Recycling of Electrical Cables—Current Challenges and Future Prospects
Abstract
:1. Introduction
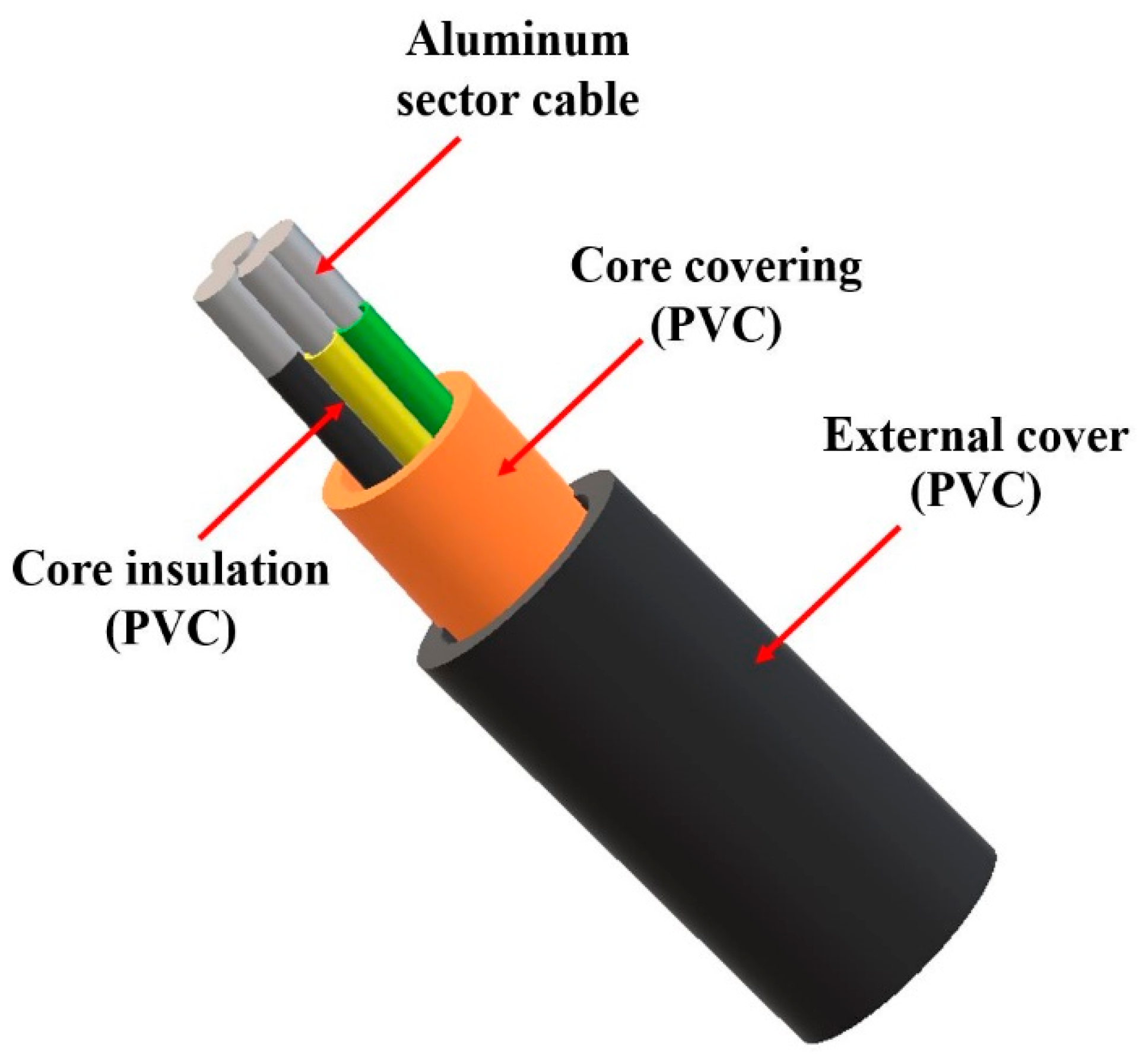
2. Electrical Cables
2.1. Structure of Electric Cables
2.2. Characteristics of Waste from Electrical Cables
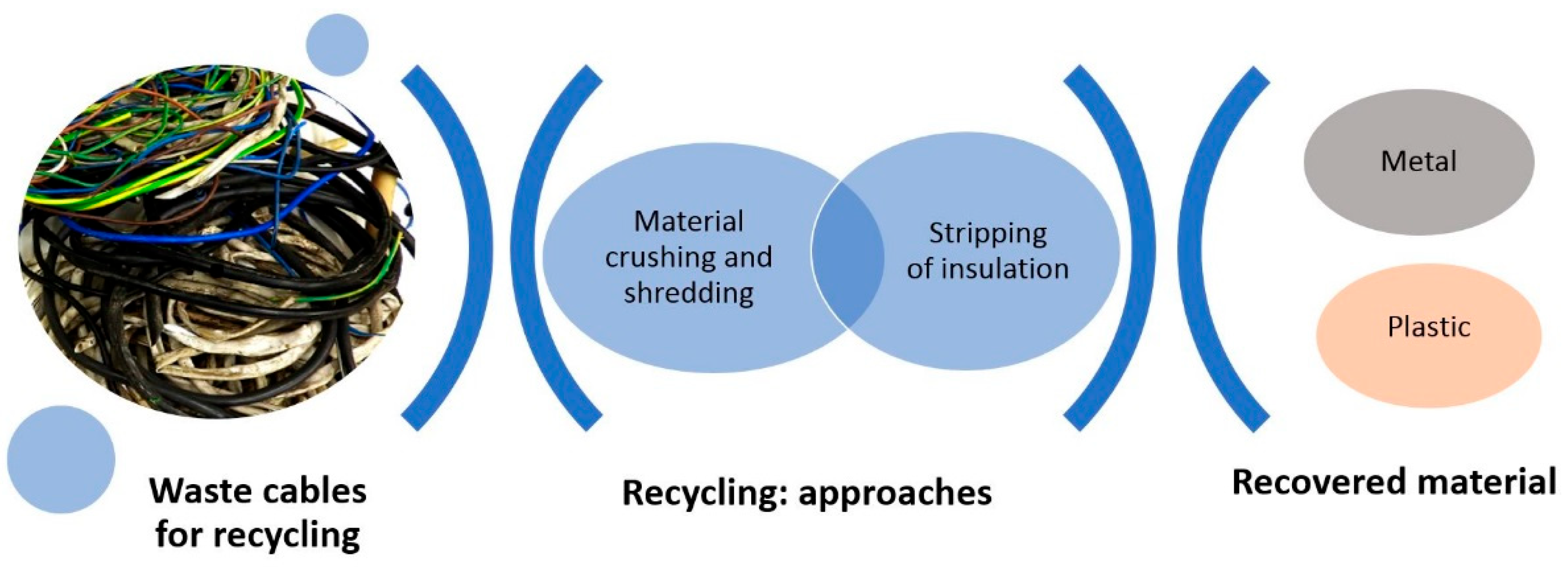
3. Recycling of Electric Cables
3.1. Dismantling
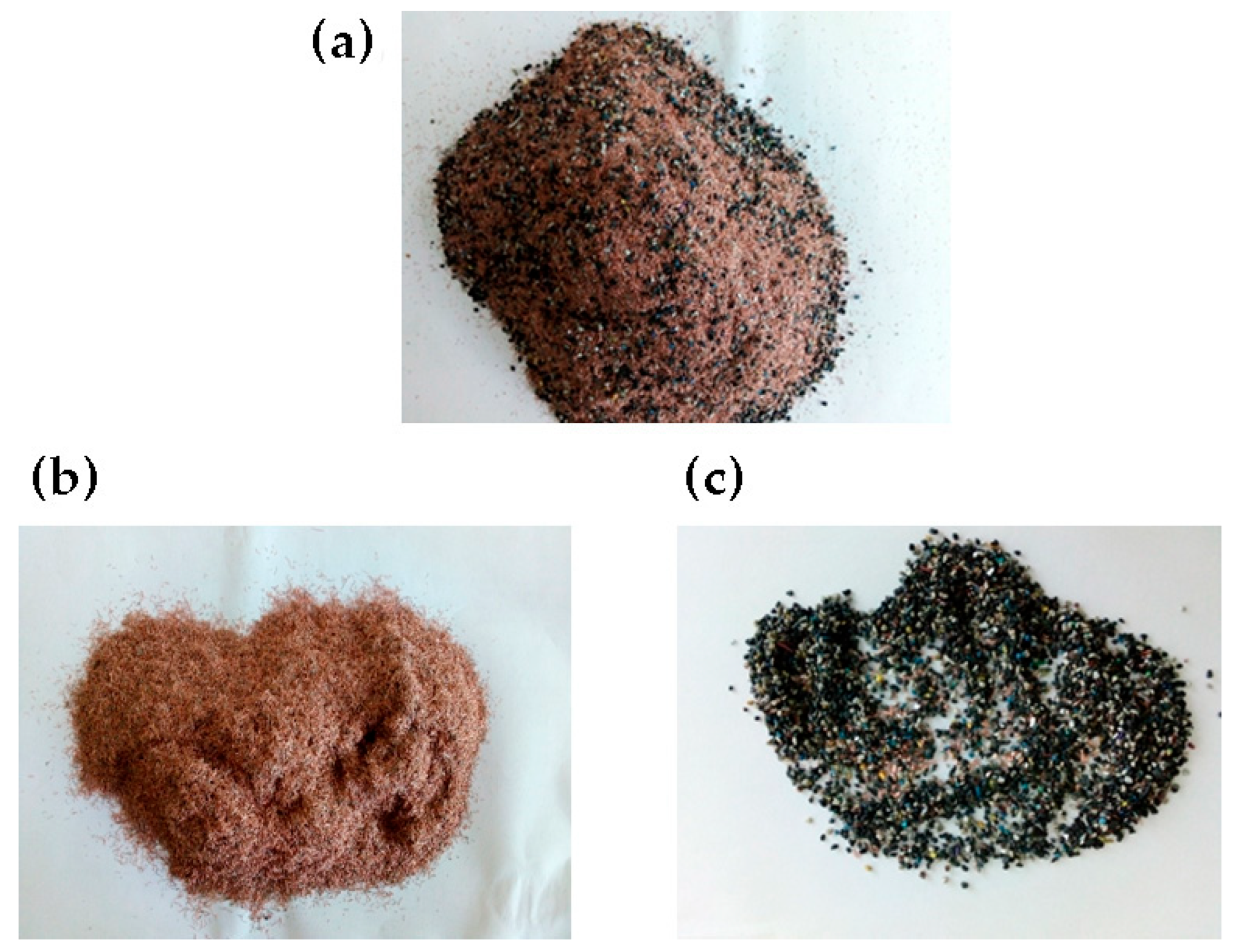
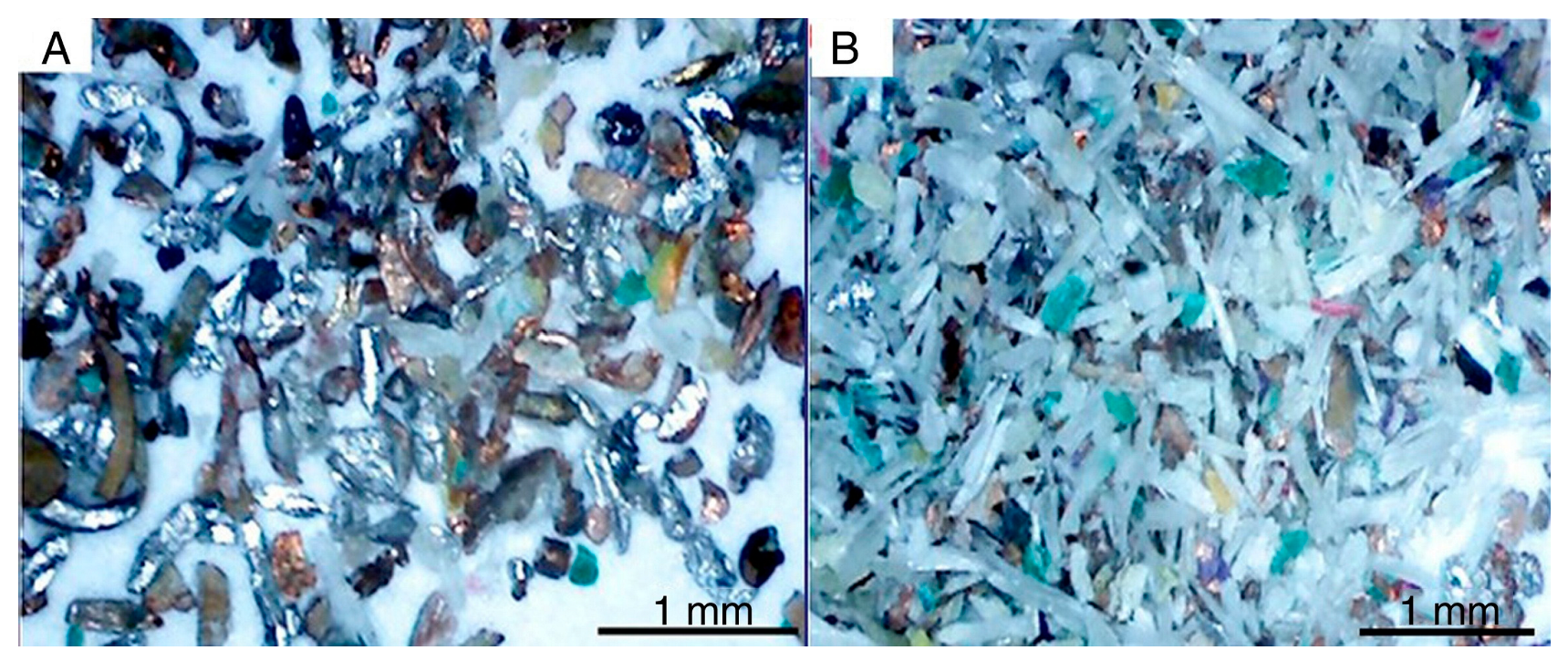
3.2. The Release of Metallic and Non-Metallic Fractions (Shredding/Crushing)
- (a)
- The type and thickness of insulation—thicker insulation may require more power and time to remove, affecting efficiency;
- (b)
- The type of wires and cables—different types may require different settings and stripper speeds, impacting the work pace;
- (c)
- Automation—a higher degree of automation, such as adjustable cutting depths, conveyor systems, or computer control, can increase the efficiency of the wire stripper;
- (d)
- Operator experience—the skills and experience of the operator can affect work efficiency;
- (e)
- Length of wires/cables—processing longer segments of material may require more time;
- (f)
- Breakdowns and downtime—the frequency of breakdowns, maintenance, or technical interruptions can affect the overall machine productivity;
- (g)
- Number and type of operators—teamwork or employing a greater number of operators can increase efficiency.
