Biomechanical Assessment of Endodontically Treated Molars Restored by Endocrowns Made from Different CAD/CAM Materials
Abstract
:1. Introduction
2. Materials and Methods
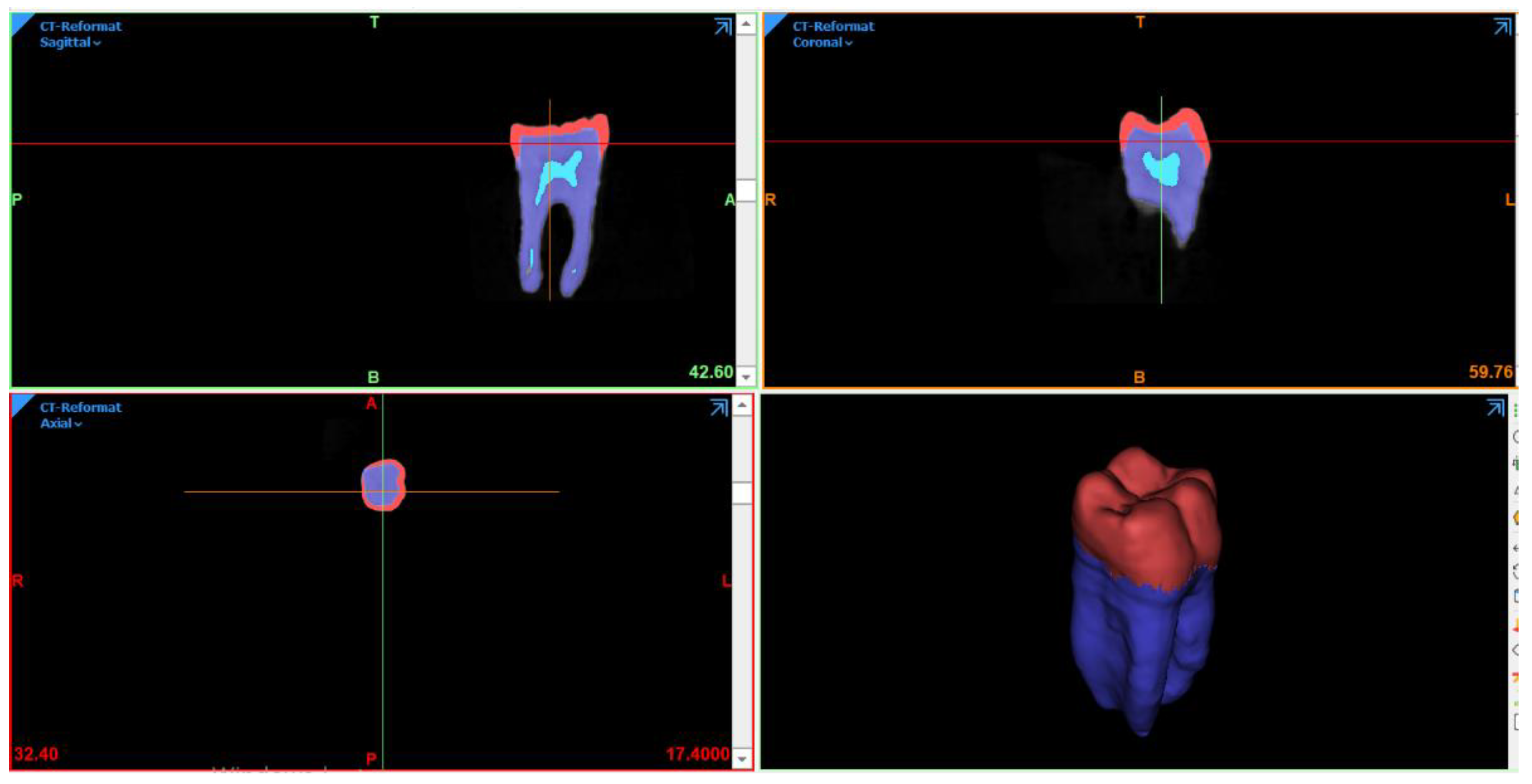
2.1. FEA Modeling
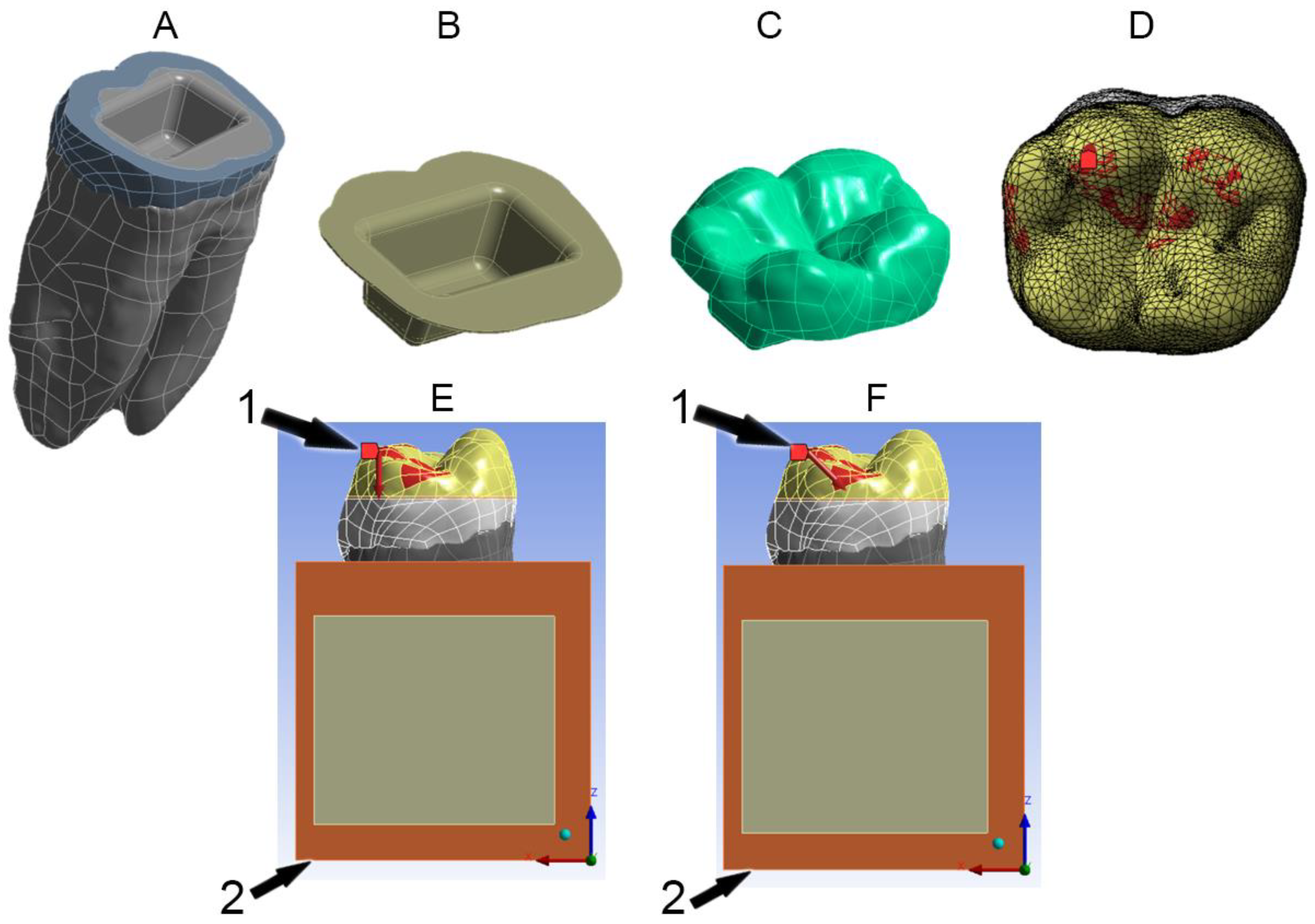
2.2. Preparation Design
2.3. Material Properties
2.4. Boundary Conditions
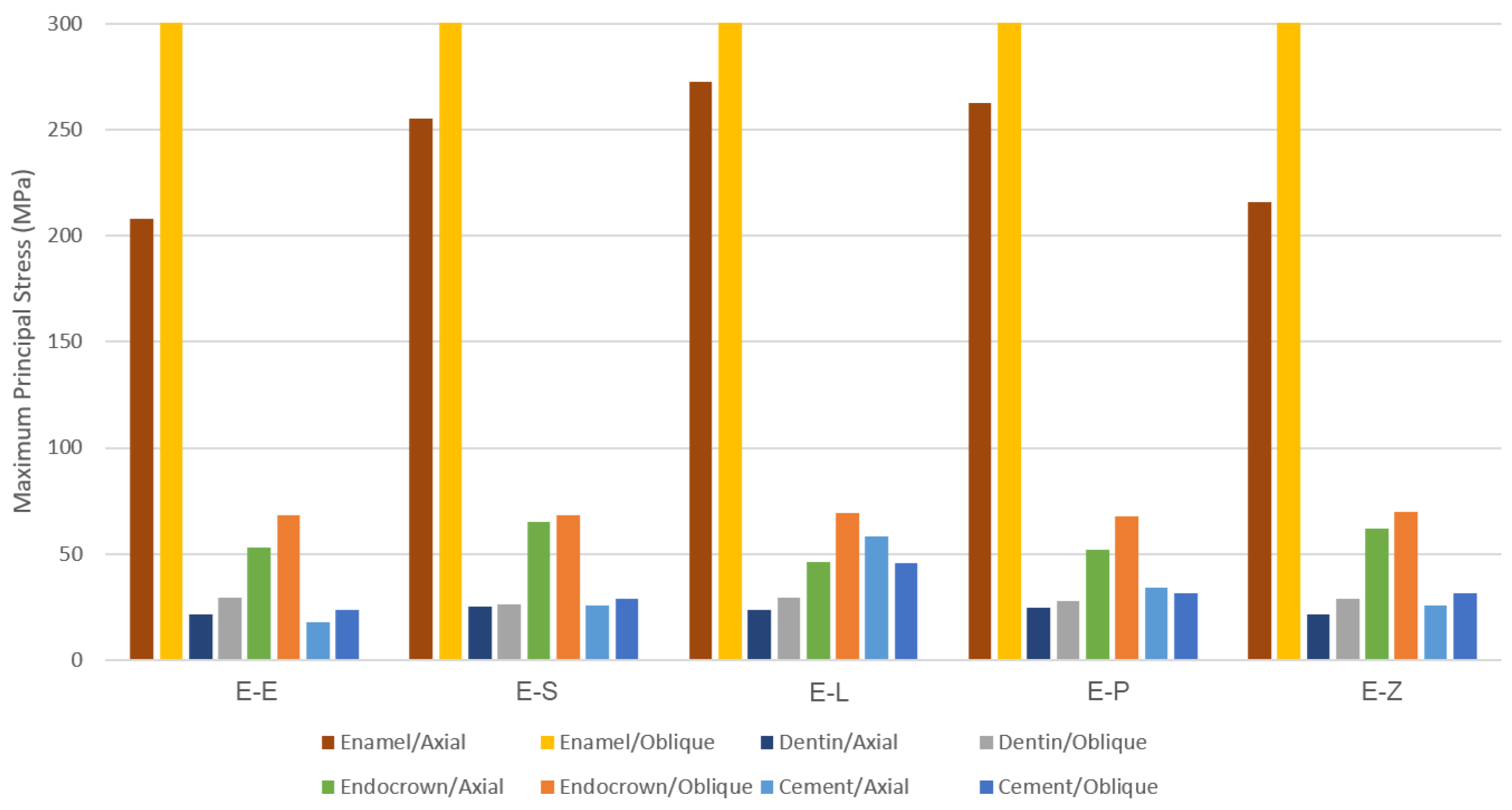
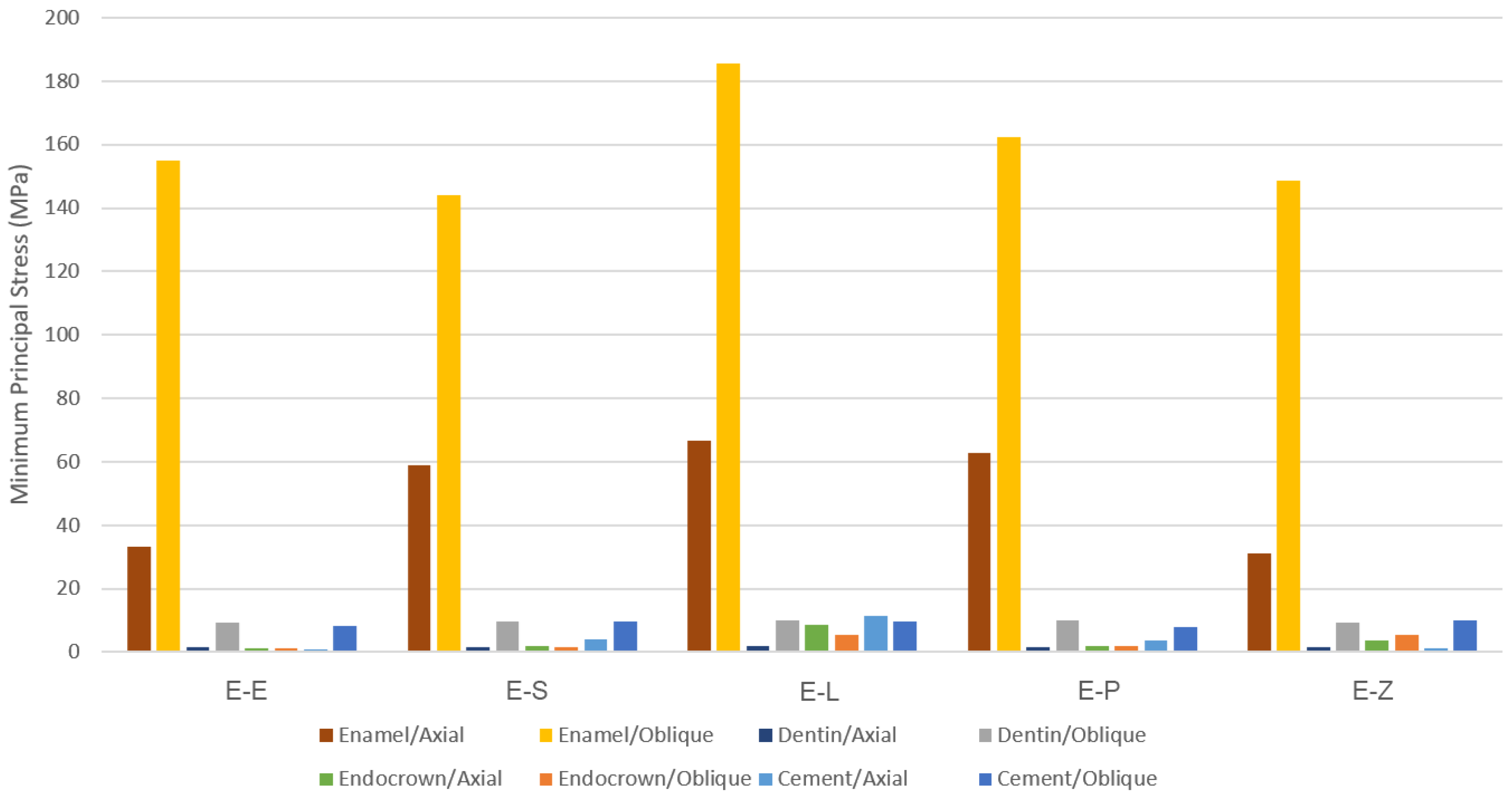
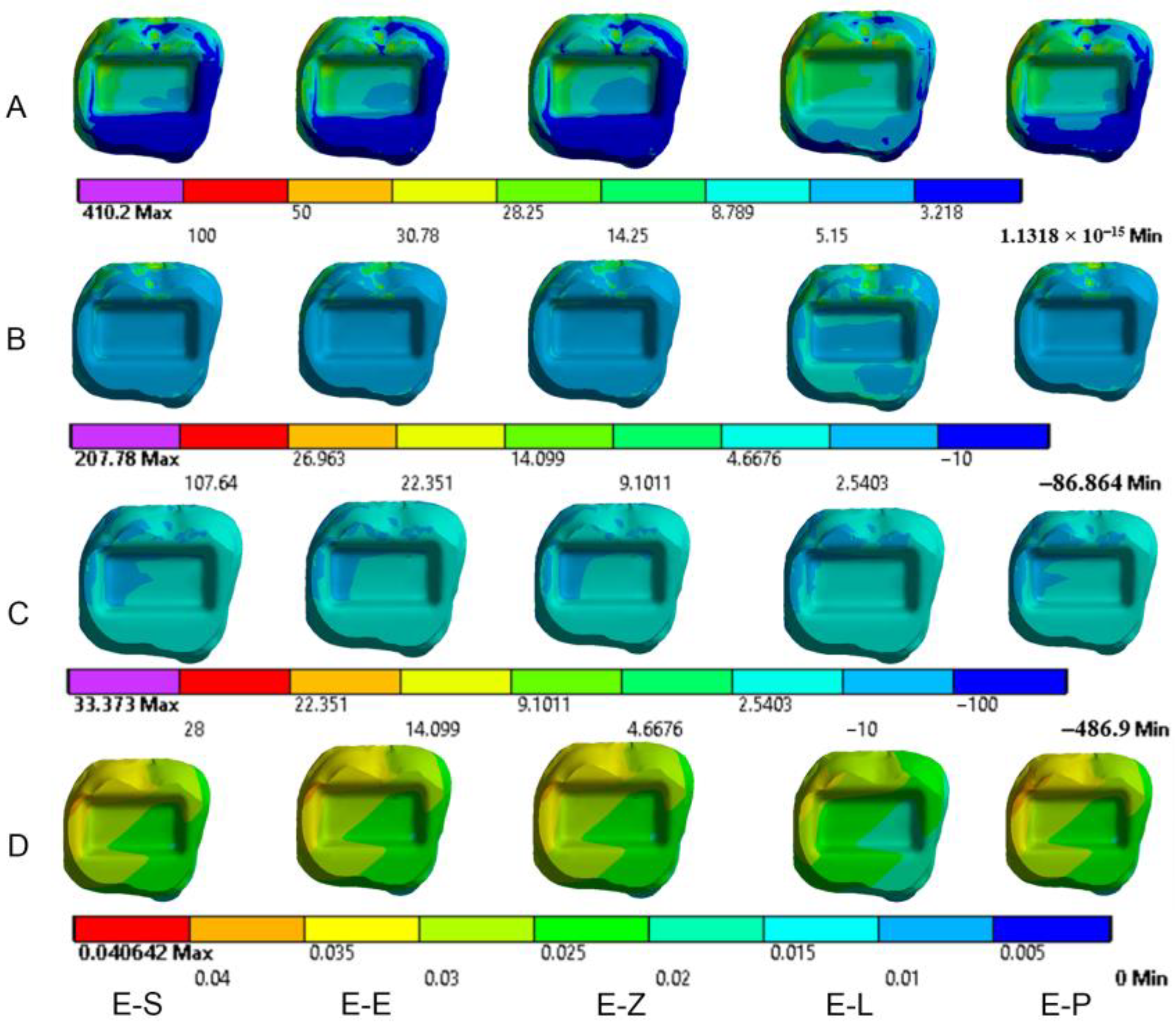
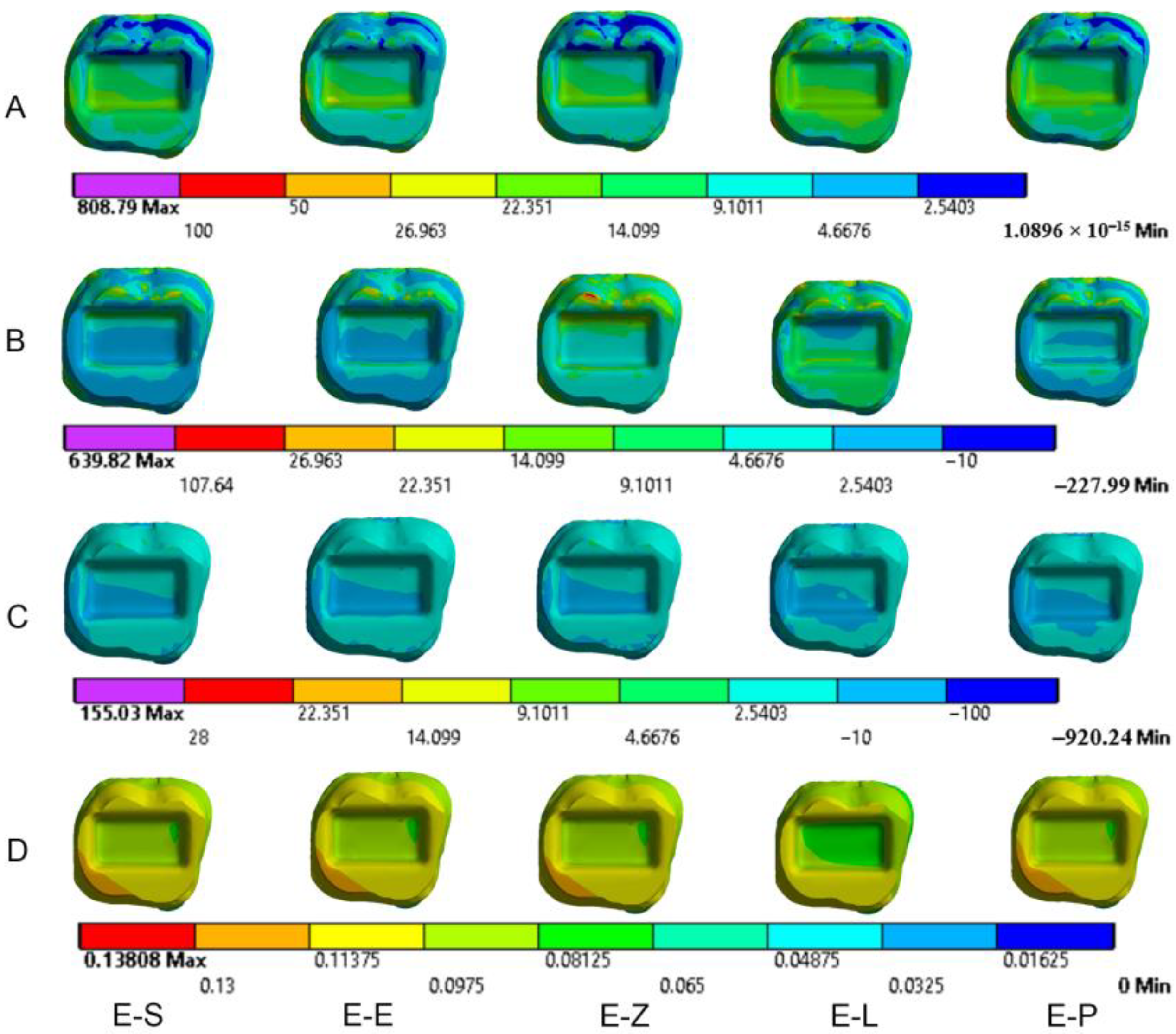
3. Results
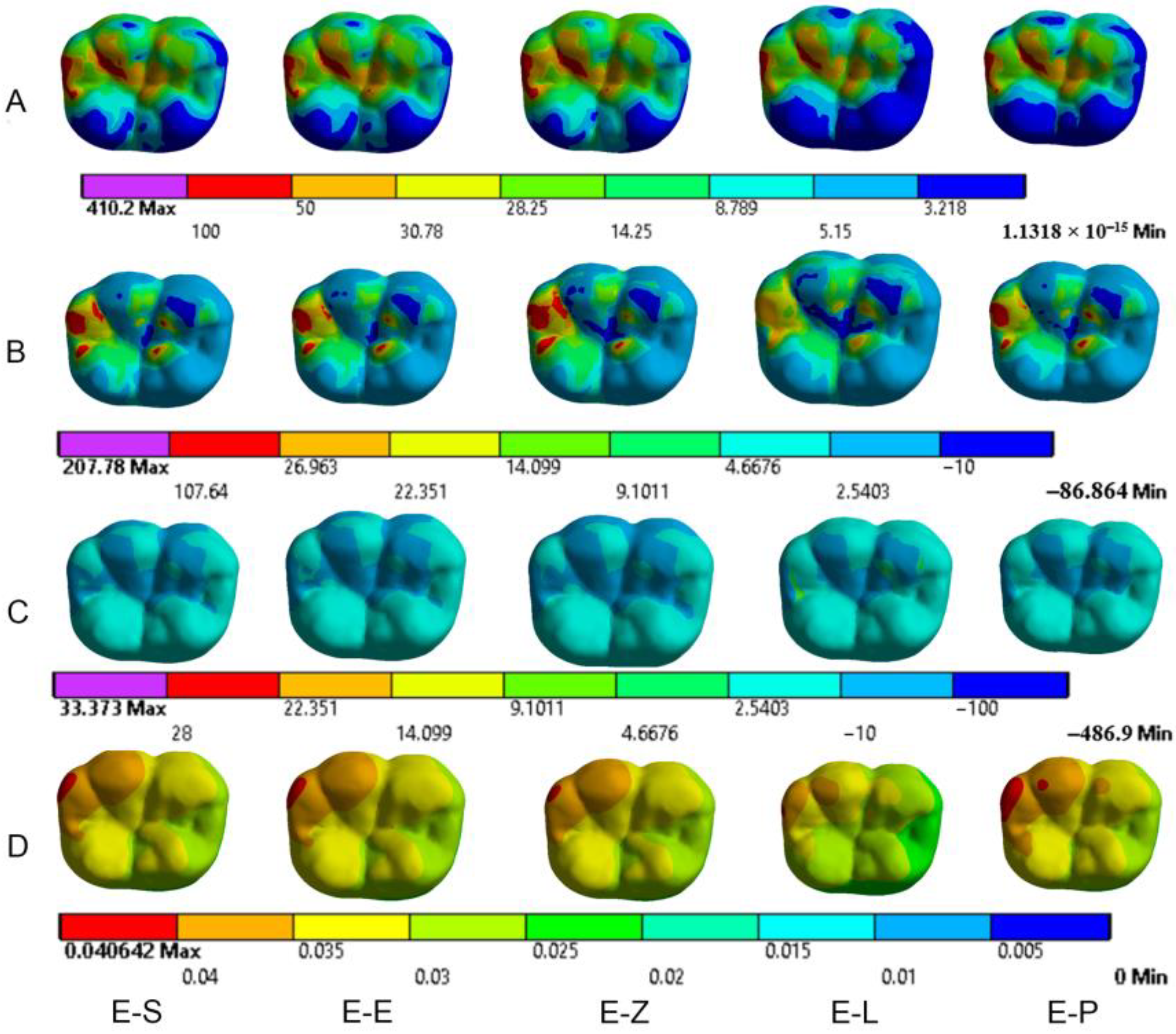
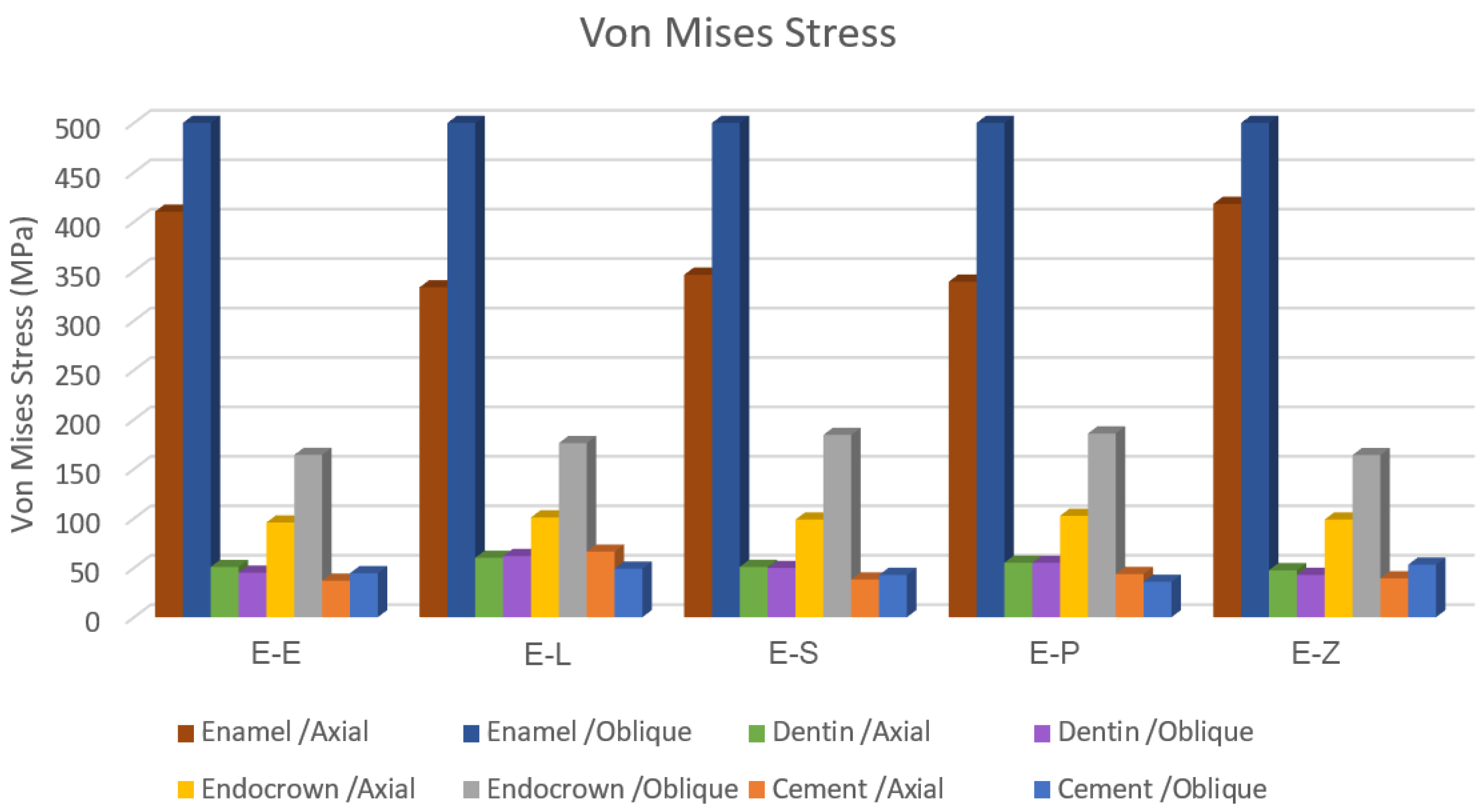
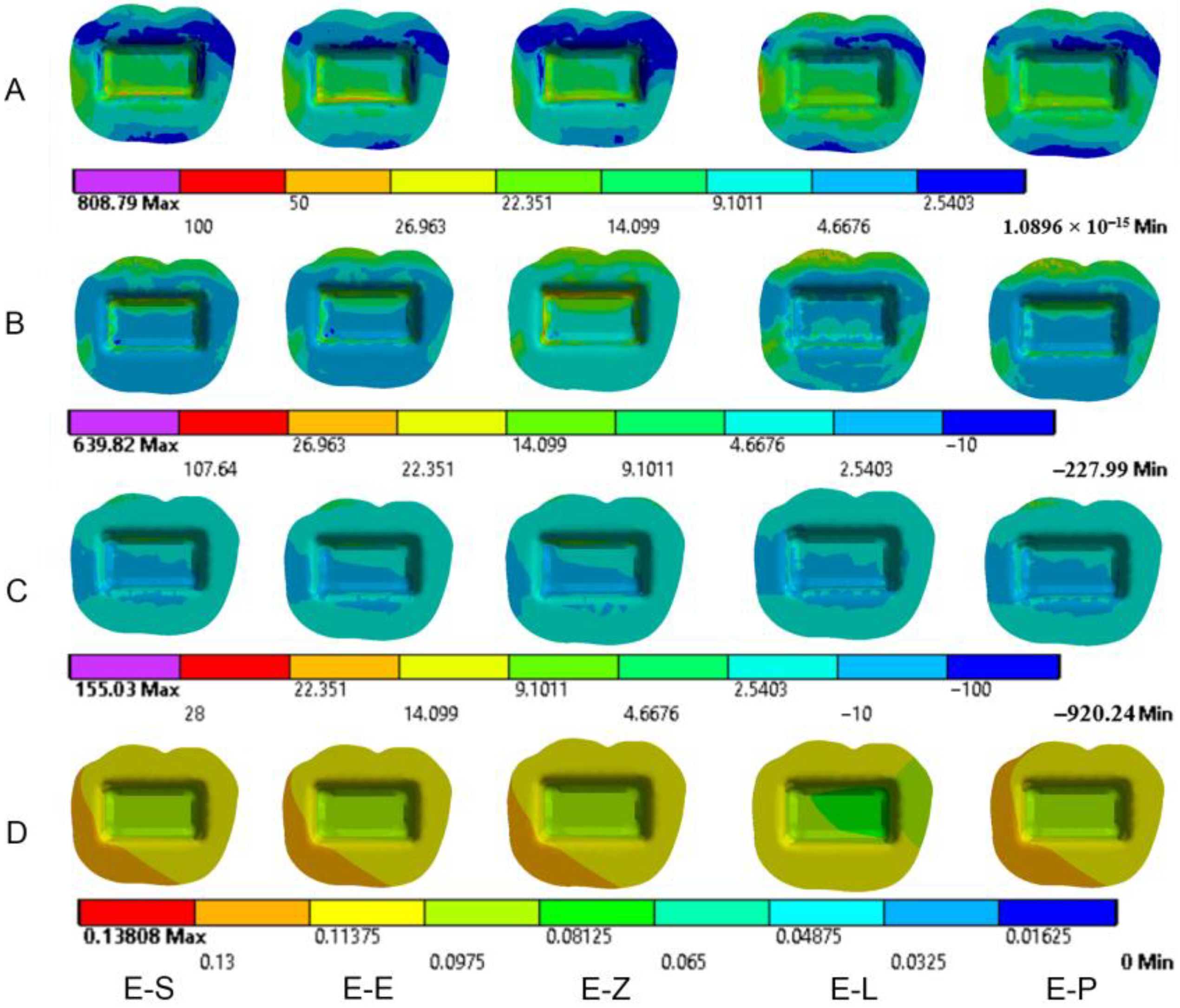
3.1. Endocrowns
3.2. Dental Structures
3.3. Cement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, R.S.; Robbins, J.W. Post Placement and Restoration of Endodontically Treated Teeth: A Literature Review. J. Endod. 