Effect of Cutting Fluid on Machined Surface Integrity and Corrosion Property of Nickel Based Superalloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Workpiece Material and Cutting Fluids
2.2. Milling Experiments
2.3. Electrochemical Tests
3. Results and Discussion
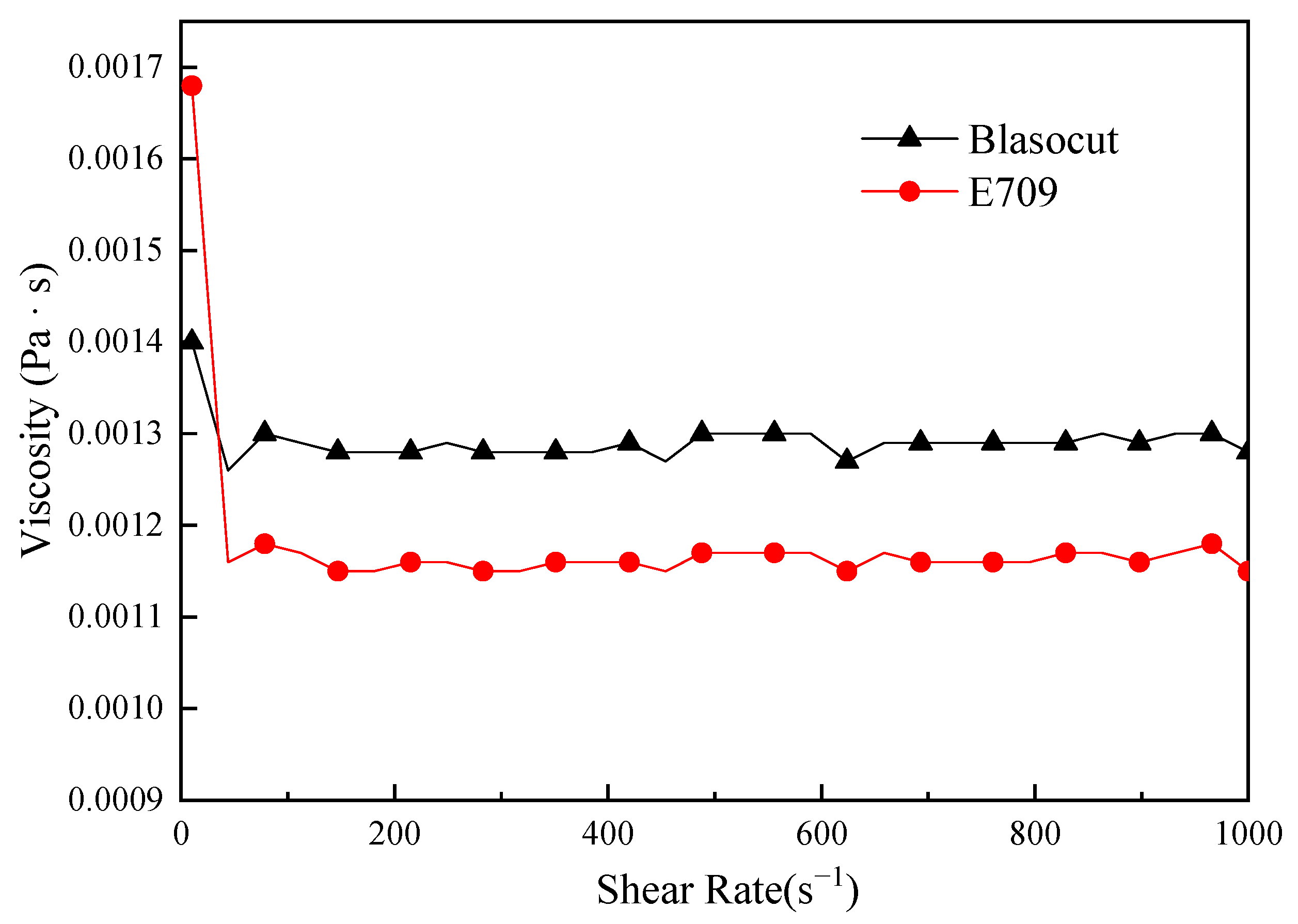
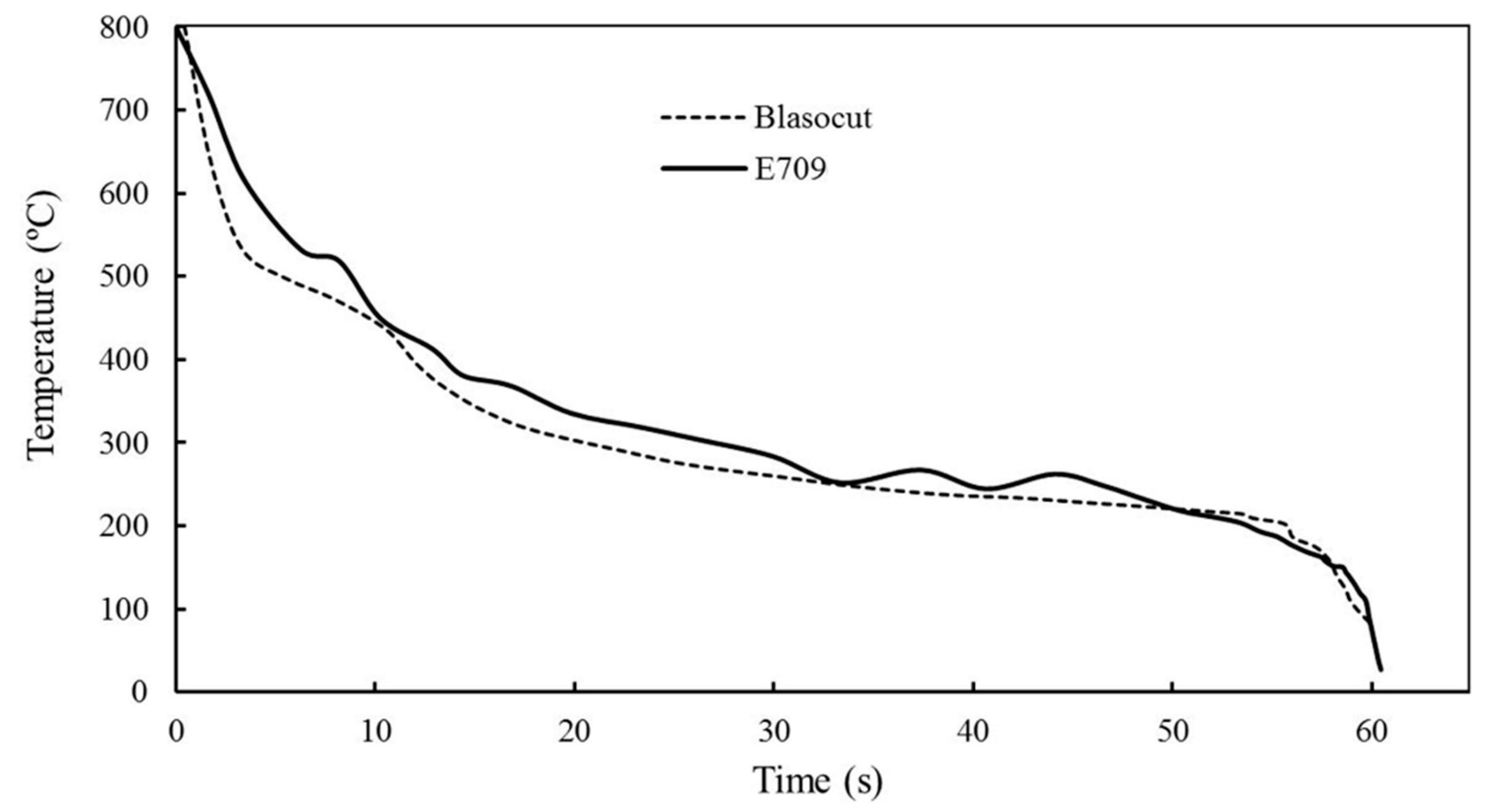
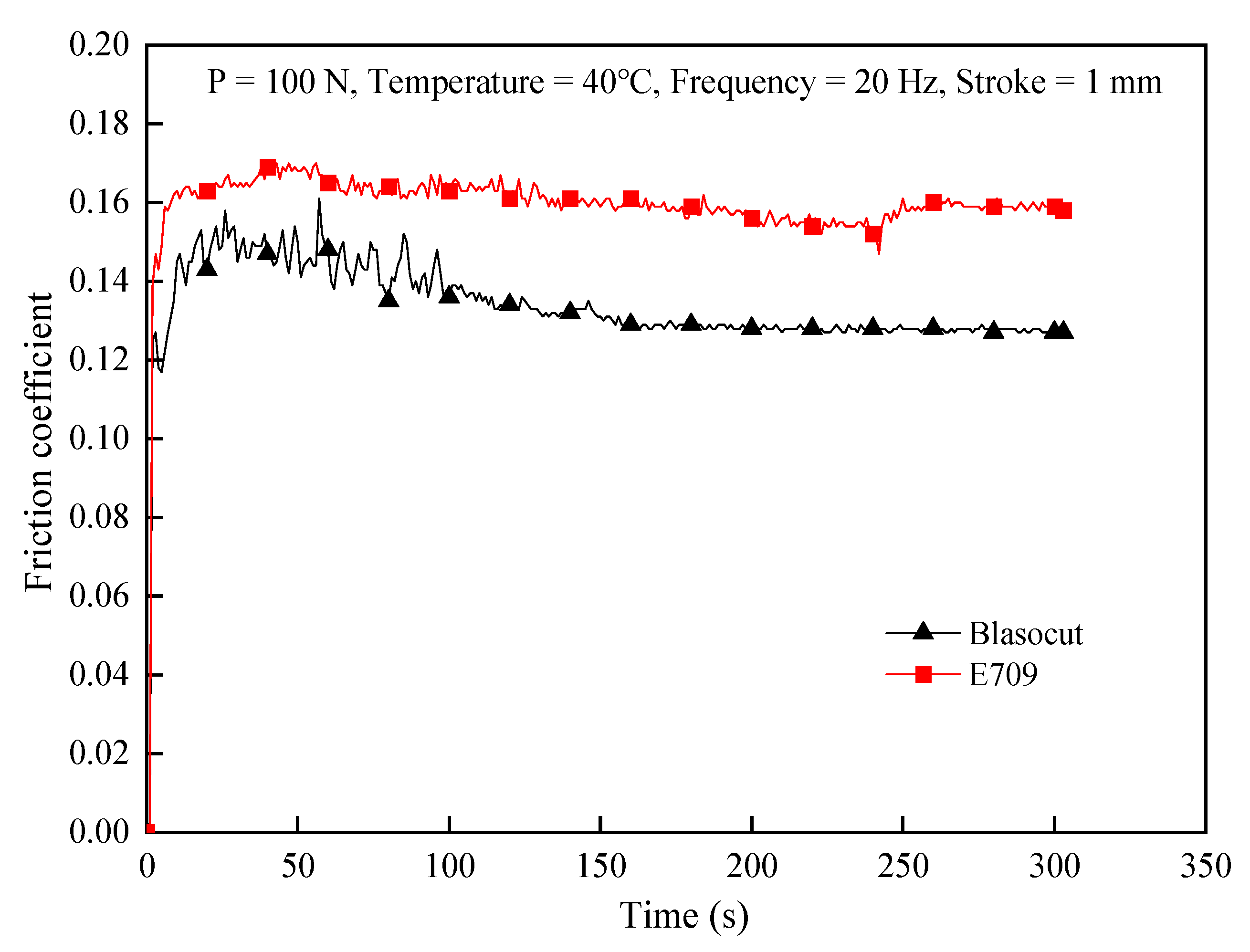
3.1. Physical & Chemical Properties of Cutting Fluids
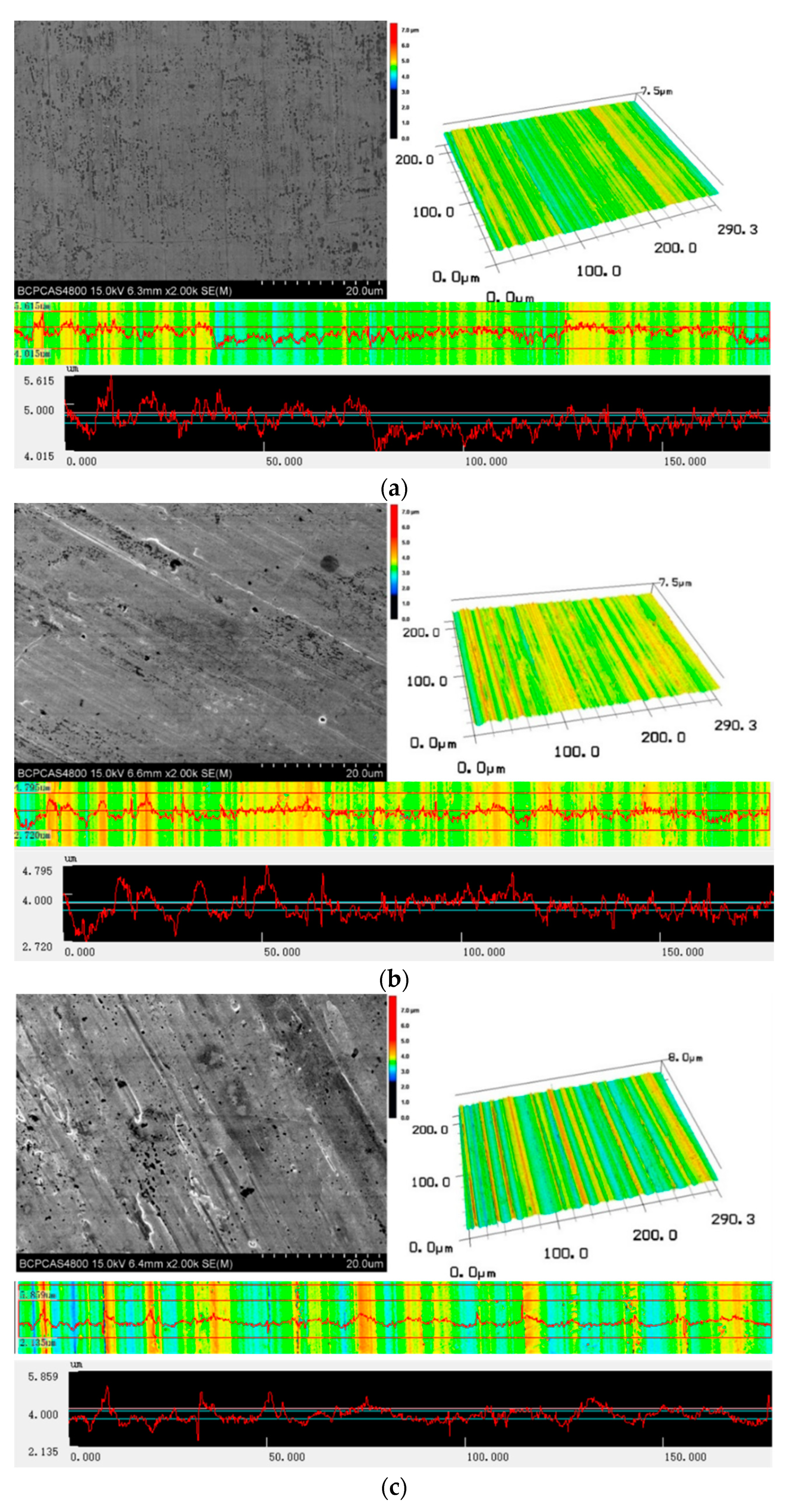
3.2. Surface Topography and Roughness
3.3. Residual Stress
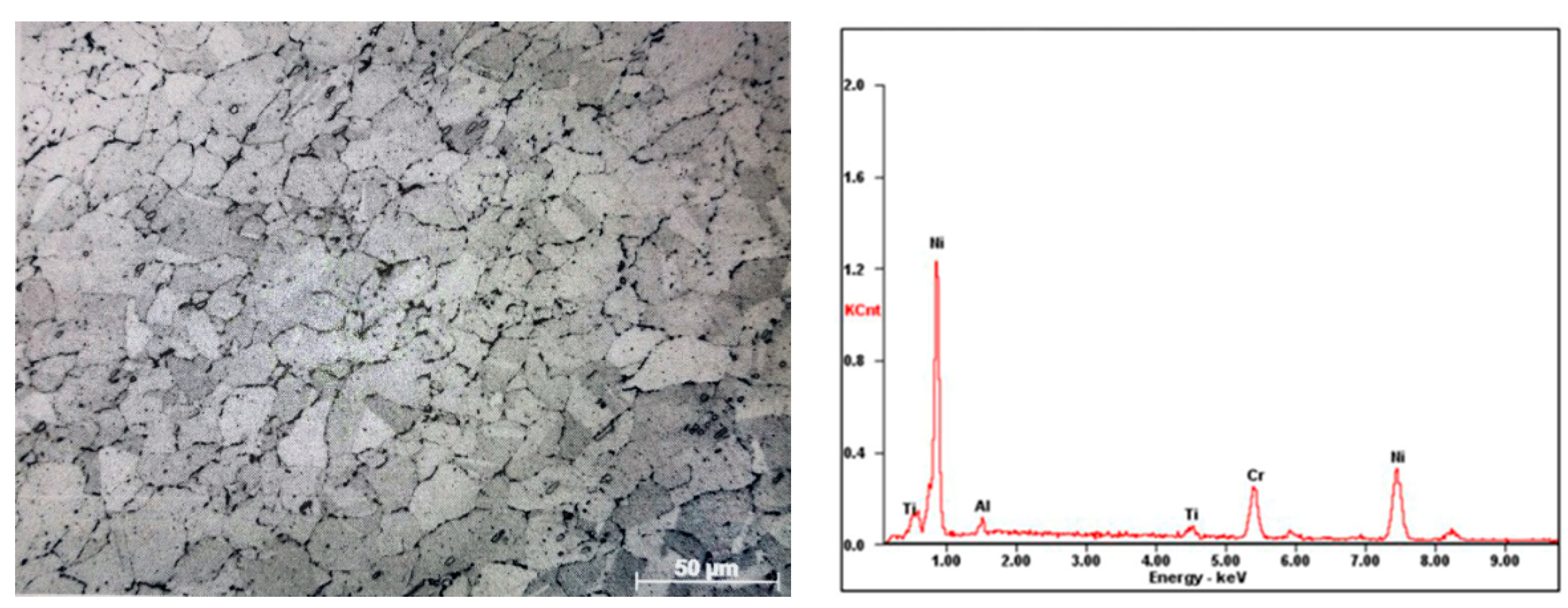
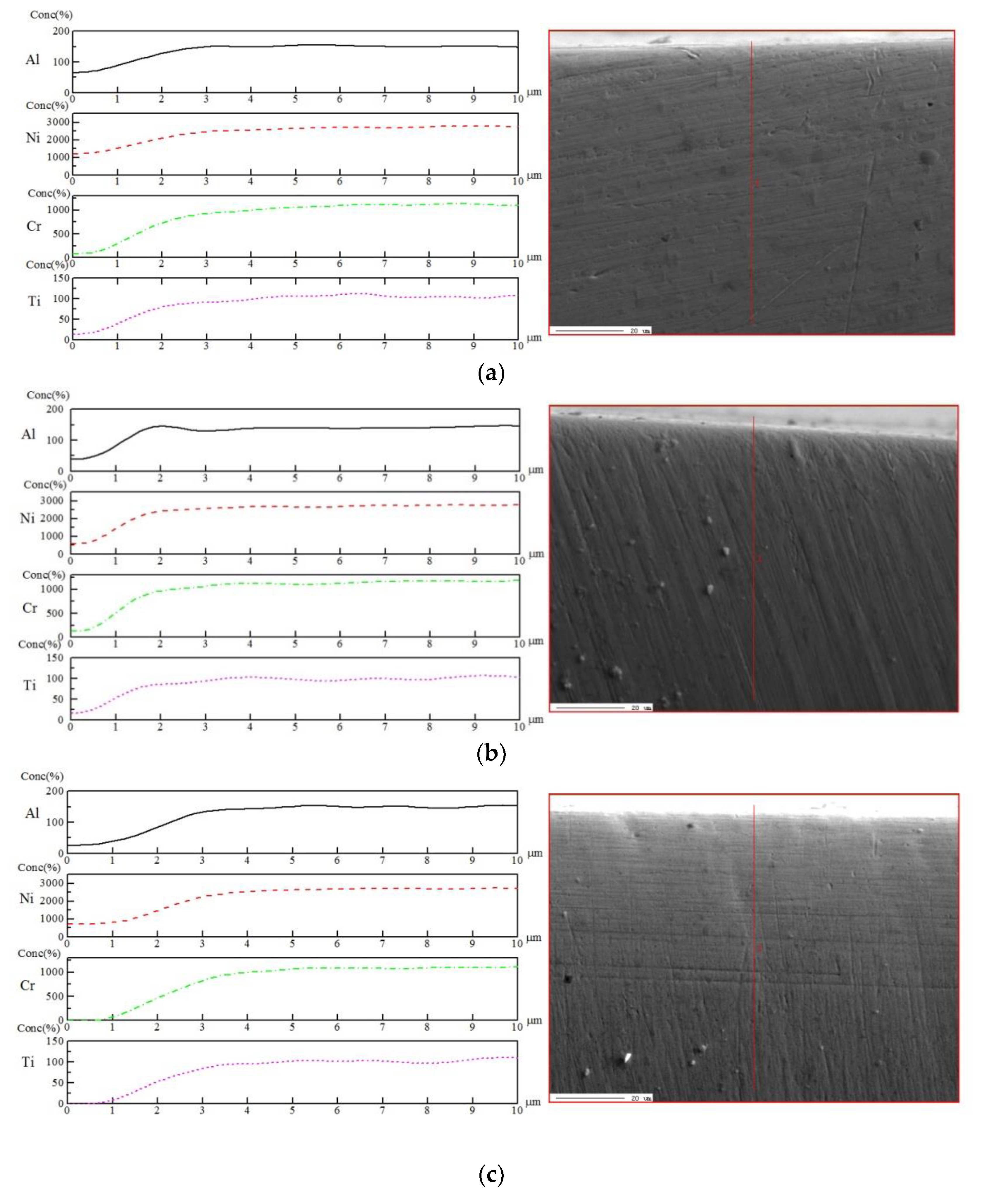
3.4. Surface Element Distribution
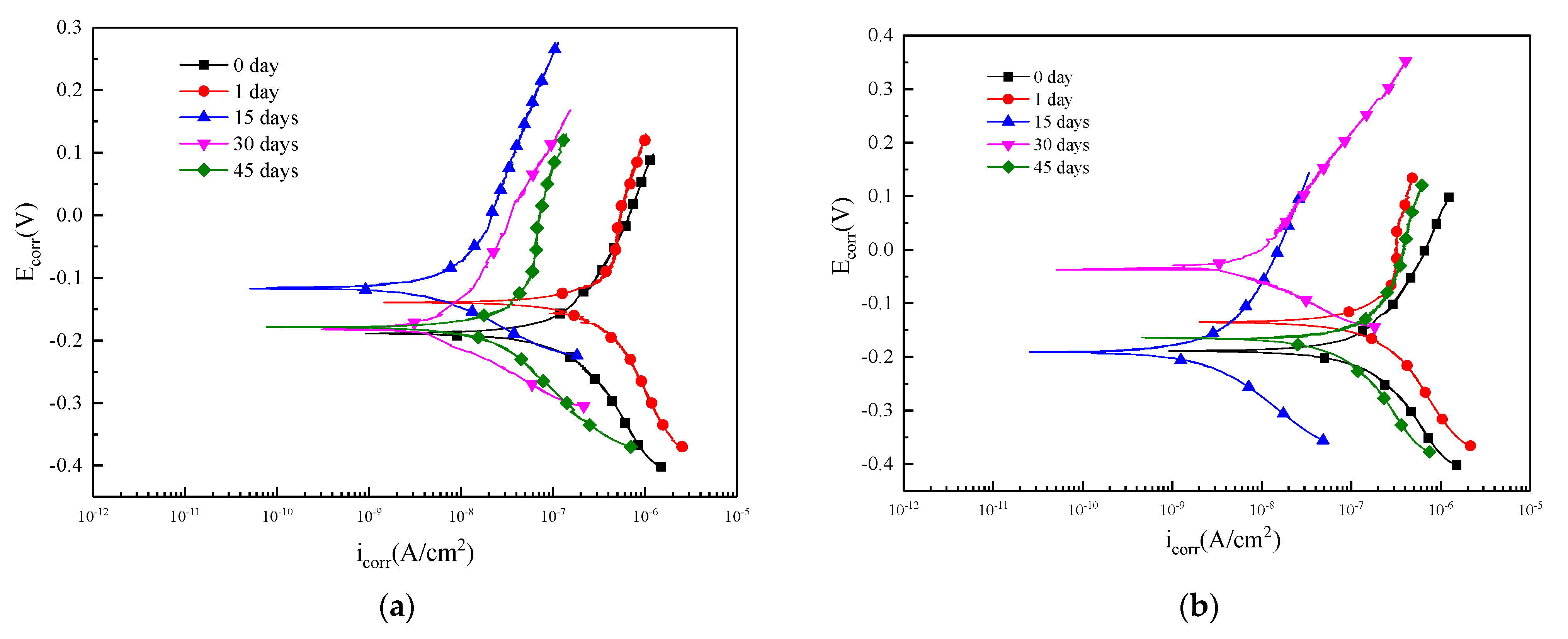
3.5. Potentiodynamic Polarization
3.6. Electrochemical Impedance Spectroscopy
3.7. Micro Morphology of Corroded Surface
4. Conclusions
- (1)
- The main difference of these two cutting fluids was their lubrication property, such as the reduction in friction and the ability to form a lubricating film.
- (2)
- The machined surface topography and roughness of E709 were between Blasocut and water. The machined surface roughness, under Blasocut and E709, were almost the same, while that of water was slightly larger.
- (3)
- The residual stresses along both the x and y directions of Blasocut were smaller than those of E709 or water, and there were even residual compressive stresses along the y-direction.
- (4)
- According to the self-corrosion current density and self-corrosion potential, the surface corrosion of the specimens in Blasocut was much slower than that of E709.
- (5)
- The surface in E709, for 45 days, changed from an unstable pitting stage to a stable pitting stage with pitting corrosion.
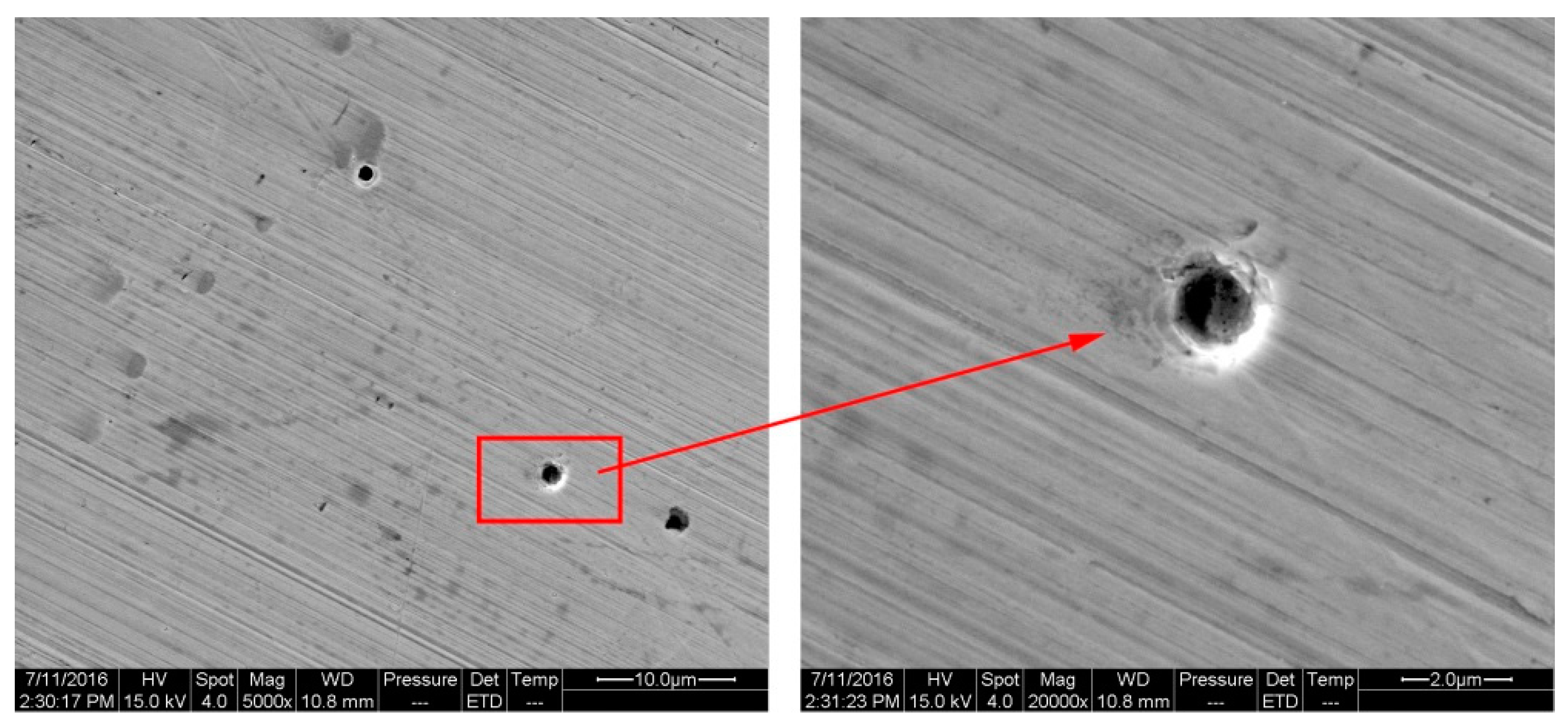
