Improved DC Dielectric Performance of Cross-Linked Polyethylene Modified by Free Radical-Initiated Grafting BMIE
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterization and Measurement
2.3. Molecular Model and Theoretical Methodology
3. Results and Discussion
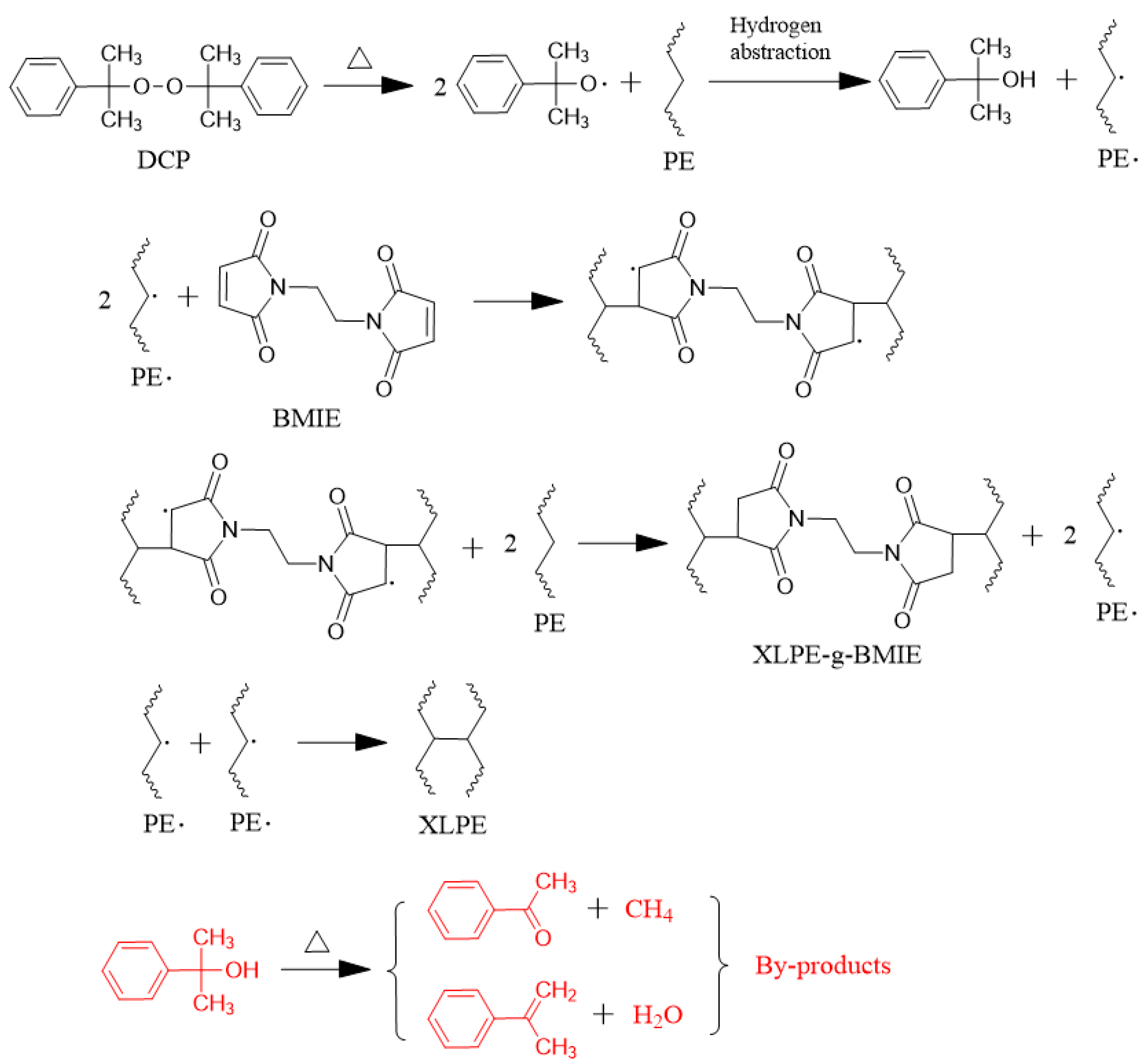
3.1. Testing and Characterization of Crosslinking
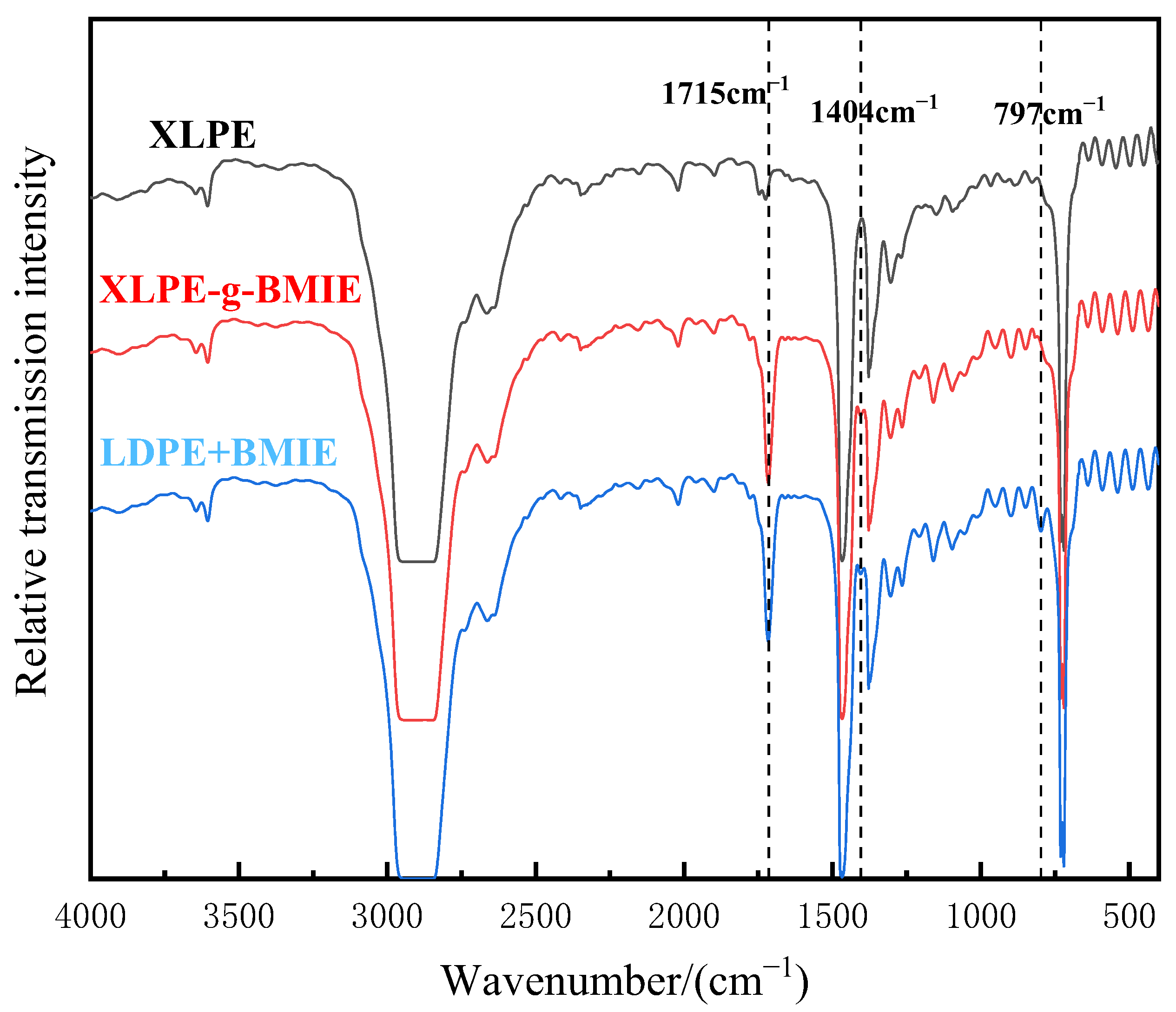
3.2. Molecular Structure Characterization
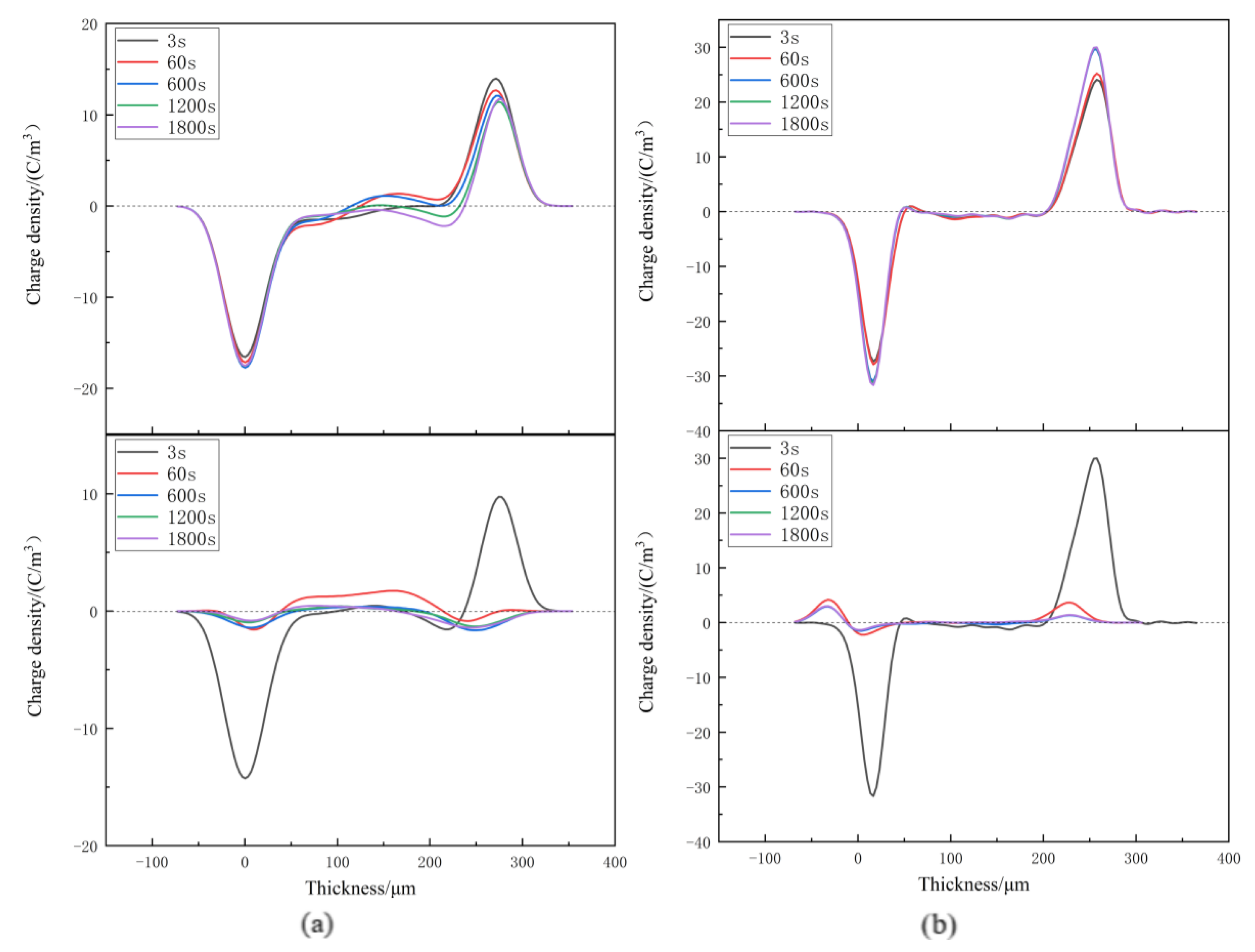
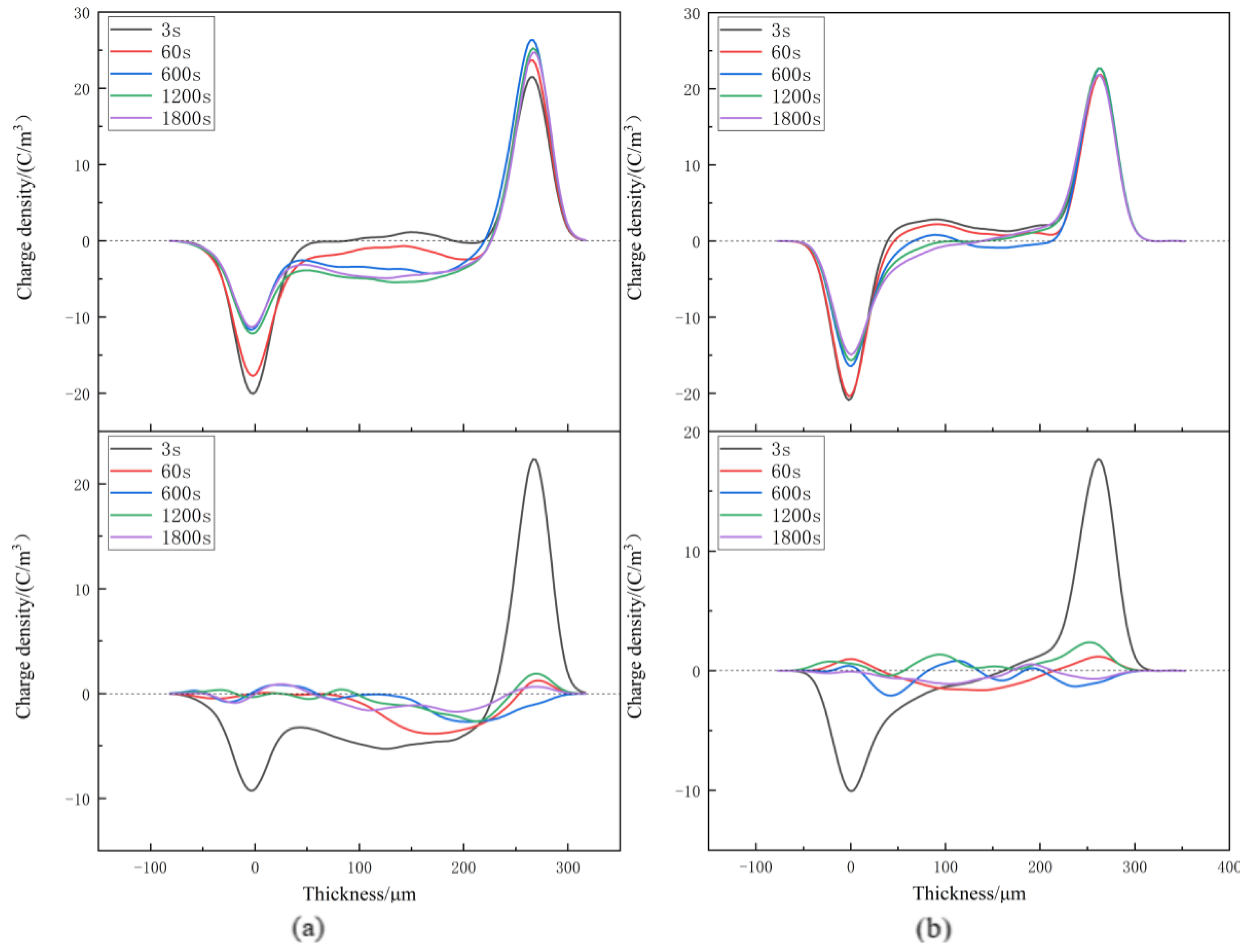
3.3. Space Charge Characteristics
3.4. Electric Current Density
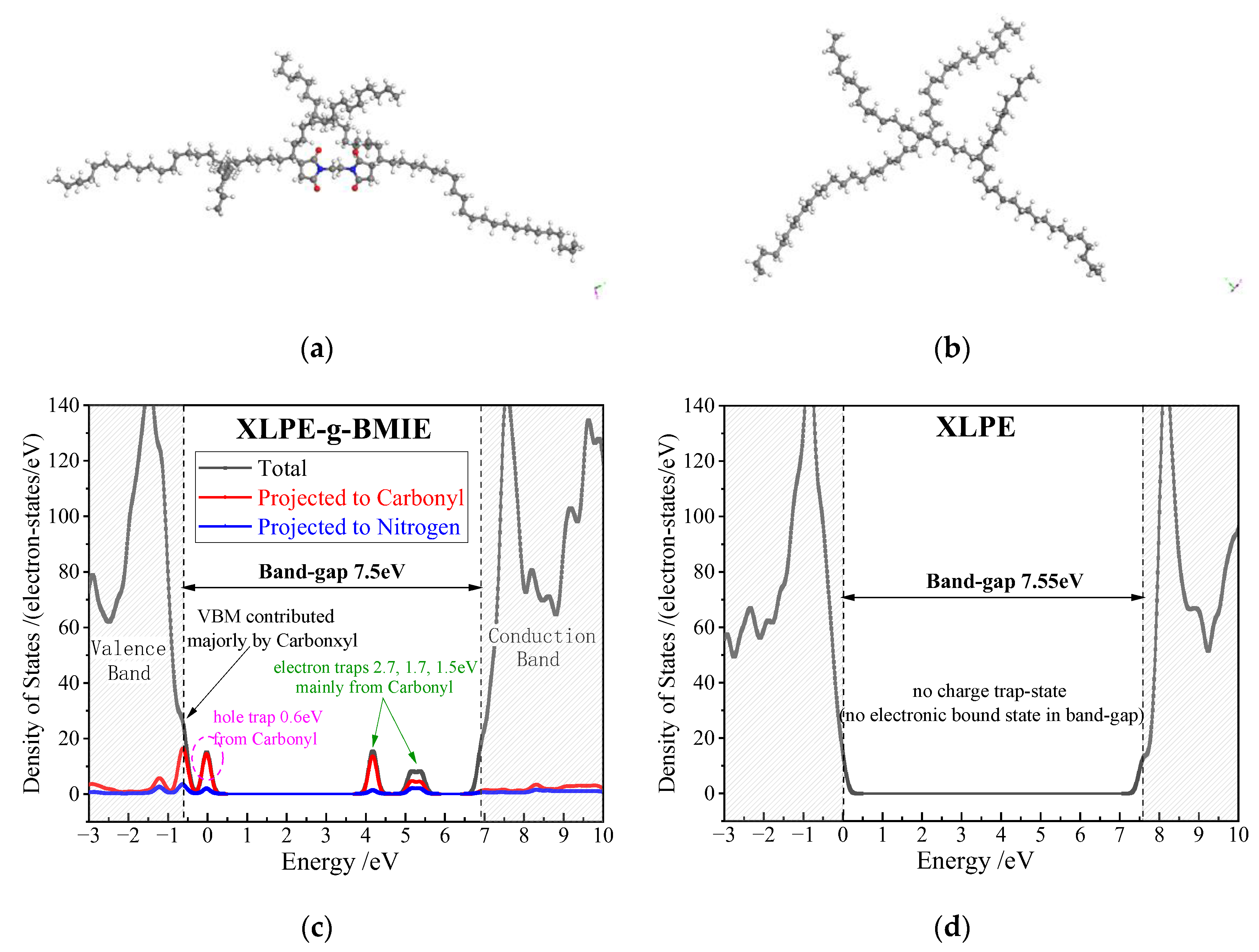
3.5. Electric Charge Traps
3.6. DC Breakdown Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.Y. Research of global clean energy resource and power grid interconnection. Chin. Soc. Electr. Eng. 2016, 36, 5103–5110. [Google Scholar]
