Amino-Acid-Substituted Perylene Diimide as the Organic Cathode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of PDI_AAs
2.4. Assembly of Coin Cells
2.5. Computational Calculation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Lou, Z.; Jiang, K.; Shen, G. Device Configurations and Future Prospects of Flexible/Stretchable Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1805596. [Google Scholar] [CrossRef]
- Ba, Z.; Wang, Z.; Luo, M.; Li, H.B.; Li, Y.; Huang, T.; Dong, J.; Zhang, Q.; Zhao, X. Benzoquinone-Based Polyimide Derivatives as High-Capacity and Stable Organic Cathodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, W.; Son, D.Y.; Ono, L.K.; Qi, Y. Lithium-Ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Wang, K.; Wan, J.; Xiang, Y.; Zhu, J.; Leng, Q.; Wang, M.; Xu, L.; Yang, Y. Recent Advances and Historical Developments of High Voltage Lithium Cobalt Oxide Materials for Rechargeable Li-Ion Batteries. J. Power Sources 2020, 460, 228062. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, B.; Lu, Y. Progress and Perspective of High-Voltage Lithium Cobalt Oxide in Lithium-Ion Batteries. J. Energy Chem. 2022, 74, 283–308. [Google Scholar] [CrossRef]
- Sun, T.; Xie, J.; Guo, W.; Li, D.S.; Zhang, Q. Covalent–Organic Frameworks: Advanced Organic Electrode Materials for Rechargeable Batteries. Adv. Energy Mater. 2020, 10, 1904199. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wang, H.; Yao, C.J.; Nie, H.J.; Wang, K.Z.; Zhong, Y.W.; Chen, P.; Mei, S.; Zhang, Q.; et al. Recent Progress in Carbonyl-Based Organic Polymers as Promising Electrode Materials for Lithium-Ion Batteries (LIBs). J. Mater. Chem. A 2020, 8, 11906–11922. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Q. Recent Progress in Rechargeable Lithium Batteries with Organic Materials as Promising Electrodes. J. Mater. Chem. A 2016, 4, 7091–7106. [Google Scholar] [CrossRef]