- (6)
- The bright circles around the small hole on the corrosion surface were caused by the layer-by-layer corrosion of the metal, which accorded with the characteristics of pitting holes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, M.K.; Song, Q.; Liu, Z.; Sarikaya, M.; Jamil, M.; Mia, M.; Singla, A.K.; Khan, A.M.; Khanna, N.; Pimenov, D.Y. Environment and economic burden of sustainable cooling/lubrication methods in machining of Inconel-800. J. Clean. Prod. 2021, 287, 125074. [Google Scholar] [CrossRef]
- Khanna, N.; Agrawal, C.; Pimenov, D.Y.; Singla, A.K.; Machado, A.R.; da Silva, L.R.R.; Gupta, M.K.; Sarikaya, M.; Krolczyk, G.M. Review on design and development of cryogenic machining setups for heat resistant alloys and composites. J. Manuf. Process. 2021, 68, 398–422. [Google Scholar] [CrossRef]
- Baig, A.; Jaffery, S.H.I.; Khan, M.A.; Alruqi, M. Statistical Analysis of Surface Roughness, Burr Formation and Tool Wear in High Speed Micro Milling of Inconel 600 Alloy under Cryogenic, Wet and Dry Conditions. Micromachines 2022, 14, 13. [Google Scholar] [CrossRef]
- Habrat, W.; Krupa, K.; Markopoulos, A.P.; Karkalos, N.E. Thermo-mechanical aspects of cutting forces and tool wear in the laser-assisted turning of Ti-6Al-4V titanium alloy using AlTiN coated cutting tools. Int. J. Adv. Manuf. Technol. 2021, 115, 759–775. [Google Scholar] [CrossRef]
- Salur, E.; Kuntoğlu, M.; Aslan, A.; Pimenov, D.Y. The Effects of MQL and Dry Environments on Tool Wear, Cutting Temperature, and Power Consumption during End Milling of AISI 1040 Steel. Metals 2021, 11, 1674. [Google Scholar] [CrossRef]
- Debnath, S.; Reddy, M.M.; Yi, Q.S. Influence of cutting fluid conditions and cutting parameters on surface roughness and tool wear in turning process using Taguchi method. Measurement 2016, 78, 111–119. [Google Scholar] [CrossRef]
