The Influence of Titanium Dioxide Nanosheet on Water Permeability of Silicone Rubber after Nitrogen Dioxide Aging Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparations
2.3. Characterization
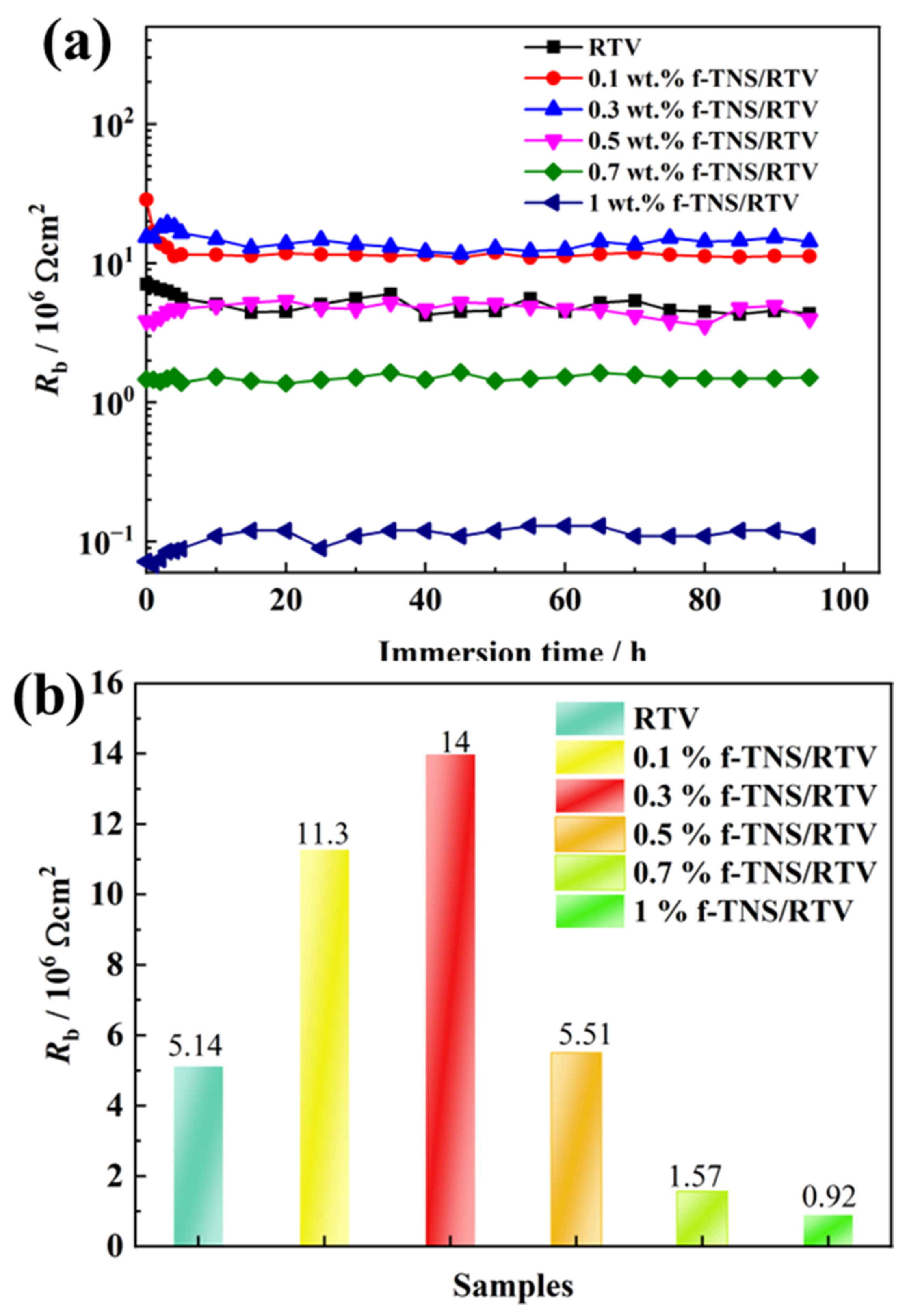
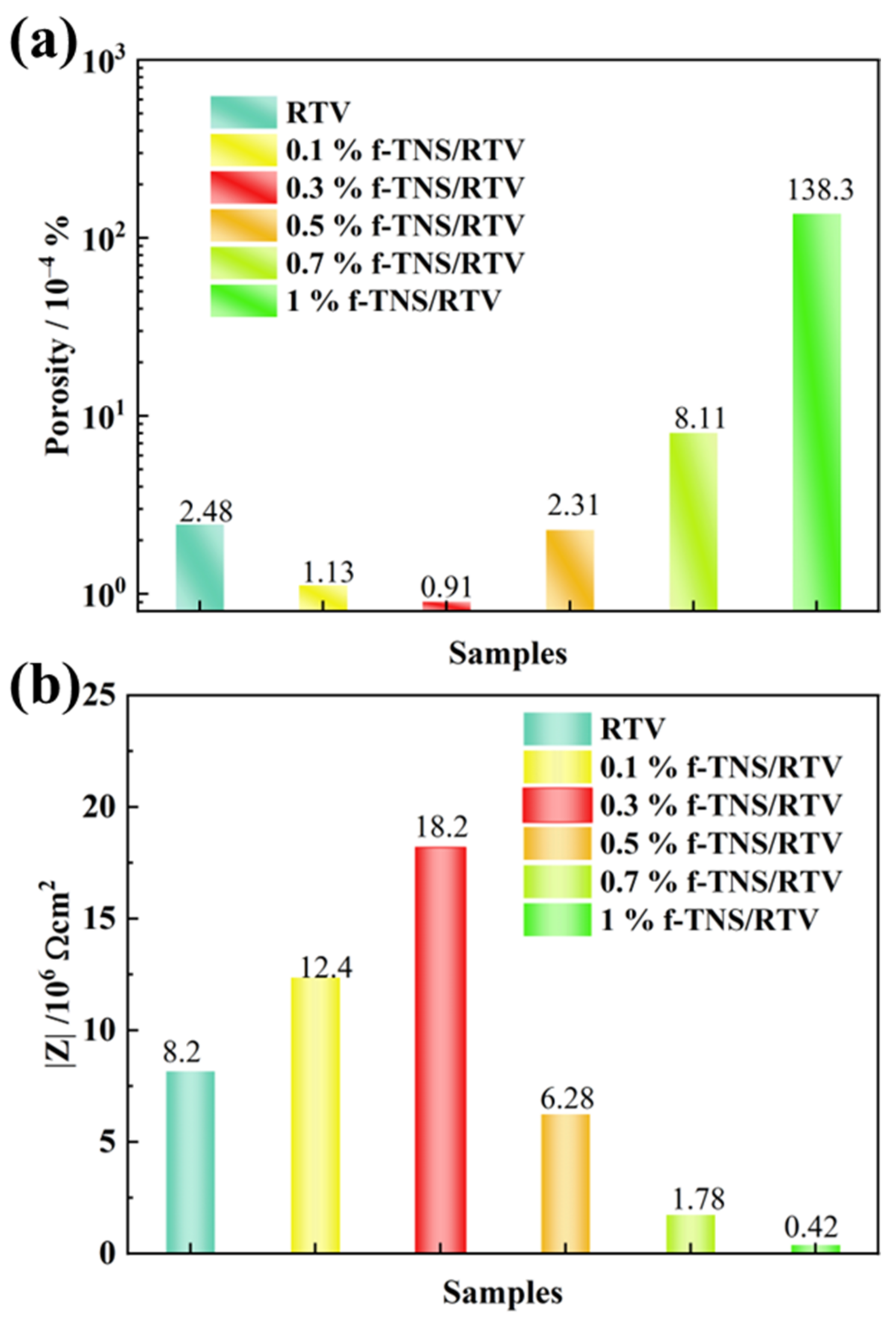
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Guan, Z.C.; Ha, Z.D. The developments of room temperature vulcanized silicone rubber coating and its application in China. In Proceedings of the 1st International Conference and Exhibition on Transmission and Distribution in the Asia Pacific Region, Yokohama, Japan, 6–10 October 2002; pp. 2203–2206. [Google Scholar]
- Cherney, E.A.; Gorur, R.S. RTV silicone rubber coatings for outdoor insulators. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 605–611. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Yan, C.; Hou, T.Q.; Dou, H.L.; Shen, H. Multifunctional Ti3C2Tx MXene-Based Composite Coatings with Superhydrophobic Anti-icing and Photothermal Deicing Properties. ACS Appl. Mater. Interfaces 2022, 14, 26077–26087. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Alam, M.N.; Manikkavel, A.; Song, M.; Lee, D.J.; Park, S.S. Silicone Rubber Composites Reinforced by Carbon Nanofillers and Their Hybrids for Various Applications: A Review. Polymers 2021, 13, 2322. