.
2.3.2. Bond Strength Testing of the Asphalt Mastic–Aggregate Interface
2.3.3. Water Absorption of Asphalt Mastics
2.3.4. Water Attack Testing of the Asphalt Mastic–Aggregate Interface
3. Results and Discussion
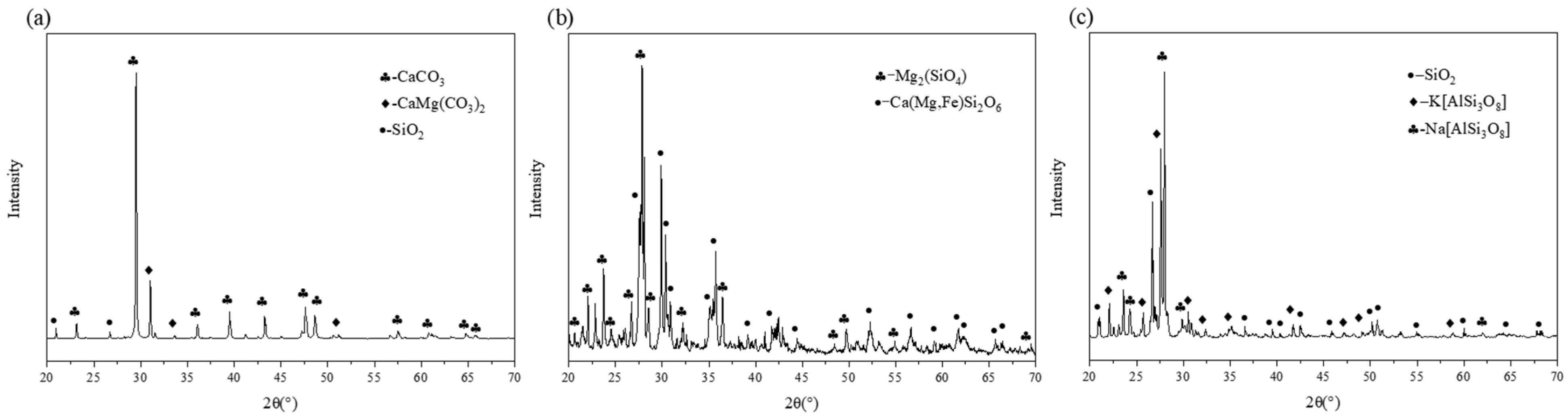
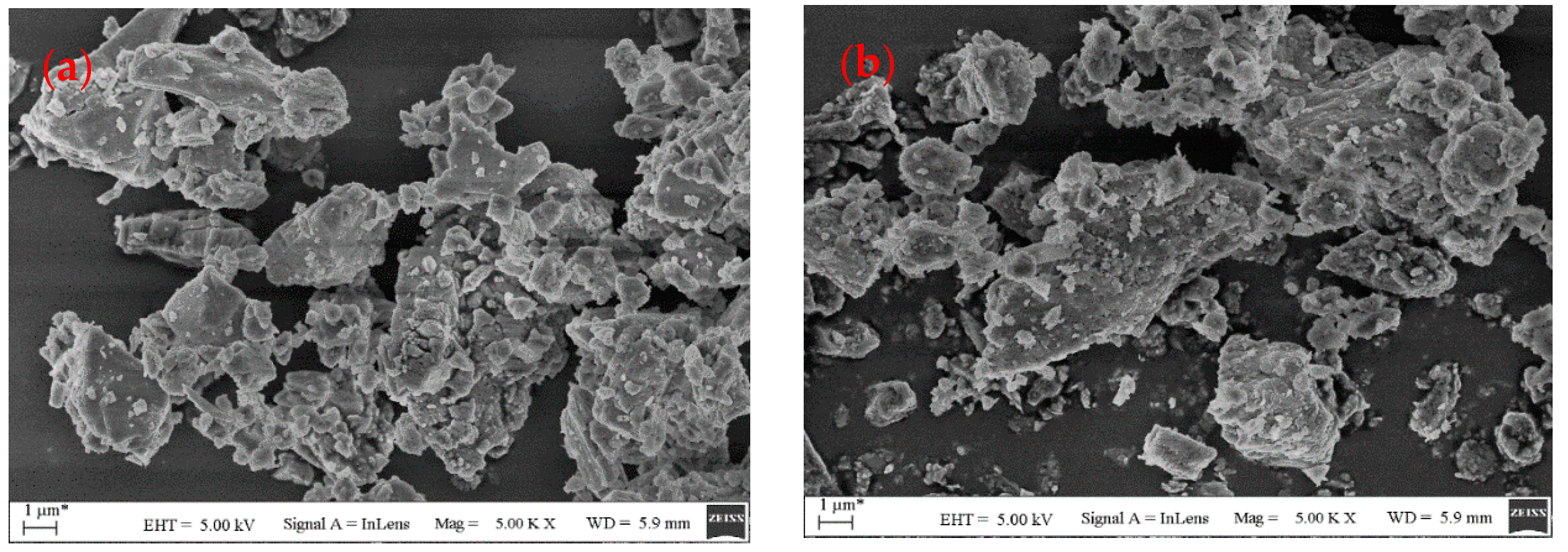
3.1. Physical Features of Three Types of Filler
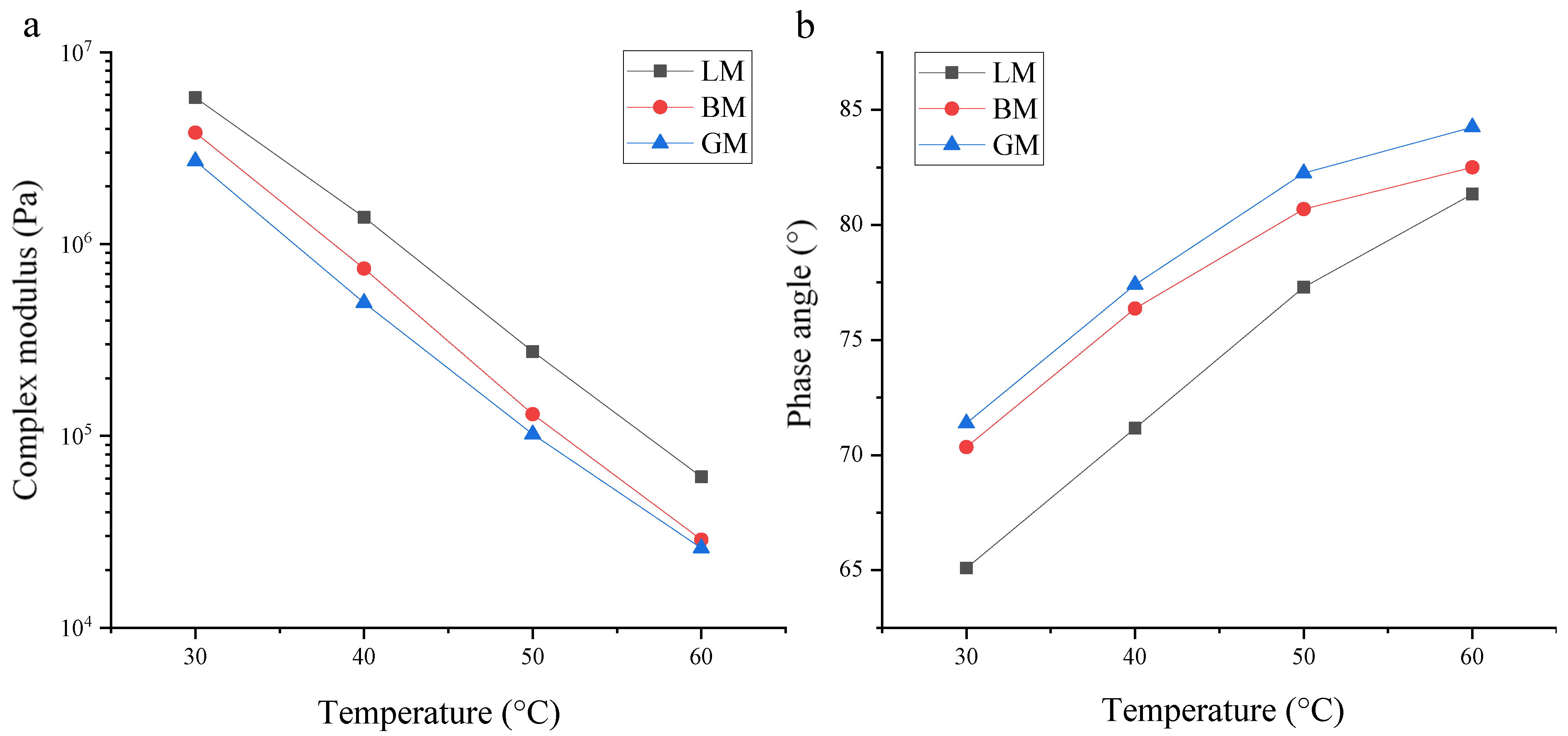
3.2. Rheological Properties of Asphalt Mastics at High Temperature
3.3. Rheological Properties of Asphalt Mastics at Low Temperature
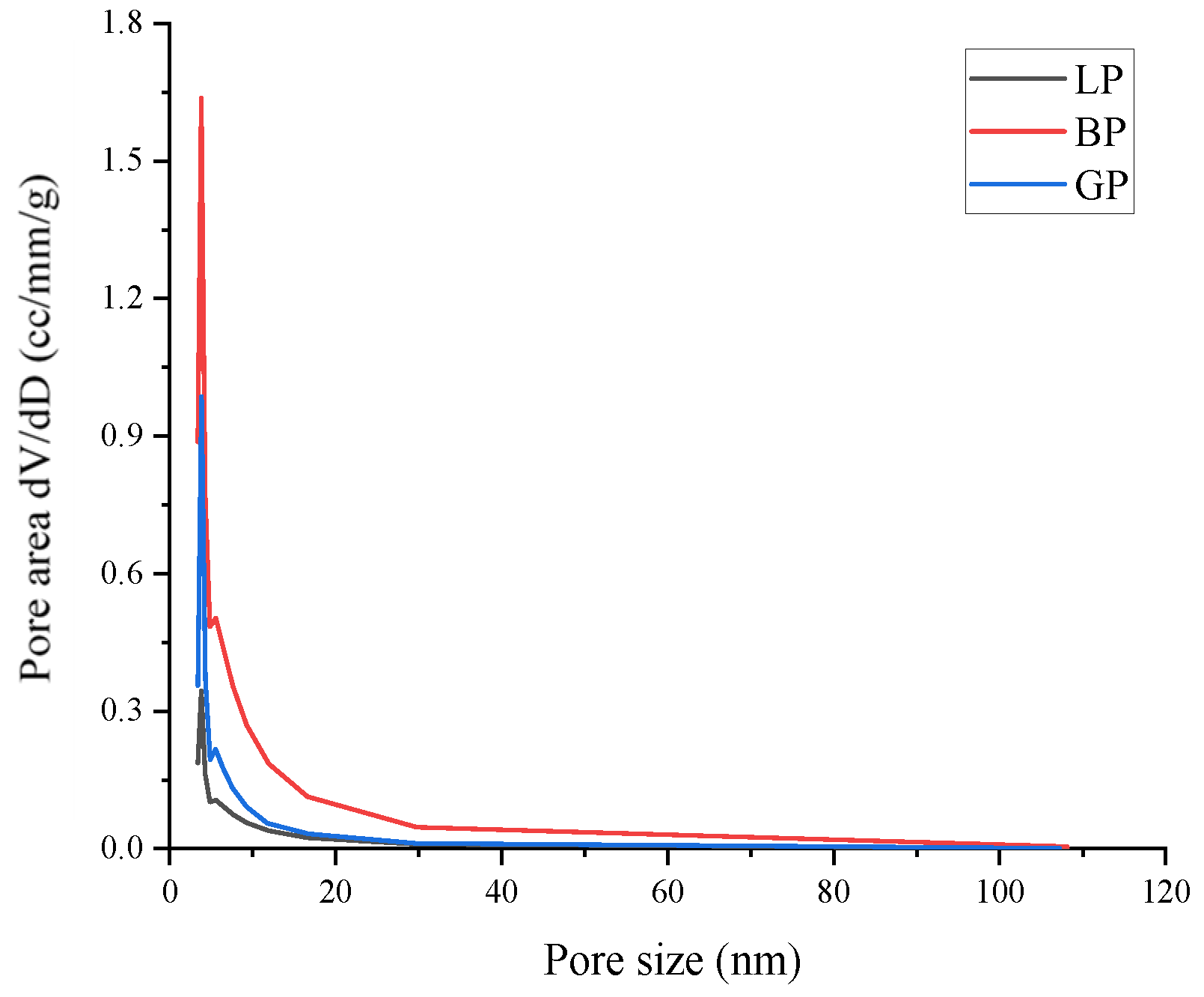
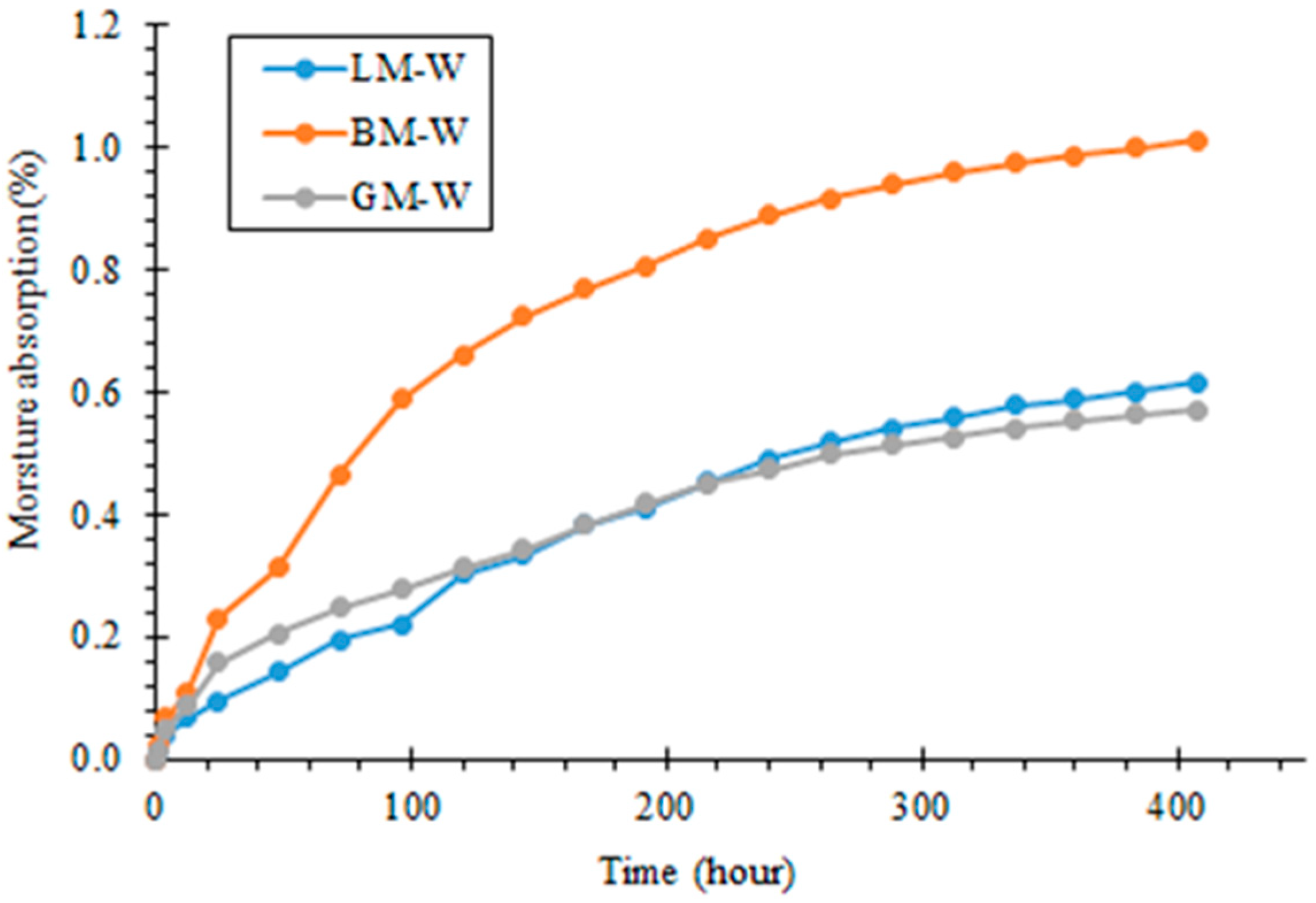
3.4. Water Diffusion of Asphalt Mastics
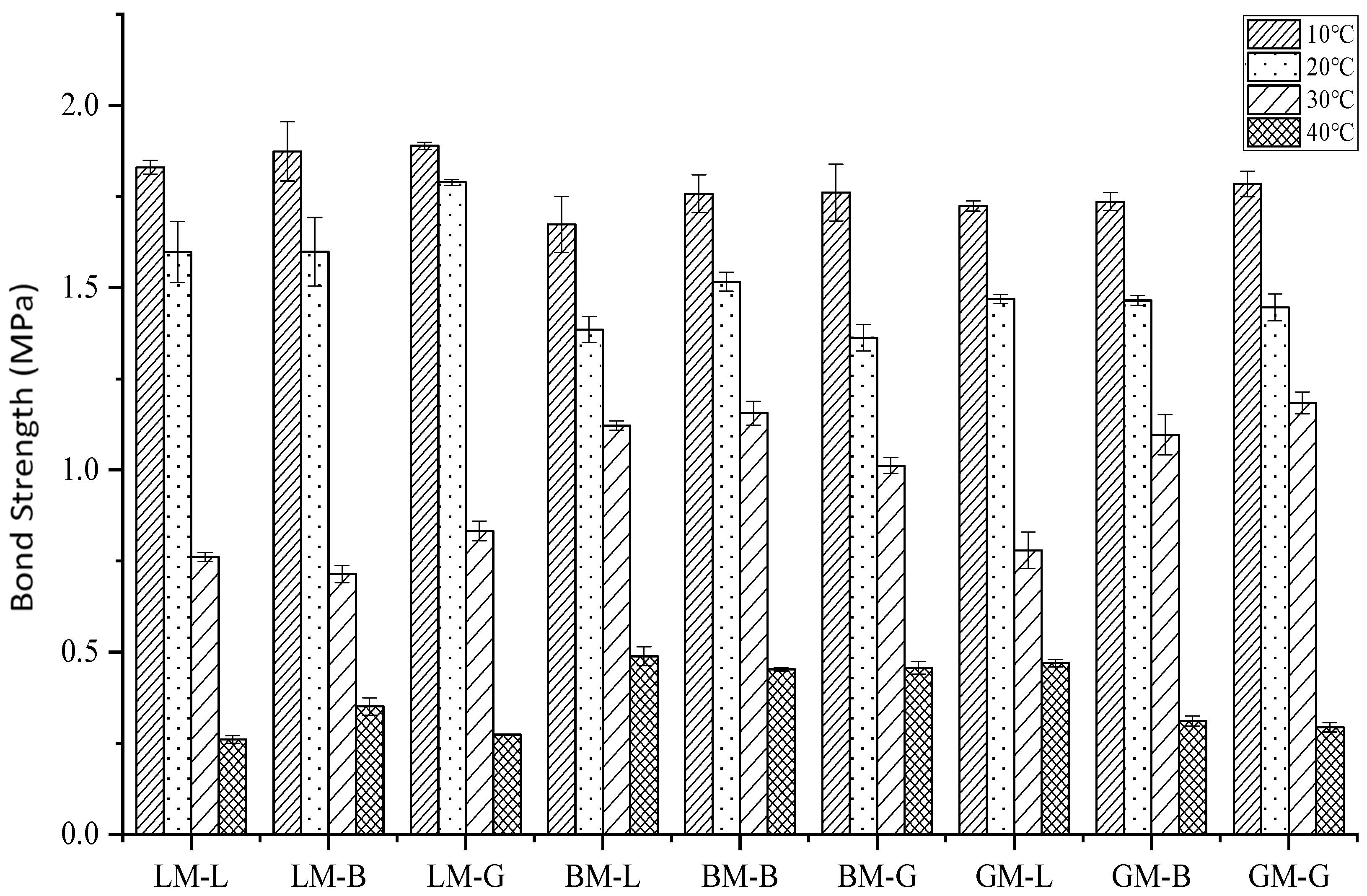
3.5. Bond Strength of the Asphalt Mastic–Aggregate Interface
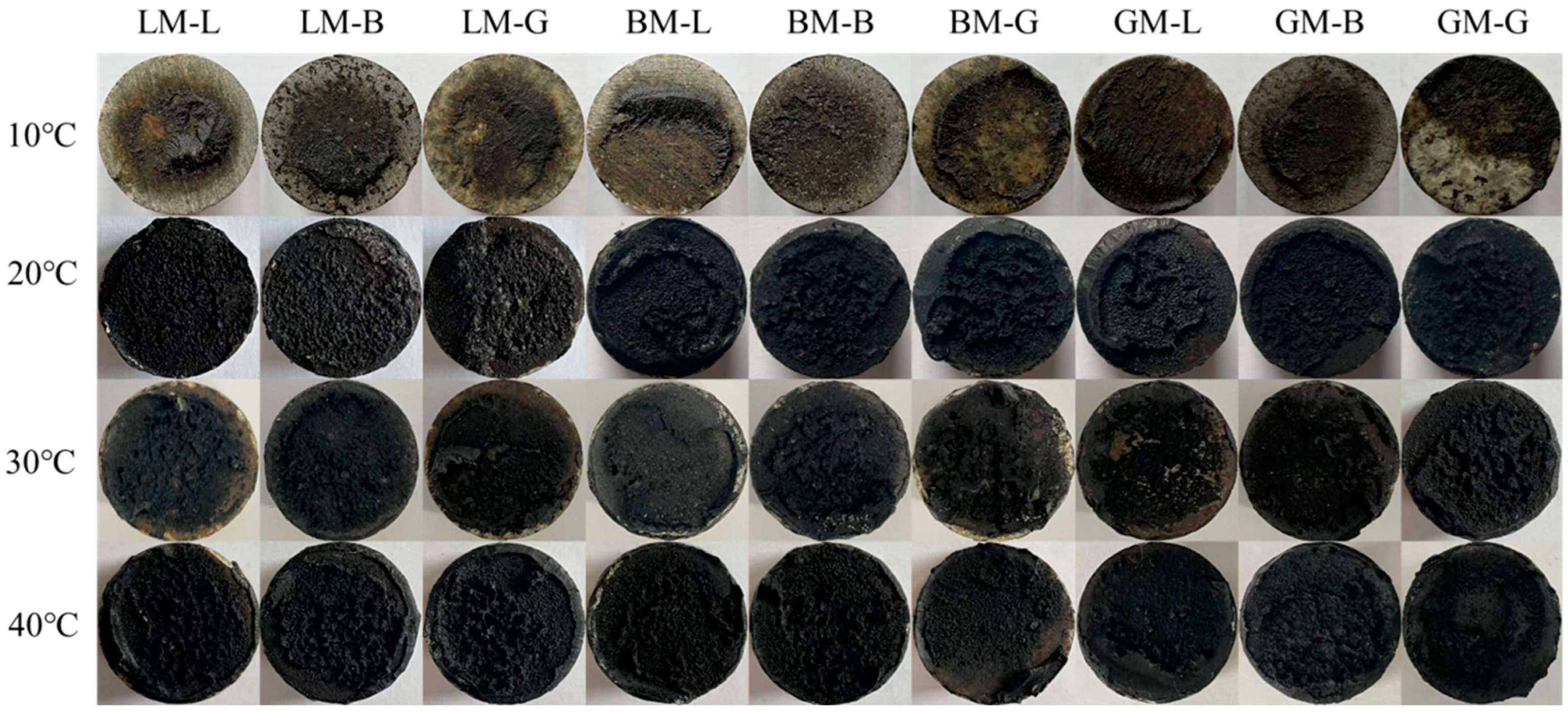
3.5.1. Influence of Temperature
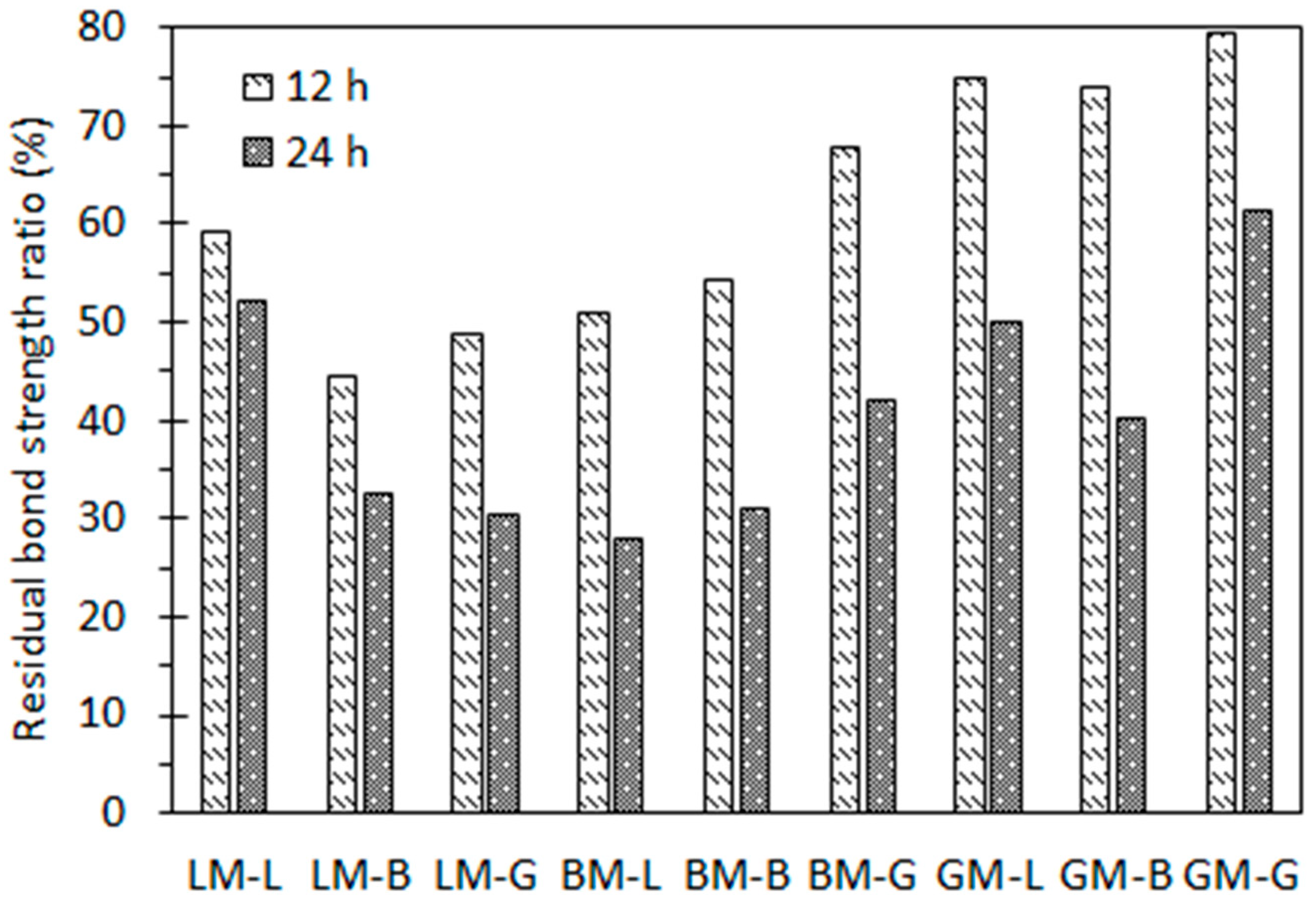
3.5.2. Influence of Water Immersion without Pressure
3.5.3. Influence of Water Pressure
4. Conclusions
- The mineral filler type influenced the complex modulus and low-temperature performance index of the asphalt mastic, and the difference in pore volume and specific surface area changed the content of the structural asphalt components in the asphalt mastics, thereby affecting the phase angle. The specific surface area of the basalt filler was the largest, resulting in a high content of structural asphalt components, and its mastic had a relatively higher stiffness modulus at −18 °C and −12 °C. The chemical composition of the filler was the primary factor in determining rheological behavior and the morphology was the secondary factor in influencing the properties of asphalt mastics;
