Microwave-Prepared Quantum Dots and Their Potential Applications as Adsorbents and Chemosensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cellulose Extraction
2.2.2. Preparation of Quantum Dots (QDs)
- (a)
- Cellulose, chitosan, and biochar.
- (b)
- Cellulose and chitosan.
- (c)
- Chitosan and biochar.
- (d)
- Cellulose and biochar.
2.2.3. Adsorption Study of Metal Ions
2.2.4. Characterization and Analysis
Fluorescence Spectroscopy
UV Spectroscopy
Fourier-Transform Infrared Spectroscopy (FT-IR)
X-ray Diffraction
Raman Analysis
SEM/EDX
Thermogravimetric Analysis (TGA/DTG)
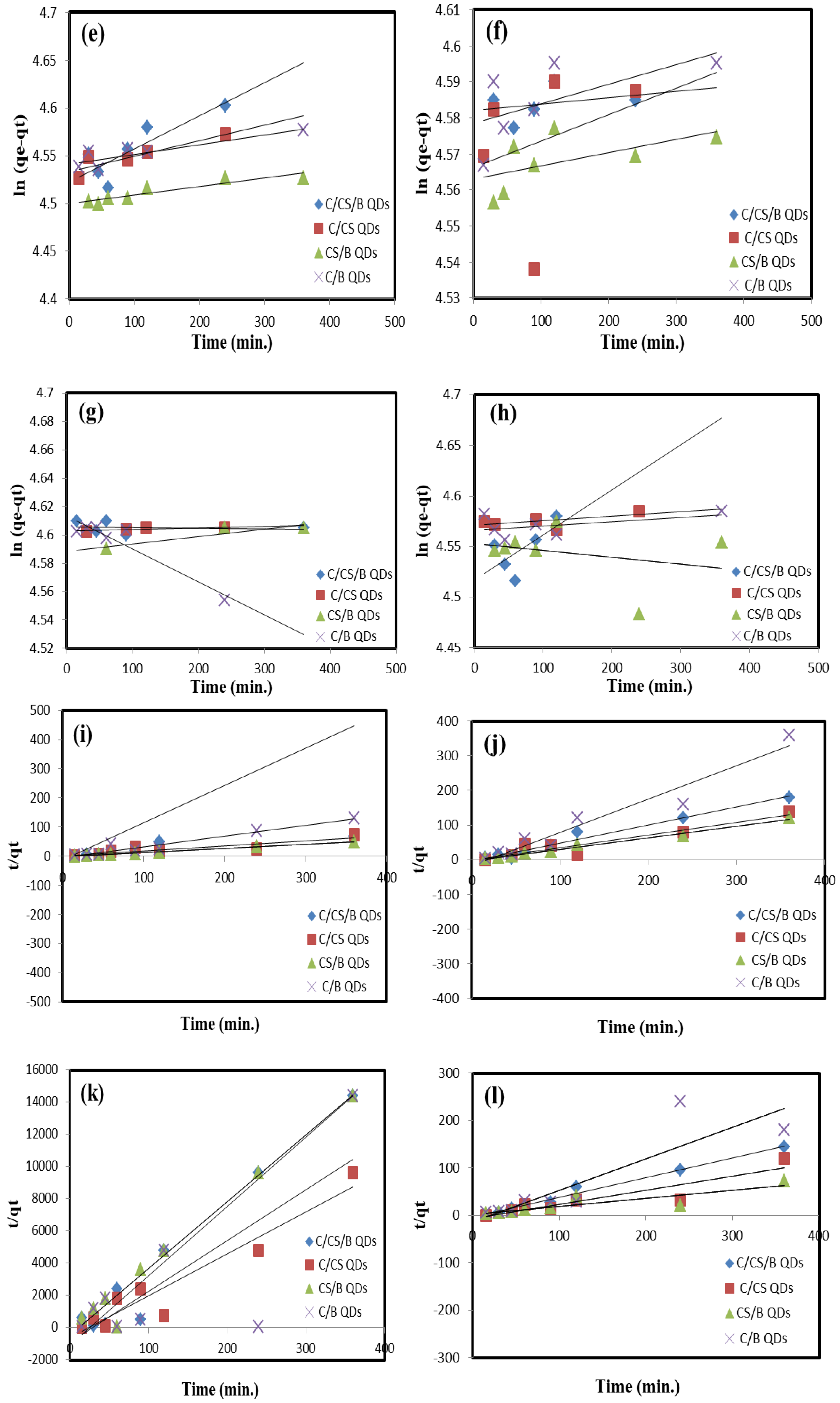
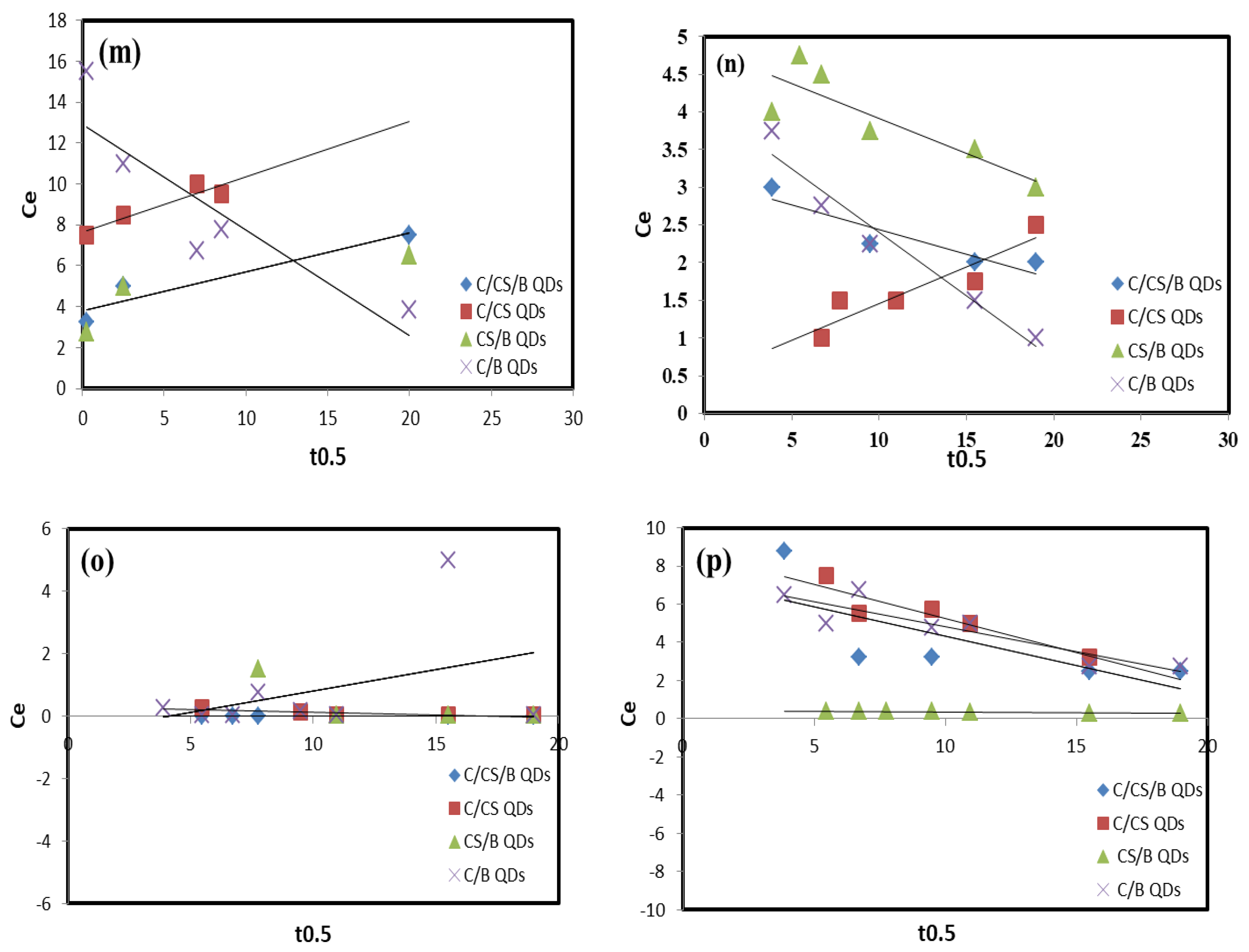
Kinetics and Isotherm Studies
Statistical Section
3. Results
3.1. Caharacteizations