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, A.; Alam, M.N.; Park, S.S. Effect of graphite nanoplatelets surface area on mechanical properties of room-temperature vulcanized silicone rubber nanocomposites. J. Appl. Polym. Sci. 2022, 139, e52503. [Google Scholar] [CrossRef]
- Li, Y.W.; Song, Z.B.; Tian, Y.; Yang, C.; Liu, F.L.; Bai, H.; Zhao, F.P.; Liu, X.W.; Yang, H. Study on the Algae Contamination and Its Effects on the Properties of RTV-Coated Insulators. Energies 2022, 15, 5216. [Google Scholar] [CrossRef]
- Wang, S.W.; Zou, Q.G.; Zhao, X.G.; Chen, J.Y.; Li, L.; Chen, J.W.; Xie, Y.; Yang, K. Predicting the DC pollution flashover voltage on the insulation surfaces with superhydrophobicity. Colloid Surf. A Physicochem. Eng. Asp. 2022, 646, 128987. [Google Scholar] [CrossRef]
- Zhou, K.; Li, T.H.; Chen, Z.L.; Yang, D.; Tao, W.B. The Relationship between Microstructure and Hydrophobicity of Service-aged Silicone Rubber Insulators. In Proceedings of the International Conference on Condition Monitoring and Diagnosis (CMD), Xi’an, China, 25–28 September 2016; pp. 952–955. [Google Scholar]
- Wang, X.Y.; Zhou, J.; Meng, R.; Zhao, Y.; Guo, W.H. Preparation and properties of room-temperature-vulcanized silicone rubber using modified dopamine as a crosslinking agent. Mater. Res. Express 2022, 9, 045304. [Google Scholar] [CrossRef]