- Shadike, Z.; Tan, S.; Wang, Q.C.; Lin, R.; Hu, E.; Qu, D.; Yang, X.Q. Review on Organosulfur Materials for Rechargeable Lithium Batteries. Mater. Horizons 2021, 8, 471–500. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yin, Y.J.; Hei, J.P.; Wan, X.J.; Li, M.L.; Cui, Y. Molecular Engineering of Aromatic Imides for Organic Secondary Batteries. Small 2021, 17, 2005752. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Sun, X.-G.; Dai, S. Organic Cathode Materials for Lithium-Ion Batteries: Past, Present, and Future. Adv. Energy Sustain. Res. 2021, 2, 2000044. [Google Scholar] [CrossRef]
- Wang, X.; Tang, W.; Hu, Y.; Liu, W.; Yan, Y.; Xu, L.; Fan, C. Insoluble Small-Molecule Organic Cathodes for Highly Efficient Pure-Organic Li-Ion Batteries. Green Chem. 2021, 23, 6090–6100. [Google Scholar] [CrossRef]
- Han, C.; Li, H.; Shi, R.; Zhang, T.; Tong, J.; Li, J.; Li, B. Organic Quinones towards Advanced Electrochemical Energy Storage: Recent Advances and Challenges. J. Mater. Chem. A 2019, 7, 23378–23415. [Google Scholar] [CrossRef]
- Häupler, B.; Wild, A.; Schubert, U.S. Carbonyls: Powerful Organic Materials for Secondary Batteries. Adv. Energy Mater. 2015, 5, 1402034. [Google Scholar] [CrossRef]
- Yokoji, T.; Matsubara, H.; Satoh, M. Rechargeable Organic Lithium-Ion Batteries Using Electron-Deficient Benzoquinones as Positive-Electrode Materials with High Discharge Voltages. J. Mater. Chem. A 2014, 2, 19347–19354. [Google Scholar] [CrossRef] [Green Version]
