Local Electrochemical Corrosion of 6061 Aluminum Alloy with Nano-SiO2/MAO Composite Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens Preparation
2.2. Surface Characteristics
2.3. Electrochemical Experiments
2.3.1. Global Electrochemical Measurement
2.3.2. Local Electrochemical Measurement
2.4. Numerical Simulation Scheme for Long-Period Corrosion Prediction
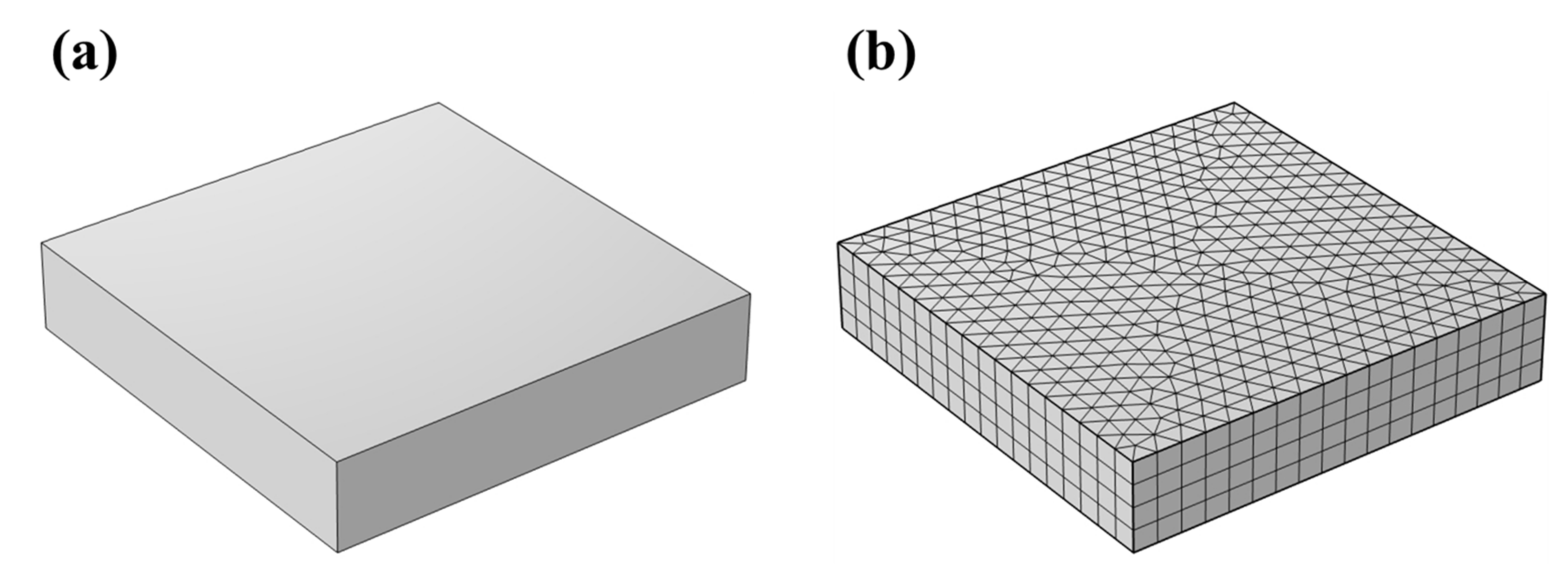
2.4.1. Models and Grids
2.4.2. Control Equations and Boundary Conditions
3. Results and Discussion
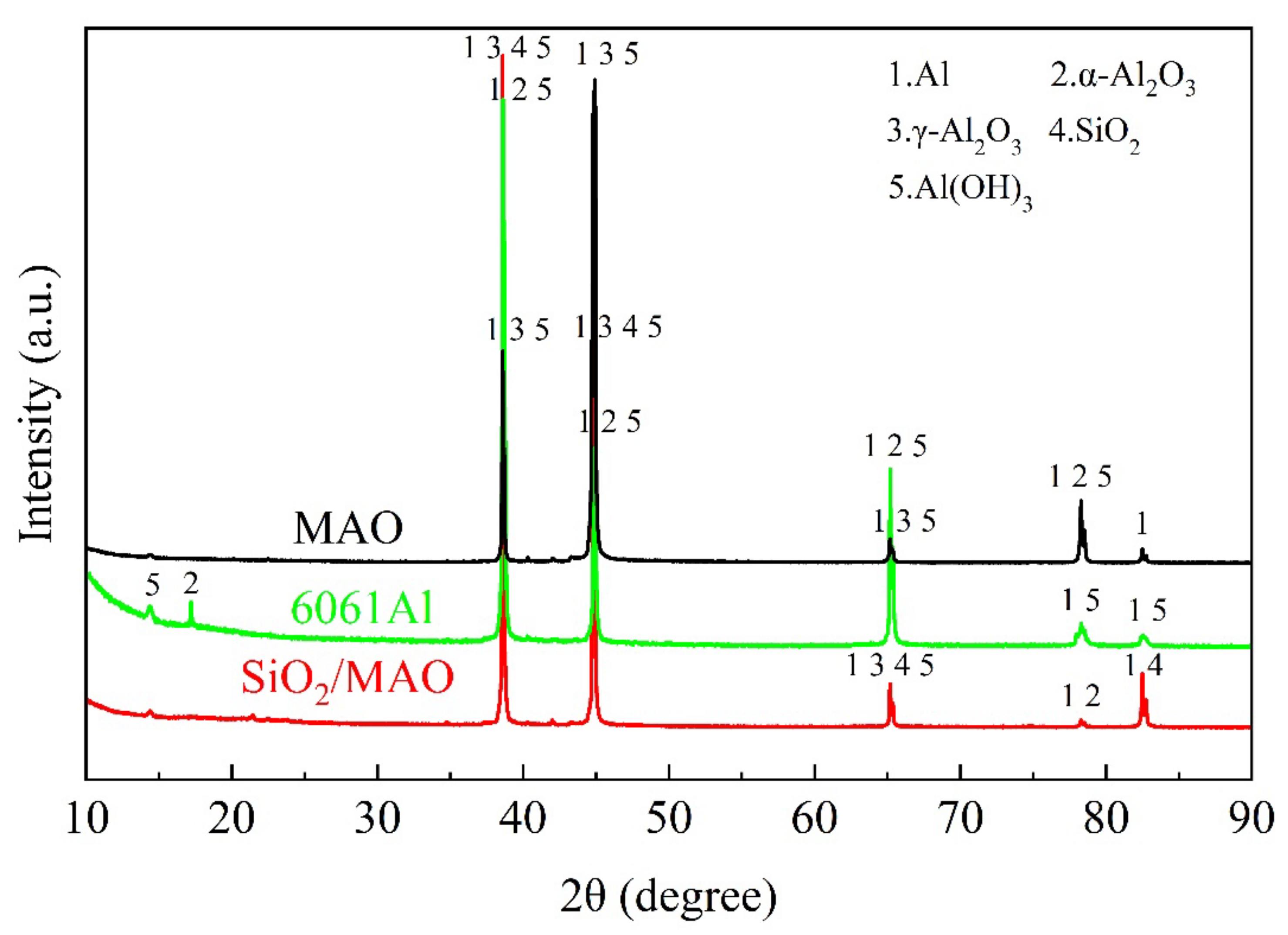
3.1. Characterization of MAO Coatings
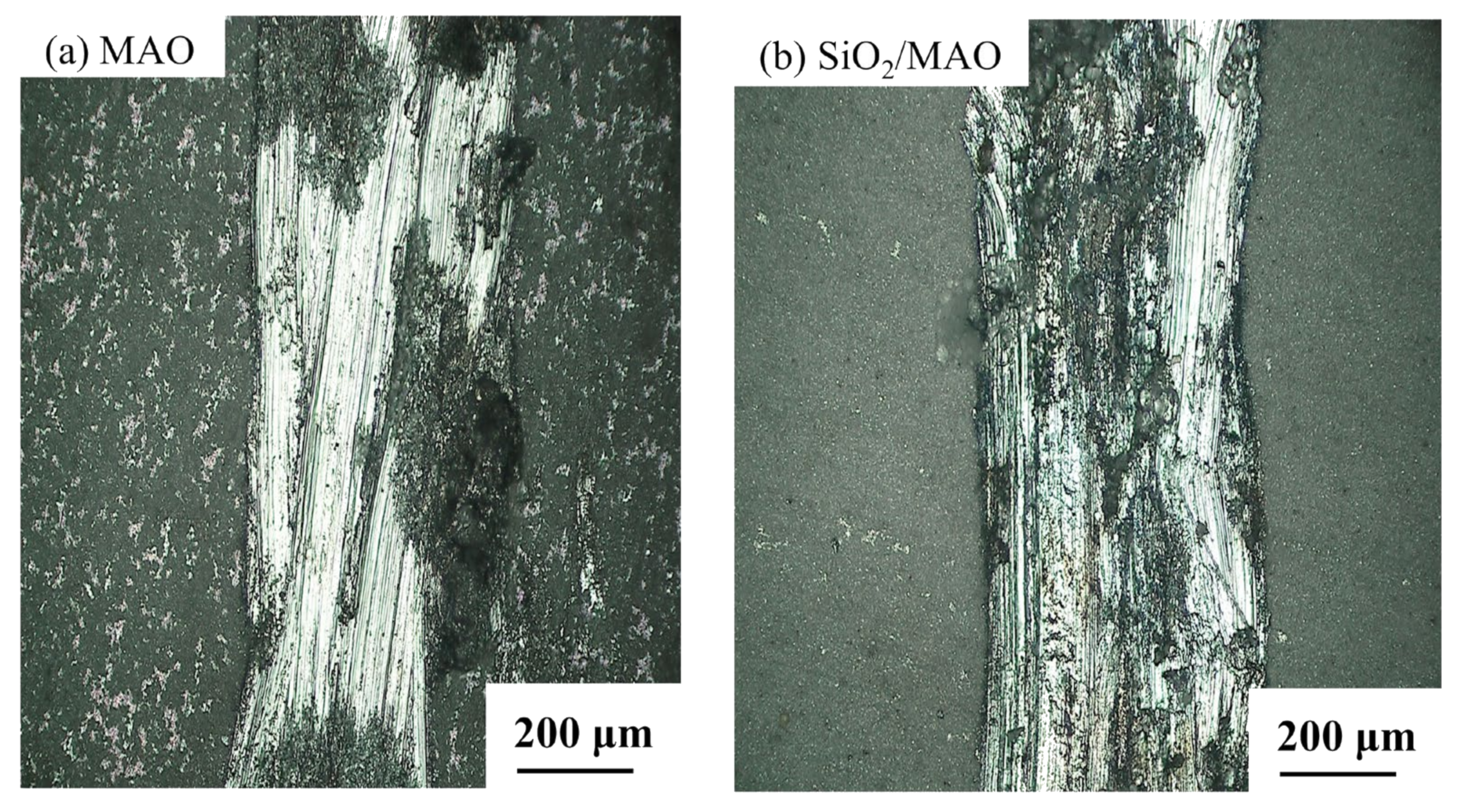