3.3. Screening and Classification of Waste
3.4. Chemical Composition Determination
- (a)
- Flammability and flame color;
- (b)
- Odor and smoke development;
- (c)
- Residue formation (e.g., ash or non-ash);
- (d)
- Behavior of the material when exposed to fire, such as dripping or self-extinguishing properties.
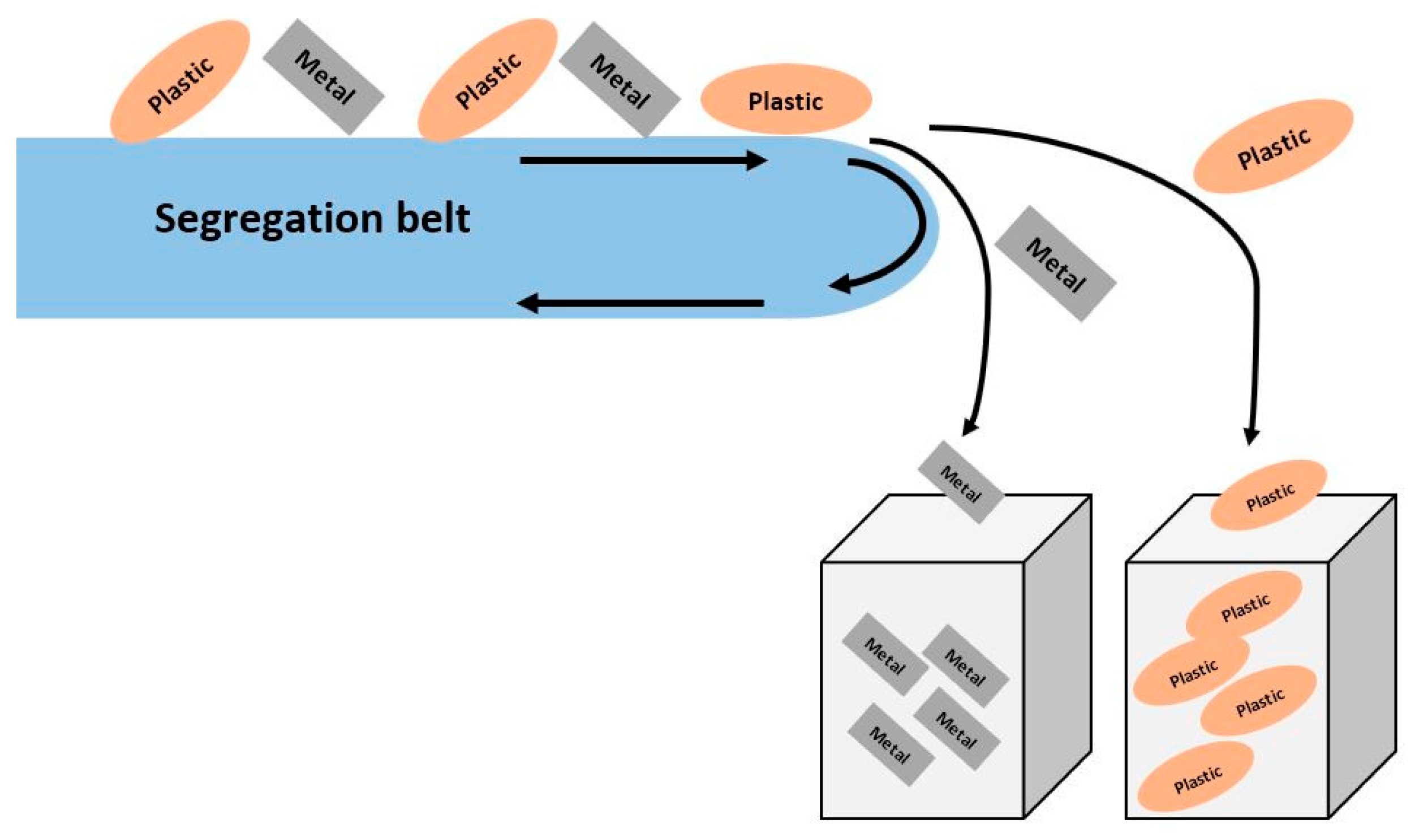
3.5. Gravity Separation
3.6. Magnetic Separation
3.7. Electrostatic Separation
- Ionizing electrode: rotor distance = 25 cm, electrode inclination angle: 80°;
- Static electrode: rotor distance = 25 cm, inclination angle: 52.5°;
- Rotor revolutions per minute: 85;
- Voltage: 45–46 kV.
3.8. Feedstock Recycling
4. New Methods of Recycling Electrical Cables and Their Challenges
- (a)
- Separation and sorting: This area is centered on the precise separation of different cable components using electrodynamic and eddy current separators, allowing for automatic recognition and sorting of materials.
- (b)
- Pollution management: This area places a strong emphasis on safe and environmentally friendly management of pollutants and chemicals that may be present in cable insulations. Cleaning and monitoring systems are employed to minimize the impact on the environment and the health of workers.
4.1. Chemical Recycling
4.2. Technologies Utilizing Artificial Intelligence and Automation
4.3. Microorganism-Utilizing Technologies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naik, S.; Eswari, J.S. Electrical waste management: Recent advances challenges and future outlook. Total Environ. Res. Themes 2022, 1–2, 100002. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Markandeya; Patel, N.; Pandey, M.; Pandey, A.K.; Dubey, A.K.; Kumar, A.; Bhardwaj, A.K.; Chaudhary, V.K. Concepts of circular economy for sustainable management of electronic wastes: Challenges and management options. Environ. Sci. Pollut. Res. 2023, 30, 48654–48675. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J. Occupational health hazards related to informal recycling of E-waste in India: An overview. Indian. J. Occup. Environ. Med. 2015, 19, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Narro, G.; Prasertcharoensuk, P.; Diaz-Silvarrey, L.S.; Dixon, L.; Phan, A.N. Chemical recycling of mixed plastic waste via catalytic pyrolysis. J. Environ. Chem. Eng. 2022, 10, 108494. [Google Scholar] [CrossRef]
- Huang, J.; Veksha, A.; Chan, W.P.; Giannis, A.; Lisak, G. Chemical recycling of plastic waste for sustainable material management: A prospective review on catalysts and processes. Renew. Sust. Energ. Rev. 2022, 154, 111866. [Google Scholar] [CrossRef]
- European Environment Agency. Europes Consumption in a Circular Economy: The Benefits of Longer-Lasting Electronics. Available online: https://www.eea.europa.eu/publications/europe2019s-consumption-in-a-circular (accessed on 30 June 2023).
