Effect of Mg Addition and PMMA Coating on the Biodegradation Behaviour of Extruded Zn Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Processing and Characterization
2.2. Grafted PMMA on Zn-1.89Mg
2.3. PMMA Coating Characterization
2.4. Electrochemical Measurements
2.5. Static Immersion Test
2.6. Characterization of Surfaces after Corrosion
2.7. Statistical Analysis
3. Results
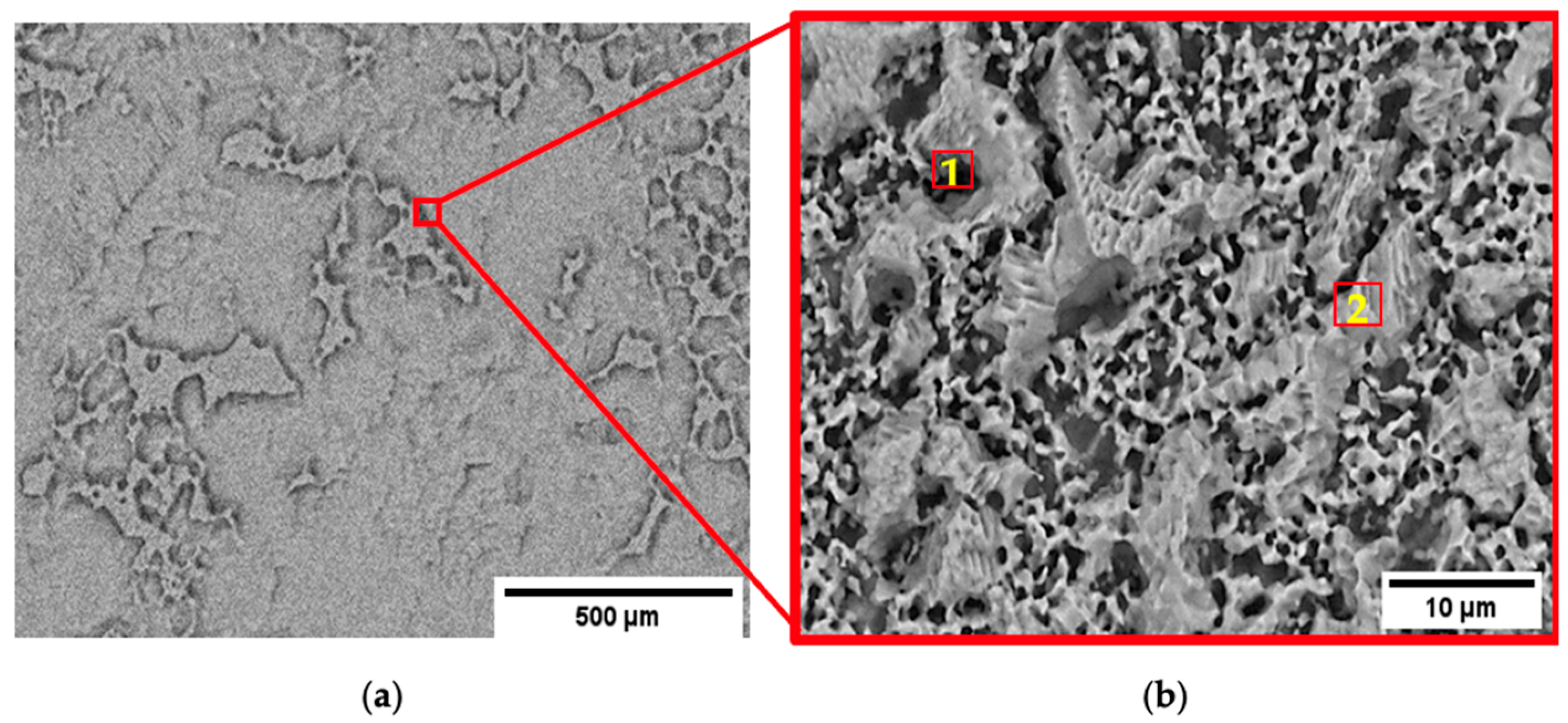
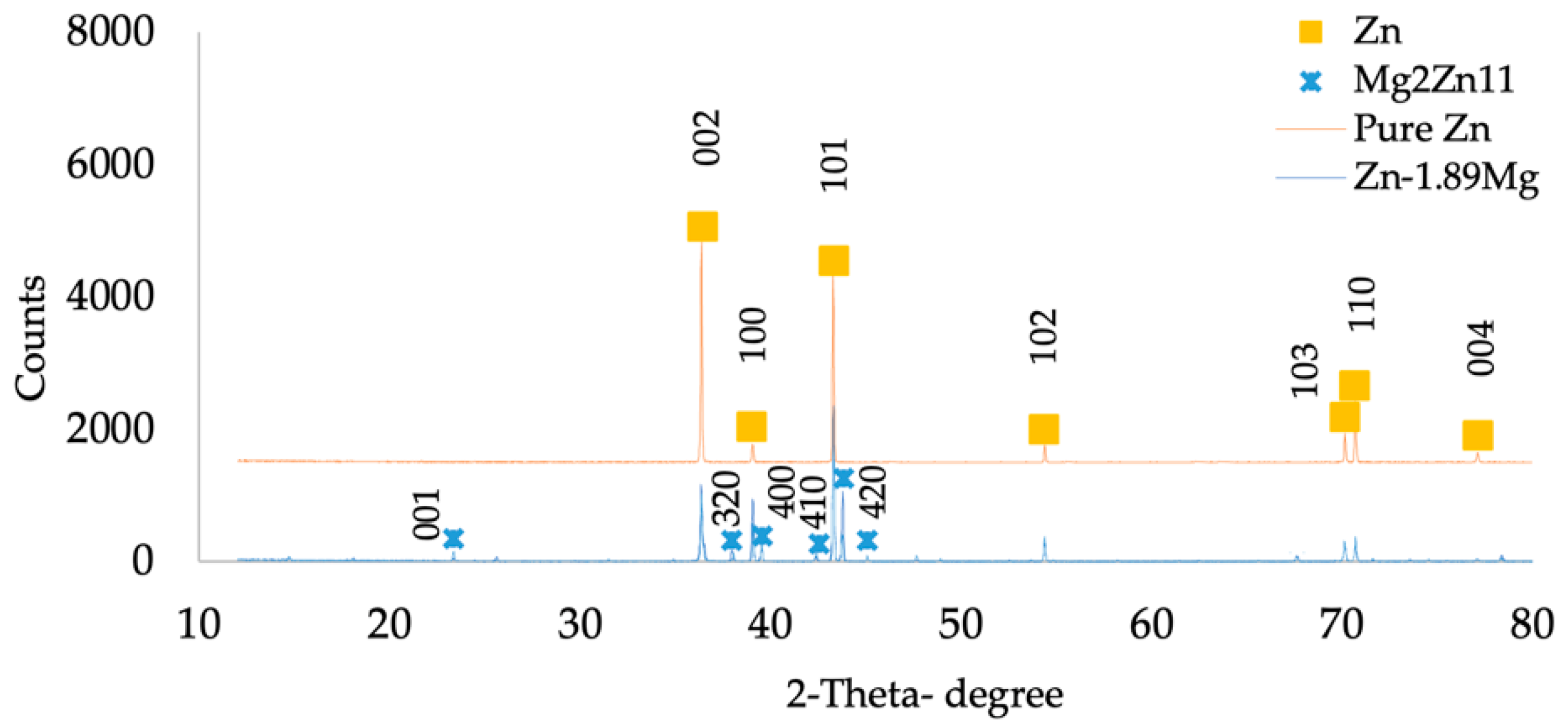
3.1. Microstructure of Pure Zn and Zn-1.89Mg
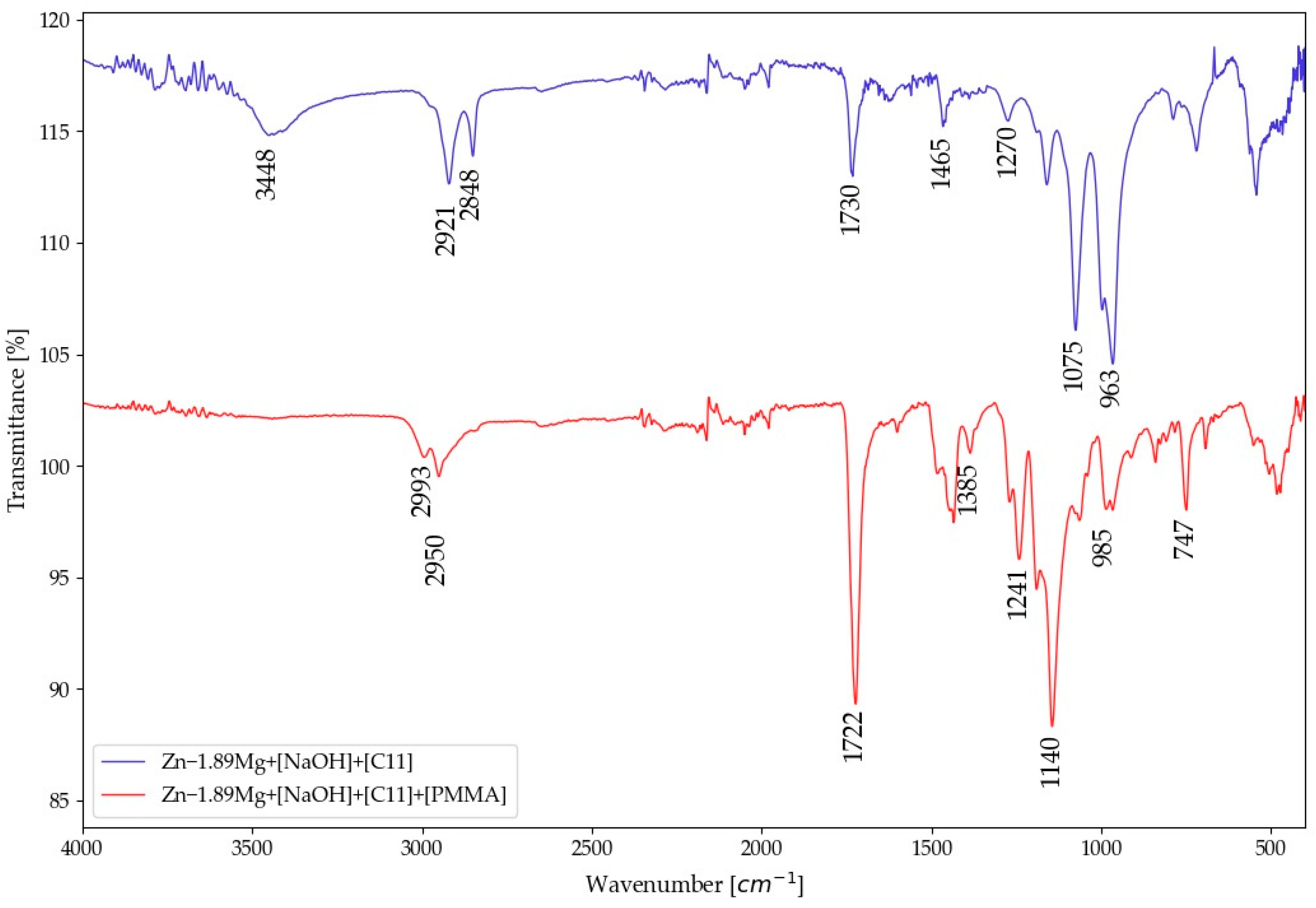
3.2. Surface of PMMA-Coated Zn-1.89Mg
3.3. Electrochemical Behaviour
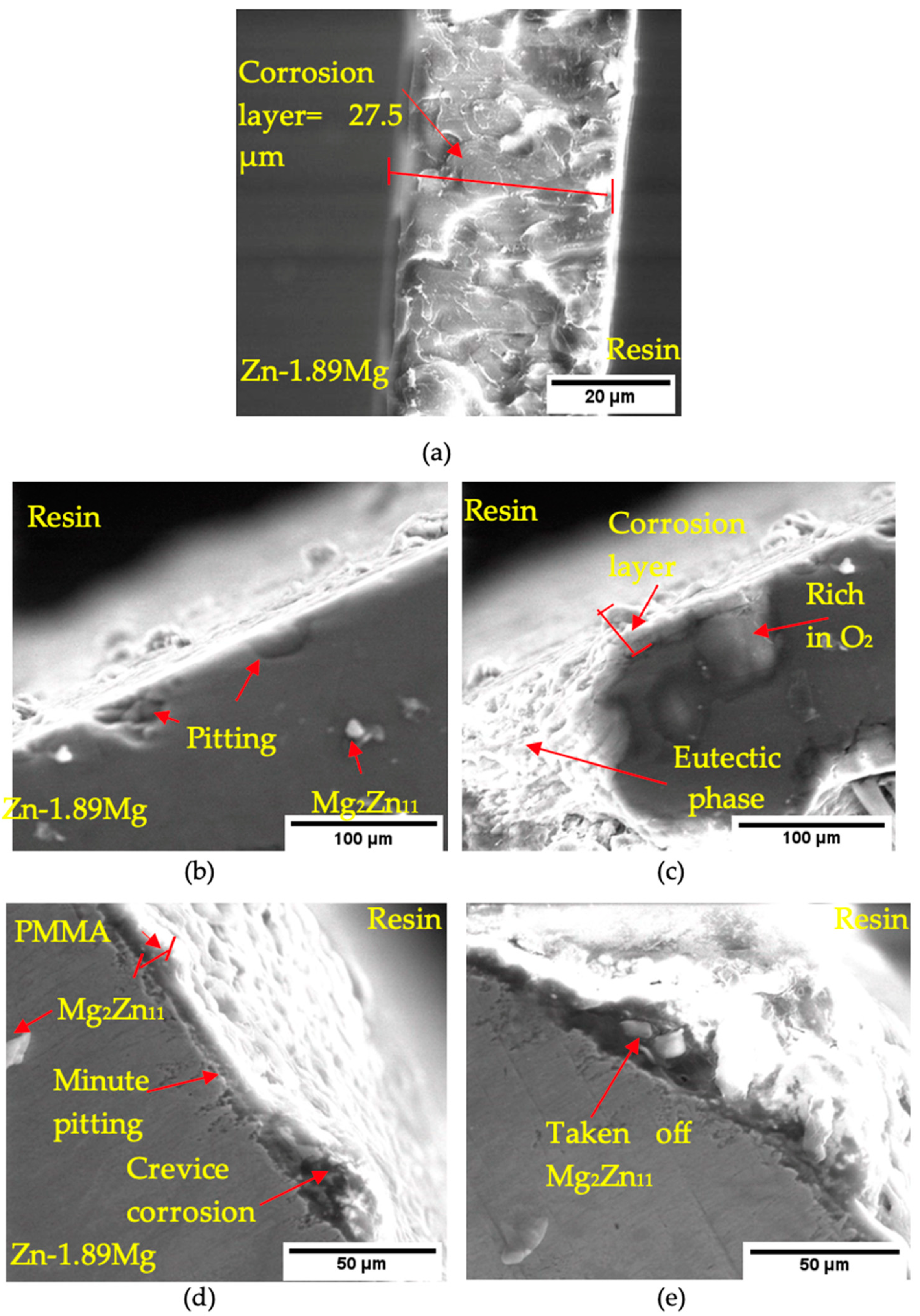
3.4. Immersion Test
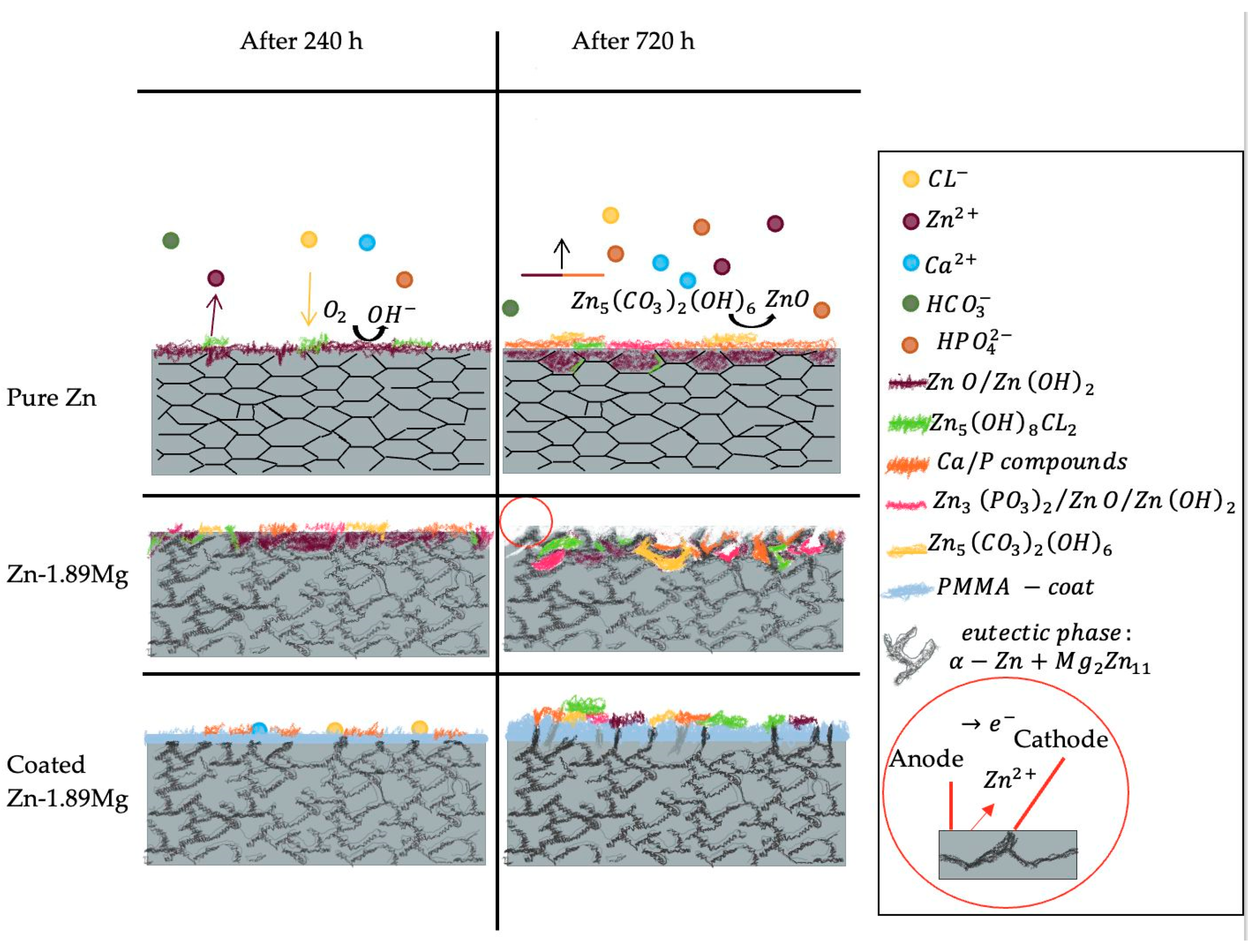
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Zheng, Y.; Chen, X.-H.; Yang, J.-A.; Pan, H.; Chen, D.; Wang, L.; Zhang, J.; Zhu, D.; Wu, S.; et al. Fundamental Theory of Biodegradable Metals—Definition, Criteria, and Design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Zhuo, X.; Wu, Y.; Ju, J.; Liu, H.; Jiang, J.; Hu, Z.; Bai, J.; Xue, F. Recent progress of novel biodegradable zinc alloys: From the perspective of strengthening and toughening. J. Mater. Res. Technol. 2022, 17, 244–269. [Google Scholar] [CrossRef]