- Braham-Bouchnak, T.; Germain, G.; Morel, A.; Furet, B. Influence of High-Pressure Coolant Assistance on the Machinability of the Titanium AlloyTi555–3. Mach. Sci. Technol. 2015, 19, 134–151. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, J.; Xiao, M.; Zhang, X.; Li, J.; Jiang, W.; Yan, J.; Qin, Z.; Zhang, S.; He, W.; et al. Ethylenediaminetetraacetic acid induces surface erosion of zero-valent iron for enhanced hexavalent chromium removal. Appl. Surf. Sci. 2020, 525, 146593. [Google Scholar] [CrossRef]
- Kumar, A.; Melkote, S.N. The chemo-mechanical effect of cutting fluid on material removal in diamond scribing of silicon. Appl. Phys. Lett. 2017, 111, 011901. [Google Scholar] [CrossRef]
- Manikanta, J.E.; Raju, B.; Prasad, C.; Sankar, B.S.S.P. Machining Performance on SS304 using nontoxic, biodegradable Vegetable-Based Cutting Fluids. Chem. Data Collect. 2022, 42, 100961. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Singh, R.K.; Dixit, A.R. Tribological Investigation of TiO2 Nanoparticle based Cutting Fluid in Machining under Minimum Quantity Lubrication (MQL). Mater. Today Proc. 2016, 3, 2155–2162. [Google Scholar] [CrossRef]
- Wang, S.; Kou, H. Cooling effectiveness of cutting fluid in creep feed grinding. Int. Commun. Heat Mass Transf. 1997, 24, 771–783. [Google Scholar] [CrossRef]
- Machado, A.R.; Wallbank, J.; Pashby, I.R.; Ezugwu, E.O. Tool performance and chip control when machining ti6a14v and inconel 901 using high pressure coolant supply. Mach. Sci. Technol. 1998, 2, 1–12. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Chang, H.-Y. Experimental study of surface roughness in slot end milling AL2014-T6. Int. J. Mach. Tools Manuf. 2004, 44, 51–57. [Google Scholar] [CrossRef]
