Characteristics and Functional Application of Cellulose Fibers Extracted from Cow Dung Wastes
Abstract
:1. Introduction
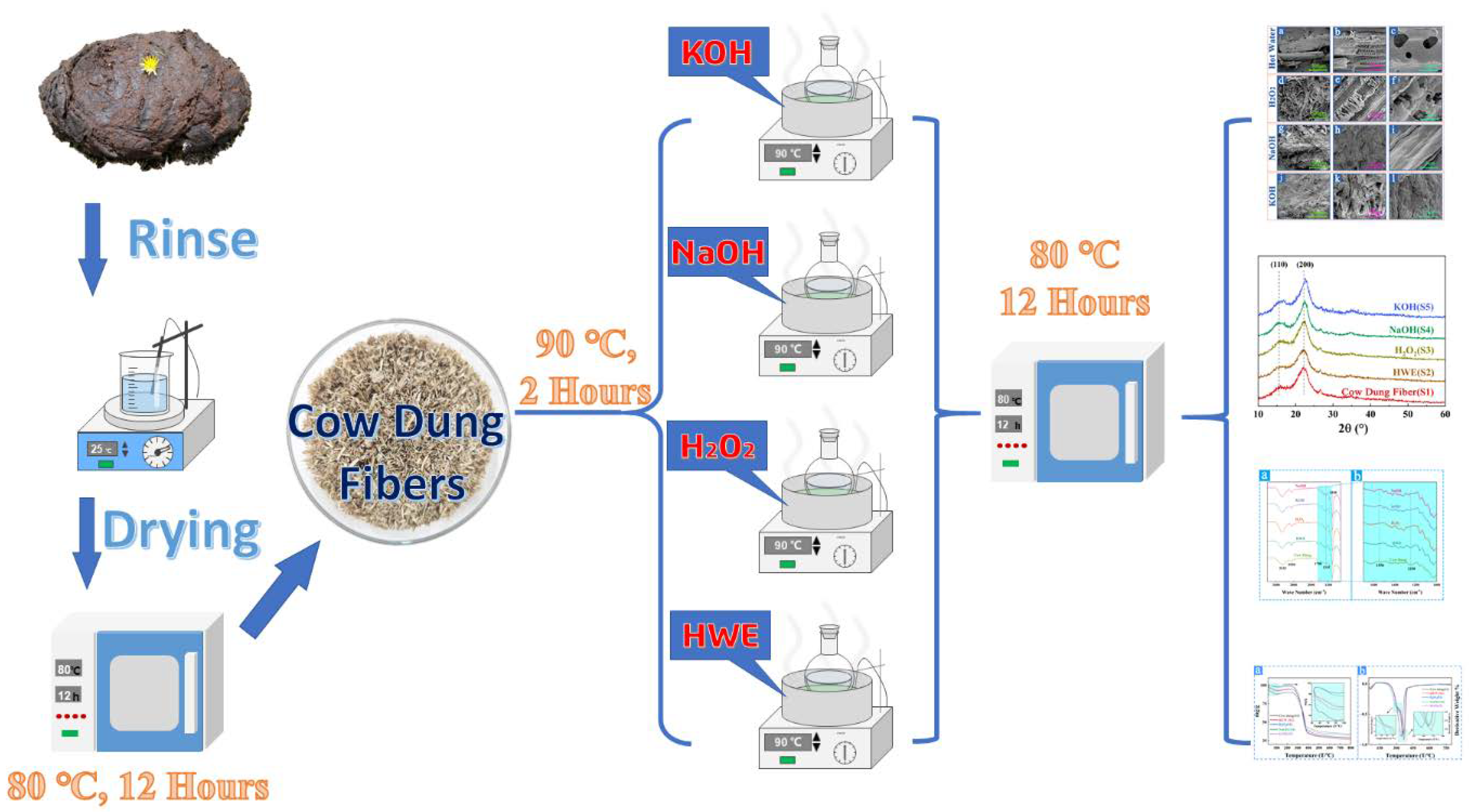
2. Experimental Procedure
2.1. Materials
2.2. Experimental Process
2.2.1. Hot Water Extraction (S2)
2.2.2. Hydrogen Peroxide Extraction (S3)
2.2.3. Sodium Hydroxide Extraction (S4)
2.2.4. Potassium Hydroxide Extraction (S5)
2.3. Characterization
Composition Analysis
- Fourier transform infrared spectroscopy (FTIR)
- X-ray diffraction (XRD)
- Thermogravimetric analysis (TGA)
- Scanning electron microscopy (SEM)
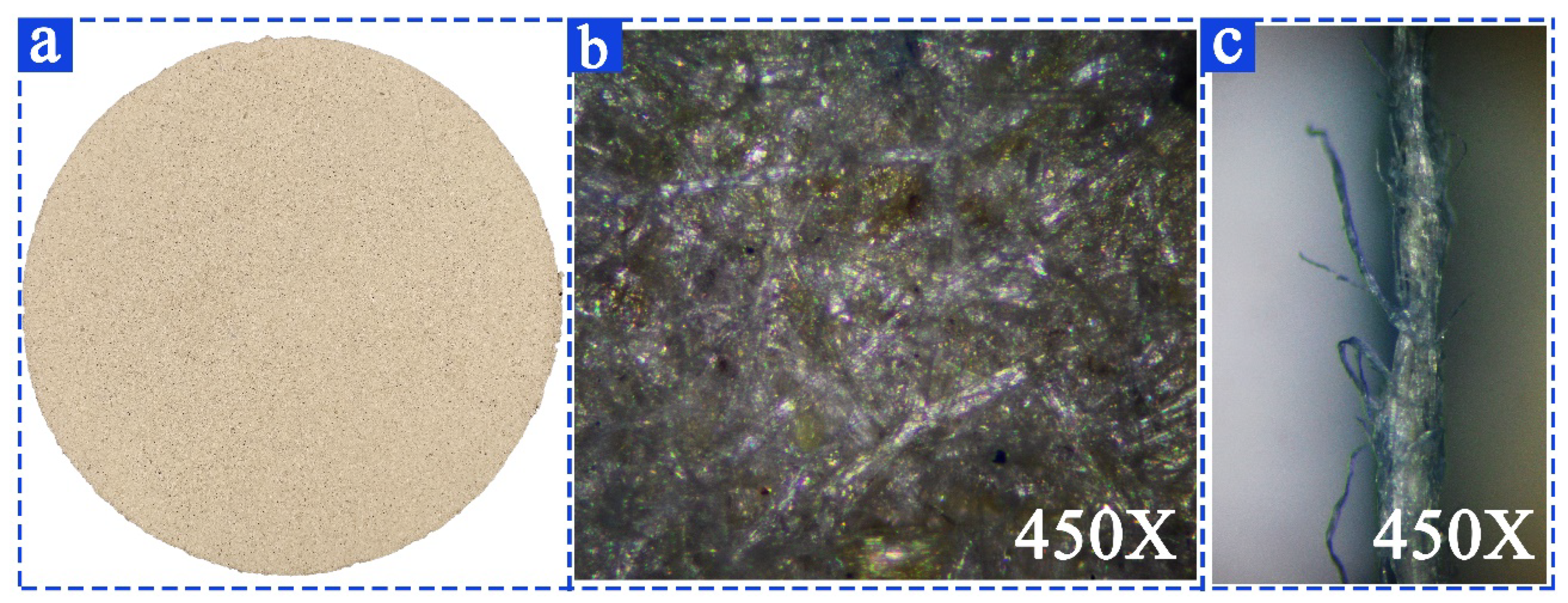
2.4. Preparation and Testing of Cow Dung Paper
2.4.1. Preparation of Cow Dung Paper
2.4.2. Testing of Cow Dung Paper
3. Results and Discussions
3.1. Characterization of Cow Dung
3.1.1. Chemical Composition
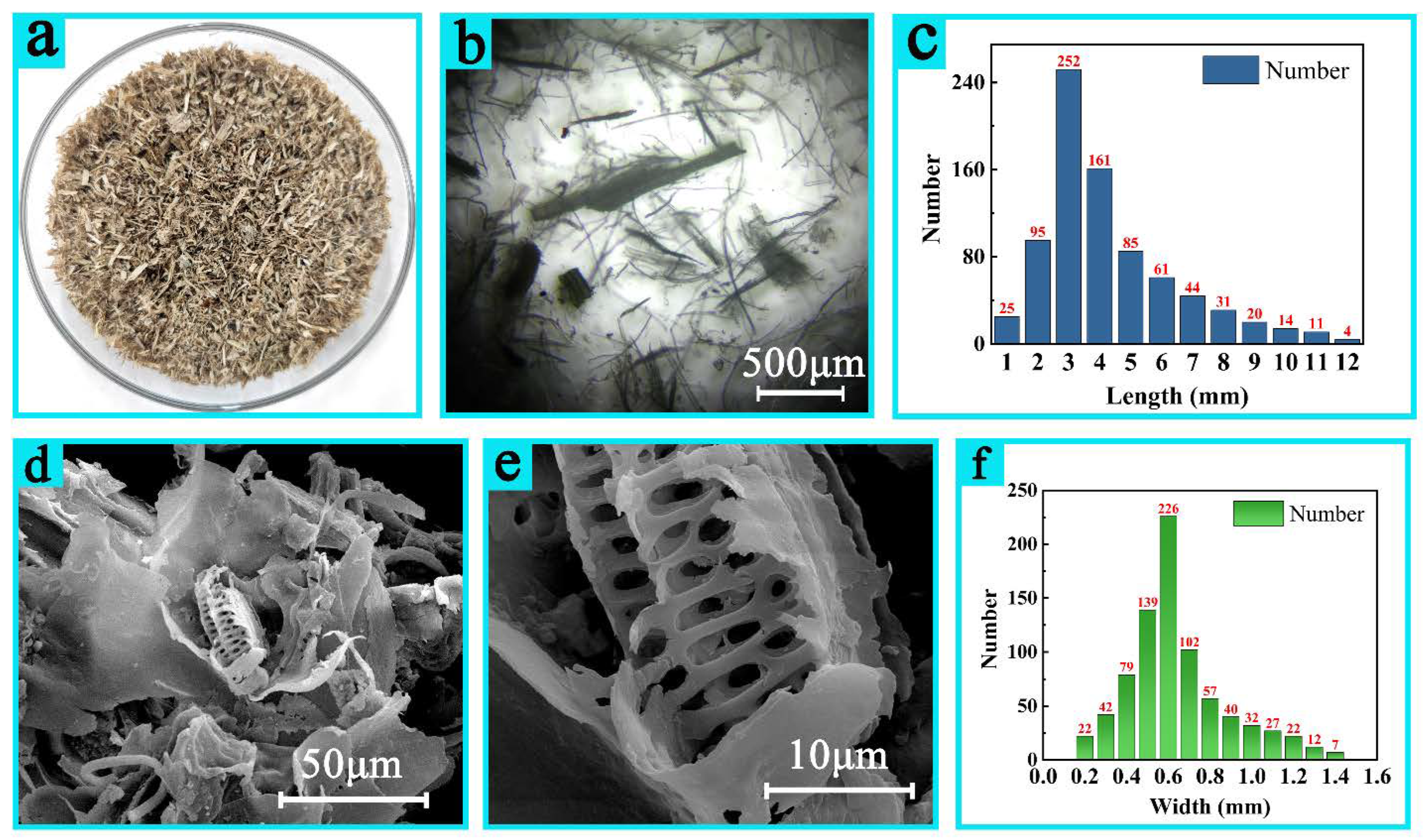
3.1.2. Morphology
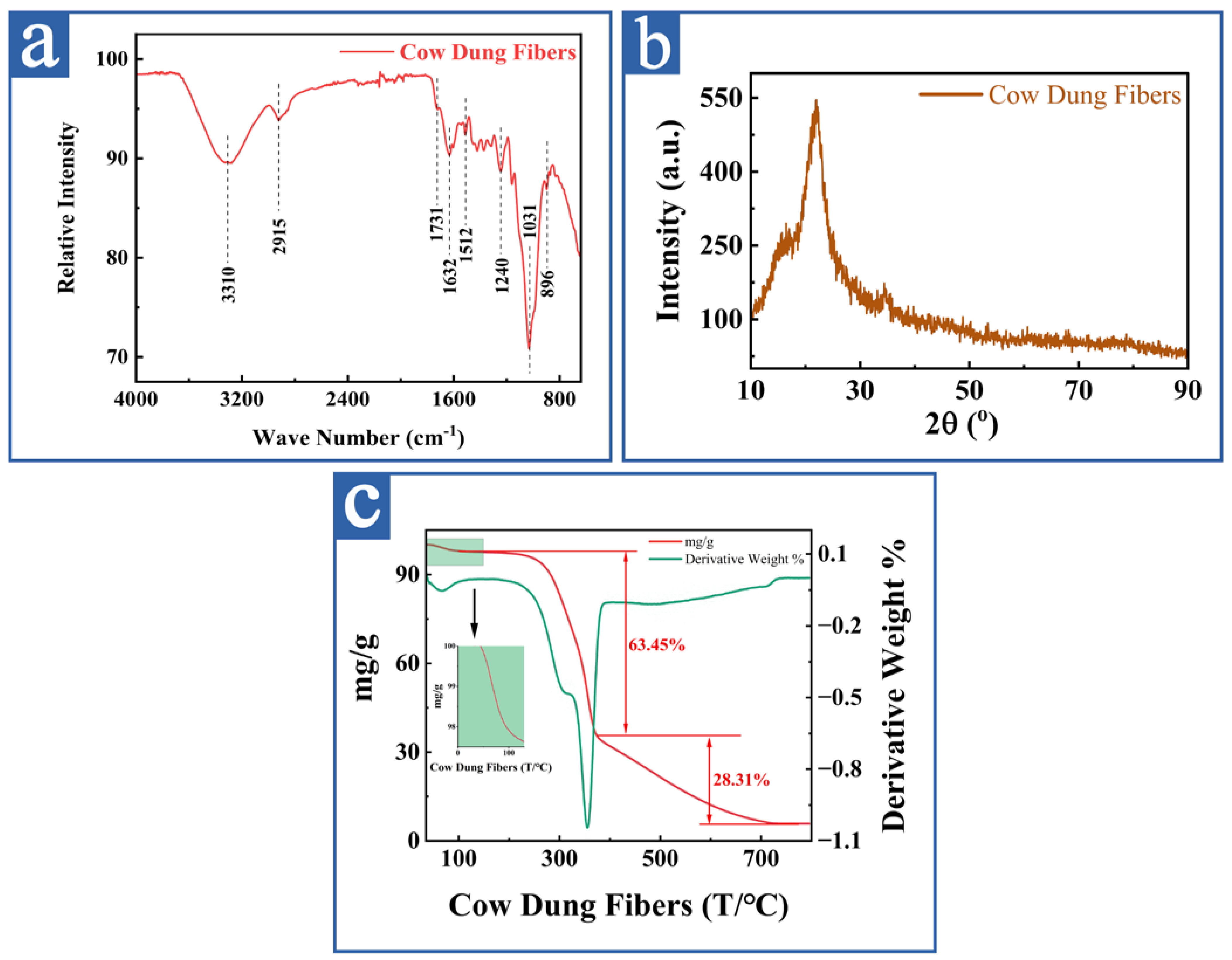
3.1.3. Structure and Thermal Stability Analyses
- FTIR analysis of cow dung fibers
- XRD analysis of cow dung fibers
- Thermogravimetric analysis
3.2. Extraction Treatments
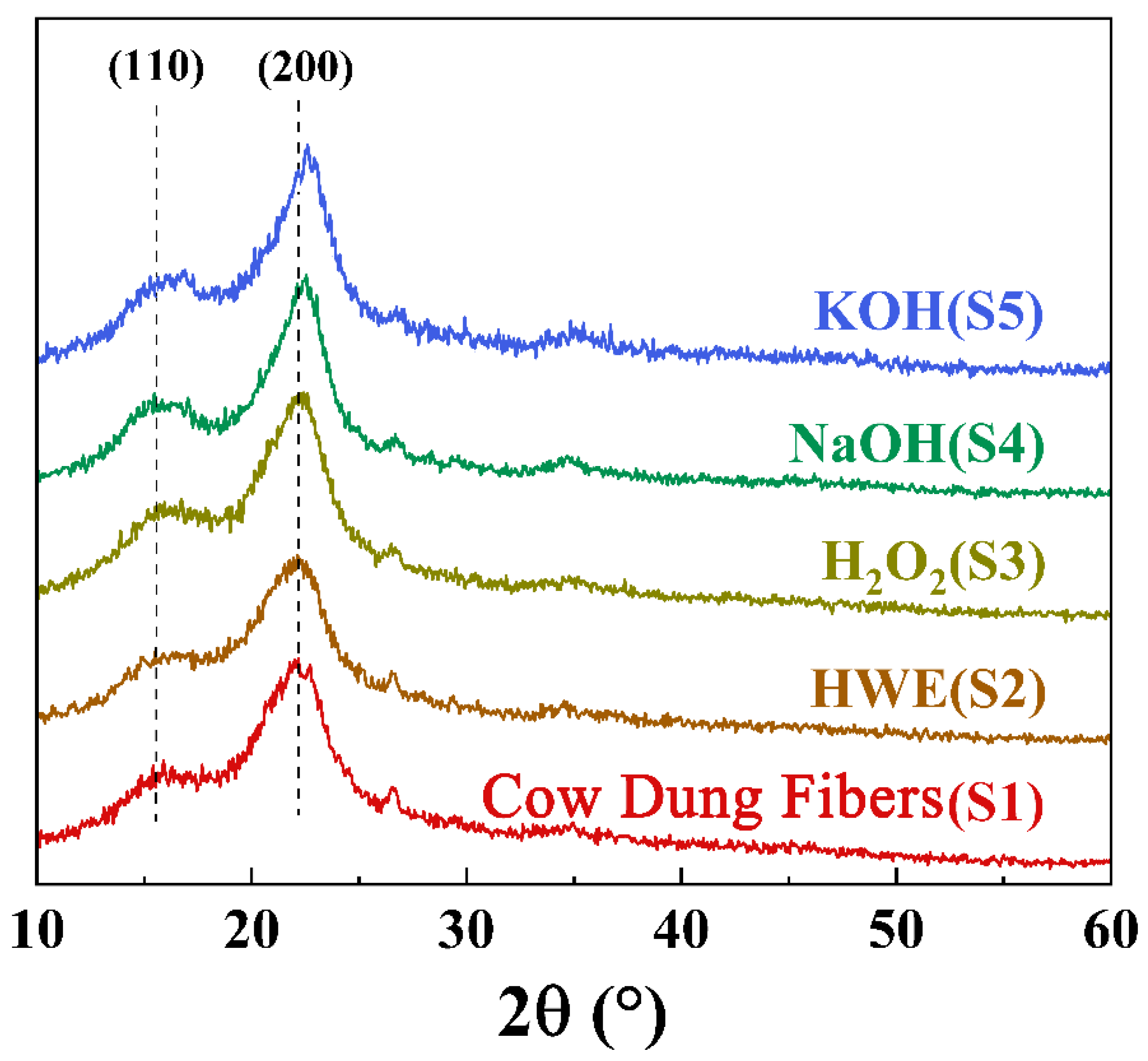
3.2.1. The XRD Results and Analyses of the Samples from Different Extraction Treatments
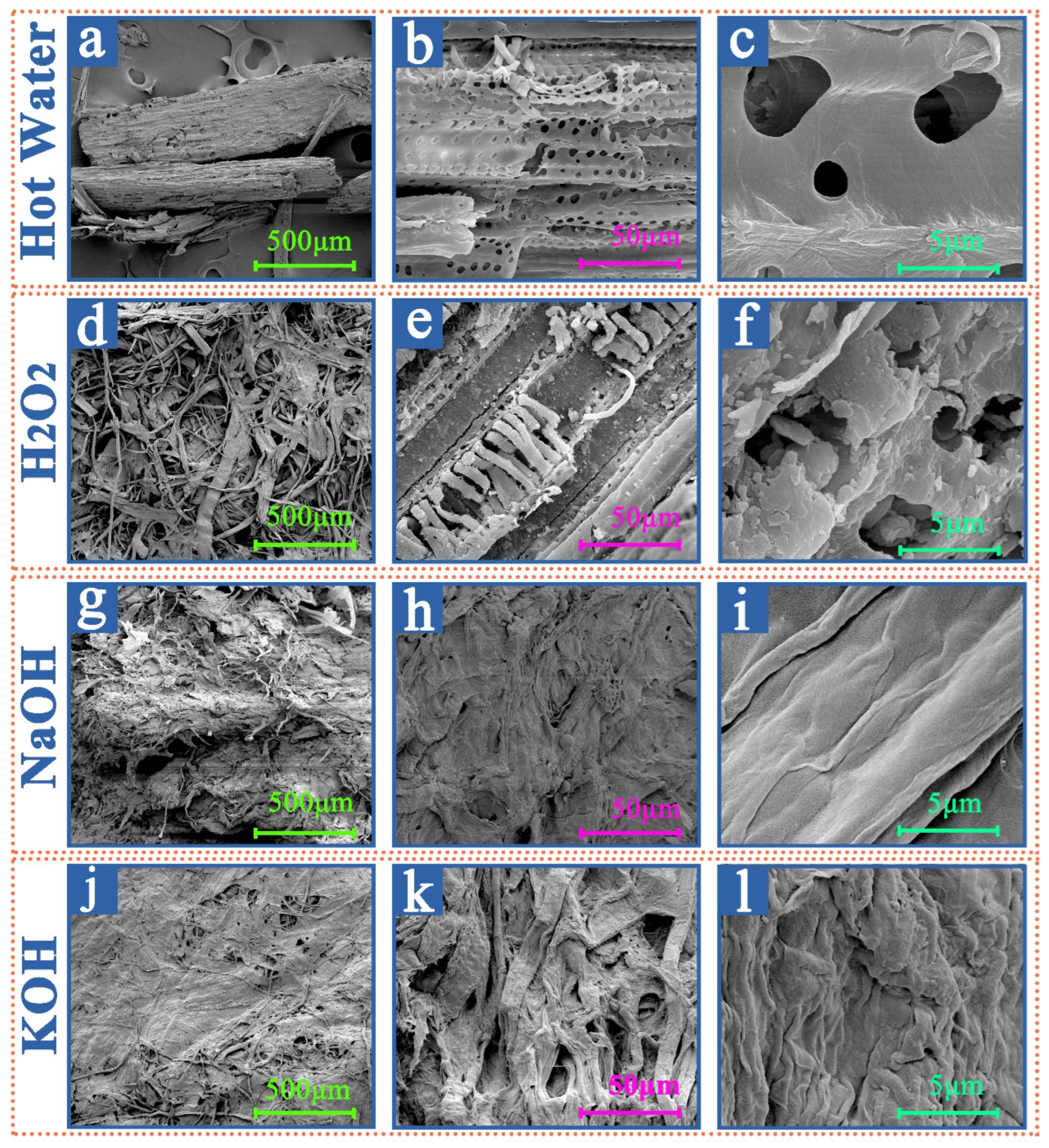
3.2.2. SEM Results and Analyses of the Samples from Different Extraction Treatments
3.2.3. FTIR Results and Analyses of the Samples from Different Extraction Treatments
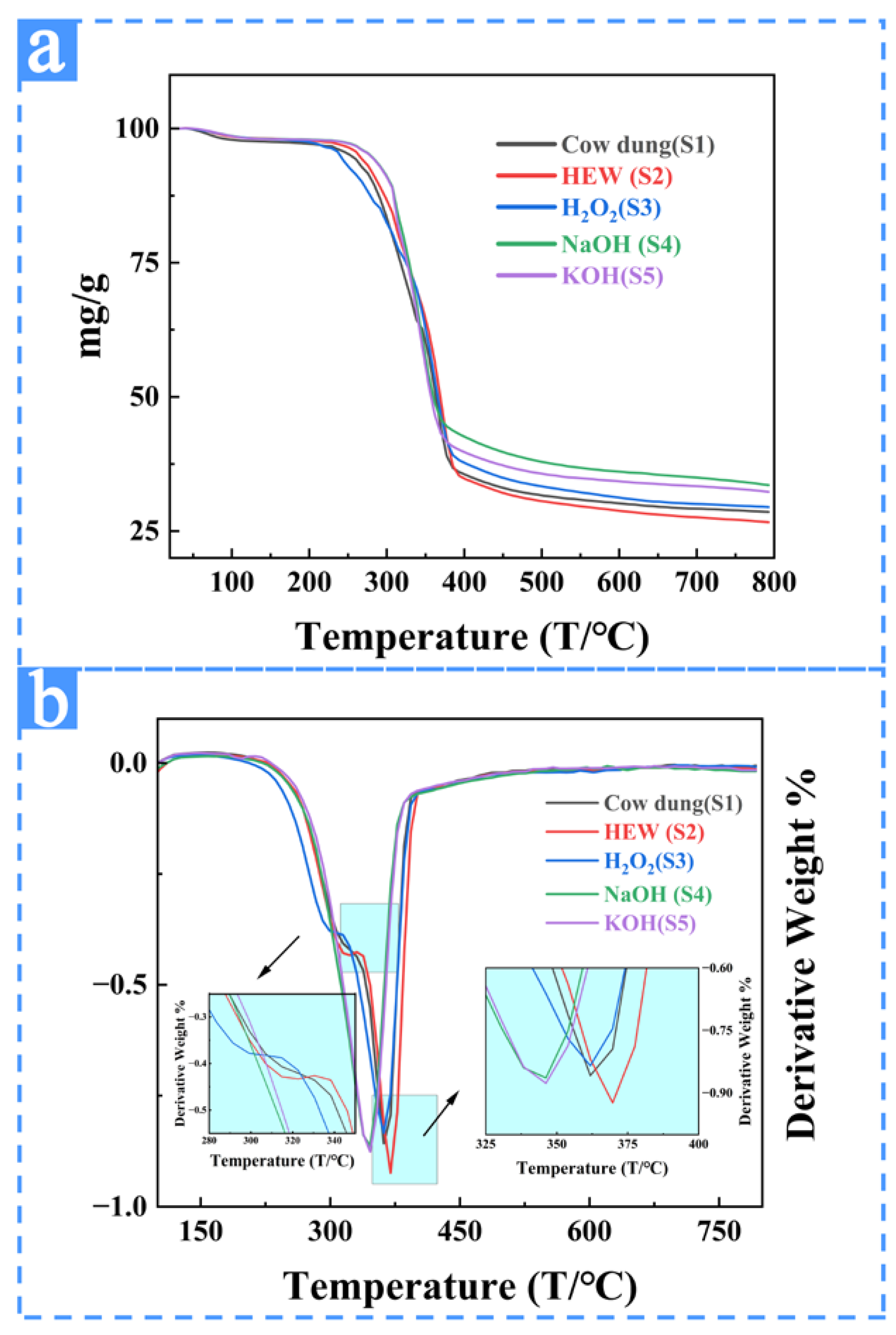
3.2.4. TGA Results and Analyses of the Samples from Different Extraction Treatments
3.3. Characterization of Cow Dung Paper
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sumesh, K.R.; Kanthavel, K.; Kavimani, V. Peanut Oil Cake-Derived Cellulose Fiber: Extraction, Application of Mechanical and Thermal Properties in Pineapple/Flax Natural Fiber Composites. Int. J. Biol. Macromol. 2020, 150, 775–785. [Google Scholar] [CrossRef]