3.1.1. Surface and Cross-Section Morphology
3.1.2. Three-Dimensional Micropore Distribution
3.1.3. Phase Analysis
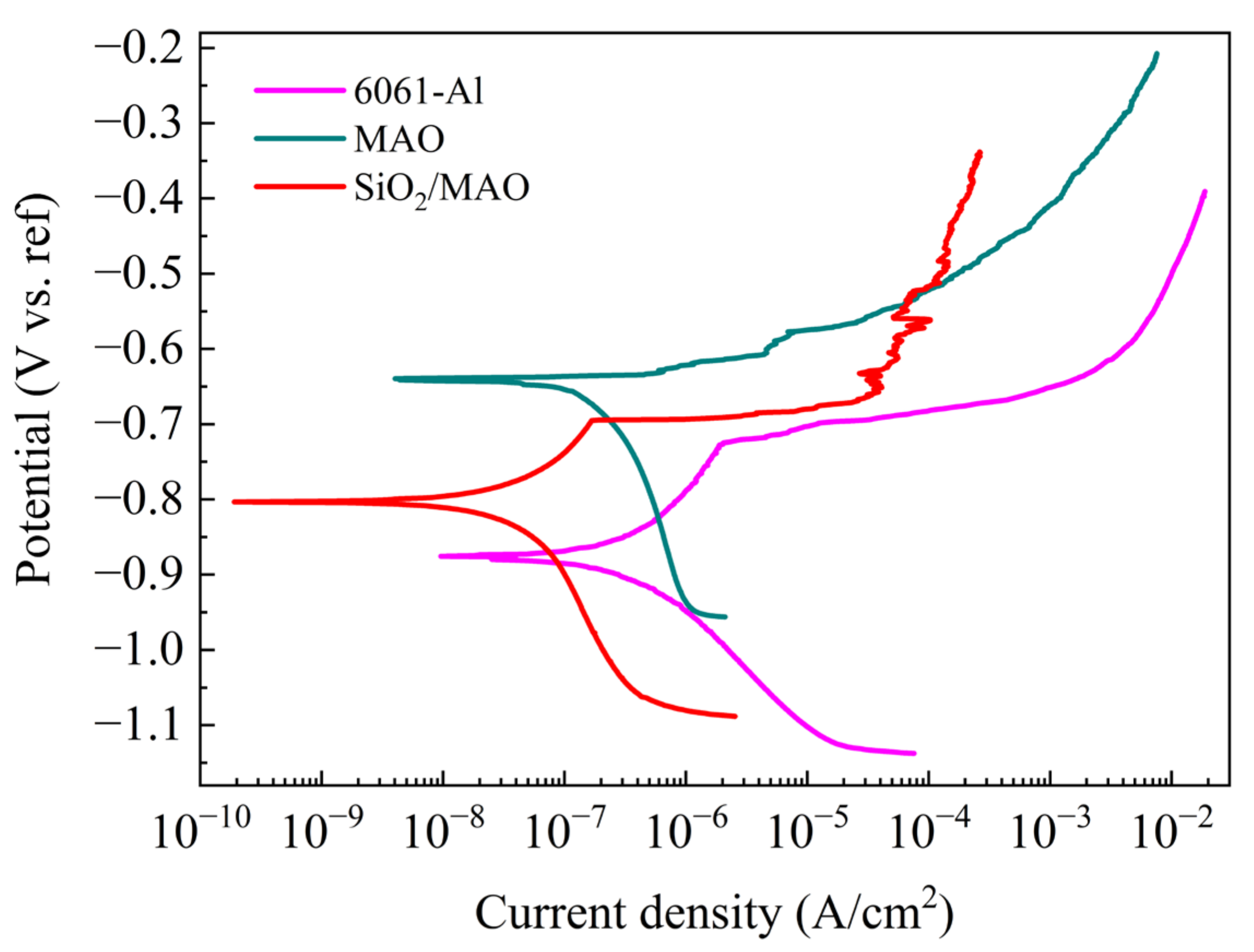
3.2. Global Electrochemical Behavior
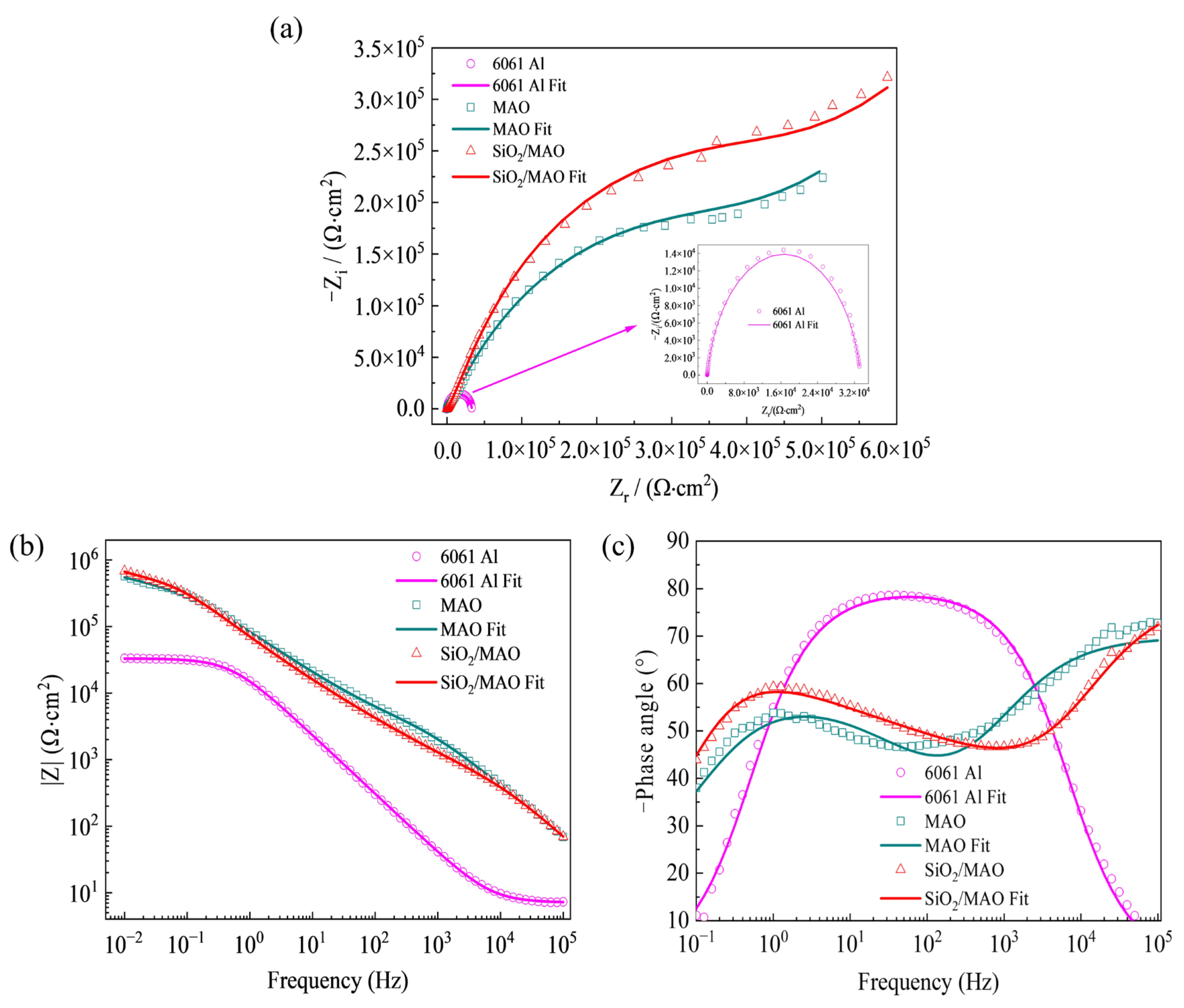
3.2.1. Electrochemical Analysis
3.2.2. Corrosion Products
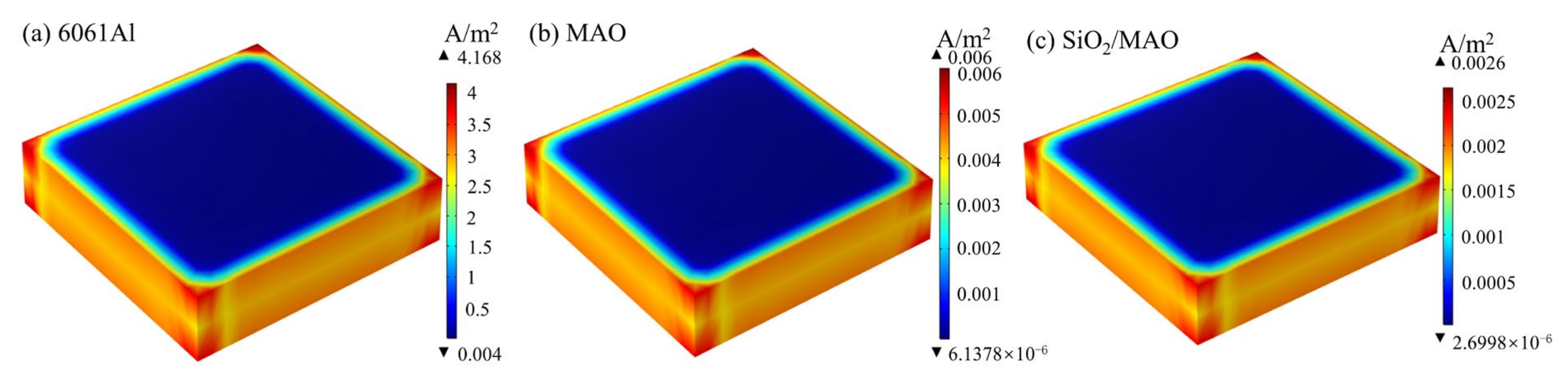