- Moura, R.; Silva, M.; Machado, L.; Sales, W. The effect of application of cutting fluid with solid lubricant in suspension during cutting of Ti-6Al-4V alloy. Wear 2015, 332–333, 762–771. [Google Scholar] [CrossRef]
- Tan, X.; Liu, F.; Cao, H.; Zhang, H. A decision-making framework model of cutting fluid selection for green manufacturing and a case study. J. Mater. Process. Technol. 2002, 129, 467–470. [Google Scholar] [CrossRef]
- Williams, J.; Tabor, D. The role of lubricants in machining. Wear 1977, 43, 275–292. [Google Scholar] [CrossRef]
- Godlevski, V.A.; Volkov, A.V.; Latyshev, V.N.; Maurin, L.N. The kinetics of lubricant penetration action during machining. Lubr. Sci. 2010, 9, 127–140. [Google Scholar] [CrossRef]
- Williams, J.A. The Action of Lubricants in Metal Cutting. ARCHIVE J. Mech. Eng. Sci. 1977, 19, 202–212. [Google Scholar] [CrossRef]
- Priarone, P.C.; Robiglio, M.; Settineri, L.; Tebaldo, V. Effectiveness of Minimizing Cutting Fluid Use when Turning Difficult-to-cut Alloys. Procedia CIRP 2015, 29, 341–346. [Google Scholar] [CrossRef]
- Amigo, F.J.; Urbikain, G.; Pereira, O.; Fernández-Lucio, P.; Fernández-Valdivielso, A.; de Lacalle, L.L. Combination of high feed turning with cryogenic cooling on Haynes 263 and Inconel 718 superalloys. J. Manuf. Process 2020, 58, 208–222. [Google Scholar] [CrossRef]
- Gajrani, K.K.; Ram, D.; Sankar, M.R. Biodegradation and hard machining performance comparison of eco-friendly cutting fluid and mineral oil using flood cooling and minimum quantity cutting fluid techniques. J. Clean. Prod. 2017, 165, 1420–1435. [Google Scholar] [CrossRef]