- Sit, K.; Pradhan, A.K.; Chatterjee, B.; Dalai, S. A Review on Characteristics and Assessment Techniques of High Voltage Silicone Rubber Insulator. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1889–1903. [Google Scholar] [CrossRef]
- Mahmood, A.; Alam, S. RTV-SiR based composites aged in a multi-stressed environment under AC and bipolar DC voltages. J. Elastomers Plast. 2022, 54, 1091–1111. [Google Scholar] [CrossRef]
- Mishra, P.; Paul, M.; Vinod, P.; Sarathi, R.; Kornhuber, S. Performance evaluation of room temperature vulcanized silicone rubber nanocomposites aged in strong aqueous solutions. Polym. Eng. Sci. 2022, 62, 1619–1630. [Google Scholar] [CrossRef]
- Englert, M.; Minister, F.; Moussaoui, A.; Pisula, W. Mechanical properties of thermo-oxidative aged silicone rubber thermally stabilized by titanium oxide based fillers. Polym. Test 2022, 115, 107726. [Google Scholar] [CrossRef]
- Wu, J.; Dong, J.Y.; Wang, Y.S.; Gond, B.K. Thermal oxidation ageing effects on silicone rubber sealing performance. Polym. Degrad. Stabil. 2017, 135, 43–53. [Google Scholar] [CrossRef]
- Li, J.g.; Lu, Y.K. Status Quo and Prospect of Research on Contamination Characteristics of Insulators. Guangdong Electr. Power 2018, 31, 18–23. [Google Scholar] [CrossRef]
- Bleszynski, M.; Kumosa, M. Silicone Rubber RTV-1 Aging in the Presence of Aqueous Salt. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2822–2829. [Google Scholar] [CrossRef]