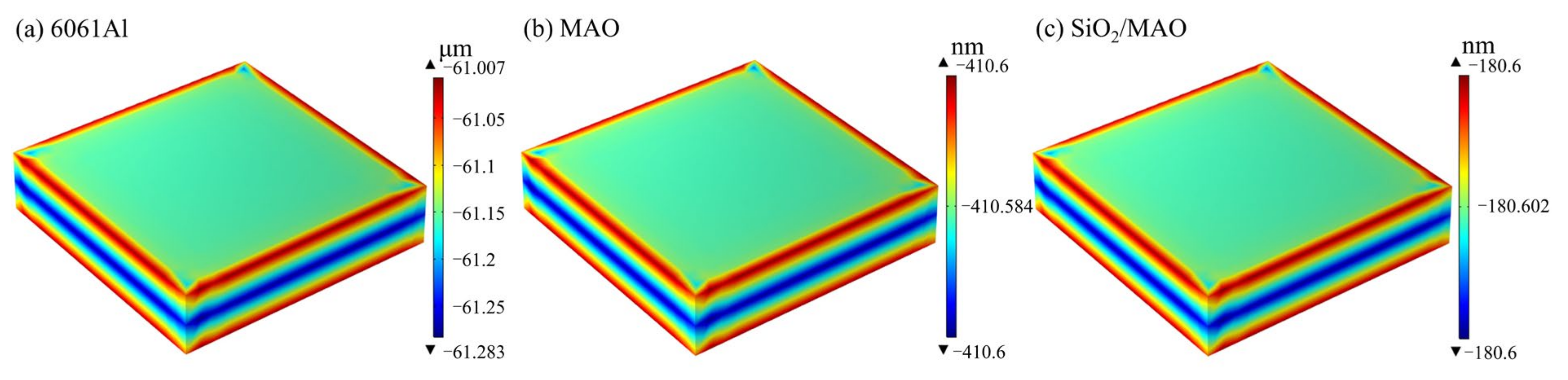
3.3. The Results of Numerical Simulation Analysis for Long-Period Corrosion Prediction
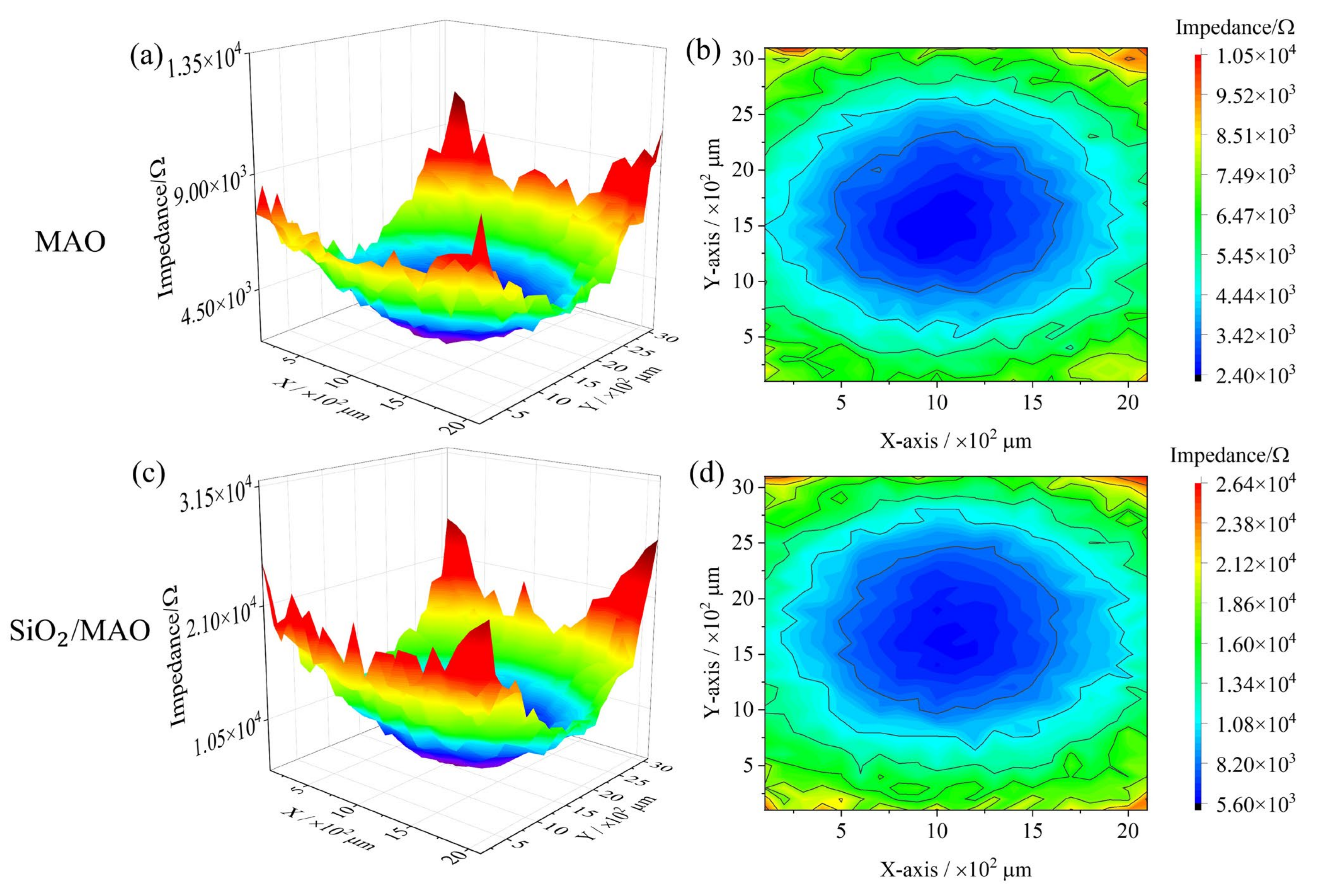
3.4. Local Electrochemical Behavior
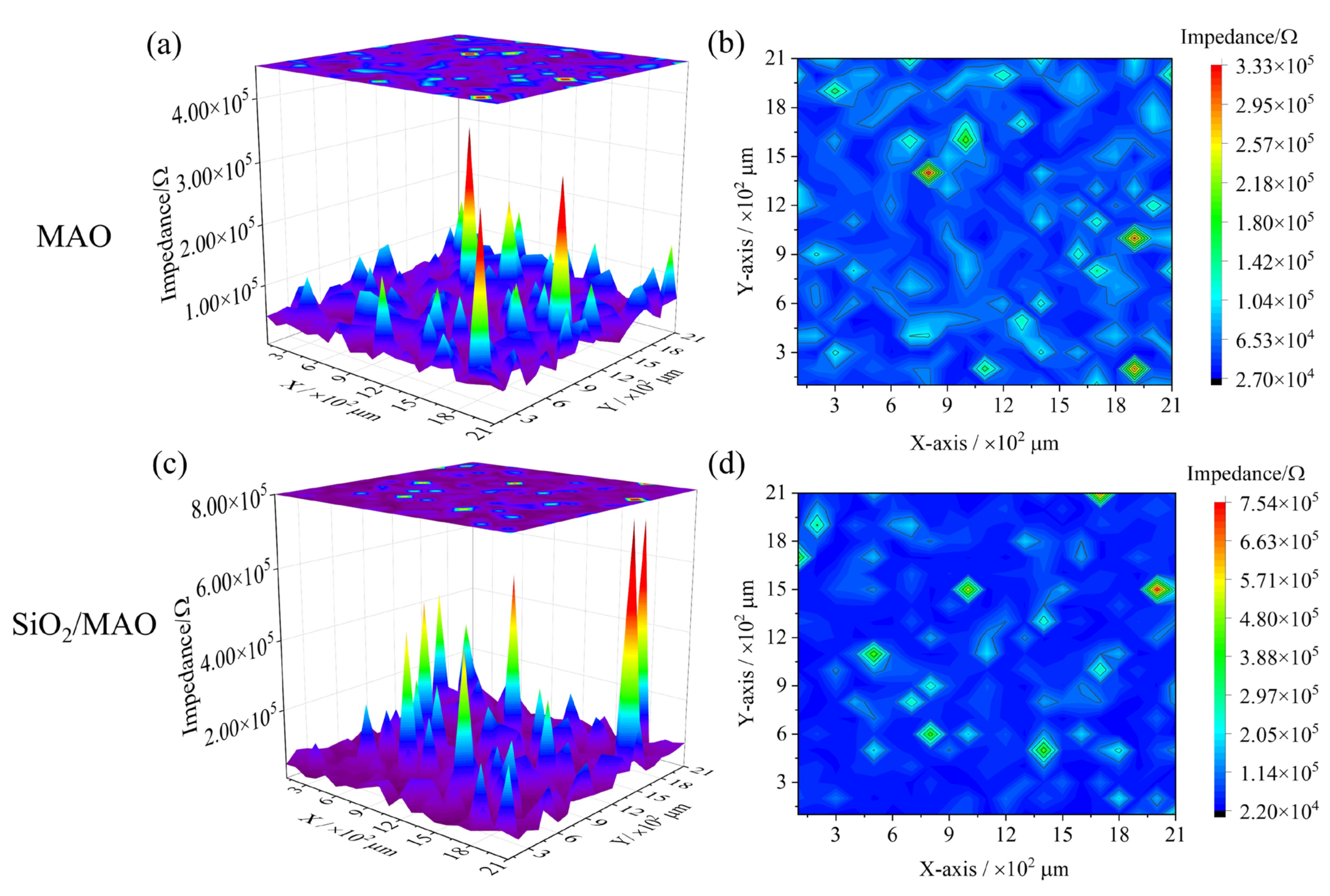
3.4.1. Local Impedance Distribution
3.4.2. Local Corrosion Morphology
3.5. Corrosion Mechanism Model
4. Conclusions
- (1)
- The main composition of the MAO coating and nano-SiO2/MAO composite coating is Al2O3, and the thickness of nano-SiO2/MAO composite coating reaches 9.6 μm. Nano-SiO2/MAO composite coating has fewer micropores on the surface, indicating that nano-SiO2 particles have a good sealing effect.