2004, 30, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Dong, X.-Y.; He, S.; Pan, X.; Tang, L. Effect of Post Placement on the Restoration of Endodontically Treated Teeth: A Systematic Review. Int. J. Prosthodont. 2015, 28, 475–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedrez-Porto, J.A.; da Rosa, W.L.D.O.; da Silva, A.F.; Münchow, E.A.; Pereira-Cenci, T. Endocrown Restorations: A Systematic Review and Meta-Analysis. J. Dent. 2016, 52, 8–14. [Google Scholar] [CrossRef]
- Dartora, N.R.; Maurício Moris, I.C.; Poole, S.F.; Bacchi, A.; Sousa-Neto, M.D.; Silva-Sousa, Y.T.; Gomes, E.A. Mechanical Behavior of Endocrowns Fabricated with Different CAD-CAM Ceramic Systems. J. Prosthet. Dent. 2021, 125, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Taha, D.; Spintzyk, S.; Sabet, A.; Wahsh, M.; Salah, T. Assessment of Marginal Adaptation and Fracture Resistance of Endocrown Restorations Utilizing Different Machinable Blocks Subjected to Thermomechanical Aging. J. Esthet. Restor. Dent. 2018, 30, 319–328. [Google Scholar] [CrossRef]
- Biacchi, G.R.; Basting, R.T. Comparison of Fracture Strength of Endocrowns and Glass Fiber Post-Retained Conventional Crowns. Oper. Dent. 2012, 37, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Belleflamme, M.M.; Geerts, S.O.; Louwette, M.M.; Grenade, C.F.; Vanheusden, A.J.; Mainjot, A.K. No Post-No Core Approach to Restore Severely Damaged Posterior Teeth: An up to 10-Year Retrospective Study of Documented Endocrown Cases. J. Dent. 2017, 63, 1–7. [Google Scholar] [CrossRef]
- El-Damanhoury, H.M.; Haj-Ali, R.N.; Platt, J.A. Fracture Resistance and Microleakage of Endocrowns Utilizing Three CAD-CAM Blocks. Oper. Dent. 2015, 40, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Lin, Z.; Zheng, Z. Effect