- Gajrani, K.K.; Suvin, P.; Kailas, S.V.; Sankar, M.R. Hard machining performance of indigenously developed green cutting fluid using flood cooling and minimum quantity cutting fluid. J. Clean. Prod. 2019, 206, 108–123. [Google Scholar] [CrossRef]
- Zeilmann, R.; Vacaro, T.; Bordin, F.; Soares, R. Effects of the cutting fluid reduction on the dimensional quality of holes. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2014, 229, 1314–1323. [Google Scholar] [CrossRef]
- Zhong, W.; Zhao, D.; Wang, X. A comparative study on dry milling and little quantity lubricant milling based on vibration signals. Int. J. Mach. Tools Manuf. 2010, 50, 1057–1064. [Google Scholar] [CrossRef]
- Wang, L.; Su, J.; Nie, X. Corrosion and tribological properties and impact fatigue behaviors of TiN- and DLC-coated stainless steels in a simulated body fluid environment. Surf. Coat. Technol. 2010, 205, 1599–1605. [Google Scholar] [CrossRef]
- Yosri, A.; Zayed, A.; Saad-Eldeen, S.; Leheta, H. Influence of stress concentration on fatigue life of corroded specimens under uniaxial cyclic loading. Alex Eng. J. 2021, 60, 5205–5216. [Google Scholar] [CrossRef]
- Yan, P.; Rong, Y.; Wang, X.; Zhu, J.; Jiao, L.; Liang, Z. Effect of cutting fluid on precision machined surface integrity of heat-resistant stainless steel. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2018, 232, 1535–1548. [Google Scholar] [CrossRef]
- Gómez-Merino, A.; Jiménez-Galea, J.; Rubio-Hernández, F.; Santos-Ráez, I. Experimental assessment of thermal and rheological properties of coconut oil-silica as green additives in drilling performance based on minimum quantity of cutting fluids. J. Clean. Prod. 2022, 368, 133104. [Google Scholar] [CrossRef]