- Li, L.; Liu, G.; Pan, D.; Wang, W.; Wu, Y.; Zuo, T. Overview of the recycling technology for copper-containing cables. Resour. Conserv. Recycl. 2017, 126, 132–140. [Google Scholar] [CrossRef]
- Hartmannova, V.; Replacement of Electrical Wiring in the Apartment, House, Apartment Building. What Is the Price of a Permit? Available online: https://www.topbyvanie.sk/magazin/vymena-elektrickych-rozvodov (accessed on 30 June 2023).
- Seif, R.; Salem, F.Z.; Allam, N.K. E-waste recycled materials as efficient catalyst for renewable energy technologies and better environmental sustainability. Environ. Dev. Sustain. 2023, 1–36. [Google Scholar] [CrossRef]
- Garside, M. Global Copper Production by Region 2012–2017. Available online: https://www.statista.com/statistics/612963/copper-production-worldwide-by-region (accessed on 30 June 2023).
- International Aluminium. Aluminium Consumption Recycling. Available online: https://international-aluminium.org/new-test-article/ (accessed on 30 June 2023).
- Shamsudin, S.; Lajis, M.A.; Zhong, Z.W. Evolutionary in Solid State Recycling Techniques of Aluminium: A review. Procedia CIRP 2016, 40, 256–261. [Google Scholar] [CrossRef]
- Ding, N.; Gao, F.; Wang, Z.; Xianzheng, G. Comparative analysis of primary aluminium and recycled aluminium energy consumption and greenhouse gas emission. Chin. J. Nonferrous Met. 2012, 22, 2908–2915. [Google Scholar]
- Report on the Environmental Benefits of Recycling—2016 Edition. Available online: https://www.bir.org/publications/facts-figures/download/172/174/36?method=view (accessed on 7 August 2023).
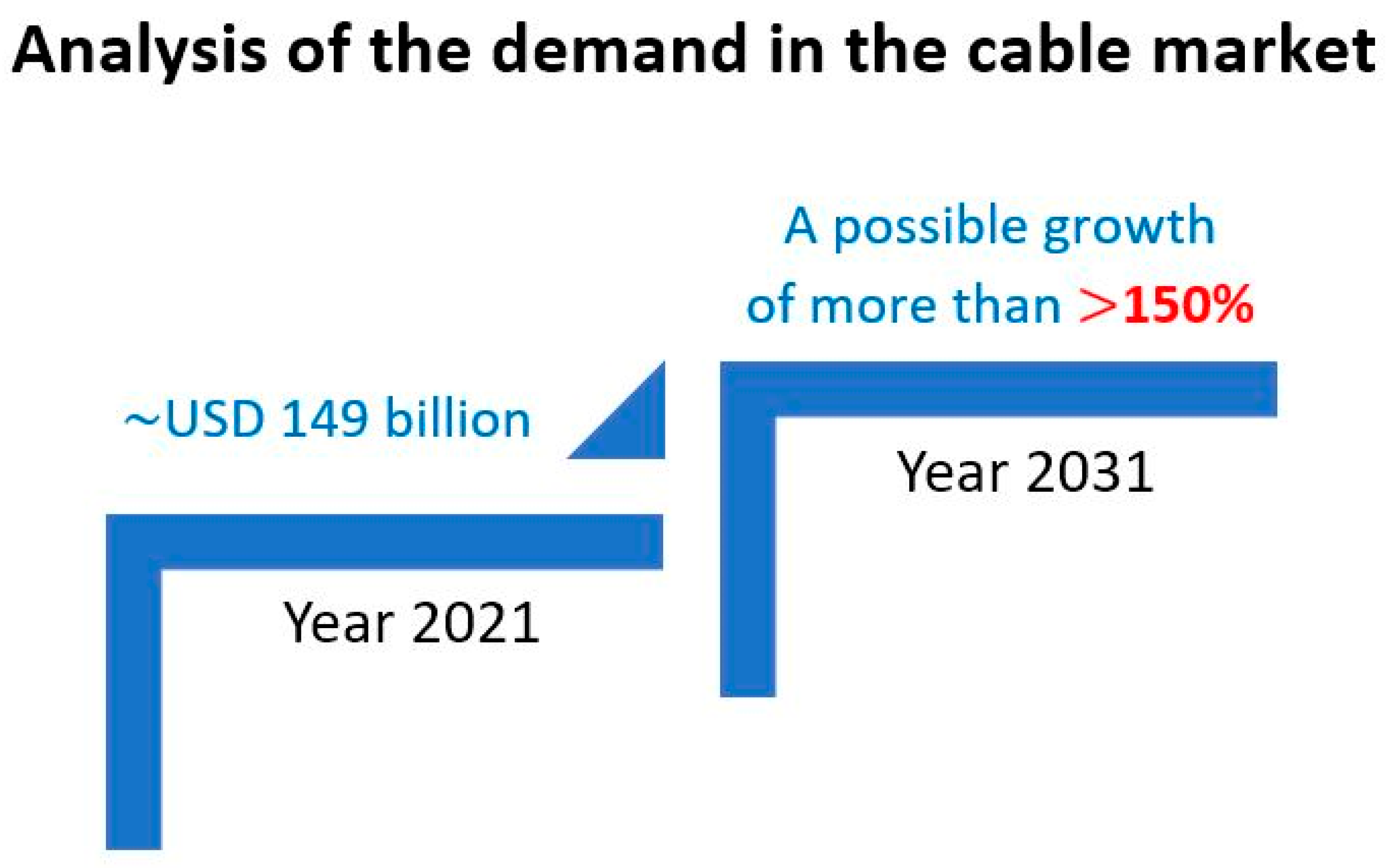
- Research and Markets the World’s Largest Market Research Store. Power Cable Market by Distribution Type, Voltage Rating, End—Use: Global Opportunity Analysis and Industry Forecast, 2021–2031. Available online: https://www.researchandmarkets.com/reports/5457351/power-cable-market-by-distribution-type (accessed on 1 August 2023).
- Oncioiu, I.; Petrescu, A.G.; Grecu, E.; Petrescu, M. Optimizing the Renewable Energy Potential: Myth or Future Trend in Romania. Energies 2017, 10, 759. [Google Scholar] [CrossRef]
- Geis-Schroer, J.; Hubschneider, S.; Held, L.; Gielnik, F.; Armbruster, M.; Suriyah, M.; Leibfried, T. Modeling of German Low Voltage Cables with Ground Return Path. Energies 2023, 14, 1265. [Google Scholar] [CrossRef]
- Xu, J.; Kumagi, S.; Kameda, T.; Saito, Y.; Takahashi, K.; Hayashi, H.; Yoshioka, T. Separation of copper and polyvinyl chloride from thin waste electric cables: A combined PVC-swelling and centrifugal approach. Waste Manag. 2019, 89, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Top Cable. Electrical Cable Types, Sizes and Installation. Available online: https://www.topcable.com/blog-electric-cable/en/electrical-cable-types (accessed on 30 June 2023).