- (2)
- The global electrochemical test results showed that the electrochemical impedance film value and corrosion current density of nano-SiO2/MAO composite coating increased by 9% and decreased by 87% compared with MAO coating, respectively. The local electrochemical test results showed that the local impedance of MAO coating ranges from 2.40 × 103 to 1.05 × 104 Ω, while the local impedance of nano-SiO2/MAO composite coating ranges from 5.60 × 103 to 2.64 × 104 Ω, which more than doubled the local impedance in the condition of defective coating. It can be seen that nano-SiO2/MAO composite coating still shows high corrosion resistance in the case of defects.
- (3)
- Based on the results of the global electrochemical experiments, the COMSOL simulation program was established to simulate the long-period corrosion experiments of the coatings and to predict the annual corrosion thinning rate of the coatings by using the Nernst–Planck equation and the Butler–Volmer equation. The numerical simulation results show that the nano-SiO2/MAO composite coating has the lowest corrosion current density of 0.0026 A/m2, the slowest corrosion thinning rate of 18.06 nm/a, and the strongest durability, which can satisfy the service life requirement of the surface coating of the battery pack.
- (4)
- The corrosion mechanism model of nano-SiO2/MAO composite coating was established. When the coating is damaged, the 6061Al substrate is corroded by oxygen, and the γ phase of the composite coating reacts with Cl− in the corrosion solution to finally form the corrosion product Al(OH)3. As the corrosion time grows, the coating collapses inward due to its own weight and eventually forms pitting pits.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, J.; Kim, T.; Kim, D.; Kim, D.; Kim, K. Castability and mechanical properties of new 7xxx aluminum alloys for automotive chassis/body applications. J. Alloys Compd. 2017, 698, 577–590. [Google Scholar] [CrossRef]
- Piao, N.; Wang, L.; Anwar, T.; Feng, X.N.; Sheng, S.E.; Tian, G.Y.; Wang, J.L.; Tang, Y.P.; He, X.M. Corrosion resistance mechanism of chromate conversion coated aluminium current collector in lithium-ion batteries. Corros. Sci. 2019, 158, 108100. [Google Scholar] [CrossRef]
- Sobolev, A.; Peretz, T.; Borodianskiy, K. Synthesis and growth mechanism of ceramic coatings on an Al-Cu alloy using plasma electrolytic oxidation in molten salt. J. Alloys Compd. 2021, 869, 159309. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Shore, D.; Avelar-Batista Wilson, J.C.; Matthews, A.; Yerokhin, A. Adhesive bond strength of PEO coated AA6060-T6. Surf. Coat. Technol. 2021, 428, 127898. [Google Scholar] [CrossRef]
- Sobolev, A.; Bograchev, D.; Borodianskiy, K.; Zinigrad, M. Kinetics and mechanism of corrosion of oxide coating fabricated on aluminum alloy by the plasma electrolytic oxidation in molten salt. Corros. Sci. 2022, 208, 110604. [Google Scholar] [CrossRef]
- Venugopal, A.; Panda, R.; Manwatkar, S.; Sreekumar, K.; Krishna, L.R.; Sundararajan, G. Effect of micro arc oxidation treatment on localized corrosion behaviour of AA7075 aluminum alloy in 3.5% NaCl solution. J. Trans. Nonferr. Metal. Soc. 2012, 22, 700–710. [Google Scholar] [CrossRef]
- Lin, L.; Sirong, Y.; Enyang, L.; Yan, Z.; Bingying, W.; Yafeng, N.; Kai, Z.; Guang, Z.; Quan, L. Preparation and characterization of micro-arc oxidation coating on hollow glass microspheres/Mg alloy degradable composite. J. Mater. Chem. Phys. 2021, 271, 124935. [Google Scholar]
- Shen, D.; Li, G.; Guo, C.; Zou, J.; Cai, J.; He, D.; Ma, H.; Liu, F. Microstructure and corrosion behavior of micro-arc oxidation coating on 6061 aluminum alloy pre-treated by high-temperature oxidation. J. Appl. Surf. Sci. 2013, 287, 451–456. [Google Scholar] [CrossRef]
- Kaseem, M.; Kamil, M.P.; Kwon, J.H.; Ko, Y.G. Effect of sodium benzoate on corrosion behavior of 6061 Al alloy processed by plasma electrolytic oxidation. J. Surf. Coat. Technol. 2015, 283, 268–273. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, B.; Zhao, X.; Wang, B. Binary Additives Enhance Micro Arc Oxidation Coating on 6061Al Alloy with Improved Corrosion resistance Property. J. Coat. 2020, 10, 128. [Google Scholar] [CrossRef]