- Suárez, A.; De Lacalle, L.N.L.; Polvorosa, R.; Veiga, F.; Wretland, A. Effects of high-pressure cooling on the wear patterns on turning inserts used on alloy IN718. Mater. Manuf. Process. 2017, 32, 678–686. [Google Scholar] [CrossRef]
- González, H.; Fernández-Lucio, P.; Pereira, O.; Calleja, A.; Fernández-Valdivielso, A.; De Lacalle, L.L. Abrasive tool behavior comparing lubri-cooling techniques for Super Abrasive Machining full-slotting in Inconel®718. Procedia Manuf. 2019, 41, 642–649. [Google Scholar] [CrossRef]
- Pereira, O.; Urbikain, G.; Rodríguez, A.; Fernández-Valdivielso, A.; Calleja, A.; Ayesta, I.; de Lacalle, L. Internal cryolubrication approach for Inconel 718 milling. Procedia Manuf. 2017, 13, 89–93. [Google Scholar] [CrossRef]
- Kuram, E.; Ozcelik, B.; Bayramoglu, M.; Demirbas, E.; Simsek, B.T. Optimization of cutting fluids and cutting parameters during end milling by using D-optimal design of experiments. J. Clean. Prod. 2013, 42, 159–166. [Google Scholar] [CrossRef]
- Karmiris-Obratański, P.; Karkalo, N.; Kudelski, R.; Markopoulos, A. Experimental study on the effect of the cooling method on surface topography and workpiece integrity during trochoidal end milling of Incoloy 800. Tribol. Int. 2022, 176, 107899. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zu Ren, C.; Chen, G.; Han, B.; Wang, Y. Effect of Various Cooling Strategies on Surface Roughness of High Speed Milling Ti-6Al-4V. Mater. Sci. Forum 2011, 697–698, 49–52. [Google Scholar] [CrossRef]
- Suárez, A.; Veiga, F.; Polvorosa, R.; Artaza, T.; Holmberg, J.; de Lacalle, L.L.; Wretland, A. Surface integrity and fatigue of non-conventional machined Alloy 718. J. Manuf. Process. 2019, 48, 44–50. [Google Scholar] [CrossRef]
- Aurrekoetxea, M.; López de Lacalle, L.N.L.; Llanos, I. Machining Stresses and Initial Geometry on Bulk Residual Stresses Characterization by On-Machine Layer Removal. Materials 2020, 13, 1445. [Google Scholar] [CrossRef] [PubMed]