- Gharehkhani, S.; Sadeghinezhad, E.; Kazi, S.N.; Yarmand, H.; Badarudin, A.; Safaei, M.R.; Zubir, M.N.M. Basic Effects of Pulp Refining on Fiber Properties—A Review. Carbohydr. Polym. 2015, 115, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Khawas, P.; Deka, S.C. Isolation and Characterization of Cellulose Nanofibers from Culinary Banana Peel Using High-Intensity Ultrasonication Combined with Chemical Treatment. Carbohydr. Polym. 2016, 137, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.D.; Alshammari, B.A.; Saba, N.; Alothman, O.Y.; Sanjay, M.R.; Almutairi, Z.; Jawaid, M. Characterization of Natural Fiber Obtained from Different Parts of Date Palm Tree (Phoenix Dactylifera L.). Int. J. Biol. Macromol. 2019, 135, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Vilaseca, F.; Del Rey, R.; Serrat, R.; Alba, J.; Mutje, P.; Espinach, F.X. Macro and Micro-Mechanics Behavior of Stifness in Alkaline Treated Hemp Core Fibres Polypropylene-Based Composites. Compos. Part B Eng. 2018, 144, 118–125. [Google Scholar] [CrossRef]
- Jeetah, P.; Jaffur, N. Coconut Husk, a Lignocellulosic Biomass, as a Promising Engineering Material for Non-Wood Paper Production. J. Nat. Fibers 2021, 19, 5622–5636. [Google Scholar] [CrossRef]
- Zykova, A.K.; Pantyukhov, P.V.; Mastalygina, E.E.; Chaverri-Ramos, C.; Nikolaeva, S.G.; Saavedra-Arias, J.J.; Popov, A.A.; Wortman, S.E.; Poletto, M. Biocomposites of Low-Density Polyethylene Plus Wood Flour or Flax Straw: Biodegradation Kinetics across Three Environments. Polymers 2021, 13, 2138. [Google Scholar] [CrossRef]
- Wang, H.Q.; Li, J.C.; Zeng, X.H.; Tang, X.; Sun, Y.; Lei, T.Z.; Lin, L. Extraction of Cellulose Nanocrystals Using a Recyclable Deep Eutectic Solvent. Cellulose 2020, 27, 1301–1314. [Google Scholar] [CrossRef]
- Raymond, P.; Mshandete, A.M.; Kajumulo Kivaisi, A. Production of Oxidative and Hydrolytic Enzymes by Coprinus cinereus (Schaeff.) Gray from Sisal Wastes Supplemented with Cow Dung Manure. Biotechnol. Res. Int. 2015, 2015, 650543. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Comprehensive Cellulose Chemistry: Volume I: Fundamentals and Analytical Methods; Wiley: Hoboken, NJ, USA, 1998; p. 37. [Google Scholar]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A Review of Recent Developments in Natural Fibre Composites and Their Mechanical Performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Liu, Y.; Song, Y.; Han, J.; Pan, H. Rosin Modified Cellulose Nanofiber as a Reinforcing and Co-Antimicrobial Agents in Polylactic Acid /Chitosan Composite Film for Food Packaging. Carbohydr. Polym. 