- Chen, C.; Fan, S.; Niu, J.; Huang, H.; Jin, Z.; Kong, L.; Zhu, D.; Yuan, G. Alloying design strategy for biodegradable zinc alloys based on first-principles study of solid solution strengthening. Mater. Des. 2021, 204, 109676. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef] [Green Version]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- El Mahallawy, N.; Diaa, A.A.; Akdesir, M.; Palkowski, H. Microstructure and mechanical properties of Mg-6Sn and Mg-6Zn alloys prepared by different processing techniques: A comparative study. Mater. Werkst. 2016, 47, 37–46. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Loffredo, S.; Demir, A.; Previtali, B.; Mantovani, D.; Beanland, R.; Vedani, M. Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef] [PubMed]
- Wątroba, M.; Mech, K.; Bednarczyk, W.; Kawałko, J.; Marciszko-Wiąckowska, M.; Marzec, M.; Shepherd, D.E.; Bała, P. Long-term in vitro corrosion behavior of Zn-3Ag and Zn-3Ag-0.5Mg alloys considered for biodegradable implant applications. Mater. Des. 2022, 213, 110289. [Google Scholar] [CrossRef]
- Li, M.; Jiang, M.; Gao, Y.; Zheng, Y.; Liu, Z.; Zhou, C. Bioactive Materials Current status and outlook of biodegradable metals in neuroscience and their potential applications as cerebral vascular stent materials. Bioact. Mater. 2022, 11, 140–153. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, S.M.; Al-Khaled, K.; Ayadi, M.; Abbas, N.; Chammam, W. Performance analysis of biodegradable materials for orthopedic applications. Mater. Today Commun. 2022, 31, 103167. [Google Scholar] [CrossRef]