- Bleszynski, M.; Kumosa, M. Silicone rubber aging in electrolyzed aqueous salt environments. Polym. Degrad. Stabil. 2017, 146, 61–68. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhao, J.J.; Wan, X.D.; Jiang, X.L.; Hu, J.L. Mechanical Properties of High Temperature Vulcanized Silicone Rubber Aged in the Natural Environment. Polymers 2022, 14, 4439. [Google Scholar] [CrossRef]
- Zhang, J.S.; Du, S.W.; Wang, Z.; Qian, L.B.; He, C.Q.; Huang, Z.; Peng, X.Y.; Xu, H.; Fang, P.F. Prominently enhanced corrosive gas NO2 resistibility for silicone rubber composite coatings by incorporation of functional g-C3N4 nanosheets. Prog. Org. Coat. 2021, 157, 106292. [Google Scholar] [CrossRef]
- Fang, J.P.; Luo, Y.; Kuang, S.L.; Luo, K.; Xiao, Z.K.; Peng, X.Y.; Huang, Z.; Wang, Z.; Fang, P.F. Effect of NO2 Aging on the Surface Structure and Thermal Stability of Silicone Rubber with Varying Al(OH)3 Contents. Materials 2023, 16, 2540. [Google Scholar] [CrossRef]
- Ogbonna, V.E.; Popoola, P.I.; Popoola, O.M.; Adeosun, S.O. A comparative study on the failure analysis of field failed high voltage composite insulator core rods and recommendation of composite insulators: A review. Eng. Fail. Anal. 2022, 138, 106369. [Google Scholar] [CrossRef]