2018, 183, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Wahlstrom, N.; Edlund, U.; Pavia, H.; Toth, G.; Jaworski, A.; Pell, A.J.; Choong, F.X.; Shirani, H.; Nilsson, K.P.R.; Richter-Dahlfors, A. Cellulose from the Green Macroalgae Ulva Lactuca: Isolation, Characterization, Optotracing, and Production of Cellulose Nanofibrils. Cellulose 2020, 27, 3707–3725. [Google Scholar] [CrossRef] [Green Version]
- Di Giorgio, L.; Salgado, P.R.; Dufresne, A.; Mauri, A.N. Nanocelluloses from Phormium (Phormium tenax) Fibers. Cellulose 2020, 27, 4975–4990. [Google Scholar] [CrossRef]
- Kassab, Z.; Kassem, I.; Hannache, H.; Bouhfid, R.; Qaiss, A.; El Achaby, M. Tomato Plant Residue as New Renewable Source for Cellulose Production: Extraction of Cellulose Nanocrystals with Different Surface Functionalities. Cellulose 2020, 27, 4287–4303. [Google Scholar] [CrossRef]
- Khan, A.; Vijay, R.; Singaravelu, D.L.; Sanjay, M.R.; Siengchin, S.; Verpoort, F.; Alamry, K.A.; Asiri, A.M. Extraction and Characterization of Natural Fiber from Eleusine Indica Grass as Reinforcement of Sustainable Fiber Reinforced Polymer Composites. J. Nat. Fibers 2021, 18, 1742–1750. [Google Scholar] [CrossRef]
- Saeed, H.A.M.; Liu, Y.; Lucia, L.; Chen, H. Evaluation of Sudanese Sorghum and Bagasse as a Pulp and Paper Feedstock. Bioresources 2017, 12, 5212–5222. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, E.S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Non-Wood Fibers as Raw Material for Pulp and Paper Industry. Nord. Pulp Pap. Res. J. 2020, 35, 215–230. [Google Scholar] [CrossRef]
- Braun, U. Ultrasonographic Examination of the Reticulum, Rumen, Omasum, Abomasum, and Liver in Calves. Vet. Clin. N. Am. Food Anim. Pract. 2016, 32, 85–107. [Google Scholar] [CrossRef]