- When exposed to the water immersion, the moisture absorption rate of the basalt mastic was the highest, followed by the limestone and granite mastics, and the granite mastic had the lowest diffusion coefficient. This may be related to the pore features of the basalt filler, which had a high pore volume and surface area;
- The alkaline limestone aggregate exhibited strong initial adhesion with bitumen and the acid granite aggregate exhibited weak adhesion. When the complex modulus of asphalt mastic was high and its phase angle was low, it resulted in a good initial bonding behavior with aggregate in the dry state. However, this does not indicate that such an interface between alkaline limestone aggregate and asphalt mastic exhibits good durability, e.g., against water attack;
- Static and pressured water immersion conditions can accelerate the deterioration process of asphalt mastic–aggregate interfaces. For the three asphalt mastics, when the complex modulus of the asphalt mastic was low and its phase angle was high, the durability of asphalt mixtures subjected to static and pressured water immersion conditions improved. The asphalt mastic with the acid granite filler initially exhibited relatively weak adhesion in the asphalt mastic, but it showed good water attack resistance between the asphalt mastic and coarse aggregate;
- The deterioration mechanism of the interfacial bond strength of the asphalt mastic–aggregate interface under static water immersion was different from pressured water immersion. It was found that the water stability of the asphalt mastic–aggregate interface was strongly related to the properties of the aggregates and fillers and the diffusion behavior of the asphalt mastics, which influenced the rheological properties of the asphalt mastics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thives, L.P.; Ghisi, E. Asphalt mixtures emission and energy consumption: A review. Renew. Sustain. Energy Rev. 2017, 72, 473–484. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Liu, S.J.; Yao, Z.Y.; Wu, S.P.; Jiang, H.G.; Liang, M.; Qiao, Y.N. Environmental aspects and pavement properties of red mud waste as the replacement of mineral filler in asphalt mixture. Constr. Build. Mater. 2018, 180, 605–613. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Yang, Z.X.; Guo, X.M.; Chen, W.Q. Modulus prediction of asphalt concrete with imperfect bonding between aggregate-asphalt mastic. Compos. Part B Eng. 2011, 42, 1404–1411. [Google Scholar] [CrossRef]
- Kakar, M.R.; Hamzah, M.O.; Valentin, J. A review on moisture damages of hot and warm mix asphalt and related investigations. J. Clean. Prod. 2015, 99, 39–58. [Google Scholar] [CrossRef]
- Zhang, J.P.; Fan, Z.P.; Hu, D.L.; Hu, Z.; Pei, J.Z.; Kong, W.C. Evaluation of asphalt-aggregate interaction based on the rheological properties. Int. J. Pavement Eng. 2018, 19, 586–592. [Google Scholar] [CrossRef]
- Lv, D.; Zheng, C.F.; Qin, Y.; Bi, H.; Li, K.Y.; Huang, J.F. Analysing the effects of the mesoscopic characteristics of mineral powder fillers on the cohesive strength of asphalt mortars at low temperatures. Constr. Build. Mater. 2014, 65, 330–337. [Google Scholar] [CrossRef]