of Different Restorative Crown Design and Materials on Stress Distribution in Endodontically Treated Molars: A Finite Element Analysis Study. BMC Oral. Health 2020, 20, 226. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.A.; Wang, Z. Biomechanical Assessment of Restored Mandibular Molar by Endocrown in Comparison to a Glass Fiber Post-Retained Conventional Crown: 3D Finite Element Analysis: Mandibular Molar Endocrown Biomechanical Behavior: 3D FEA. J. Prosthodont. 2019, 28, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Albero, A.; Pascual, A.; Camps, I.; Grau-Benitez, M. Comparative Characterization of a Novel Cad-Cam Polymer-Infiltrated-Ceramic-Network. J. Clin. Exp. Dent. 2015, 7, e495–e500. [Google Scholar] [CrossRef] [PubMed]
- Kanat-Ertürk, B.; Saridağ, S.; Köseler, E.; Helvacioğlu-Yiğit, D.; Avcu, E.; Yildiran-Avcu, Y. Fracture Strengths of Endocrown Restorations Fabricated with Different Preparation Depths and CAD/CAM Materials. Dent. Mater. J. 2018, 37, 256–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schatz, C.; Strickstrock, M.; Roos, M.; Edelhoff, D.; Eichberger, M.; Zylla, I.-M.; Stawarczyk, B. Influence of Specimen Preparation and Test Methods on the Flexural Strength Results of Monolithic Zirconia Materials. Materials 2016, 9, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Kaizer, M.R.; Zhang, Y. Load-Bearing Capacity of Lithium Disilicate and Ultra-Translucent Zirconias. J. Mech. Behav. Biomed. Mater. 2018, 88, 170–175. [Google Scholar] [CrossRef]
- Ghodsi, S.; Jafarian, Z. A Review on Translucent Zirconia. Eur. J. Prosthodont. Restor. Dent. 2018, 26, 62–74. [Google Scholar] [CrossRef]
- Ivoclar Vivadent, Liechtenstein, IPS e.max CAD. IPS e.max CAD, Scientific Documentation. Available online: http://www.ivoclarvivadent.com/en/dental-professional-/ips-emax-cad-for-programill (accessed on 14 December 2020).
- Mclaren, E.A.; Cao, P.T. Ceramics in Dentistry-Part I: Classes of Materials. Inside Dent. 2009, 5, 94–103. [Google Scholar]
- VITA ENAMIC, Vita Zahnfabrik, Germany, Technical and Scientific Documentation. Available online: https://www.vita-zahnfabrik.com/en/VITA-ENAMIC-24970.html (accessed on 21 March 2018).
- VITA SUPRINITY® PC, Vita Zahnfabrik, Germany, Technical and scientific documentation. Available online: https://www.vita-zahnfabrik.com/en/VITA-SUPRINITY-PC-44049.html (accessed on 4 March 2022).
- 3M, ESPE, Lava Ultimate, USA. Lava™ Ultimate CAD/CAM Restorative, Technical Product Profile. Available online: https://www.3m.com/3M/en_US/p/d/b00008161/ (accessed on 4 March 2022).