- Tao, B.; Cheng, L.; Wang, J.Y.; Zhang, X.L.; Liao, R.J. A review on mechanism and application of functional coatings for overhead transmission lines. Front. Mater. 2022, 9, 995290. [Google Scholar] [CrossRef]
- Cui, Y.B.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef]
- Lange, S.; Arroval, T.; Saar, R.; Kink, I.; Aarik, J.; Krumme, A. Oxygen Barrier Properties of Al2O3- and TiO2-coated LDPE Films. Polym.-Plast. Technol. Eng. 2015, 54, 301–304. [Google Scholar] [CrossRef]
- Roilo, D.; Maestri, C.A.; Scarpa, M.; Bettotti, P.; Checchetto, R. Gas barrier and optical properties of cellulose nanofiber coatings with dispersed TiO2 nanoparticles. Surf. Coat. Technol. 2018, 343, 131–137. [Google Scholar] [CrossRef]
- Harito, C.; Bavykin, D.V.; Light, M.E.; Walsh, F.C. Titanate nanotubes and nanosheets as a mechanical reinforcement of water-soluble polyamic acid: Experimental and theoretical studies. Compos. Part B Eng. 2017, 124, 54–63. [Google Scholar] [CrossRef]
- Hara, T.; Nishimura, S.; Ozawa, S.; Abe, R.; Tokudome, Y.; Takahashi, M. Graphene oxide incorporation in lamellar organosiloxane film for improved water vapor barrier property. J. Sol-Gel Sci. Technol. 2016, 79, 405–409. [Google Scholar] [CrossRef]