- Hammons, T.J.; Lescale, V.F.; Uecker, K.; Haeusler, M.; Retzmann, D. State of the Art in Ultrahigh-Voltage Transmission. Proc. IEEE 2012, 100, 360–390. [Google Scholar] [CrossRef]
- Mauseth, F.; Haugda, H. Electric Field Simulations of High Voltage DC Extruded Cable Systems. IEEE Electr. Insul. Mag. 2017, 33, 16–21. [Google Scholar] [CrossRef]
- Saeedifard, M.; Iravani, R. Dynamic Performance of a Modular Multilevel Back-to-Back HVDC System. IEEE Trans. Power Deliv. 2010, 25, 2903–2912. [Google Scholar] [CrossRef]
- Du, B.X.; Han, C.L.; Li, J.; Li, Z.L. Research Status of Polyethylene Insulation for High Voltage Direct Current Cables. Diangong Jishu Xuebao/Trans. China Electrotech. Soc. 2019, 34, 179–191. [Google Scholar]
- Fu, M.; Chen, G.; Dissado, L.A.; Fothergill, J.C. Influence of thermal treatment and residues on space charge accumulation in XLPE for DC power cable application. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 53–64. [Google Scholar] [CrossRef]
- Zhao, X.D.; Zhao, H.; Sun, W.F. Significantly Improved Electrical Properties of Crosslinked Polyethylene Modified by UV-Initiated Grafting MAH. Polymers 2020, 12, 62. [Google Scholar] [CrossRef]
- Smith, R.C.; Liang, C.; Landry, M.; Nelson, J.K.; Schadler, L.S. The Mechanisms Leading to the Useful Electrical Properties of Polymer Nanodielectrics. IEEE Trans. Dielectr. Electr. Insul. A Publ. IEEE Dielectr. Electr. Insul. Soc. 2008, 15, 187–196. [Google Scholar] [CrossRef]
- Li, S.; Yin, G.; Chen, G.J.L.; Bai, S.; Zhong, L.; Zhang, Y.; Lei, Q. Short-term Breakdown and Long-term Failure in Nanodielectrics: A Review. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1523–1535. [Google Scholar] [CrossRef]
- Huang, X.Y.; Liu, F.; Jiang, P.K. Effect of Nanoparticle Surface Treatment on Morphology, Electrical and Water Treeing Behavior of LLDPE Composites. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1697–1704. [Google Scholar] [CrossRef]