- Khalid, M.; Ratnam, C.T.; Abdullah, L.C.; Walvekar, R.; Ching, Y.C.; Ketabchi, M.R. Mechanical and Physical Performance of Cowdung-Based Polypropylene Biocomposites. Polym. Compos. 2018, 39, 288–296. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, S.T.; Ho, A.; Hong, C.O.; Lee, C.H.; Kim, P.J. Cattle Manure Composting: Shifts in the Methanogenic Community Structure, Chemical Composition, and Consequences on Methane Production Potential in a Rice Paddy. Appl. Soil Ecol. 2018, 124, 344–350. [Google Scholar] [CrossRef]
- Cestonaro, T.; Costa, M.S.S.D.; Costa, L.A.D.; Pereira, D.C.; Rozatti, M.A.T.; Martins, M.F.L. Addition of Cattle Manure to Sheep Bedding Allows Vermicomposting Process and Improves Vermicompost Quality. Waste Manag. 2017, 61, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Song, W.; Wang, R.; Zou, J.; Yang, R.; Zhang, S. Physicochemical Properties of Biochar Derived from Anaerobically Digested Dairy Manure. Waste Manag. 2018, 79, 729–734. [Google Scholar] [CrossRef]
- Abdeshahian, P.; Lim, J.S.; Ho, W.S.; Hashim, H.; Lee, C.T. Potential of Biogas Production from Farm Animal Waste in Malaysia. Renew. Sustain. Energy Rev. 2016, 60, 714–723. [Google Scholar] [CrossRef]
- Alemayehu, G. Co-Digestion of Municipal Organic Wastes with Night Soil and Cow Dung for Biogas Production: A Review. Afr. J. Biotechnol. 2016, 15, 32–44. [Google Scholar] [CrossRef]
- Vedrtnam, A. Fabrication and Characterization of Cow Dung- Polyvinyl Alcohol Composite Film. Compos. Commun. 2018, 8, 31–35. [Google Scholar] [CrossRef]
- Jahan, M.S.; Haris, F.; Rahman, M.M.; Samaddar, P.R.; Sutradhar, S. Potassium Hydroxide Pulping of Rice Straw in Biorefinery Initiatives. Bioresour. Technol. 2016, 219, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Wang, D.; Liu, Q.; Li, K.; Li, Y.; Zhong, F.; Liu, Q.; Wang, P.; Feng, W.; Yang, X. Large-Scale Preparation of Uniform Millet Bread-like Durable Benzoxazine-Phthalonitrile Foam with Outstanding Mechanical and Thermal Properties. Polymers 2022, 14, 5410. [Google Scholar] [CrossRef]
- Mandal, T.K.; Lee, Y.R.; Parvin, N. Red Phosphorus Decorated Graphene Oxide Nanosheets: Label-Free DNA Detection. Biomater. Sci. 2020, 8, 125–131. [Google Scholar] [CrossRef]