- Wen, J.H.; Cui, Y.; Huang, D.; Li, Y.; Yu, X.C.; Zhang, X.P.; Meng, X.Y.; Zhou, Q. Mechanical, rheological, and carbon dioxide barrier properties of polyvinylidene fluoride/TiO2 composites for flexible riser applications. J. Appl. Polym. Sci. 2022, 139, e52897. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, L.Q.; Zhang, H.; Lu, Y.L. Preparation, Microstructure, and Property of Silicon Rubber/Organically Modified Montmorillonite Nanocomposites and Silicon Rubber/OMMT/Fumed Silica Ternary Nanocomposites. Polym. Compos. 2011, 32, 1245–1253. [Google Scholar] [CrossRef]
- Chen, D.; Yu, W.C.; Wei, L.; Ni, J.S.; Li, H.; Chen, Y.X.; Tian, Y.F.; Yan, S.S.; Mei, L.M.; Jiao, J. High sensitive room temperature NO2 gas sensor based on the avalanche breakdown induced by Schottky junction in TiO2-Sn3O4 nanoheterojunctions. J. Alloys Compd. 2022, 912, 165079. [Google Scholar] [CrossRef]
- Liu, S.W.; Wang, M.Y.; Liu, G.W.; Wan, N.; Ge, C.X.; Hussain, S.; Meng, H.N.; Wang, M.S.; Qiao, G.J. Enhanced NO2 gas-sensing performance of 2D Ti3C2/TiO2 nanocomposites by in-situ formation of Schottky barrier. Appl. Surf. Sci. 2021, 567, 150747. [Google Scholar] [CrossRef]
- Shi, H.W.; Liu, F.C.; Yang, L.H.; Han, E.H. Characterization of protective performance of epoxy reinforced with nanometer-sized TiO2 and SiO2. Prog. Org. Coat. 2008, 62, 359–368. [Google Scholar] [CrossRef]
- Liu, X.W.; Xiong, J.P.; Lv, Y.W.; Zuo, Y. Study on corrosion electrochemical behavior of several different coating systems by EIS. Prog. Org. Coat. 2009, 64, 497–503. [Google Scholar] [CrossRef]