- Phu, T.Q.; Cheng, K.Y.; Kai, S.J.; Liang, H.J.; Shune, C.T. High quality oxide-layers on Al-alloy by micro-arc oxidation using hybrid voltages. J. Surf. Coat. Technol. 2016, 303, 61–67. [Google Scholar]
- Wasekar, N.P.; Jyothirmayi, A.; Krishna, L.R.; Sundararajan, G. Effect of Micro Arc Oxidation Coatings on Corrosion Resistance of 6061-Al Alloy. J. Mater. Eng. Perform. 2008, 17, 708–713. [Google Scholar] [CrossRef]
- Paksoy, A.H.; Muhaffel, F.; Koca, M.; Gokce, O.; Mohammadzadeh, S.; Cimenoglu, H. Formation of a corrosion-resistant alumina coating on a 6061 aluminum alloy using a combination of micro-arc oxidation and sealing treatments. J. Mater. Tehnol. 2017, 51, 117–121. [Google Scholar] [CrossRef]
- Dudareva, N.Y. Simulation of hardened layer formation at micro-arc oxidation of aluminum specimens. J. Russ. Aeronaut. 2008, 51, 321–325. [Google Scholar] [CrossRef]
- Xu, J.L.; Liu, F.; Wang, F.P.; Yu, D.Z.; Zhao, L.C. The corrosion resistance behavior of Al2O3 coating prepared on NiTi alloy by micro-arc oxidation. J. Alloys Compd. 2008, 472, 276–280. [Google Scholar] [CrossRef]
- Wang, P.; Hu, J.; Li, R.; Gong, Z.; Liu, J.; Xiong, D.; Xiang, D.; Wei, X.; Zu, X. Effects of Indium Sulfide on the Structure and Corrosion Resistance of the Micro-arc Oxidation Coating on ZL108 Alloy. J. Rare Met. Mater. Eng. 2021, 50, 56–62. [Google Scholar]
- Chen, M.; Ou, Y.; Fu, Y.; Li, Z.; Li, J.; Liu, S. Effect of friction stirred Al-Fe-Si particles in 6061 aluminum alloy on structure and corrosion performance of MAO coating. J. Surf. Coat. Technol. 2016, 304, 85–97. [Google Scholar] [CrossRef]
- Chen, X.D.; Cai, Q.Z.; Yin, L.S. Effects of Na2WO4 Additive on Properties of Plasma Electrolytic Oxidation Coatings on 6061 Al Alloy. J. Adv. Mater. Res. 2012, 1914, 1969–1975. [Google Scholar] [CrossRef]
- Hollamby, M.J.; Dmitri, F.; Ingo, D.; Dimitriya, B.; Helmuth, M.; Dmitry, S. Hybrid polyester coating incorporating functionalized mesoporous carriers for the holistic protection of steel surfaces. J. Adv. Mater. 2011, 23, 1361–1365. [Google Scholar] [CrossRef]
- Suo, X.B.; Qiu, J.; Ma, S.N.; Wang, H.M. The Effects of Nano-SiO2 on the Chemical Composition of Layers on Aluminum Alloy by Micro-Arc Oxidation. J. Appl. Mech. Mater. 2014, 3436, 84–88. [Google Scholar] [CrossRef]
- Song, Z.X.; Yan, Z.X.; Feng, W.D. Effects of Nano-SiO2 in Electrolytes on Surface Properties of Micro-Arc Oxidation Ceramic Coatings Formed on New Casting Aluminum Alloy. J. Adv. Mater. Res. 2012, 2044, 368–372. [Google Scholar] [CrossRef]
- Yang, X.Y.; Mo, Y.; Dai, T.; Zhao, J.; Gu, Y.H. Local Electrochemical Corrosion Properties of a Nano-SiO2/MAO Composite Coating on an AM60B-Mg Alloy. Materials 2022, 15, 3999. [Google Scholar] [CrossRef] [PubMed]
- Nadimi, M.; Dehghanian, C.; Etemadmoghadam, A. Influence of SiO2 nanoparticles incorporating into ceramic coatings generated by PEO on Aluminium alloy: Morphology, adhesion, corrosion, and wear resistance. Mater. Today Commun. 2022, 31, 103587. [Google Scholar] [CrossRef]
- Wang, S.; Gu, Y.; Geng, Y.; Liang, J.; Zhao Jie Kang, J. Investigating local corrosion behavior and mechanism of MAO coated 7075 aluminum alloy. J. Alloys Compd. 2019, 826, 153976. [Google Scholar] [CrossRef]
- Galicia, G.; Pébère, N.; Tribollet, B.; Vivier, V. Local and global electrochemical impedances applied to the corrosion behaviour of an AZ91 magnesium alloy. J. Corros. Sci. 2009, 51, 1789–1794. [Google Scholar] [CrossRef]
- Ma, H.; Gu, Y.; Liu, S.; Che, J.; Yang, D. Local corrosion behavior and model of micro-arc oxidation HA coating on AZ31 magnesium alloy. J. Surf. Coat. Technol. 2017, 331, 179–188. [Google Scholar] [CrossRef]
- Dai, T.; Zhao, J.; Yang, X.; Gu, Y. Global and Local Corrosion Performance of Nano-SiC Induced Micro-arc Oxidation Coating on Magnesium Alloy. J. Mater. Eng. Perform. 2022, 31, 6747–6758. [Google Scholar] [CrossRef]