- Plesa, I.; Notingher, P.; Stancu, C.; Wiesbrock, F.; Shlogl, S. Polyethylene Nanocomposites for Power Cable Insulations. Polymers 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Phelps Dodge International Thailand. Cable Name: Phelps Dodge Cable Type 60227 IEC 01 (THW). Available online: https://www.pdcable.com/en/product-en/60227-iec-01-thw-1-2 (accessed on 7 August 2023).
- Phelps Dodge International Thailand. Cable Name: Phelps Dodge Cable Type 60227 IEC 10. Available online: https://www.pdcable.com/en/product-en/60227-iec-10-1-2/ (accessed on 7 August 2023).
- Phelps Dodge International Thailand. Cable Name: Medium Voltage Cross-Linked Polyethylene Cable. Available online: https://www.pdcable.com/en/product-en/mxlp-cts-3-core-3/ (accessed on 7 August 2023).
- Phelps Dodge International Thailand. Cable Name: Cable Name: 0.6/1(1.2) kV Fire Resistant Low Smoke & Halogen Free. Available online: https://www.pdcable.com/en/category/product-by-voltage/frc-low-smoke/ (accessed on 7 August 2023).
- Phelps Dodge International Thailand. Cable Name: Phelps Dodge Cable Type Cv-Awa. Available online: https://www.pdcable.com/en/product-en/lv/cv-awa-1-2/ (accessed on 7 August 2023).
- Phelps Dodge International Thailand. Cable Name: Medium Voltage Cross-Linked Polyethylene Cable. Available online: https://www.pdcable.com/en/producten/mxlp-awa-1-2-2/ (accessed on 7 August 2023).
- Phelps Dodge International Thailand. Cable Name: Figure 8 Al-Pe Sheathed Cable. Available online: https://www.pdcable.com/en/product-en/building-and-construction/ap8-2/ (accessed on 7 August 2023).
- Gloser, S.; Soulier, M.; Espinoza-Tercero, L.A. Dynamic Analysis of Global Copper Flows. Global Stocks, Postconsumer Material Flows, Recycling Indicators, and Uncertainty Evaluation. Environ. Sci. Technol. 2013, 47, 6564–6572. [Google Scholar] [CrossRef]
- Diaz, S.; Ortega, Z.; McCourt, M.; Kearns, M.P.; Benitez, A.N. Recycling of polymeric fraction of cable waste by rotational moulding. Waste Manag. 2018, 76, 199–206. [Google Scholar] [CrossRef]
- Arslan, F.; Celik, C.; Arslan, C. Recycling of waste electrical cables. Mater. Sci. Eng. Int. J. 2019, 3, 107–111. [Google Scholar] [CrossRef]
- Chauhan, G.; Jadhao, P.R.; Pant, K.K.; Nigam, K.D.P. Novel technologies and conventional processes for recovery of metals from waste electrical and electronic equipment: Challenges & opportunities—A review. J. Environ. Chem. Eng. 2018, 6, 1288–1304. [Google Scholar] [CrossRef]
- Lee, J.S.; Cho, K.C.; Ku, K.H.; Clio, G.; Lee, J.H. Recyclable insulation material based n polyethylene for power cable. In Proceedings of the 2012 IEEE International Conference on Condition Monitoring and Diagnosis, Bali, Indonesia, 3–27 September 2012; pp. 88–90. [Google Scholar] [CrossRef]
- Linde, E.; Gedde, U.W. Plasticizer migration from PVC cable insulation—The challenged of extrapolation methods. Polym. Degrad. Stab. 2014, 101, 24–31. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Bakhshandeh, G.R. Recycling of PVC wastes. Polym. Degrad. Stab. 2011, 96, 404–415. [Google Scholar] [CrossRef]
- Tanskanen, P. Management and recycling of electronic waste. Acta Mater. 2013, 61, 1001–1011. [Google Scholar] [CrossRef]
- Polish Standardization Committee. PN-HD 308 S2:2007. Color Marking of Wires in Cables. Available online: https://sklep.pkn.pl/pn-hd-308-s2-2007p.html (accessed on 19 June 2023).
- Utilizing Wire Color Code Chart Standards. Available online: https://www.creativesafetysupply.com/articles/wire-color-codes/ (accessed on 7 August 2023).
- Available online: https://maszynydokabli.pl/produkt/en120 (accessed on 8 August 2023).
- Blinová, L.; Godovčin, P. Importance of Recycling the Waste-Cables Containing Copper and PVC. Res. Pap. Fac. Mater. Sci. Technol. Slovak. Univ. Technol. 2021, 29, 1–21. [Google Scholar] [CrossRef]
- Taizhou Guanglong Wire Stripping Machine Manufacturing Co., LTD. Electric Copper Wire Scrap Stripping Machine. Available online: https://www.scrap-wire-stripper.com/cable-stripping-machine/45995562.html (accessed on 8 August 2023).
- Cable Recycling Machinery. Waste Cables/Wires Stripping Machine. Available online: https://www.cablerecyclingmachinery.com (accessed on 8 August 2023).
- Arasan, S.; Hasiloglu, A.S.; Akbulut, S. Shape particle of natural and crushed aggregate using image analysis. Int. J. Civ. Struct. Eng. 2010, 1, 221–233. [Google Scholar]
- Mora, C.F.; Kwan, A.K.H. Sphericity, shape factor, and convexity measurement of coarse aggregate for concrete using digital image processing. Cem. Concr. Res. 2000, 30, 351–358. [Google Scholar] [CrossRef]
- Jindal, A.B. The effect of particle shape on cellular interaction and drug delivery applications of micro- and nanoparticles. Int. J. Pharm. 2017, 532, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Prieto, S.; Blanco-Mendez, J.; Otero-Espinar, F.J. Microscopic image analysis techniques for the morphological characterization of pharmaceutical particles: Influence of the software, and the factor algorithms used in the shape factor estimation. Eur. J. Pharm. Biopharm. 2007, 67, 766–776. [Google Scholar] [CrossRef]
- Yu, W.; Hancock, B.C. Evaluation of dynamic image analysis for characterizing pharmaceutical excipient particles. Int. J. Pharm. 2008, 361, 150–157. [Google Scholar] [CrossRef]
- Nouri, A.; Sola, A. Metal particle shape: A practical perspective. Met. Powder Rep. 2018, 73, 276–282. [Google Scholar] [CrossRef]
- Zavala, J.M.R. Particle Shape Quantities and Influence on Geotechnical Properties—A Review. Ph.D. Thesis, Luleå University of Technology, Luleå, Sweden, 2012. Available online: https://www.diva-portal.org/smash/get/diva2:994837/FULLTEXT01.pdf (accessed on 16 August 2023).