- Mansfeld, F. Use of Electrochemical impedance spectroscopy for the study of corrosion protection by polymer-coatings. J. Appl. Electrochem. 1995, 25, 187–202. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Xing, J.J.; Qian, J.H.; Liu, L. Preparation of a TiO2@o-Vanillin@TEOS-APTES Nanocontainer and Its Anti-Corrosion Performance on Steel. China Pet. Process. Petrochem. Technol. 2022, 24, 36–50. [Google Scholar]
- Bondarenko, L.; Saveliev, Y.; Chernyaev, D.; Baimuratova, R.; Dzhardimalieva, G.; Dzeranov, A.; Kelbysheva, E.; Kydralieva, K. A statistical design approach to the sol-gel synthesis of (amino)organosilane hybrid nanoparticles. Phys. Chem. Chem. Phys. 2023, 25, 15862–15872. [Google Scholar] [CrossRef] [PubMed]
- Norizan, N.F.I.; Hamidon, T.S.; Hussin, M.H. Corrosion-resistant performance of mild steel employing flavonoids and phenols-rich Beta vulgaris extract-doped hybrid sol-gel coatings. J. Sol-Gel Sci. Technol. 2023, 107, 347–362. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, P.F.; Cheng, Y.L.; Gao, Y.P.; Chen, F.T.; Liu, Z.; Dai, Y.Q. Study on enhanced photocatalytic performance of cerium doped TiO2-based nanosheets. Chem. Eng. J. 2013, 219, 478–485. [Google Scholar] [CrossRef]