- Gracis, S.; Thompson, V.P.; Ferencz, J.L.; Silva, N.R.; Bonfante, E.A. A new classification system for all-ceramic and ceramic-like restorative materials. Int. J. Prosthodont. 2015, 28, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Aktas, G.; Yerlikaya, H.; Akca, K. Mechanical Failure of Endocrowns Manufactured with Different Ceramic Materials: An in Vitro Biomechanical Study: Mechanical Failure of Endocrowns. J. Prosthodont. 2018, 27, 340–346. [Google Scholar] [CrossRef]
- Sahebi, M.; Ghodsi, S.; Berahman, P.; Amini, A.; Zeighami, S. Comparison of Retention and Fracture Load of Endocrowns Made from Zirconia and Zirconium Lithium Silicate after Aging: An in Vitro Study. BMC Oral. Health 2022, 22, 41. [Google Scholar] [CrossRef]
- Hassouneh, L.; Jum’ah, A.A.; Ferrari, M.; Wood, D.J. Post-Fatigue Fracture Resistance of Premolar Teeth Restored with Endocrowns: An in Vitro Investigation. J. Dent. 2020, 100, 103426. [Google Scholar] [CrossRef]
- Haralur, S.B.; Alamrey, A.A.; Alshehri, S.A.; Alzahrani, D.S.; Alfarsi, M. Effect of Different Preparation Designs and All Ceramic Materials on Fracture Strength of Molar Endocrowns. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020947329. [Google Scholar] [CrossRef] [PubMed]
- Meijer, H.J.; Kuiper, J.H.; Starmans, F.J.; Bosman, F. Stress Distribution around Dental Implants: Influence of Superstructure, Length of Implants, and Height of Mandible. J. Prosthet. Dent. 1992, 68, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Menicucci, G.; Mossolov, A.; Mozzati, M.; Lorenzetti, M.; Preti, G. Tooth-Implant Connection: Some Biomechanical Aspects Based on Finite Element Analyses: Biomechanical Aspects of Tooth-Implant Connection. Clin. Oral. Implant. Res. 2002, 13, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Zeighami, S.; Ghodsi, S. Comparison of Marginal and Internal Adaptation in Endocrowns Milled from Translucent Zirconia and Zirconium Lithium Silicate. Int. J. Dent. 2021, 2021, 1544067. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Bai, J.; Xiang, J. Clinical Performance of CAD/CAM-Fabricated Monolithic Zirconia Endocrowns on Molars with Extensive Coronal Loss of Substance. Int. J. Comput. Dent. 2018, 21, 225–232. [Google Scholar]
- Fages, M.; Bennasar, B. The Endocrown: A Different Type of All-Ceramic Reconstruction for Molars. J. Can. Dent. Assoc. 2013, 79, d140. [Google Scholar]
- Tribst, J.P.M.; Dal Piva, A.M.D.O.; de Jager, N.; Bottino, M.A.; de Kok, P.; Kleverlaan, C.J. Full-Crown versus Endocrown Approach: A 3D-Analysis of Both Restorations and the Effect of Ferrule and Restoration Material. J. Prosthodont. 2021, 30, 335–344. [Google Scholar] [CrossRef]
- Sakaguchi, R.L.; Powers, J.M. Craig’s Restorative Dental Materials, 13th ed.; Elsevier: Mosby, MO, USA, 2012. [Google Scholar]
- Dejak, B.; Mlotkowski, A. Three-Dimensional Finite Element Analysis of Strength and Adhesion of Composite Resin versus Ceramic Inlays in Molars. J. Prosthet. Dent. 2008, 99, 131–140. [Google Scholar] [CrossRef]
- Weinstein, A.M.; Klawitter, J.J.; Cook, S.D. Implant-Bone Interface Characteristics of Bioglass Dental Implants. J. Biomed. Mater. Res. 1980, 14, 23–29. [Google Scholar] [CrossRef]
- Köycü, B.Ç.; Imirzalioğlu, P.; Oezden, U.A. Three-Dimensional Finite Element Analysis of Stress Distribution in Inlay-Restored Mandibular First Molar under Simultaneous Thermomechanical Loads. Dent. Mater. J. 2016, 35, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Holmes, D.C.; Diaz-Arnold, A.M.; Leary, J.M. Influence of Post Dimension on Stress Distribution in Dentin. J. Prosthet. Dent. 1996, 75, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Cousland, G.P.; Cui, X.Y.; Smith, A.E.; Stampfl, A.P.J.; Stampfl, C.M. Mechanical Properties of Zirconia, Doped and Undoped Yttria-Stabilized Cubic Zirconia from First-Principles. J. Phys. Chem. Solids 2018, 122, 51–71. [Google Scholar] [CrossRef]
- Belli, R.; Wendler, M.; de Ligny, D.; Cicconi, M.R.; Petschelt, A.; Peterlik, H.; Lohbauer, U. Chairside CAD/CAM Materials. Part 1: Measurement of Elastic Constants and Microstructural Characterization. Dent. Mater. 2017, 33, 84–98. [Google Scholar] [CrossRef]
- Zheng, Z.; He, Y.; Ruan, W.; Ling, Z.; Zheng, C.; Gai, Y.; Yan, W. Biomechanical Behavior of Endocrown Restorations with Different CAD-CAM Materials: A 3D Finite Element and in Vitro Analysis. J. Prosthet. Dent. 2021, 125, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Gulec, L.; Ulusoy, N. Effect of Endocrown Restorations with Different CAD/CAM Materials: 3D Finite Element and Weibull Analyses. Biomed. Res. Int. 2017, 2017, 5638683. [Google Scholar] [CrossRef] [Green Version]
- Mei, M.L.; Chen, Y.M.; Li, H.; Chu, C.H. Influence of the Indirect Restoration Design on the Fracture Resistance: A Finite Element Study. Biomed. Eng. Online 2016, 15, 3. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.; Xavier, T.; Noritomi, P.; Saavedra, G.; Borges, A. The Influence of Elastic Modulus of Inlay Materials on Stress Distribution and Fracture of Premolars. Oper. Dent. 2014, 39, E160–E170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dartora, G.; Rocha Pereira, G.K.; Varella de Carvalho, R.; Zucuni, C.P.; Valandro, L.F.; Cesar, P.F.; Caldas, R.A.; Bacchi, A. Comparison of Endocrowns Made of Lithium Disilicate Glass-Ceramic or Polymer-Infiltrated Ceramic Networks and Direct Composite Resin Restorations: Fatigue Performance and Stress Distribution. J. Mech. Behav. Biomed. Mater. 2019, 100, 103401. [Google Scholar] [CrossRef]