- Renault, S.; Geng, J.; Dolhem, F.; Poizot, P. Evaluation of Polyketones with N-Cyclic Structure as Electrode Material for Electrochemical Energy Storage: Case of Pyromellitic Diimide Dilithium Salt. Chem. Commun. 2011, 47, 2414–2416. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, R.; Nan, J.; Shu, D.; Qiu, Y.; Chou, S.L. Quinone Electrode Materials for Rechargeable Lithium/Sodium Ion Batteries. Adv. Energy Mater. 2017, 7, 1700278. [Google Scholar] [CrossRef]
- Tang, M.; Li, H.; Wang, E.; Wang, C. Carbonyl Polymeric Electrode Materials for Metal-Ion Batteries. Chin. Chem. Lett. 2018, 29, 232–244. [Google Scholar] [CrossRef]
- Zu, Y.; Xu, Y.; Ma, L.; Kang, Q.; Yao, H.; Hou, J. Carbonyl Bridge-Based p-ΠConjugated Polymers as High-Performance Electrodes of Organic Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 18457–18464. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gong, L.; Liu, X.; Zhang, P.; Li, B.; Qi, D.; Wang, K.; He, F.; Jiang, J. Mesoporous Polyimide-Linked Covalent Organic Framework with Multiple Redox-Active Sites for High-Performance Cathodic Li Storage. Angew. Chem. Int. Ed. 2022, 61, e202207043. [Google Scholar]
- Zhu, X.; Liu, X.; Deng, W.; Xiao, L.; Yang, H.; Cao, Y. Perylenediimide Dyes as a Cheap and Sustainable Cathode for Lithium Ion Batteries. Mater. Lett. 2016, 175, 191–194. [Google Scholar] [CrossRef]
- Sharma, P.; Damien, D.; Nagarajan, K.; Shaijumon, M.M.; Hariharan, M. Perylene-Polyimide-Based Organic Electrode Materials for Rechargeable Lithium Batteries. J. Phys. Chem. Lett. 2013, 4, 3192–3197. [Google Scholar] [CrossRef]
- Medabalmi, V.; Ramanujam, K. Glycination: A Simple Strategy to Enhance the Cycling Performance of Perylene Dianhydride for Secondary Li–Ion Battery Applications. ChemistrySelect 2018, 3, 10657–10662. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian Inc.: Wallingford, UK, 2016. [Google Scholar]