- Barra, B.; Breno, L.; Guerrero, Y.; Bernucci, L. Characterization of granite and limestone powders for use as fillers in bituminous mastics dosage. An. Acad. Bras. Ciênc. 2014, 86, 995–1002. [Google Scholar] [CrossRef]
- White, G. Shear creep response of an airport asphalt mastic. Int. J. Pavement Eng. 2017, 18, 567–577. [Google Scholar] [CrossRef]
- White, G. The contribution of asphalt mastic to shear resistance. In Proceedings of the 6th Eurasphalt & Eurobitume Congress, Prague, Czech Republic, 1–3 June 2016. [Google Scholar]
- Geber, R.; Simon, A.; Kocserha, I.; Buzimov, A.; Buzimov, A.Y. Microstructural and rheological analysis of fillers and asphalt mastics. J. Phys. Conf. Ser. 2017, 790, 012009. [Google Scholar] [CrossRef]
- Terrel, R.L.; Shutte, J.W. Summary Report on Water Sensitivity; Report No. SHRP-A/IR-89-003 for Strategic Highway Research Program; National Research Council: Washington, DC, USA, 1989. [Google Scholar]
- Yi, J.Y.; Pang, X.Y.; Feng, D.C.; Pei, Z.S.; Xu, M.; Xie, S.N.; Huang, Y.D. Studies on surface energy of asphalt and aggregate at different scales and bonding property of asphalt-aggregate system. Road Mater. Pavement Des. 2018, 19, 1102–1125. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, H.L.; Gao, Y.; Wang, L. Study of diffusion characteristics of asphalt-aggregate interface with molecular dynamics simulation. Int. J. Pavement Eng. 2021, 22, 319–330. [Google Scholar] [CrossRef]