- Rodríguez, A.; Serrano, L.; Moral, A.; Pérez, A.; Jiménez, L. Use of High-Boiling Point Organic Solvents for Pulping Oil Palm Empty Fruit Bunches. Bioresour. Technol. 2008, 99, 1743–1749. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Alothman, O. Isolation and Characterization of Microcrystalline Cellulose from Roselle Fibers. Int. J. Biol. Macromol. 2017, 103, 931–940. [Google Scholar] [CrossRef]
- Jaramillo-Quiceno, N.; Vélez R., J.M.; Cadena Ch., E.M.; Restrepo-Osorio, A.; Santa, J.F. Improvement of Mechanical Properties of Pineapple Leaf Fibers by Mercerization Process. Fibers Polym. 2018, 19, 2604–2611. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, Preparation and Characterization of Cellulose Fibres and Nanocrystals from Rice Husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R. Isolation and Characterization of Nanocrystalline Cellulose from Sugar Palm Fibres (Arenga Pinnata). Carbohydr. Polym. 2018, 181, 1038–1051. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of Nanocellulose Fibrils from Lignocellulosic Fibres: A Novel Approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Łojewska, J.; Miśkowiec, P.; Łojewski, T.; Proniewicz, L.M. Cellulose Oxidative and Hydrolytic Degradation: In Situ FTIR Approach. Polym. Degrad. Stab. 2005, 88, 512–520. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K. Synthesis of a Highly Fluorescence Nitrogen-Doped Carbon Quantum Dots Bioimaging Probe and Its in Vivo Clearance and Printing Applications. RSC Adv. 2016, 6, 18134–18140. [Google Scholar] [CrossRef]
- Kouadri, I.; Satha, H. Extraction and Characterization of Cellulose and Cellulose Nanofibers from Citrullus Colocynthis Seeds. Ind. Crops Prod. 2018, 124, 787–796. [Google Scholar] [CrossRef]
- Ventura-Cruz, S.; Tecante, A. Extraction and Characterization of Cellulose Nanofibers from Rose Stems (Rosa spp.). Carbohydr. Polym. 