- Llanos, I.; Aurrekoetxea, M.; Agirre, A.; de Lacalle, L.L.; Zelaieta, O. On-machine Characterization of Bulk Residual Stresses on Machining Blanks. Procedia CIRP 2019, 82, 406–410. [Google Scholar] [CrossRef]
- Cao, W.; Khadhraoui, M.; Brenier, B.; Guedou, J.Y.; Castex, L. Thermomechanical relaxation of residual stress in shot peened nickel base superalloy. Mater. Sci. Technol.-Lond. 1994, 10, 947–954. [Google Scholar] [CrossRef]
- Huang, Q.; Ren, J.X. Surface integrity and its effects on the fatigue life of the nickel-based superalloy GH33A. Int. J. Fatigue 1991, 13, 322–326. [Google Scholar] [CrossRef]
- Javidi, A.; Rieger, U.; Eichlseder, W. The effect of machining on the surface integrity and fatigue life. Int. J. Fatigue 2008, 30, 2050–2055. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]

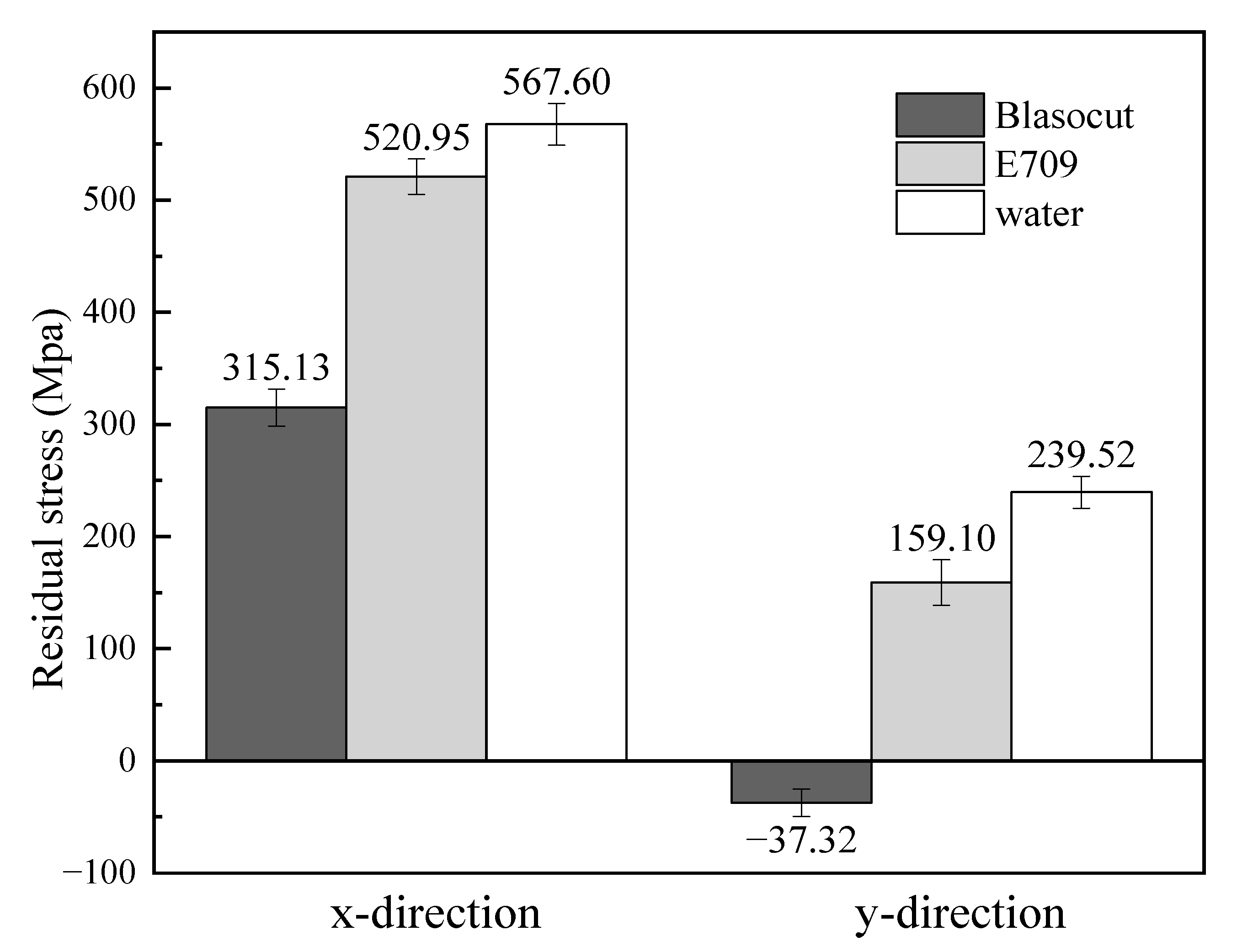


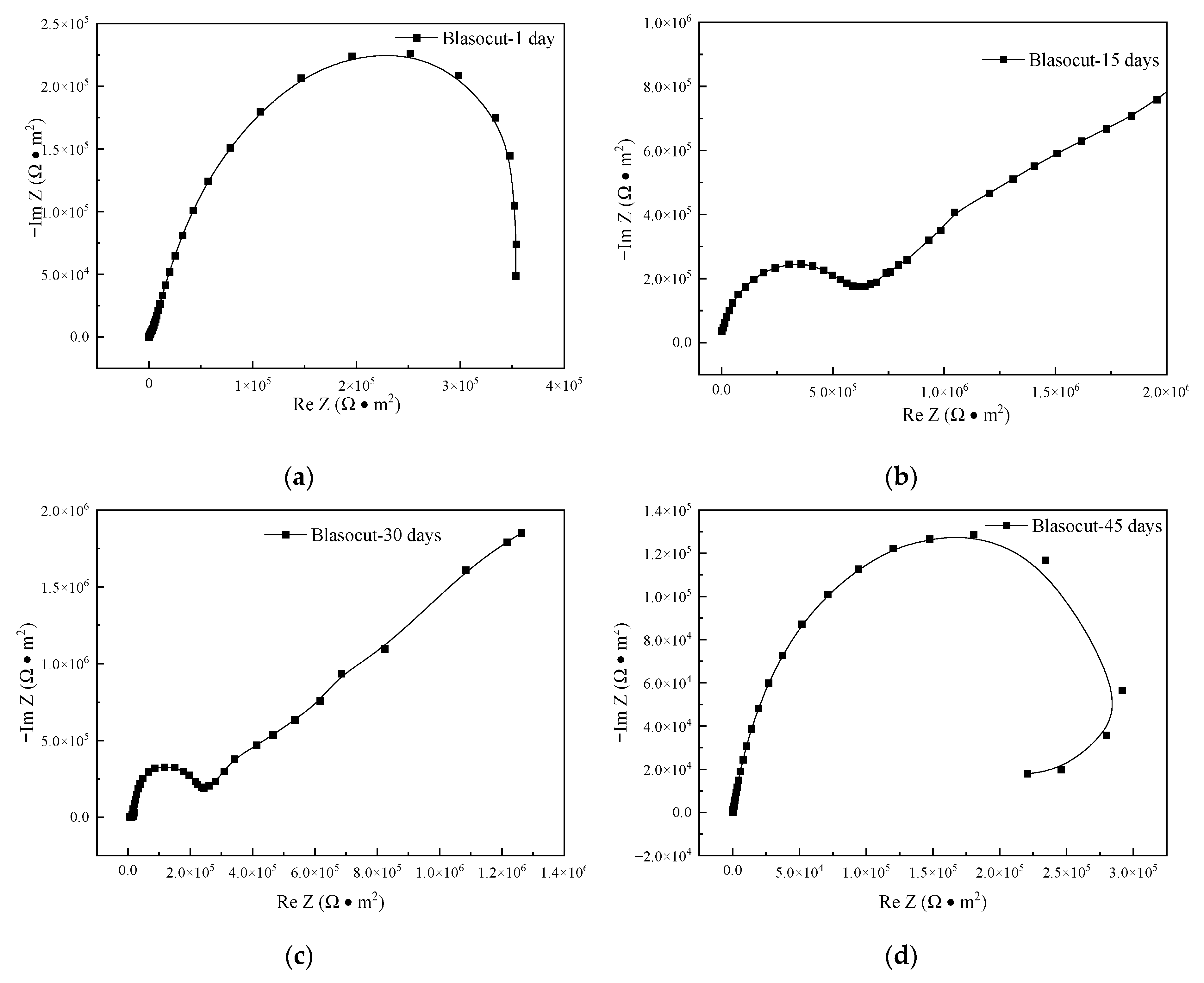
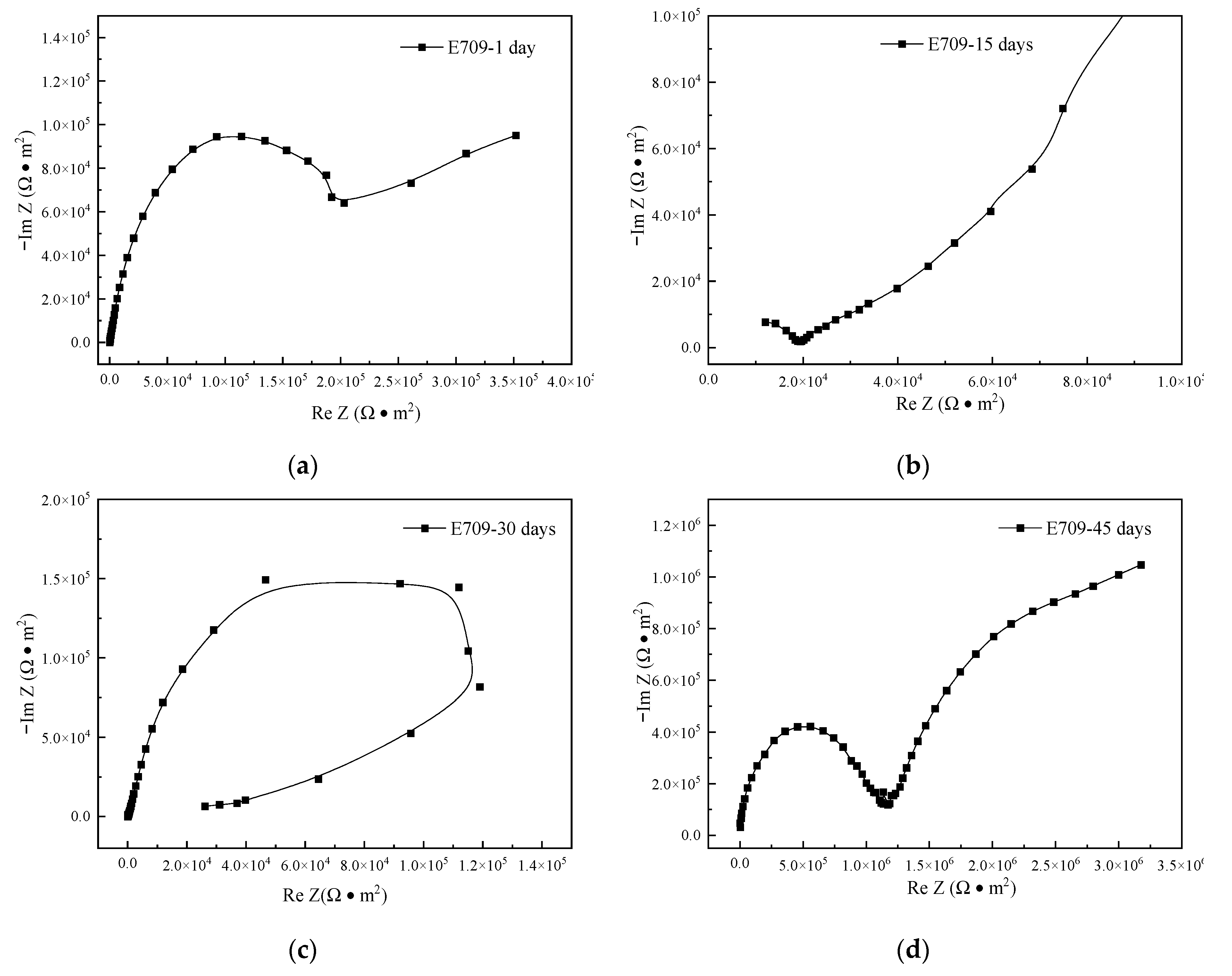
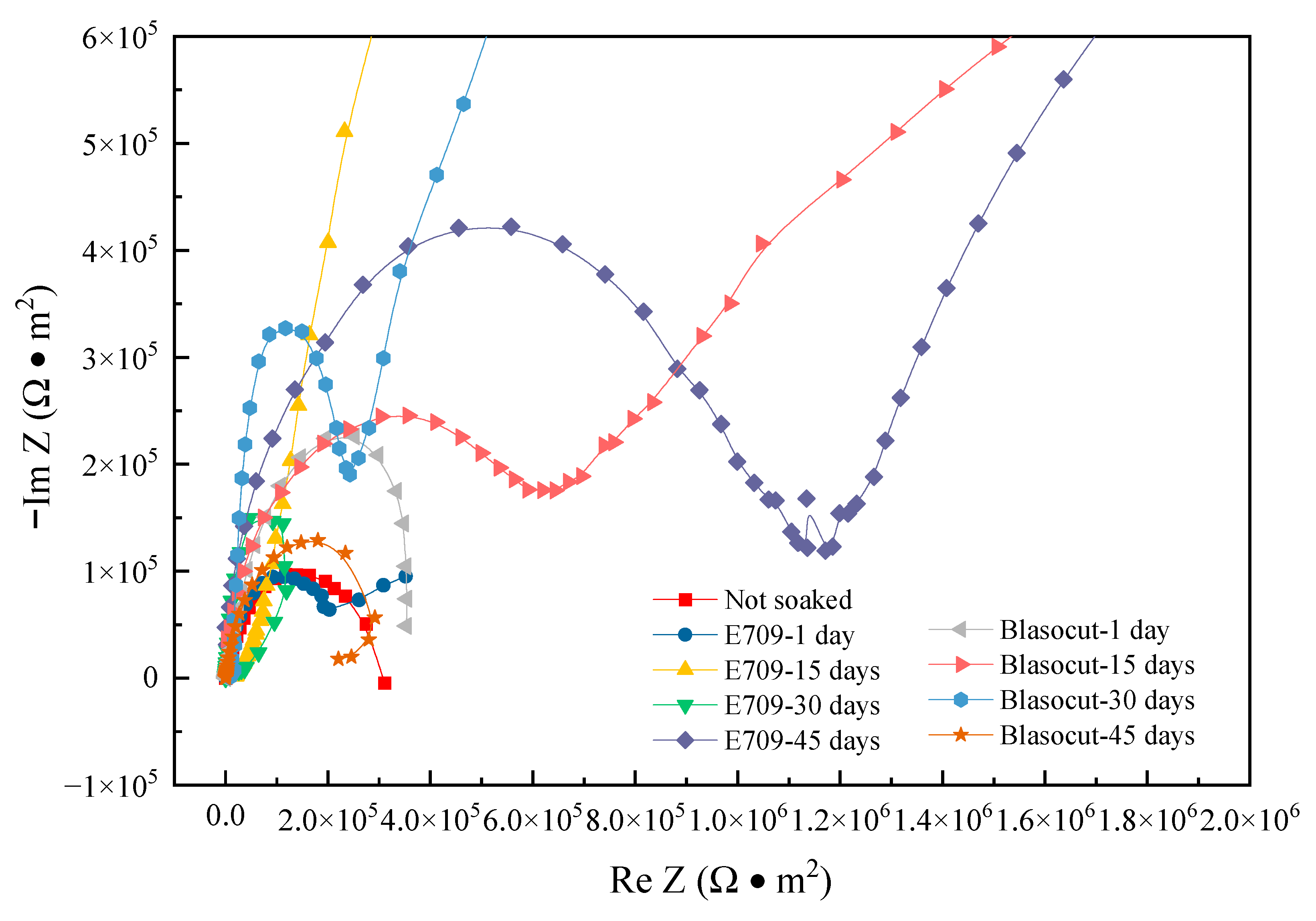


| Residual Stress (MPa) | Blasocut | E709 | Water |
|---|---|---|---|
| x-direction | 315.13 ± 16.5 | 520.95 ± 15.8 | 567.60 ± 18.5 |
| y-direction | −37.32 ± 12.4 | 159.10 ± 20.3 | 239.52 ± 14.3 |
| Time | Blasocut | E709 | ||||
|---|---|---|---|---|---|---|
| Day | icorr × 10−9 (A/cm2) | E (mV) | Corrosion Rate ×10−4 (mm/Year) | icorr × 10−9 (A/cm2) | E (mV) | Corrosion rate × 10−4 (mm/Year) |
| 0 | 102.8 ± 15.4 | −18.07 ± 1.6 | 12.02 ± 1.8 | 102.8 ± 15.4 | −18.07 ± 1.6 | 12.02 ± 1.75 |
| 1 | 342.2 ± 26.0 | −16.33 ± 1.8 | 40.03 ± 3.0 | 183.9 ± 20.0 | −13.91 ± 1.4 | 21.51 ± 2.34 |
| 15 | 6.730 ± 0.64 | −13.22 ± 1.2 | 0.79 ± 0.07 | 2.549 ± 0.34 | −19.83 ± 1.8 | 0.30 ± 0.04 |