- Little, D.N.; Allen, D.H.; Bhasin, A. Modeling and Design of Flexible Pavements and Materials; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Fakhri, M.; Javadi, S.; Sassani, A.; Torabi-Dizaji, M. Zinc Slag as a Partial or Total Replacement for Mineral Filler in Warm Mix Asphalt and Its Effects on Self-Healing Capacity and Performance Characteristics. Materials 2022, 15, 736. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, M.; Norouzi, M.A. Rheological and ageing properties of asphalt bio-binders containing lignin and waste engine oil. Constr. Build. Mater. 2022, 321, 126364. [Google Scholar] [CrossRef]
- Mehrara, A.; Khodaii, A. A review of state of the art on stripping phenomenon in asphalt concrete. Constr. Build. Mater. 2013, 38, 423–442. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Sun, C.J.; Li, P.Z.; Liang, M.; Jiang, H.G.; Yao, Z.Y. Experimental study on rheological properties and moisture susceptibility of asphalt mastic containing red mud waste as a filler substitute. Constr. Build. Mater. 2019, 211, 159–166. [Google Scholar] [CrossRef]
- Mansour, F.; Vahid, V. Effect of Liquid Nano material and hydrated lime in improving the moisture behaviour of HMA. Transp. Res. Proc. 2016, 17, 506–512. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, S.B.; Tebaldi, G.; Romeo, E. Role of mineral filler in asphalt mixture. Road Mater. Pavement Des. 2022, 23, 247–286. [Google Scholar] [CrossRef]
- Antunes, V.; Freire, A.C.; Quaresma, L.; Micaelo, R. Influence of the geometrical and physical properties of filler in the filler-bitumen interaction. Constr. Build. Mater. 2015, 76, 322–329. [Google Scholar] [CrossRef]