- Draper, E.R.; Walsh, J.J.; McDonald, T.O.; Zwijnenburg, M.A.; Cameron, P.J.; Cowan, A.J.; Adams, D.J. Air-Stable Photoconductive Films Formed from Perylene Bisimide Gelators. J. Mater. Chem. C 2014, 2, 5570–5575. [Google Scholar] [CrossRef] [Green Version]
- Muthuraj, B.; Chowdhury, S.R.; Iyer, P.K. Modulation of Amyloid-β Fibrils into Mature Microrod-Shaped Structure by Histidine Functionalized Water-Soluble Perylene Diimide. ACS Appl. Mater. Interfaces 2015, 7, 21226–21234. [Google Scholar] [CrossRef]
- Pandeeswar, M.; Govindaraju, T. Engineering Molecular Self-Assembly of Perylene Diimide through PH-Responsive Chiroptical Switching. Mol. Syst. Des. Eng. 2016, 1, 202–207. [Google Scholar] [CrossRef]
- Er, S.; Suh, C.; Marshak, M.P.; Aspuru-Guzik, A. Computational Design of Molecules for an All-Quinone Redox Flow Battery. Chem. Sci. 2015, 6, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Xue, Z.; Li, M.; Qiu, P.; Li, C.; Wang, S.; Yu, J.; Nara, H.; Na, J.; Yamauchi, Y. Electrochemical Activity of Nitrogen-Containing Groups in Organic Electrode Materials and Related Improvement Strategies. Adv. Energy Mater. 2021, 11, 2002523. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Shao, P.; Han, Y.; Gao, X.; Ma, L.; Yuan, S.; Ma, X.; Zhou, J.; Feng, X.; et al. Exfoliation of Covalent Organic Frameworks into Few-Layer Redox-Active Nanosheets as Cathode Materials for Lithium-Ion Batteries. J. Am. Chem. Soc. 2017, 139, 4258–4261. [Google Scholar] [CrossRef] [PubMed]
- Aher, J.; Graefenstein, A.; Deshmukh, G.; Subramani, K.; Krueger, B.; Haensch, M.; Schwenzel, J.; Krishnamoorthy, K.; Wittstock, G. Effect of Aromatic Rings and Substituent on the Performance of Lithium Batteries with Rylene Imide Cathodes. ChemElectroChem 2020, 7, 1160–1165. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Chen, M.; Chen, Y.; Xiao, W.; Chen, D.; Lin, M. The Impact of Vertical π-Extension on Redox Mechanisms of Aromatic Diimide Dyes. Chin. Chem. Lett. 2019, 30, 2254–2258. [Google Scholar] [CrossRef]