- Yuan, W.; Xia, D.; Wu, S.; Zheng, Y.; Guan, Z.; Rau, J.V. A review on current research status of the surface modification of Zn-based biodegradable metals. Bioact. Mater. 2022, 7, 192–216. [Google Scholar] [CrossRef] [PubMed]
- Shomali, A.A.; Guillory, R.J.; Seguin, D.; Goldman, J.; Drelich, J.W. Effect of PLLA coating on corrosion and biocompatibility of zinc in vascular environment. Surf. Innov. 2017, 5, 211–220. [Google Scholar] [CrossRef]
- Su, S.; Tang, Q.; Qu, D. In Vitro Study of Degradation and Cytocompatibility of Ceramics/PLA Composite Coating on Pure Zinc for Orthopedic Application. Front. Bioeng. Biotechnol. 2022, 10, 856986. [Google Scholar] [CrossRef] [PubMed]
- Reggente, M.; Masson, P.; Dollinger, C.; Palkowski, H.; Zafeiratos, S.; Jacomine, L.; Passeri, D.; Rossi, M.; Vrana, N.E.; Pourroy, G.; et al. Novel Alkali Activation of Titanium Substrates to Grow Thick and Covalently Bound PMMA Layers. ACS Appl. Mater. Interfaces 2018, 10, 5967–5977. [Google Scholar] [CrossRef]
- Jin, W.; Hao, Q.; Peng, X.; Chu, P.K. Enhanced corrosion resistance and biocompatibilty of PMMA-coated ZK60 magnesium alloy. Mater. Lett. 2016, 173, 178–181. [Google Scholar] [CrossRef]