- Antunes, V.; Freire, A.C.; Quaresma, L.; Micaelo, R. Effect of the chemical composition of fillers in the filler-bitumen interaction. Constr. Build. Mater. 2016, 104, 85–91. [Google Scholar] [CrossRef]
- Apeagyei, A.K.; Grenfell, J.R.A.; Airey, G.D. Evaluation of Moisture Sorption and Diffusion Characteristics of Asphalt Mastics Using Manual and Automated Gravimetric Sorption Techniques. J. Mater. Civ. Eng. 2014, 26, 04014045. [Google Scholar] [CrossRef]
- Apeagyei, A.K.; Grenfell, J.R.A.; Airey, G.D. Influence of aggregate absorption and diffusion properties on moisture damage in asphalt mixtures. Road Mater. Pavement Des. 2015, 16, 404–422. [Google Scholar] [CrossRef]
- Gao, J.Q.; Chen, H.; Ji, T.J.; Liu, H.Y. Measurement of dynamic hydraulic pressure in asphalt pavement using fiber Bragg grating. Transducer Microsyst. Technol. 2009, 28, 59–61. [Google Scholar]
- Gao, J.Q.; Guo, C.C.; Liu, Y.T. Measurement of pore water pressure in asphalt pavement and its effects on permeability. Measurement 2015, 62, 81–87. [Google Scholar] [CrossRef]
- Choudhary, J.; Kumar, B.; Gupta, A. Utilization of solid waste materials as alternative fillers in asphalt mixes: A review. Constr. Build. Mater. 2020, 234, 117271. [Google Scholar] [CrossRef]
- Faheem, A.F.; Bahia, H.U. Modelling of Asphalt Mastic in Terms of Filler-Bitumen Interaction. Road Mater. Pavement Des. 2010, 11, 281–303. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Yao, Z.Y.; Wang, K.; Wang, F.; Jiang, H.G.; Liang, M.; Wei, J.C.; Airey, G. Sustainable utilization of bauxite residue (Red Mud) as a road material in pavements: A critical review. Constr. Build. Mater. 2021, 270, 121419. [Google Scholar] [CrossRef]