- Bhosale, M.E.; Krishnamoorthy, K. Chemically Reduced Organic Small-Molecule-Based Lithium Battery with Improved Efficiency. Chem. Mater. 2015, 27, 2121–2126. [Google Scholar] [CrossRef]
- Veerababu, M.; Kothandaraman, R. Rational Functionalization of Perylene Diimide for Stable Capacity and Long-Term Cycling Performance for Li-Ion Batteries. Electrochim. Acta 2017, 232, 244–253. [Google Scholar]
- Ju, Z.; King, S.T.; Xu, X.; Zhang, X.; Raigama, K.U.; Takeuchi, K.J.; Marschilok, A.C.; Wang, L.; Takeuchi, E.S.; Yu, G. Vertically Assembled Nanosheet Networks for High-Density Thick Battery Electrodes. Proc. Natl. Acad. Sci. USA 2022, 119, e2212779119. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Q.; Zhong, J.; Chen, M.; Deng, H.; Cao, J.; Wang, L.; Peng, L.; Zhu, J.; Lu, B. 3D Holey Graphene/Polyacrylonitrile Sulfur Composite Architecture for High Loading Lithium Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2100448. [Google Scholar] [CrossRef]
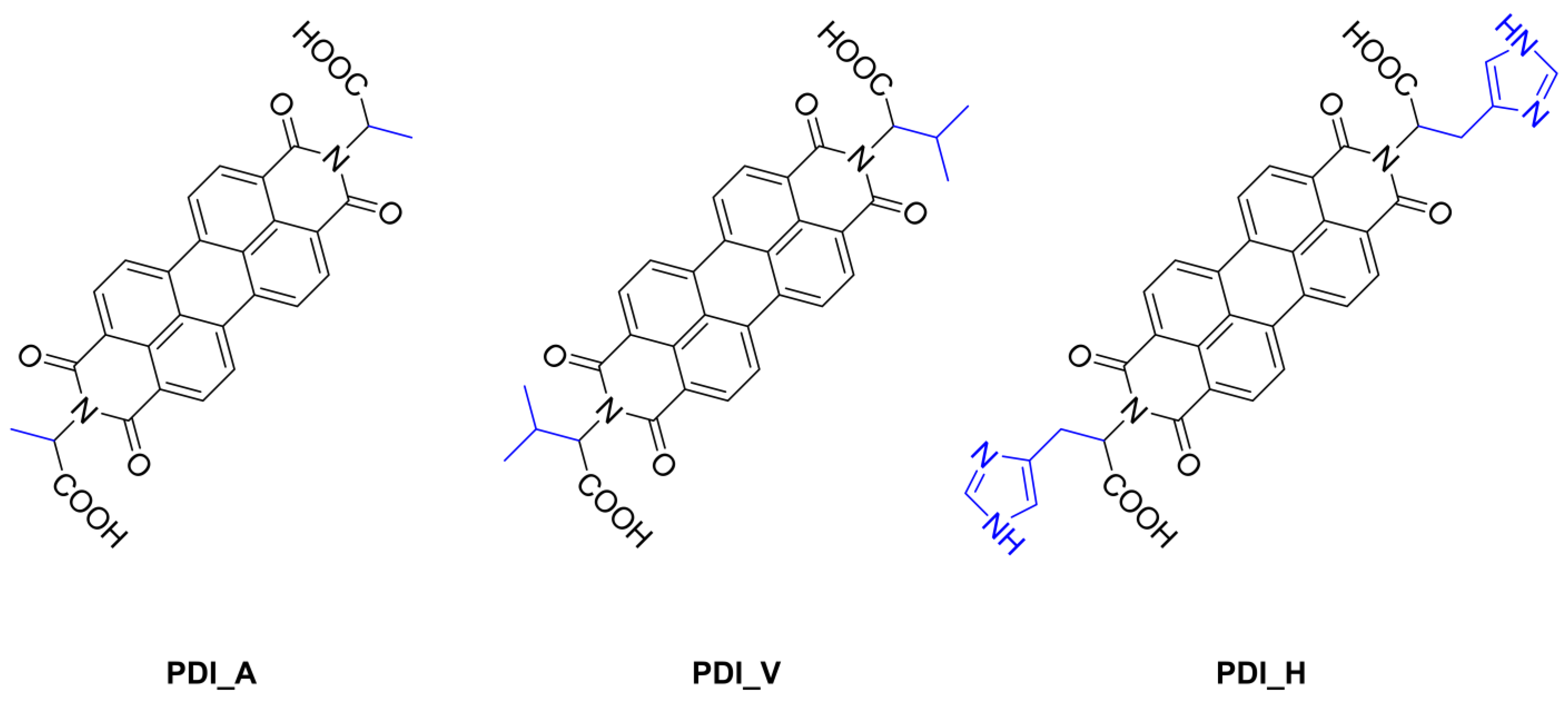

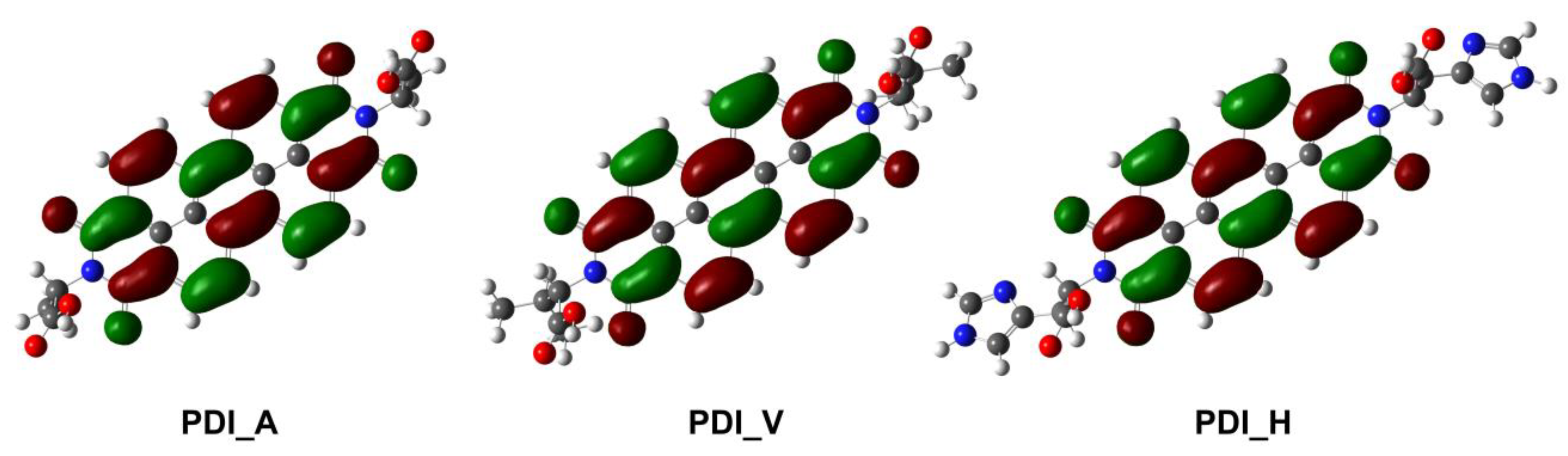
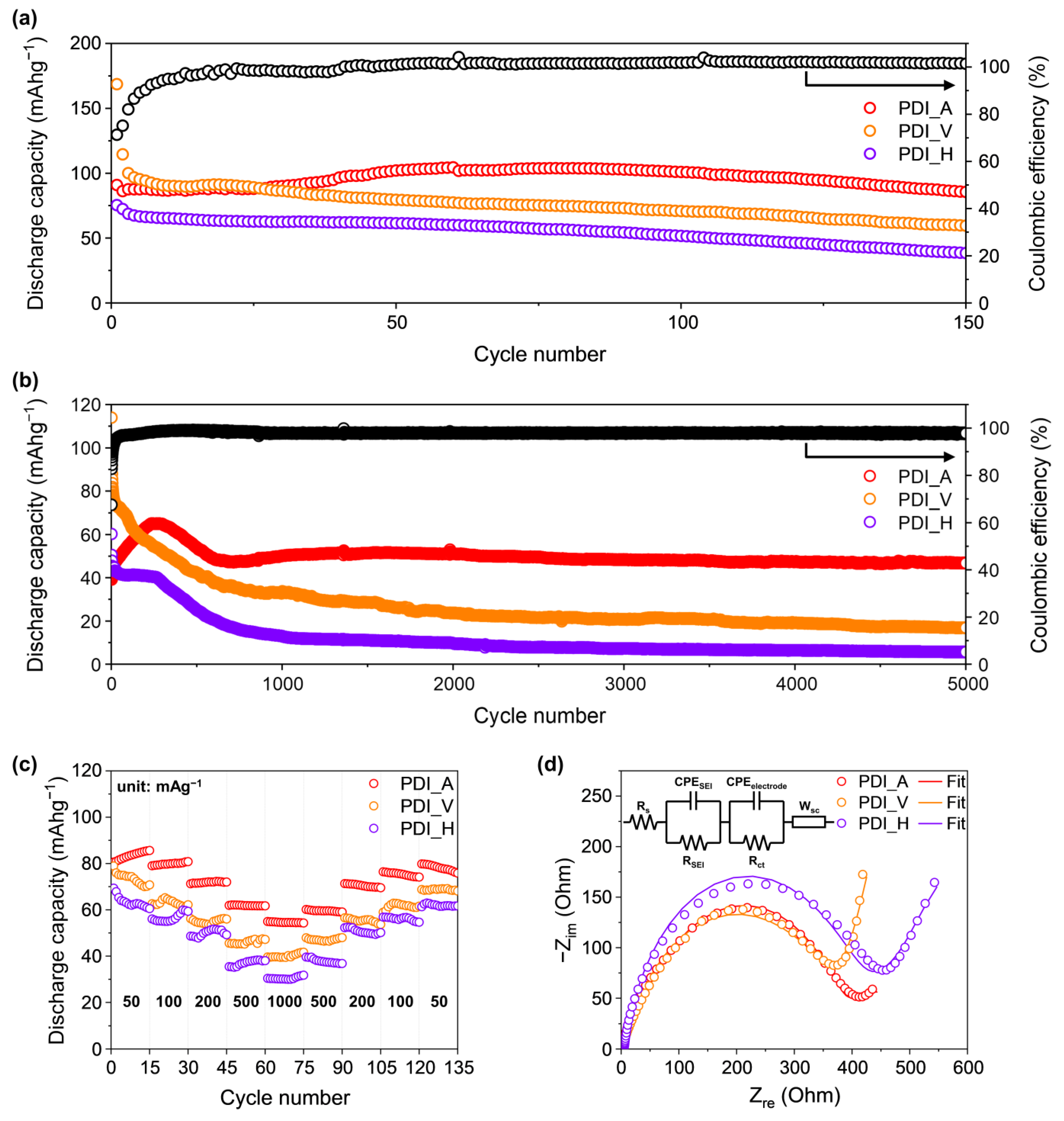

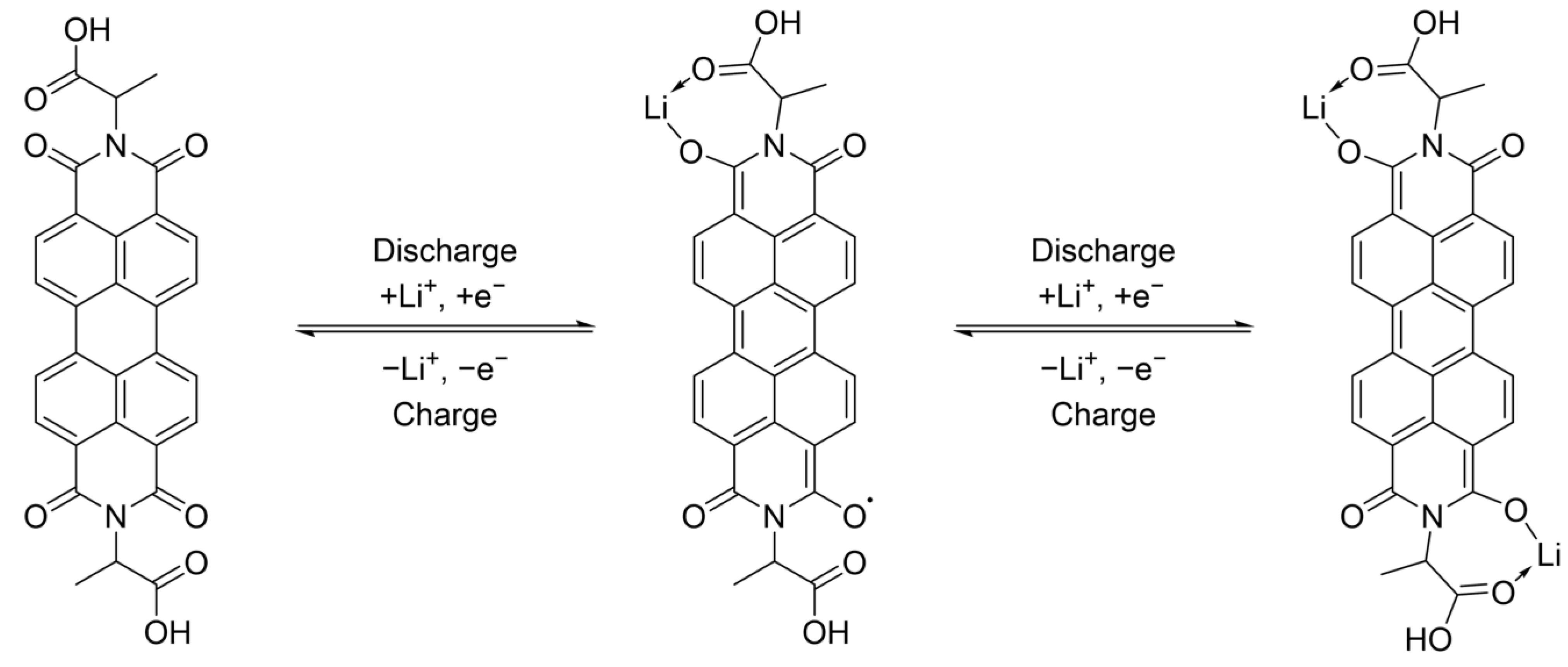
| PDI_A | PDI_V | PDI_H | |
|---|---|---|---|
| LUMO level (eV) | −3.99 | −3.97 | −3.88 |
| Discharge potential (V vs. Li/Li+) | 2.13 | 2.07 | 1.75 |
| Chemical Structure | Theoretical Capacity (mAhg−1) | Initial Discharge Capacity (mAhg−1) | Discharge Capacity (mAhg−1) /Cycle | Current Density (mAg−1) | Retention (%) | Reference |
|---|---|---|---|---|---|---|
 | 61 | 55 | 48/300th | 1 C = 61 | 87 | [33] |