- Lau, K.Y.; Vaughan, A.S.; Hosier, I.L.; Holt, A.F. On the dielectric response of silica-based polyethylene nanocomposites. J. Phys. D Appl. Phys. A Europhys. J. 2013, 46, 095303. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.-K.; Han, J.H.; Suh, K.S. Space charge and electrical conduction in Maleic Anhydride-grafted polyethylene. IEEE Trans. Dielectr. Electr. Insul. 1995, 2, 1132–1139. [Google Scholar] [CrossRef]
- Suh, K.S.; Yoon, H.G.; Lee, C.R.; Okamoto, T.; Takada, T. Space charge behavior of acrylic monomer-grafted polyethylene. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 282–287. [Google Scholar] [CrossRef]
- Zha, J.W.; Wu, Y.H.; Wang, S.J.; Wu, D.H.; Yan, H.D.; Dang, Z.M. Improvement of space charge suppression of polypropylene for potential application in HVDC cables. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2337–2343. [Google Scholar] [CrossRef]
- Meunier, M.; Quirke, N. Molecular modeling of electron trapping in polymer insulators. J. Chem. Phys. 2000, 113, 369–376. [Google Scholar] [CrossRef]
- Meunier, M.; Quirke, N.; Aslanides, A. Molecular modeling of electron traps in polymer insulators: Chemical defects and impurities. J. Chem. Phys. 2001, 115, 2876–2881. [Google Scholar] [CrossRef]
- Wen, P. Research on Formula and Technology Optimization with DCP Cross-Linked LDPE Cable Insulation Materials. Master’s Thesis, Lanzhou University of Technology, Lanzhou, China, 2020. [Google Scholar]
- Yuan, B.; Mao, Y.T.; Li, W.K.; Zhang, Z.; Deng, Z.J.; Zhang, B.S. Effect of Co-crosslinking Agent on Properties of Peroxide Crosslinked Polyethylene Insulating Material. Insul. Mater. 2020, 53, 34–40. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, C.; Shang, Y.; Zhao, H.; Li, Z. Theoretical Study on the Hydrogen Addition Reactions to Bismaleimide in the Ultra-violet Radiation Cross-linking Process of Polyethylene. J. Mol. Graph. Model. 2020, 100, 107679. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Zhao, H.; Wang, X.; Han, B.; Li, Z. Theoretical study on the reaction of maleic anhydride in the UV radiation cross-linking process of polyethylene. Polymer 2017, 133, 232–239. [Google Scholar] [CrossRef]
- GB/T 2951.21-2008; Common Test Methods for Insulating and Sheathing Materials of Electric and Optical Cables—Part 21: Methods Specific to Elastomeric Compounds—Ozone Resistance, Hot Set and Mineral Oil Immersion Tests. General Administration of Quality Supervision. Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- ASTM D 2765-2011; Standard Test Methods for Determination of Gel Content and Swell Ratio of Crosslinked Ethylene Plastics. American Society for Testing and Materials: West Conshohocken, PA, USA, 2011.
- IEC 60243; Electric Strength of Insulating Materials—Test Methods—Part 1: Tests at Power Frequencies. International Electrotechnical Commission: Geneva, Switzerland, 2013.