- Majumder, O.; Singh Bankoti, A.K.; Kaur, T.; Thirugnanam, A.; Mondal, A.K. The influence of silane and silane-PMMA coatings on the in vitro biodegradation behavior of AE42 magnesium alloy for cardiovascular stent applications. RSC Adv. 2016, 6, 107344–107354. [Google Scholar] [CrossRef]
- Gonsalves, T.C.; de Freitas, B.X.; Nunes, C.A.; Florenzano, F.H. Reduction of Magnesium Corrosion Rate by PMMA-co-PMAA Films. Mater. Res. 2021, 24, e20200361. [Google Scholar] [CrossRef]
- Schott, T.; Liautaud, F.; Kriegel, S.; Faerber, J.; He, W.; Masson, P.; Pourroy, G.; Carradò, A. Stability of PMMA-grafted/Ti hybrid biomaterial interface in corrosive media. Pure Appl. Chem. 2019, 91, 1617–1629. [Google Scholar] [CrossRef]
- Reggente, M.; Kriegel, S.; He, W.; Masson, P.; Pourroy, G.; Mura, F.; Faerber, J.; Passeri, D.; Rossi, M.; Palkowski, H.; et al. How alkali-activated Ti surfaces affect the growth of tethered PMMA chains: A close-up study on the PMMA thickness and surface morphology. Pure Appl. Chem. 2019, 91, 1687–1694. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Baboian, R. (Ed.) Corrosion Tests and Standards: Application and Interpretation; ASTM International: West Conshohocken, PA, USA, 2005; Volume 20. [Google Scholar]
- Jarzębska, A.; Bieda, M.; Maj, Ł.; Chulist, R.; Wojtas, D.; Strąg, M.; Sułkowski, B.; Przybysz, S.; Pachla, W.; Sztwiertnia, K. Controlled Grain Refinement of Biodegradable Zn-Mg Alloy: The Effect of Magnesium Alloying and Multi-Pass Hydrostatic Extrusion Preceded by Hot Extrusion. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 6784–6796. [Google Scholar] [CrossRef]