2019, 220, 53–59. [Google Scholar] [CrossRef]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose Nanowhiskers from Coconut Husk Fibers: Effect of Preparation Conditions on Their Thermal and Morphological Behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Saurabh, C.K.; Mustapha, A.; Masri, M.M.; Owolabi, A.F.; Syakir, M.I.; Dungani, R.; Paridah, M.T.; Jawaid, M.; Abdul Khalil, H.P.S. Isolation and Characterization of Cellulose Nanofibers from Gigantochloa scortechinii as a Reinforcement Material. J. Nanomater. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, L.F.d.M.S.; Hidalgo Falla, M.D.P.; Luz, S.M.D. The Influence of Potassium Hydroxide Concentration and Reaction Time on the Extraction Cellulosic Jute Fibers. J. Nat. Fibers 2021, 19, 1–13. [Google Scholar] [CrossRef]
- Leite, A.L.M.P.; Zanon, C.D.; Menegalli, F.C. Isolation and Characterization of Cellulose Nanofibers from Cassava Root Bagasse and Peelings. Carbohydr. Polym. 2017, 157, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Vishnu Vardhini, K.J.; Murugan, R. Effect of Laccase and Xylanase Enzyme Treatment on Chemical and Mechanical Properties of Banana Fiber. J. Nat. Fibers 2017, 14, 217–227. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Luffa cylindrica as a lignocellulosic source of fiber, microfibrillated cellulose, and cellulose nanocrystals. BioResources 2010, 5, 727–740. [Google Scholar]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and Application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Dhlamini, M.S.; Mothudi, B.M.; Kommula, V.P.; Zhang, J.; Zhang, J.; Rajulu, A.V. Extraction and Characterization of Cellulose Single Fibers from Native African Napier Grass. Carbohydr. Polym. 2018, 188, 85–91. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of Cellulose and Preparation of Nanocellulose from Sisal Fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Reddy, D.J.P.; Rajulu, A.V. Thermal Properties of Napier Grass Fibers. Mater. Lett. 2009, 63, 2390–2392. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Moreno, L.M.; Gorinstein, S.; Medina, O.J.; Palacios, J.; Mu?oz, E.J. Valorization of Garlic Crops Residues as Precursors of Cellulosic Materials. Waste Biomass Valorization 2020, 11, 4767–4779. [Google Scholar] [CrossRef]