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, 864–871. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Rigby, D.; Roe, R.J. Molecular dynamics simulation of polymer liquid and glass. II. Short range order and orientation correlation. J. Chem. Phys. 1988, 89, 5280–5290. [Google Scholar] [CrossRef]
- Peverati, R.; Truhlar, D.G. M11-L: A Local Density Functional That Provides Improved Accuracy for Electronic Structure Calculations in Chemistry and Physics. J. Phys. Chem. Lett. 2017, 3, 117–124. [Google Scholar] [CrossRef]
- Zhou, T.C.; Chen, G.; Liao, R.J.; Xu, Z. Charge trapping and detrapping in polymeric materials: Trapping parameters. J. Appl. Phys. 2011, 110, 043724(1)–043724(6). [Google Scholar] [CrossRef]
- Fukunaga, K. Innovative PEA space charge measurement systems for industrial applications. IEEE Electr. Insul. Mag. 2004, 20, 18–26. [Google Scholar] [CrossRef]
- Fukunaga, K. Progress and Prospects in PEA Space Charge Measurement Techniques—[Feature Article]. IEEE Electr. Insul. Mag. 2008, 24, 26–37. [Google Scholar] [CrossRef]
- Hussaini, H.; Adam, A.A. Review of Space-charge Measurement using Pulsed Electro Acoustic Method: Advantages and Limitations. Int. J. Eng. Res. Appl. 2015, 5, 90–95. [Google Scholar]
- Imburgia, A.; Miceli, R.; Sanseverino, E.R.; Romano, P.; Viola, F. Review of space charge measurement systems: Acoustic, thermal and optical methods. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3126–3142. [Google Scholar] [CrossRef]
- Takada, T. Acoustic and optical methods for measuring electric charge distributions in dielectrics. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 519–547. [Google Scholar] [CrossRef]




| Electronic Hamiltonian | Scheme | Condition and Parameter |
|---|---|---|
| Exchange correlation energy | Meta-generalized gradient approximation | M11-L [28] |
| Integration accuracy | 2000 grid points/atom | |
| Tolerance | 1 × 10−6 eV/atom | |
| SCF | Multipolar expansion | Octupole |
| Charge density mixing | Charge = 0.3, DIIS = 5 | |
| Core treatment | All-electron | |
| Numerical basis set | DNP | Basis file 4.4 |
| Orbital cutoff | Global | 5.0 Å |
| Heat Extension Rate (%) | Gel Content (%) | |
|---|---|---|
| XLPE | 36 | 85.5 |
| XLPE-g-BMIE | 34 | 85.8 |
| XLPE | XLPE-g-BMIE | |||
|---|---|---|---|---|
| 20 °C | kD (A/(kVmm)) | Ⅰ | 0.84 | 0.94 |
| Ⅱ | 3.83 | 3.53 | ||
| Ⅲ | ||||
| EΩ (kV/mm) | 10 | 35 | ||
| 40 °C | kD (A/(kVmm)) | Ⅰ | 0.88 | 1.00 |
| Ⅱ | 3.75 | 3.42 | ||
| Ⅲ | ||||
| EΩ (kV/mm) | 10 | 30 | ||
| 60 °C | kD (A/(kVmm)) | Ⅰ | 1.11 | |
| Ⅱ | 3.33 | 3.56 | ||
| Ⅲ | 1.01 | |||
| EΩ | 40 | 20 | ||
| 80 °C | kD (A/(kVmm)) | Ⅰ | 1.18 | |
| Ⅱ | 3.23 | 3.46 | ||
| Ⅲ | 1.52 | |||
| EΩ (kV/mm) | 30 | 15 | ||
| 20 °C | 40 °C | 60 °C | 80 °C | |
|---|---|---|---|---|
| XLPE | 342.1 | 311.3 | 271.1 | 231.6 |
| XLPE-g-BMIE | 385.4 | 355.7 | 310.3 | 294.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Wang, X.; Jin, J.; Zhang, H.; Han, W. Improved DC Dielectric Performance of Cross-Linked Polyethylene Modified by Free Radical-Initiated Grafting BMIE. Materials 2023, 16, 6659. https://doi.org/10.3390/ma16206659
Li P, Wang X, Jin J, Zhang H, Han W. Improved DC Dielectric Performance of Cross-Linked Polyethylene Modified by Free Radical-Initiated Grafting BMIE. Materials. 2023; 16(20):6659. https://doi.org/10.3390/ma16206659
Chicago/Turabian StyleLi, Peng, Xuan Wang, Jin Jin, Hui Zhang, and Wei Han. 2023. "Improved DC Dielectric Performance of Cross-Linked Polyethylene Modified by Free Radical-Initiated Grafting BMIE" Materials 16, no. 20: 6659. https://doi.org/10.3390/ma16206659
APA StyleLi, P., Wang, X., Jin, J., Zhang, H., & Han, W. (2023). Improved DC Dielectric Performance of Cross-Linked Polyethylene Modified by Free Radical-Initiated Grafting BMIE. Materials, 16(20), 6659. https://doi.org/10.3390/ma16206659