- Ye, L.; Huang, H.; Sun, C.; Zhuo, X.; Dong, Q.; Liu, H.; Ju, J.; Xue, F.; Bai, J.; Jiang, J. Effect of grain size and volume fraction of eutectic structure on mechanical properties and corrosion behavior of as-cast Zn–Mg binary alloys. J. Mater. Res. Technol. 2022, 16, 1673–1685. [Google Scholar] [CrossRef]
- Minet, I.; Delhalle, J.; Hevesi, L.; Mekhalif, Z. Surface-initiated ATRP of PMMA, PS and diblock PS-b-PMMA copolymers from stainless steel modified by 11-(2-bromoisobutyrate)-undecyl-1-phosphonic acid. J. Colloid Interface Sci. 2009, 332, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Luschtinetz, R.; Seifert, G.; Jaehne, E.; Adler, H.-J.P. Infrared Spectra of Alkylphosphonic Acid Bound to Aluminium Surfaces. Macromol. Symp. 2007, 254, 248–253. [Google Scholar] [CrossRef]
- El-Mahallawy, N.; Palkowski, H.; Breitinger, H.-G.; Klingner, A.; Shoeib, M.; Diaa, A. Microstructure, mechanical properties, cytotoxicity, and bio-corrosion of micro-alloyed Mg–xSn–0.04Mn alloys for biodegradable orthopedic applications: Effect of processing techniques. J. Mater. Res. 2021, 36, 1456–1474. [Google Scholar] [CrossRef]