- Kai, Z.; Sirong, Y. Preparation of wear and corrosion resistant micro-arc oxidation coating on 7N01 aluminum alloy. J. Surf. Coat. Technol. 2019, 388, 125453. [Google Scholar]
- Wei, T.; Yan, F.; Tian, J. Characterization and wear- and corrosion-resistance of microarc oxidation ceramic coatings on aluminum alloy. J. Alloys Compd. 2004, 389, 169–176. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Zhou, Y.; Ouyang, J.; Guo, L.; Jia, D. Corrosion evaluation of microarc oxidation coatings formed on 2024 aluminium alloy. J. Corros. Sci. 2010, 52, 2687–2696. [Google Scholar] [CrossRef]
- Ming, M. Preparation and Corrosion Resistance of Micro-arc Oxidation/Self-Assembly Composite Film on 6061 Aluminum Alloy. Int. J. Electrochem. Sci. 2019, 14, 11731–11743. [Google Scholar]
- Wei, S.; Wang, Y.; Wen, Y.; Zhan, X.; Kong, D. Study on the Properties of Micro—Arc Oxidation Self—Assembled Composite coatings on Magnesium Alloy. Int. J. Electrochem. Sci. 2017, 12, 11875–11891. [Google Scholar]
- Cao, F.; Lin, L.; Zhang, Z.; Zhang, J.; Cao, C. Environmental friendly plasma electrolytic oxidation of AM60 magnesium alloy and its corrosion resistance. J. Trans. Nonferr. Met. Soc. 2008, 18, 240–247. [Google Scholar] [CrossRef]
- Xia, S.J.; Yue, R.; Rateick, R.G., Jr.; Birss, V.I. Electrochemical Studies of AC/DC Anodized Mg Alloy in NaCl Solution. J. Electrochem. Soc. 2004, 151, B179–B187. [Google Scholar] [CrossRef]
- Wang, G.; Tuo, X.; Kou, L.; Zhao, W.; Zhu, X. Research on corrosion performance of 6061aluminum alloy in salt spray environment. Mater. Werkst. 2020, 51, 1686–1699. [Google Scholar] [CrossRef]
- Yu, X.F.; Zhang, G.Q.; Zhang, Z.X.; Wang, Y.G. Research on corrosion resistance of anodized and sealed 6061 aluminum alloy in 3.5% sodium chloride solution. Mater. Today Commun. 2023, 18, 100092. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, Z.; Tang, S.; Dong, W.; Tong, Q.; Li, W. Influence of graphene particles on the micro-arc oxidation behaviors of 6063 aluminum alloy and the coating properties. J. Appl. Surf. Sci. 2017, 423, 939–950. [Google Scholar] [CrossRef]
- Khamaj, J.A. Cyclic polarization analysis of corrosion behavior of ceramic coating on 6061 Al/SiCp composite for marine applications. J. Prot. Met. Phys. Chem. Surf. 2016, 52, 886–893. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Du, H.; Zhao, Y. Corrosion resistance of micro-arc oxidation coatings formed on aluminum alloy with addition of Al2O3. J. Mater. Res. Express 2018, 5, 036527. [Google Scholar] [CrossRef]




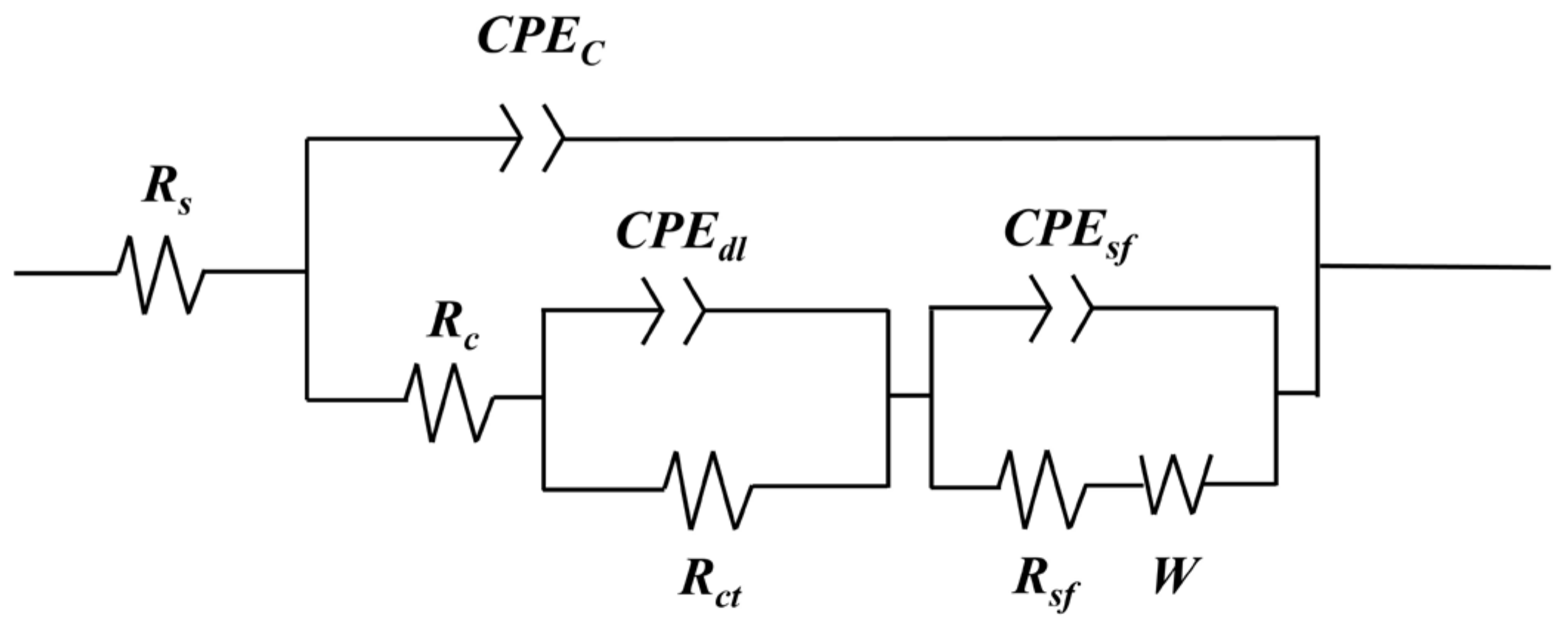



| Specimen | Rs (Ω·cm2) | CPEc (Ω−1·cm−2·s−n) | Rc (Ω·cm2) | CPEdl (Ω−1·cm−2·s−n) | Rct (Ω·cm2) | CPEsf (Ω−1·cm−2·s−n) | Rsf (Ω·cm2) | W (Ω·cm2) | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| 6061Al | 7.31 | - | - | 1.08 × 10−5 | 3.48 × 104 | - | - | - | 7.63 × 10−4 |
| MAO | 20.40 | 1.43 × 10−7 | 8.84 × 102 | 4.71 × 10−6 | 2.63 × 105 | 9.37 × 10−6 | 3.72 × 103 | 7.43 × 103 | 2.91 × 10−4 |
| SiO2/MAO | 21.58 | 1.71 × 10−7 | 8.66 × 102 | 5.47 × 10−6 | 2.87 × 105 | 1.89 × 10−6 | 3.66 × 103 | 7.64 × 103 | 2.95 × 10−4 |
| Specimen | 6061Al | MAO | SiO2/MAO |
|---|---|---|---|
| Ecorr | −0.860 | −0.750 | −0.710 |
| Icorr | 2.020 | 0.758 | 0.504 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhang, H.; Yang, X.; Gu, Y.; Liu, Y. Local Electrochemical Corrosion of 6061 Aluminum Alloy with Nano-SiO2/MAO Composite Coating. Materials 2023, 16, 6721. https://doi.org/10.3390/ma16206721
Zhao J, Zhang H, Yang X, Gu Y, Liu Y. Local Electrochemical Corrosion of 6061 Aluminum Alloy with Nano-SiO2/MAO Composite Coating. Materials. 2023; 16(20):6721. https://doi.org/10.3390/ma16206721
Chicago/Turabian StyleZhao, Jie, Hongwei Zhang, Xiaoyu Yang, Yanhong Gu, and Yida Liu. 2023. "Local Electrochemical Corrosion of 6061 Aluminum Alloy with Nano-SiO2/MAO Composite Coating" Materials 16, no. 20: 6721. https://doi.org/10.3390/ma16206721
APA StyleZhao, J., Zhang, H., Yang, X., Gu, Y., & Liu, Y. (2023). Local Electrochemical Corrosion of 6061 Aluminum Alloy with Nano-SiO2/MAO Composite Coating. Materials, 16(20), 6721. https://doi.org/10.3390/ma16206721