- Chen, F.T.; Liu, Z.; Liu, Y.; Fang, P.F.; Dai, Y.Q. Enhanced adsorption and photocatalytic degradation of high-concentration methylene blue on Ag2O-modified TiO2-based nanosheet. Chem. Eng. J. 2013, 221, 283–291. [Google Scholar] [CrossRef]

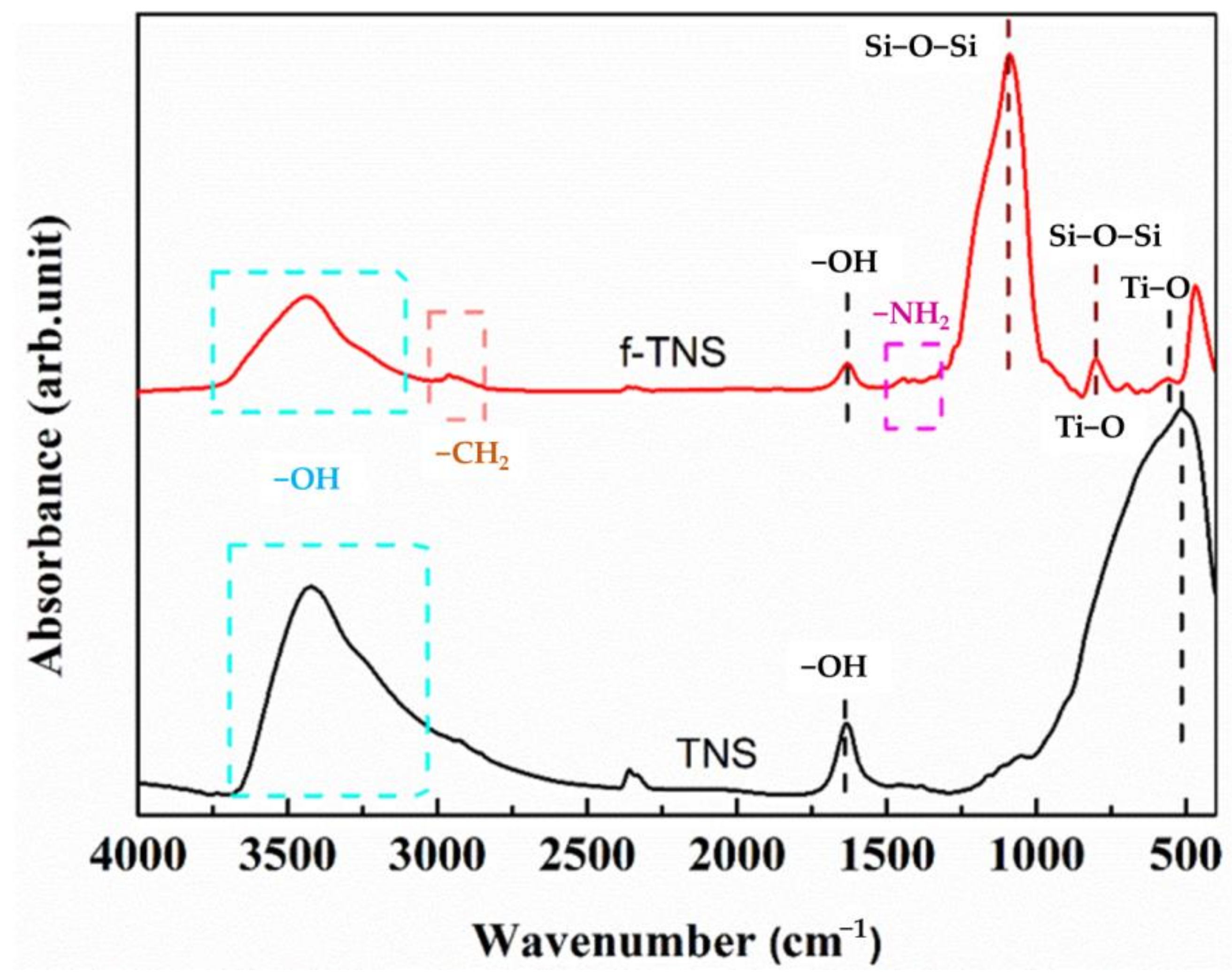
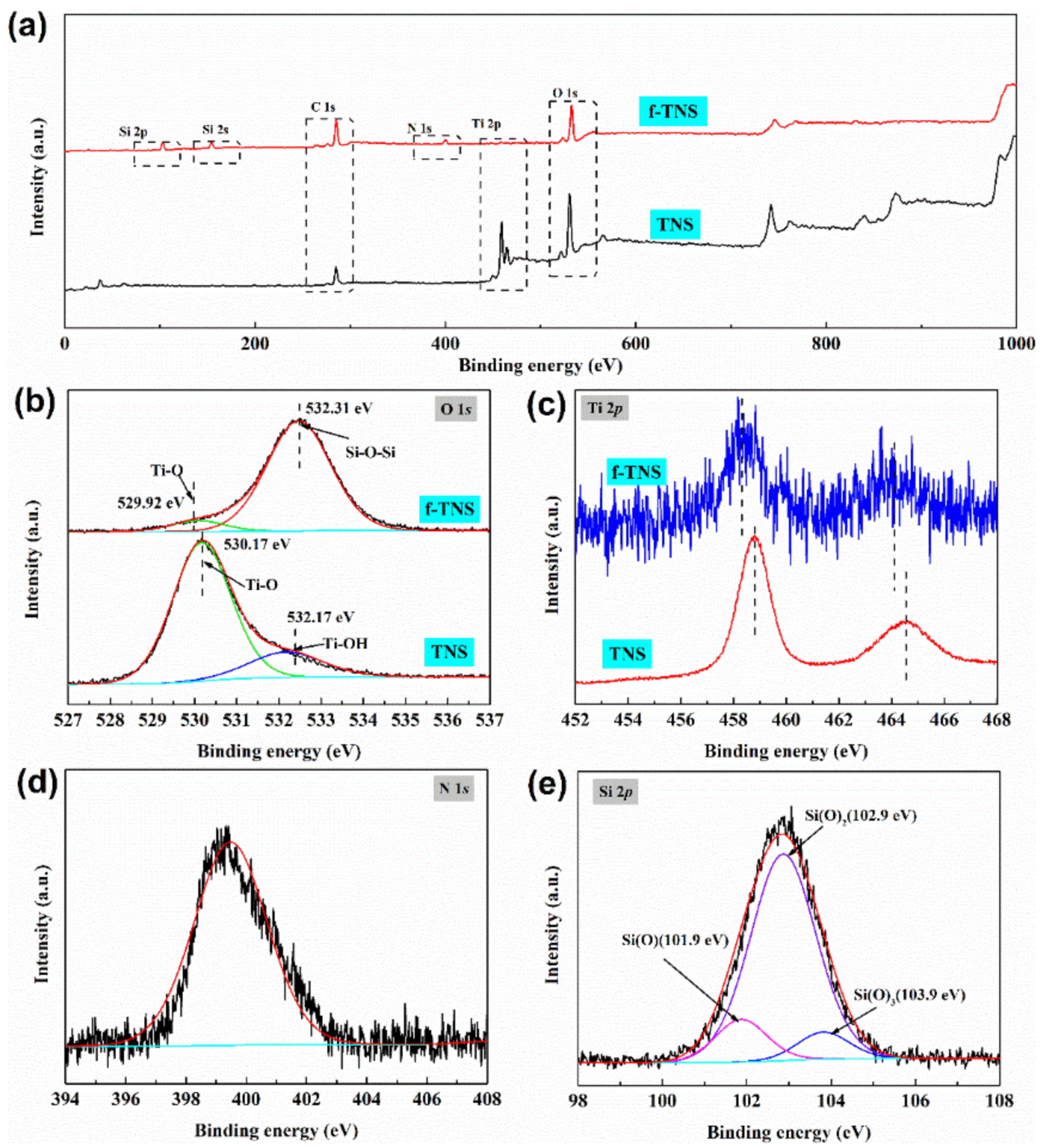
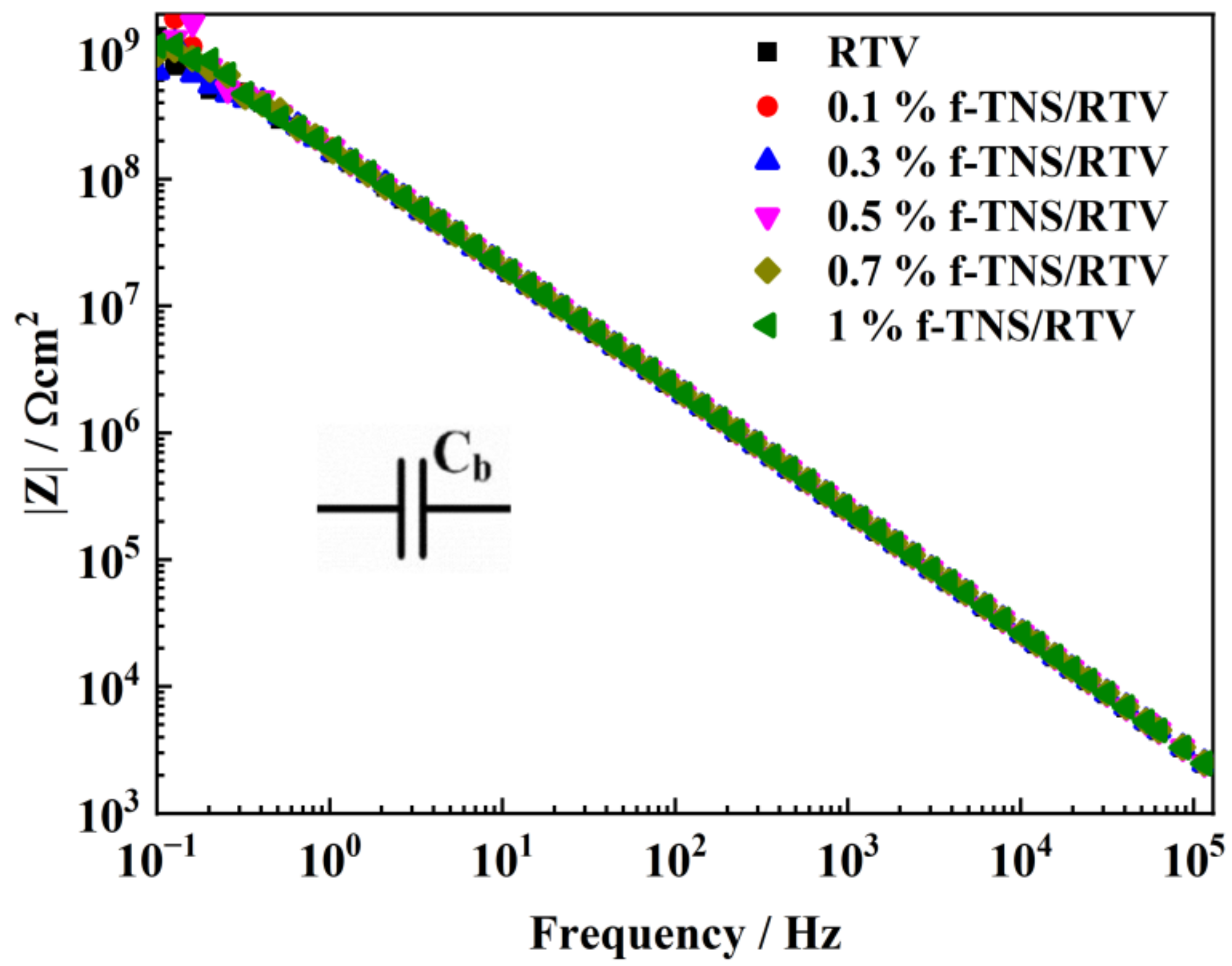

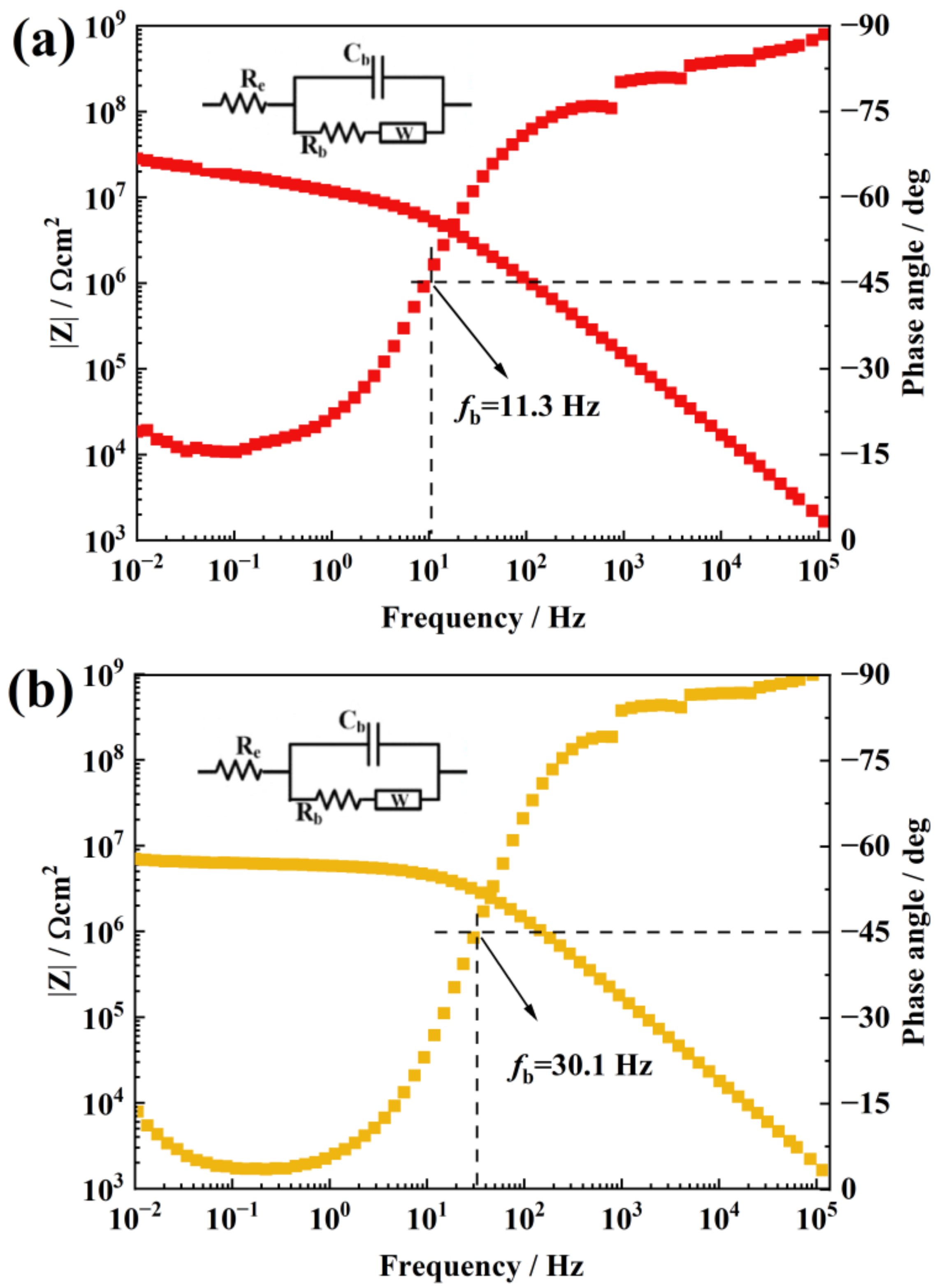


| C/at % | N/at % | O/at % | Ti/at % | Si/at % | |
|---|---|---|---|---|---|
| TNS | 34.51 | / | 46.91 | 18.58 | / |
| f-TNS | 49.11 | 4.25 | 27.19 | 1.58 | 17.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Zhang, J.; Fang, J.; Wang, Z.; Huang, Z.; Kuang, S.; He, C. The Influence of Titanium Dioxide Nanosheet on Water Permeability of Silicone Rubber after Nitrogen Dioxide Aging Treatment. Materials 2023, 16, 7138. https://doi.org/10.3390/ma16227138
Peng X, Zhang J, Fang J, Wang Z, Huang Z, Kuang S, He C. The Influence of Titanium Dioxide Nanosheet on Water Permeability of Silicone Rubber after Nitrogen Dioxide Aging Treatment. Materials. 2023; 16(22):7138. https://doi.org/10.3390/ma16227138
Chicago/Turabian StylePeng, Xiangyang, Jinshuai Zhang, Jiapeng Fang, Zheng Wang, Zhen Huang, Shilong Kuang, and Chunqing He. 2023. "The Influence of Titanium Dioxide Nanosheet on Water Permeability of Silicone Rubber after Nitrogen Dioxide Aging Treatment" Materials 16, no. 22: 7138. https://doi.org/10.3390/ma16227138