- Vasconcelos, K.L.; Bhasin, A.; Little, D.N.; Lytton, R.L. Experimental Measurement of Water Diffusion through Fine Aggregate Mixtures. J. Mater. Civ. Eng. 2011, 23, 445–452. [Google Scholar] [CrossRef]






| Items | Results | Unit | Requirement |
|---|---|---|---|
| Needle penetration (25 °C) | 68.3 | 0.1 mm | 60–80 |
| Ductility (10 °C) | 40.1 | cm | no less than 25 |
| Ductility (15 °C) | >150 | cm | no less than 100 |
| Softening point | 48.2 | °C | no less than 45 |
| Viscosity (135 °C) | 0.450 | Pa·s | - |
| Density (15 °C) | 1.035 | g/cm3 | - |
| Solubility | 99.6 | % | no less than 99.5 |
| Property | LP | BP | GP |
| Density/g/cm3 | 2.67 | 2.87 | 2.69 |
| Items | LP | BP | GP |
|---|---|---|---|
| D10 | 2.75 | 3.27 | 3.89 |
| D50 | 13.08 | 26.17 | 26.16 |
| D90 | 52.33 | 62.33 | 62.23 |
| Oxide | Filler Type | ||
|---|---|---|---|
| LP | BP | GP | |
| CaO | 82.828 | 9.879 | 2.877 |
| SiO2 | 5.843 | 45.812 | 63.87 |
| Al2O3 | 4.15 | 18.552 | 16.854 |
| Fe2O3 | 0.573 | 11.656 | 3.116 |
| MgO | 4.786 | 5.954 | 0.692 |
| K2O | 0.351 | 1.893 | 5.252 |
| Na2O | / | 2.581 | 6.023 |
| Other | 1.469 | 3.673 | 1.316 |
| Properties | Limestone | Basalt | Granite | Requirement |
|---|---|---|---|---|
| Density/g/cm3 | 2.816 | 3.111 | 3.070 | no less than 2.6 |
| Crushing value/% | 23.1 | 10.2 | 19.8 | no less than 26 |
| Abrasion value/% | 20.2 | 16.6 | 14.3 | no less than 28 |