- Rodriguez, J.M.; Edeskar, T.; Knutsson, S. Particle Shape Quantities and Measurement Techniques—A review. EJGE 2013, 18, 169–198. [Google Scholar]
- Lu, J.; Xu, J.; Kumagi, S.; Kameda, T.; Saito, Y.; Yoshioka, T. Separation mechanism of polyvinyl chloride and copper components from swollen electric cables by mechanical agitation. Waste Manag. 2019, 93, 54–62. [Google Scholar] [CrossRef]
- Tateishi, I.; Furukawa, M.; Katsumata, H.; Kaneco, S. Metal and Molecular Vapor Separation Analysis for Direct Determination of Mn and Cu by Atomic Absorption Detection, Free of Background Absorption. Sustain. Chem. 2022, 3, 475–481. [Google Scholar] [CrossRef]
- Sudip, P.; Saikia, A.; Majhi, V.; Pandey, V.K. Introduction to Biomedical Instrumentation and Its Applications; Chapter 5—Analytical instruments; Academic Press: London, UK, 2022; ISBN 9780128219713. [Google Scholar]
- Vanhaecke, F.; Degryse, P. Isotopic Analysis. Fundamentals and Applications Using ICP-MS; Single Collector Inductively Coupled Plasma Mass Spectrometry; Wiley-VCH: Weinham, Germany, 2012. [Google Scholar]
- Senila, M.; Cadar, O.; Senila, L.; Böringer, S.; Seaudeau-Pirouley, K.; Ruiu, A.; Lacroix-Desmazes, P. Performance Parameters of Inductively Coupled Plasma Optical Emission Spectrometry and Graphite Furnace Atomic Absorption Spectrometry Techniques for Pd and Pt Determination in Automotive Catalysts. Materials 2020, 13, 5136. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.; Beal, M.; Maley, J.; Brinkmann, M. Qualitative and quantitative analysis of microplastics and microfiber contamination in effluents of the City of Saskatoon wastewater treatment plant. Environ. Sci. Pollut. Res. 2021, 28, 32545–32553. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Joshi, G.M.; Mukherjee, A.; Paramanandam, T. Electrical properties and thermal degradation of poly(vinyl chloride)/polyvinylidene fluoride/ZnO polymer nanocomposites. Polym. Int. 2016, 65, 1098–1106. [Google Scholar] [CrossRef]
- Alim, A.A.A.; Baharum, A.; Shirajuddin, S.S.M.; Annuar, F.H. Blending of Low-Density Polyethylene and Poly(Butylene Succinate) (LDPE/PBS) with Polyethylene–Graft–Maleic Anhydride (PE–g–MA) as a Compatibilizer on the Phase Morphology, Mechanical and Thermal Properties. Polymers 2023, 15, 261. [Google Scholar] [CrossRef]
- Ibrahim, H.; Ramadan, M.; Ahmed, H.A.M.; Ahmed, S.M.; Abualsoud, R.M.; Motawie, A.M.; Thermal Degradation of Four Types of Plastic Solid Waste HDPE, LDPE, PP, PS. 2018. Available online: https://www.researchgate.net/publication/326625592_Thermal_Degradation_of_Four_Types_of_Plastic_Waste (accessed on 19 June 2023). preprint.
- Muhammad, C.; Onwudili, J.A.; Williams, P.T. Thermal Degradation of Real-World Waste Plastics and Simulated Mixed Plastics in a Two-Stage Pyrolysis–Catalysis Reactor for Fuel Production. Energy Fuels 2015, 29, 2601–2609. [Google Scholar] [CrossRef]
- Dodbiba, G.; Fujita, T. Progress in separating plastic materials for recycling. Phys. Separ. Sci. Eng. 2004, 13, 165–182. [Google Scholar] [CrossRef]
- Shent, H.; Pugh, R.J.; Forssberg, E. A review of plastics waste recycling and the flotation of plastics. Resour. Conserv. Recycl. 1999, 25, 85–109. [Google Scholar] [CrossRef]
- Tsuchimoto, I.; Kajikawa, Y. Recycling of Plastic Waste: A Systematic Review Using Bibliometric Analysis. Sustainability 2022, 14, 16340. [Google Scholar] [CrossRef]
- The International Bromine Council. Impact of Brominated Flame Retardants on the Recycling of WEEE Plastics. Available online: https://www.bsef.com (accessed on 24 August 2023).
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Wang, C.Q.; Wang, H.; Fu, J.G.; Liu, Y.N. Flotation Separation of waste plastics for recycling—A review. Waste Manag. 2015, 41, 28–38. [Google Scholar] [CrossRef]
- Yenial, Ü.; Burat, F.; Yüce, A.E.; Guney, A.; Kangal, O. Separation of PET and PVC by Flotation Technique Without Using Alkaline Treatment. Miner. Process. Extr. Metall. Rev. 2013, 34, 412–421. [Google Scholar] [CrossRef]
- Phengsaart, T.; Srichonphaisan, P.; Kertbundit, C.; Soonthornwiphat, N.; Sinthugoot, S.; Phumkokrux, N.; Juntarasakul, O.; Maneeintr, K.; Numprasanthai, A.; Park, I.; et al. Conventional and recent advances in gravity separation technologies for coal cleaning: A systematic and critical review. Heliyon 2023, 9, e13083. [Google Scholar] [CrossRef] [PubMed]
- Aketi, V.A.; Vakamalla, T.; Narasimha, M.; Sreedhar, G.E.; Shivakumar, R.; Kumar, R. Computational fluid dynamic study on the effect of near gravity material on dense medium cyclone treating coal using discrete phase model and algebraic slip mixture multiphase model. J. Comput. Multiph. Flows 2016, 9, 58–70. [Google Scholar] [CrossRef]
- Plastic Recovery Systems: Process for the Separation and Recovery of Plastics. International Patent No. WO91 04100, 4 April 1991.
- Bertram, A.; Unkelbach, K.H. High duty plastics recycling with the aid of sorting centrifuges. In Proceedings of the XX International Mineral Processing Congress, Aachen, Germany, 21–26 September 1997; Volume 5, pp. 373–382. [Google Scholar]
- Negari, M.S.; Movahed, S.O.; Ahmadpour, A. Separation of polyvinylchloride (PVC), polystyrene (PS) and polyethylene terephthalate (PET) granules using various chemical agents by flotation technique. Sep. Purif. Technol. 2018, 194, 368–376. [Google Scholar] [CrossRef]
- Araujo, A.C.; Viana, P.R.M.; Peres, A.E.C. Reagents in iron ores flotation. Miner. Eng. 2005, 18, 219–224. [Google Scholar] [CrossRef]
- Pearse, M.J. An overview of the use of chemical reagents in mineral processing. Miner. Eng. 2005, 18, 139–149. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.L.; Bai, Y.; Guo, C.; Zhang, L. Application of dissolved air flotation on separation of waste plastics ABS and PS. Waste Manag. 2012, 32, 1297–1305. [Google Scholar] [CrossRef]
- Fraunholcz, N. Separation of waste plastics by froth flotation—A review, part I. Miner. Eng. 2004, 17, 261–268. [Google Scholar] [CrossRef]
- Kokklic, O.; Mohammadi-Jam, S.; Chu, P.; Marion, C.; Yang, Y.; Waters, K.E. Separation of plastic wastes using froth flotation—An overview. Adv. Colloid. Interface Sci. 2022, 308, 102769. [Google Scholar] [CrossRef]
- Marques, G.A.; Tenório, J.A.S. Use of froth flotation to separate PVC/PET mixtures. Waste Manag. 2000, 20, 265–269. [Google Scholar] [CrossRef]
- Burat, F.; Guney, A.; Kangal, M.O. Selective separation of virgin and post-consumer polymers (PET and PVC) by flotation method. Waste Manag. 2009, 29, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Metallurgist Process Equipment. Magnetic Separators. Available online: https://www.911metallurgist.com/equipment/magnetic-separators (accessed on 23 August 2023).