 | 64.3 | 44.8 | 28.6/100th | 1 C = 64.3 | 64 | [34] |
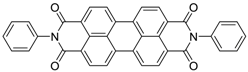 | 98 | 97 | 40/50th | 170 | 41 | [35] |
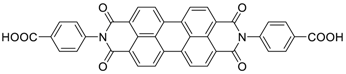 | 85 | 82 | 72/200th | 850 | 88 | [35] |
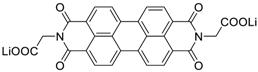 | 103 | 102 101 81 | 101/100th 92/150th 87/1000th | 17 100 200 | 99 91 >100 | [36] |
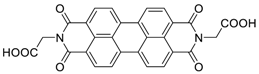 | 110 | 35 | 70/2000th | 200 | >100 | [25] |
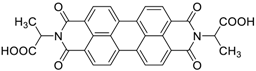 | 100 | 91 48 | 86/150th 47/5000th | 50 500 | 95 98 | This work |
| PDI_A | PDI_V | PDI_H | |
|---|---|---|---|
| Rct (Ω) | 338 | 376 | 394 |
| DLi (cm2s−1) | 4.55 × 10−11 | 8.18 × 10−9 | 8.32 × 10−12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, H.; Nam, W.; Kim, G.; Moon, J.H.; Jin, Y.; Kwon, S.-R.; Lee, J.-H.; Choi, J. Amino-Acid-Substituted Perylene Diimide as the Organic Cathode Materials for Lithium-Ion Batteries. Materials 2023, 16, 839. https://doi.org/10.3390/ma16020839
Seong H, Nam W, Kim G, Moon JH, Jin Y, Kwon S-R, Lee J-H, Choi J. Amino-Acid-Substituted Perylene Diimide as the Organic Cathode Materials for Lithium-Ion Batteries. Materials. 2023; 16(2):839. https://doi.org/10.3390/ma16020839
Chicago/Turabian StyleSeong, Honggyu, Wonbin Nam, Geongil Kim, Joon Ha Moon, Youngho Jin, Seung-Ryong Kwon, Joon-Hwa Lee, and Jaewon Choi. 2023. "Amino-Acid-Substituted Perylene Diimide as the Organic Cathode Materials for Lithium-Ion Batteries" Materials 16, no. 2: 839. https://doi.org/10.3390/ma16020839
APA StyleSeong, H., Nam, W., Kim, G., Moon, J. H., Jin, Y., Kwon, S.-R., Lee, J.-H., & Choi, J. (2023). Amino-Acid-Substituted Perylene Diimide as the Organic Cathode Materials for Lithium-Ion Batteries. Materials, 16(2), 839. https://doi.org/10.3390/ma16020839