- Dong, H.; Lin, F.; Boccaccini, A.R.; Virtanen, S. Corrosion behavior of biodegradable metals in two different simulated physiological solutions: Comparison of Mg, Zn and Fe. Corros. Sci. 2021, 182, 109278. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zheng, R.-N.; Zheng, Y.-Q.; Chen, L.-S. Microstructure characteristics, degradation behaviors and film formation mechanism of Zn-1Mg-0.25Nd-xSn in the Kokubo’s electrolyte. Mater. Charact. 2021, 174, 111034. [Google Scholar] [CrossRef]
- Hybasek, V.; Kubasek, J.; Capek, J.; Alferi, D.; Pinc, J.; Jiru, J.; Fojt, J. Influence of model environment complexity on corrosion mechanism of biodegradable zinc alloys. Corros. Sci. 2021, 187, 109520. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.; Shen, D.; Cheng, Y.; Chen, D.; Zheng, Y. Exploring the biodegradation of pure Zn under simulated inflammatory condition. Corros. Sci. 2021, 189, 109606. [Google Scholar] [CrossRef]
- Huang, S.; Wu, W.; Su, Y.; Qiao, L.; Yan, Y. Insight into the corrosion behaviour and degradation mechanism of pure zinc in simulated body fluid. Corros. Sci. 2020, 178, 109071. [Google Scholar] [CrossRef]
- N’Diaye, M.; Pascaretti-Grizon, F.; Massin, P.; Baslé, M.F.; Chappard, D. Water Absorption of Poly(methyl methacrylate) Measured by Vertical Interference Microscopy. Langmuir 2012, 28, 11609–11614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Kent, D.; Zhan, H.; Doan, N.; Dargusch, M.; Wang, G. Dynamic recrystallization of pure zinc during high strain-rate compression at ambient temperature. Mater. Sci. Eng. A 2020, 784, 139325. [Google Scholar] [CrossRef]