- Kallel, F.; Bettaieb, F.; Khiari, R.; García, A.; Bras, J.; Chaabouni, S.E. Isolation and Structural Characterization of Cellulose Nanocrystals Extracted from Garlic Straw Residues. Ind. Crops Prod. 2016, 87, 287–296. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothen, L.A.; Cintil, J.; Thomas, S.; John, M.J.; Anandjiwala, R.; Narine, S.S. Environmental Friendly Method for the Extraction of Coir Fibre and Isolation of Nanofibre. Carbohydr. Polym. 2013, 92, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- El Achaby, M.; El Miri, N.; Aboulkas, A.; Zahouily, M.; Bilal, E.; Barakat, A.; Solhy, A. Processing and Properties of Eco-Friendly Bio-Nanocomposite Films Filled with Cellulose Nanocrystals from Sugarcane Bagasse. Int. J. Biol. Macromol. 2017, 96, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Elanthikkal, S.; Gopalakrishnapanicker, U.; Varghese, S.; Guthrie, J.T. Cellulose Microfibres Produced from Banana Plant Wastes: Isolation and Characterization. Carbohydr. Polym. 2010, 80, 852–859. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Structure and Properties of High Quality Natural Cellulose Fibers from Cornstalks. Polymer 2005, 46, 5494–5500. [Google Scholar] [CrossRef]
- Ebringerová, A.; Heinze, T. Xylan and Xylan Derivatives—Biopolymers with Valuable Properties, 1. Naturally Occurring Xylans Structures, Isolation Procedures and Properties. Macromol. Rapid Commun. 2000, 21, 542–556. [Google Scholar] [CrossRef]
- Ateş, S.; Deniz, İ.; Kirci, H.; AtiK, C.; Okan, O.T. Comparison of Pulping and Bleaching Behaviors of Some Agricultural Residues. Turk. J. Agric. For. 2015, 39, 144–153. [Google Scholar] [CrossRef]

| CW | HW | NaOH (1%) | OS | Ash | Lign | Hol | Cell | |
|---|---|---|---|---|---|---|---|---|
| Cow Dung | 4.2 | 4.8 | 23.7 | 2.28 | 5.79 | 21.31 | 69.86 | 41.6 |
| Bagasse [18] | 10.6 | 11.3 | 15.5 | 5.0 | 15.2 | 64.1 | 43.0 | |
| Cotton stalks [31] | 3.33 | 20.34 | 1.42 | 2.17 | 24.45 | 14.38 | 58.48 | |
| Softwood [2] | - | - | - | - | - | 25–31 | 65–74 | 40–45 |
| Hardwood [2] | - | - | - | - | - | 16–24 | 67–82 | 43–47 |
| Length (mm) | 1 | 2 | 3 | 4 | 5 | 6 | 6 | 8 | 9 | 10 | 11 | 12 | -- |
| Proportion | 3.11% | 11.83% | 31.28% | 20.05% | 10.59% | 7.6% | 5.48% | 3.86% | 2.49% | 1.74% | 1.37% | 0.5% | -- |