| 30 | 6.242 ± 0.58 | −19.02 ± 1.3 | 0.73 ± 0.07 | 6.917 ± 0.65 | −4.020 ± 1.4 | 0.81 ± 0.08 |
| 45 | 30.75 ± 3.2 | −20.89 ± 1.5 | 3.60 ± 0.37 | 99.75 ± 6.6 | −20.70 ± 2.3 | 11.67 ± 0.77 |
| Time (Day) | Blasocut | E709 | ||||||
|---|---|---|---|---|---|---|---|---|
| Rs/Ω∙cm2 | R1/Ω∙cm2 | Q1/μF∙cm−2 | n1 | Rs/Ω∙cm2 | R1/Ω∙cm2 | Q1/μF∙cm−2 | n1 | |
| 0 | 12.19 | 2.65 × 104 | 39.23 | 0.63 | 5.94 | 4.69 × 104 | 20.98 | 0.63 |
| 1 | 13.26 | 4.43 × 107 | 5.32 | 0.85 | 8.17 | 4.77 × 105 | 8.28 | 0.73 |
| 15 | 5.68 | 3.80 × 105 | 11.28 | 0.70 | 5.83 | 8.34 × 105 | 8.22 | 0.76 |
| 30 | 3.11 | 3.34 × 107 | 6.45 | 0.81 | 5.01 | 1.45 × 106 | 6.28 | 0.77 |
| 45 | 12.19 | 2.65 × 104 | 39.23 | 0.63 | 8.55 | 1.11 × 105 | 17.5 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Yan, P.; Zhu, J.; Wang, Y.; Zhao, W.; Jiao, L.; Wang, X. Effect of Cutting Fluid on Machined Surface Integrity and Corrosion Property of Nickel Based Superalloy. Materials 2023, 16, 843. https://doi.org/10.3390/ma16020843
Chen S, Yan P, Zhu J, Wang Y, Zhao W, Jiao L, Wang X. Effect of Cutting Fluid on Machined Surface Integrity and Corrosion Property of Nickel Based Superalloy. Materials. 2023; 16(2):843. https://doi.org/10.3390/ma16020843
Chicago/Turabian StyleChen, Shiqi, Pei Yan, Junyi Zhu, Yubin Wang, Wenxiang Zhao, Li Jiao, and Xibin Wang. 2023. "Effect of Cutting Fluid on Machined Surface Integrity and Corrosion Property of Nickel Based Superalloy" Materials 16, no. 2: 843. https://doi.org/10.3390/ma16020843
APA StyleChen, S., Yan, P., Zhu, J., Wang, Y., Zhao, W., Jiao, L., & Wang, X. (2023). Effect of Cutting Fluid on Machined Surface Integrity and Corrosion Property of Nickel Based Superalloy. Materials, 16(2), 843. https://doi.org/10.3390/ma16020843