- Pachla, W.; Przybysz, S.; Jarzębska, A.; Bieda, M.; Sztwiertnia, K.; Kulczyk, M.; Skiba, J. Structural and mechanical aspects of hypoeutectic Zn–Mg binary alloys for biodegradable vascular stent applications. Bioact. Mater. 2021, 6, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zhang, D.; Lin, J.; Dai, Y.; Luan, Y.; Sun, Q.; Shi, Z.; Wang, K.; Gao, Y.; Lin, J.; et al. Development of biodegradable Zn–1Mg–0.1RE (RE = Er, Dy, and Ho) alloys for biomedical applications. Acta Biomater. 2020, 117, 384–399. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Xiong, P.; Li, W.; Huang, H.-H.; Zheng, Y. Comparative studies of Tris-HCl, HEPES and NaHCO3/CO2 buffer systems on the biodegradation behaviour of pure Zn in NaCl and SBF solutions. Corros. Sci. 2019, 157, 205–219. [Google Scholar] [CrossRef]
- Huang, S.; Chen, L.; Jiang, B.; Qiao, L.; Yan, Y. Early electrochemical characteristics and corrosion behaviors of pure zinc in simulated body fluid. J. Electroanal. Chem. 2021, 886, 115145. [Google Scholar] [CrossRef]
- Guo, P.; Zhu, X.; Yang, L.; Deng, L.; Zhang, Q.; Li, B.Q.; Cho, K.; Sun, W.; Ren, T.; Song, Z. Ultrafine- and uniform-grained biodegradable Zn-0.5Mn alloy: Grain refinement mechanism, corrosion behavior, and biocompatibility in vivo. Mater. Sci. Eng. C 2021, 118, 111391. [Google Scholar] [CrossRef]