| Width (mm) | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | 1.1 | 1.2 | 1.3 | 1.4 |
| Proportion | 2.76% | 5.21% | 9.82% | 17.18% | 27.91% | 12.58% | 7.06% | 4.91% | 3.99% | 3.37% | 2.76% | 1.53% | 0.92% |
| Sample | Thermal Stability (°C) | Peak 1 | Peak 2 | Peak 3 | Weight Loss |
|---|---|---|---|---|---|
| 100 °C | |||||
| S1 | 275 °C | 65 °C/10% | 315 °C/13.5% | 362 °C/25.1% | 12 |
| S2 | 275 °C | 70 °C/4.9% | 322 °C/18.3% | 370 °C/27.3% | 6.9 |
| S3 | 282 °C | 65 °C/0.6% | 300 °C/12.6% | 362 °C/33.4% | 1.8 |
| S4 | 290 °C | 70 °C/0.8% | -- | 346 °C/39% | 1.9 |
| S5 | 292 °C | 60 °C/0.6% | -- | 346 °C/39.7% | 1.7 |
| Raw Materials | °SR (Schopper-Riegler’s Degree) | Grammage (g/m2) | Density (kg/m3) | Bulkiness (g/cm3) | Tear Index (mNm2/g) | Burst Index (kPam2/g) | Breaking Length (km) | Tensile Index (Nm/g) |
|---|---|---|---|---|---|---|---|---|
| Cow dung | 42.0 | 62.5 | 518..67 | 1.93 | 4.83 | 2.48 | 2.73 | 26.72 |
| Wheat straw | 57.5 | 67.2 | 896.06 | 1.12 | 3.58 | 4.84 | 7.95 | 78.00 |
| Corn stalk | 41.5 | 68.68 | 735.89 | 1.36 | 5.36 | 3.16 | 5.92 | 58.01 |
| Rice straw | 65.5 | 69.76 | 611.02 | 1.64 | 4.89 | 3.42 | 6.02 | 59.00 |
| Cotton stalk | 45.0 | 75.40 | 591.40 | 1.69 | 4.75 | 2.11 | 3.79 | 37.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Li, L.; Zhao, W.; Wang, M.; Yang, W.; Tian, Y.; Zheng, R.; Deng, S.; Mu, Y.; Zhu, X. Characteristics and Functional Application of Cellulose Fibers Extracted from Cow Dung Wastes. Materials 2023, 16, 648. https://doi.org/10.3390/ma16020648
Yang X, Li L, Zhao W, Wang M, Yang W, Tian Y, Zheng R, Deng S, Mu Y, Zhu X. Characteristics and Functional Application of Cellulose Fibers Extracted from Cow Dung Wastes. Materials. 2023; 16(2):648. https://doi.org/10.3390/ma16020648
Chicago/Turabian StyleYang, Xiangjun, Lu Li, Wuyun Zhao, Mengyang Wang, Wanxia Yang, Yuhang Tian, Ruizhe Zheng, Shuhang Deng, Yongsong Mu, and Xiaodong Zhu. 2023. "Characteristics and Functional Application of Cellulose Fibers Extracted from Cow Dung Wastes" Materials 16, no. 2: 648. https://doi.org/10.3390/ma16020648