- Sarkar, R. Investigation of Structural Integrity of Normal& Extreme Loading Conditions. Master’s Thesis, Homi Bhabha National Institute, Mumbai, India, 2012. [Google Scholar]
- Govare, N.; Contrepois, M. Endocrowns: A Systematic Review. J. Prosthet. Dent. 2020, 123, 411–418.e9. [Google Scholar] [CrossRef]
- Ausiello, P.; Rengo, S.; Davidson, C.L.; Watts, D.C. Stress Distributions in Adhesively Cemented Ceramic and Resin-Composite Class II Inlay Restorations: A 3D-FEA Study. Dent. Mater. 2004, 20, 862–872. [Google Scholar] [CrossRef]
- Gresnigt, M.M.M.; Özcan, M.; van den Houten, M.L.A.; Schipper, L.; Cune, M.S. Fracture Strength, Failure Type and Weibull Characteristics of Lithium Disilicate and Multiphase Resin Composite Endocrowns under Axial and Lateral Forces. Dent. Mater. 2016, 32, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Hasan, I.; Frentzen, M.; Utz, K.-H.; Hoyer, D.; Langenbach, A.; Bourauel, C. Finite Element Analysis of Adhesive Endo-Crowns of Molars at Different Height Levels of Buccally Applied Load. J. Dent. Biomech. 2012, 3, 1758736012455421. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; Iserte-Vilar, J.L.; González-Lluch, C. Interpreting Finite Element Results for Brittle Materials in Endodontic Restorations. Biomed. Eng. Online 2011, 10, 44. [Google Scholar] [CrossRef]
- Holberg, C.; Winterhalder, P.; Wichelhaus, A.; Hickel, R.; Huth, K. Fracture Risk of Lithium-Disilicate Ceramic Inlays: A Finite Element Analysis. Dent. Mater. 2013, 29, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.O.; Xavier, T.A.; Platt, J.A.; Borges, A.L.S. Effect of Base and Inlay Restorative Material on the Stress Distribution and Fracture Resistance of Weakened Premolars. Oper. Dent. 2015, 40, E158–E166. [Google Scholar] [CrossRef] [PubMed]



| Model | Material | Abbreviation | Chemical Composition (%wt) * | Example | Manufacturer |
|---|---|---|---|---|---|
| E-E | Lithium disilicate glass ceramic | LDS | SiO2 (80.0), Li2O (19), K2O (13), P2O5 (11), ZrO2 (8), ZnO (8), Al2O3 (5), MgO (5) and coloring oxides | IPS e.max CAD | Ivoclar Vivadent GmbH, Germany |
| E-P | Polymer-infiltrated ceramic network | PICN | Ceramic part (86 wt%): SiO2 (63), Al2O3 (23), Na2O (6), B2O3 (2), ZrO2 (<1), CaO (<1). Polymer part (14 wt%): UDMA and TEGDMA | VITA Enamic | VITA Zahnfabrik, Germany |
| E-S | Zirconia-reinforced lithium silicate glass ceramic | ZLS | SiO2 (64), Li2O (21), K2O (4), P2O5 (8), Al2O3 (4), ZrO2 (12), CeO2 (4), La2O3 (0.1) and pigments (6) | VITA Suprinity | VITA Zahnfabrik, Germany |
| E-L | Resin nanoceramic | RNC | Nanomer and nanocluster fillers (nanoceramic material 80% wt). Nanoclusters (0.6–10 µm) of 20 nm silica and zirconia 4–11 nm. | Lava Ultimate | 3M ESPE, USA |
| E-Z | Translucent zirconia | - | ZrO2, HfO2, Y2O3 (> 90), Al2O3 (<0.5) and other oxides (≤1) ** | DD Bio zx2 | Dental Direkt GmbH, Germany |
| Model * | Elements | Nodes |
|---|---|---|
| E-E | 56,274 | 110,644 |
| E-L | 64,796 | 126,213 |
| E-S | 64,796 | 126,213 |
| E-P | 64,796 | 126,213 |
| E-Z | 50,626 | 100,495 |
| Material | Young’s Modulus (GPa) | Poisson Ratio | UTS * (MPa) | UCS ** (MPa) |
|---|---|---|---|---|
| Enamel | 84 | 0.33 | - | - |
| Dentin | 18.6 | 0.30 | - | - |
| Periodontal ligament | 0.069 | 0.45 | - | - |
| Resin cement | 8.3 | 0.35 | 45 | 178 |
| Cortical bone | 13.7 | 0.30 | - | - |
| Trabecular bone | 1.37 | 0.30 | - | - |
| Gutta percha | 0.69 | 0.45 | - | - |
| Translucent zirconia | 210 | 0.307 | 745 | 904 |
| Zirconia-reinforced lithium silicate ceramic | 102.9 | 0.208 | 459 ‡ | 676 ‡ |
| Lithium disilicate glass ceramic | 83 | 0.21 | 173 | 448 |
| Polymer-infiltrated network ceramic | 30 | 0.23 | 100 | 370 |
| Resin nanoceramic | 12.7 | 0.47 | 100 | 516 |
| Model | Axial Loading | Oblique Loading |
|---|---|---|
| E-E | 0.30 | 0.39 |
| E-S ** | 0.14 | 0.15 |
| E-L | 0.48 | 0.70 |
| E-P | 0.52 | 0.68 |
| E-Z | 0.08 | 0.09 |
| Model | Axial Loading | Oblique Loading |
|---|---|---|
| E-E | 0.40 | 0.57 |
| E-S | 0.59 | 0.69 |
| E-L | 1.35 | 1.06 |
| E-P | 0.78 | 0.73 |
| E-Z | 0.57 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwich, M.A.; Aljareh, A.; Alhouri, N.; Szávai, S.; Nazha, H.M.; Duvigneau, F.; Juhre, D. Biomechanical Assessment of Endodontically Treated Molars Restored by Endocrowns Made from Different CAD/CAM Materials. Materials 2023, 16, 764. https://doi.org/10.3390/ma16020764
Darwich MA, Aljareh A, Alhouri N, Szávai S, Nazha HM, Duvigneau F, Juhre D. Biomechanical Assessment of Endodontically Treated Molars Restored by Endocrowns Made from Different CAD/CAM Materials. Materials. 2023; 16(2):764. https://doi.org/10.3390/ma16020764
Chicago/Turabian StyleDarwich, Mhd Ayham, Abeer Aljareh, Nabil Alhouri, Szabolcs Szávai, Hasan Mhd Nazha, Fabian Duvigneau, and Daniel Juhre. 2023. "Biomechanical Assessment of Endodontically Treated Molars Restored by Endocrowns Made from Different CAD/CAM Materials" Materials 16, no. 2: 764. https://doi.org/10.3390/ma16020764