| Adhesion grade | 5 | 5 | 3 | - |
| Type of Interface | Name |
|---|---|
| Limestone mastic–Limestone | LM–L |
| Limestone mastic–Basalt | LM–B |
| Limestone mastic–Granite | LM–G |
| Basalt mastic–Limestone | BM–L |
| Basalt mastic–Basalt | BM–B |
| Basalt mastic–Granite | BM–G |
| Granite mastic–Limestone | GM–L |
| Granite mastic–Basalt | GM–B |
| Granite mastic–Granite | GM–G |
| Pore Features | LP | BP | GP |
|---|---|---|---|
| Pore volume/cm3/g | 0.007 | 0.034 | 0.009 |
| Average pore size/nm | 3.059 | 3.810 | 3.819 |
| Surface area/m2/g | 1.899 | 9.008 | 3.42 |
| Type of Mastic | Condition | D/(cm2/s) | R2 |
|---|---|---|---|
| LP mastic | Water Immersion | 1.054 × 10−10 | 0.8396 |
| BP mastic | 1.078 × 10−10 | 0.8912 | |
| GP mastic | 0.938 × 10−10 | 0.8725 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
E, G.; Zhang, J.; Shen, Q.; Ji, P.; Wang, J.; Xiao, Y. Influence of Filler Type and Rheological Properties of Asphalt Mastic on the Asphalt Mastic–Aggregate Interaction. Materials 2023, 16, 574. https://doi.org/10.3390/ma16020574
E G, Zhang J, Shen Q, Ji P, Wang J, Xiao Y. Influence of Filler Type and Rheological Properties of Asphalt Mastic on the Asphalt Mastic–Aggregate Interaction. Materials. 2023; 16(2):574. https://doi.org/10.3390/ma16020574
Chicago/Turabian StyleE, Guangxun, Jizhe Zhang, Quanjun Shen, Ping Ji, Jing Wang, and Yushuai Xiao. 2023. "Influence of Filler Type and Rheological Properties of Asphalt Mastic on the Asphalt Mastic–Aggregate Interaction" Materials 16, no. 2: 574. https://doi.org/10.3390/ma16020574