- Zhan, H.; Wang, L.; Wang, T.; Yu, J. The Influence and Compensation Method of Eccentricity for Cylindrical Specimens in Eddy Current Displacement Measurement. Sensor 2020, 20, 6608. [Google Scholar] [CrossRef] [PubMed]
- Borovik, S.; Kuteynikova, M.; Sekisov, Y. Reducing the Impact of Influence Factors on the Measurement Results from Single-Coil Eddy Current Sensors. Sensors 2023, 23, 351. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.R.; Nagel, J.R.; Rajamani, R.K. Eddy current separation for recovery of non-ferrous metallic particles: A comprehensive review. Miner. Eng. 2019, 133, 149–159. [Google Scholar] [CrossRef]
- Hadi, P.; Ning, C.; Ouyang, W.; Xu, M.; Lin, C.S.K.; Mckay, G. Toward environmentally-benign utilization of nonmetallic fraction of waste printed circuit boards as modifier and precursor. Waste Manag. 2015, 35, 236–246. [Google Scholar] [CrossRef]
- Wang, D.; Ma, X.; Zhi, X.; Zhang, S. Research Review of Scrap Metals Eddy Current Separation Technology. Sens. Transducers 2013, 158, 242–248. [Google Scholar]
- Fenercioğlu, A.; Barutcu, H. Separation of Granule Non-Ferrous Metals in Shredded Cable Waste with Eddy Current Separator. In Proceedings of the World Congress on Mechanical, Chemical and Material Engineering (MCM 2015), Barcelona, Spain, 20–21 July 2015. Paper No. 250. [Google Scholar]
- Kang, H.Y.; Schoenung, J.M. Electronic Waste Recycling: A Review of U.S. Infrastructure and Technology Options. Resour. Conserv. Recycl. 2005, 45, 368–400. [Google Scholar] [CrossRef]
- Xue, M.; Li, J.; Xu, Z. Management strategies on the industrialization road of state-of-the-art technologies for e-waste recycling: The case study of electrostatic separation—A review. Waste Manag. Res. 2013, 31, 130–140. [Google Scholar] [CrossRef]
- Veit, H.M.; Diehl, T.R.; Salami, A.P.; Rodrigues, J.S.; Bernardes, A.M.; Tenorio, J.A.S. Utilization of magnetic and electrostatic separation in the recycling of printed circuit boards scrap. Waste Manag. 2005, 25, 67–74. [Google Scholar] [CrossRef]
- Dascalescu, L.; Iuga, A.; Morar, R.; Neamtu, V.; Suarasan, I.; Samuila, A.; Rafiroiu, D. Corona and electrostatic electrodes for high-tension separators. J. Electrostat. 1993, 29, 211–225. [Google Scholar] [CrossRef]
- Zhang, S.; Forssberg, E. Optimization of electrodynamic separation for metals recovery from electronic scrap. Resour. Conserv. Recycl. 1998, 22, 143–162. [Google Scholar] [CrossRef]
- Fang, J.C.; Wen, T. A Wide Linear Range Eddy Current Displacement Sensor Equipped with Dual-Coil Probe. Applied in the Magnetic Suspension Flywheel. Sensors 2012, 12, 10693–10706. [Google Scholar] [CrossRef] [PubMed]
- Garforth, A.A.; Ali, S.; Hernández-Martínez, J.; Akah, A. Feedstock recycling of polymer wastes. Curr. Opin. Solid State Mater. 2004, 8, 419–425. [Google Scholar] [CrossRef]
- Kameda, T.; Ono, M.; Grause, G.; Mizoguchi, T.; Yoshioka, T. Ball Mill-Assisted Dechlorination of Flexible and Rigid Poly (Vinyl Chloride) in NaOH/EG Solution. Ind. Eng. Chem. Res. 2008, 47, 8619–8624. [Google Scholar] [CrossRef]
- Lu, J.; Borjigin, S.; Kumagai, S.; Kameda, T.; Saito, Y.; Yoshioka, T. Practical Dechlorination of Polyvinyl Chloride Wastes in NaOH/Ethylene Glycol Using an up-Scale Ball Mill Reactor and Validation by Discrete Element Method Simulations. Waste Manag. 2019, 99, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, K.; Skórczewska, K. A Brief Review of Poly(Vinyl Chloride) (PVC) Recycling. Polymers 2022, 14, 3035. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, K.; Mabuchi, K. Formation characteristics of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and coplanar PCBs in fly ash from a gasification—Melting process of municipal solid waste. J. Mater. Cycles Waste Manag. 2001, 3, 38–47. [Google Scholar]
- Kalali, E.N.; Lotfian, S.; Shabestari, M.E.; Khayatzadeh, S.; Zhao, C.; Nezhad, H.Y. A critical review of the current progress of plastic waste recycling technology in structural materials. Curr. Opin. Green Sustain. Chem. 2023, 40, 100763. [Google Scholar] [CrossRef]
- Lehr, A.L.; Heider, K.L.; Aboagye, E.A.; Chea, J.D.; Stengel, J.P.; Benavides, P.T.; Yenkie, K.M. Design of solvent-assisted plastics recycling: Integrated economics and environmental impacts analysis. Front. Sustain. 2022, 3, 989720. [Google Scholar] [CrossRef]
- Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef]
- Beghetto, V.; Sole, R.; Buranello, C.; Al-Abkal, M.; Facchin, M. Recent Advancements in Plastic Packaging Recycling: A Mini-Review. Materials 2021, 14, 4782. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, D.; Gros, J.; Fangerow, N.; Werner, T.; Colloseus, C.; Taha, I. Recyclebot—Using robots for sustainable plastic recycling. Procedia CIRP 2023, 116, 275–280. [Google Scholar] [CrossRef]
- Álvarez-de-los-Mozos, E.; Rentería-Bilbao, A.; Díaz-Martín, F. WEEE Recycling and Circular Economy Assisted by Collaborative Robots. Appl. Sci. 2020, 10, 4800. [Google Scholar] [CrossRef]
- Friedrich, K.; Koinig, G.; Fritz, T.; Pomberger, R.; Vollprecht, D. Sensor-based and Robot Sorting Processes and their Role in Achieving European Recycling Goals—A Review. Academ. J. Polym. Sci. 2022, 5, 555668. [Google Scholar] [CrossRef]
- Kshirsagar, P.R.; Kumar, N.; Almulihi, A.H.; Alassery, F.; Khan, A.I.; Islam, S.; Rothe, J.P.; Jagannadham, D.B.V.; Dekeba, K. Artificial Intelligence-Based Robotic Technique for Reusable Waste Materials. Comput. Intell. Neurosci. 2022, 2022, 2073482. [Google Scholar] [CrossRef]
- Madhav, A.V.S.; Rajaraman, R.; Harini, S.; Kiliroor, C.C. Application of artificial intelligence to enhance collection of E-waste: A potential solution for household WEEE collection and segregation in India. Waste Manag. Res. 2022, 40, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Abdulhasan, M.J.; Kumar, N.M.; Abdulkareem, K.H.; Mostafa, S.A.; Maashi, M.S.; Khalid, L.S.; Abdulaali, H.S.; Chopra, S.S. Automated waste-sorting and recycling classification using artificial neural network and features fusion: A digital-enabled circular economy vision for smart cities. Multimed. Tools Appl. 2022, 1–16. [Google Scholar] [CrossRef]