| Alloy | Tafel Fitting | EIS Circuit Fitting | |||||
|---|---|---|---|---|---|---|---|
| Βa | Βc | Ecorr | Icorr | Corr. Rate | Rs | Rct | |
| (V/Dec) | (V/Dec) | (V) | (μA/cm2) | (mm·y−1) | Ω·cm2 | Ω·cm2 | |
| Pure Zn | 0.46 ± 0.02 | 0.08 ± 0.01 | −0.89 ± 0.00 | 0.4 ± 0.1 | 0.12 ± 0.03 | 160 ± 24 | 840 ± 35 |
| Zn-1.89Mg | 0.34 ± 0.02 | 0.12 ± 0.04 | −1.22 ± 0.01 | 1.2 ± 0.3 | 0.37 ± 0.14 | 195 ± 22 | 546 ± 15 |
| PMMA Coated | 0.32 ± 0.03 | 0.10 ± 0.01 | −1.00 ± 0.01 | 1.1 ± 0.1 | 0.22 ± 0.01 | 55 ± 10 | 692 ± 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaa, A.A.; El-Mahallawy, N.; Shoeib, M.; Lallemand, N.; Mouillard, F.; Masson, P.; Carradò, A. Effect of Mg Addition and PMMA Coating on the Biodegradation Behaviour of Extruded Zn Material. Materials 2023, 16, 707. https://doi.org/10.3390/ma16020707
Diaa AA, El-Mahallawy N, Shoeib M, Lallemand N, Mouillard F, Masson P, Carradò A. Effect of Mg Addition and PMMA Coating on the Biodegradation Behaviour of Extruded Zn Material. Materials. 2023; 16(2):707. https://doi.org/10.3390/ma16020707
Chicago/Turabian StyleDiaa, Alia A., Nahed El-Mahallawy, Madiha Shoeib, Nicolas Lallemand, Flavien Mouillard, Patrick Masson, and Adele Carradò. 2023. "Effect of Mg Addition and PMMA Coating on the Biodegradation Behaviour of Extruded Zn Material" Materials 16, no. 2: 707. https://doi.org/10.3390/ma16020707