- Ziouzios, D.; Baras, N.; Balafas, V.; Dasygenis, M.; Stimoniaris, A. Intelligent and Real-Time Detection and Classification Algorithm for Recycled Materials Using Convolutional Neural Networks. Recycling 2022, 7, 9. [Google Scholar] [CrossRef]
- Fang, B.; Yu, J.; Chen, Z.; Osman, A.I.; Farghali, M.; Ihara, I.; Hamza, E.H.; Rooney, D.W.; Yap, P.S. Artificial intelligence for waste management in smart cities: A review. Environ. Chem. Lett. 2023, 21, 1959–1989. [Google Scholar] [CrossRef]
- Basak, S.N.; Meena, S.S. Microbial biodegradation of plastics: Challenges, opportunities, and a critical perspective. Front. Environ. Sci. Eng. 2022, 16, 161. [Google Scholar] [CrossRef]
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and Biotechnological Perspectives on Microbial Degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, A.K.; Bhatt, K. Biodegradation of polythenes by bacteria isolated from soil. Int. J. Res. Dev. Pharm. Life Sci. 2016, 5, 2056–2062. [Google Scholar]
- Kyaw, B.M.; Champakalakshim, R.; Sakharkar, M.K.; Lim, C.S.; Sakharkar, K.R. Biodegradation of Low Density Polythene (LDPE) by Pseudomonas Species. Indian J. Microbiol. 2012, 52, 411–419. [Google Scholar] [CrossRef]
- Rad, M.M.; Moghimi, H.; Azin, E. Biodegradation of thermo-oxidative pretreated low-density polyethylene (LDPE) and polyvinyl chloride (PVC) microplastics by Achromobacter denitrificans Ebl13. Mar. Pollut. Bull. 2022, 181, 113830. [Google Scholar] [CrossRef]
- Khan, S.; Ali, S.A.; Ali, A.S. Biodegradation of low density polyethylene (LDPE) by mesophilic fungus ‘ Penicillium citrinum’ isolated from soils of plastic waste dump yard, Bhopal, India. Environ. Technol. 2023, 44, 2300–2314. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.K.; Sharma, K.K.; Chandra, H. Utilization of Bacillus cereus strain CGK5 associated with cow feces in the degradation of commercially available high-density polyethylene (HDPE). Arch. Microbiol. 2023, 205, 101. [Google Scholar] [CrossRef] [PubMed]
- Orlando, M.; Molla, G.; Castellani, P.; Pirillo, V.; Torretta, V.; Ferronato, N. Microbial Enzyme Biotechnology to Reach Plastic Waste Circularity: Current Status, Problems and Perspectives. Int. J. Mol. Sci. 2023, 24, 3877. [Google Scholar] [CrossRef] [PubMed]
- Kusenberg, M.; Eschenbacher, A.; Djokic, M.R.; Zayoud, A.; Ragaert, K.; Meester, S.D.; Van Geem, K.M. Opportunities and challenges for the application of post-consumer plastic waste pyrolysis oils as steam cracker feedstocks: To decontaminate or not to decontaminate? Waste Manag. 2022, 138, 83–115. [Google Scholar] [CrossRef]



| Cable | Type | Construction | Application | Ref. |
|---|---|---|---|---|
| Phelps Dodge Cable | 60227 IEC 01 | Insulation: PVC. Core: annealed copper (stranded or solid). | Building wiring. | [21,22,23,24,25,26,27] |
| 60227 IEC 10 | Insulation: PVC. Core: annealed copper (stranded or solid). Depending on requirements can be concentric or compact. | Surface mounting exposed. Permissible wet and dry conditions. | ||
| Medium Voltage Crosslinked Polyethylene | Insulation: crosslinked polyethylene. Core: copper-stranded wire, metallic shield. | Cabling of urban networks, large agglomerations. Possible use in underground conditions. | ||
| 0.6/1 (1.2) kV Fire Resistant Low and Halogen Free | Insulation: crosslinked polyethylene. Core: two variants—copper wire round concentric or round compact. | Provision of energy for air canal, cable resources, underground. | ||
| CV-AWA | Insulation: crosslinked polyethylene. Core: two variants—concentric stranded annealed copper or compact round stranded annealed copper. | Providing energy in dry and wet conditions. For air canals and underground excavations. | ||
| Medium Voltage Crosslinked Polyethylene Cable | Insulation: crosslinked polyethylene. Core: round and compact copper wire. | Urban networks, ducts, underground. Aerial installations. | ||
| AL-PE Sheathed Cable | Insulation: PP or PE. Core: solid annealed copper. | Distribution and telephone lines. |
| Type of Material | Temp. [°C] | Liquid [%] | Char [%] | Gases [%] | Density at 20 °C, [g/cm3] |
|---|---|---|---|---|---|
| LDPE | 280–420 | 92,16 | 2.1% | 35 | 0.7821 |
| HDPE | 310–450 | 90,06 | 1.6 | 48 | 0.7962 |
| PP | 260–430 | 89,98 | 3.65 | 45 | 0.7816 |
| PS | 260–330 | 82,04 | 16.07 | 64 | 0.9127 |
| Reagent | Medium Content (Frother) | Configuration of Use Mixture Plastic | Reagent Concentration [% vol] | Flotation Recovery [%] |
|---|---|---|---|---|
| Methanol | Methyl Isobutyl Carbinol (190 mg/L) | PET and PVC | 5 | 95 PET |
| Alkyl | Methyl Isobutyl Carbinol with NaOH, HCl pH regulator, pH = 10 | PVC, PC | n/a | 97 PC |
| Calcium lignosulfonate | Pine oil (three to five drops), pH = 12 | PVC and PET | 0.03 | 95 PVC |
| Epoxidized linseed oil (ELO) | Methyl Isobutyl Carbinol, pH = 12.4 | PET, PVC | 0.03 | 93 PET |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wędrychowicz, M.; Kurowiak, J.; Skrzekut, T.; Noga, P. Recycling of Electrical Cables—Current Challenges and Future Prospects. Materials 2023, 16, 6632. https://doi.org/10.3390/ma16206632
Wędrychowicz M, Kurowiak J, Skrzekut T, Noga P. Recycling of Electrical Cables—Current Challenges and Future Prospects. Materials. 2023; 16(20):6632. https://doi.org/10.3390/ma16206632
Chicago/Turabian StyleWędrychowicz, Maciej, Jagoda Kurowiak, Tomasz Skrzekut, and Piotr Noga. 2023. "Recycling of Electrical Cables—Current Challenges and Future Prospects" Materials 16, no. 20: 6632. https://doi.org/10.3390/ma16206632