3.1.1. FTIR Spectroscopy
3.1.2. Raman Analysis
3.1.3. X-ray Diffraction Study
3.1.4. Morphological Analysis
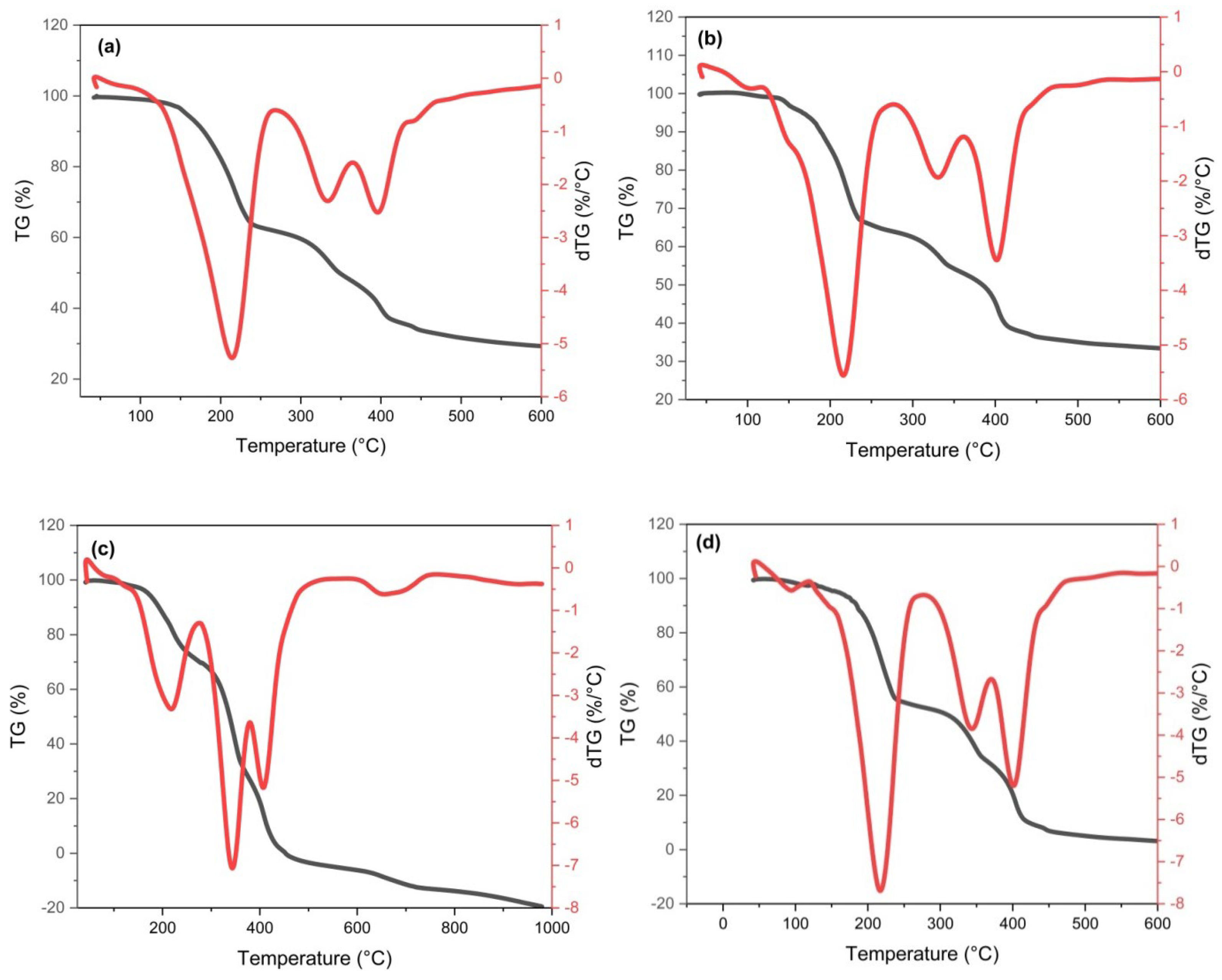
3.1.5. Thermal Study
3.1.6. Fluorescence Microscopy
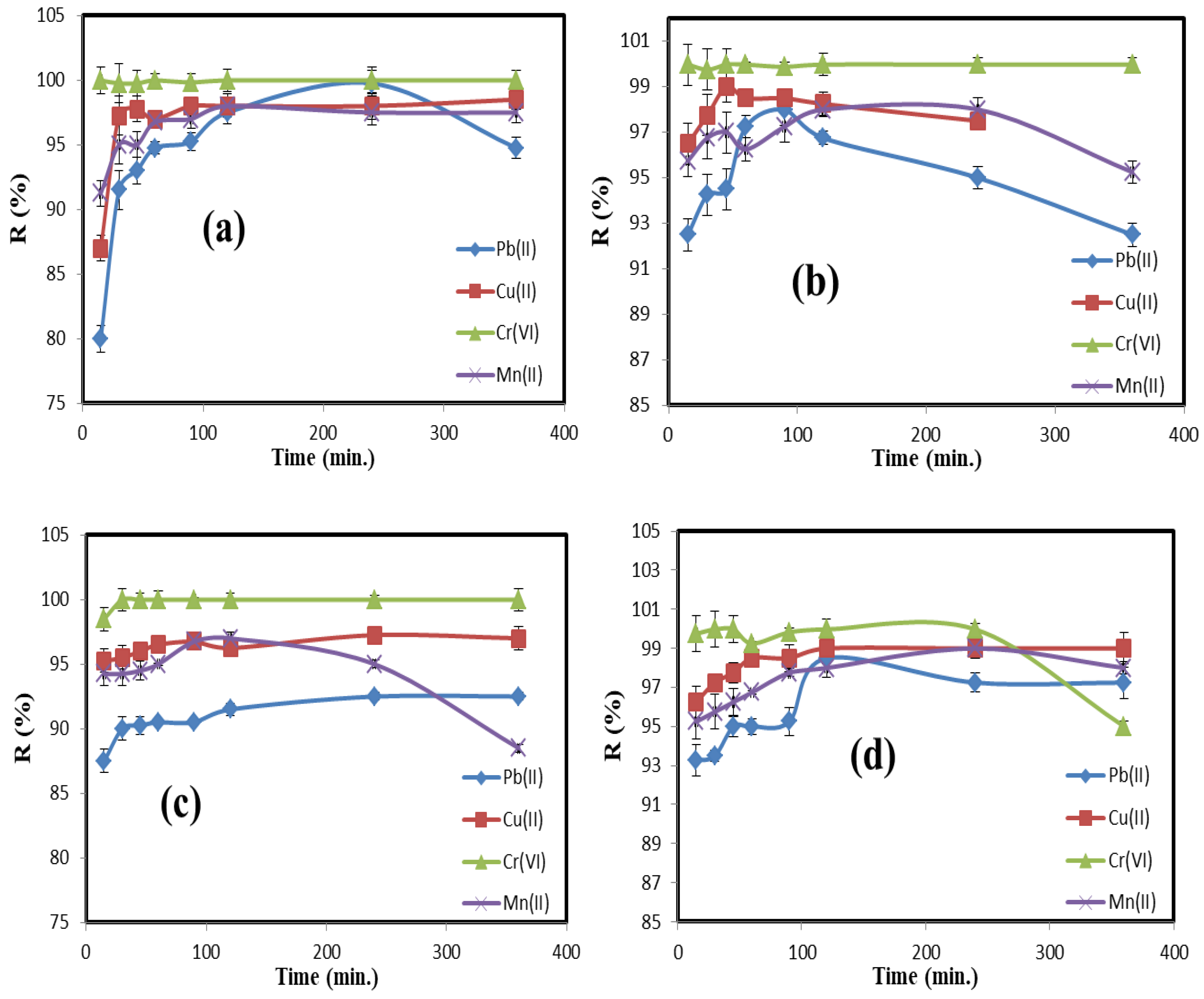
3.2. Adsorption Study
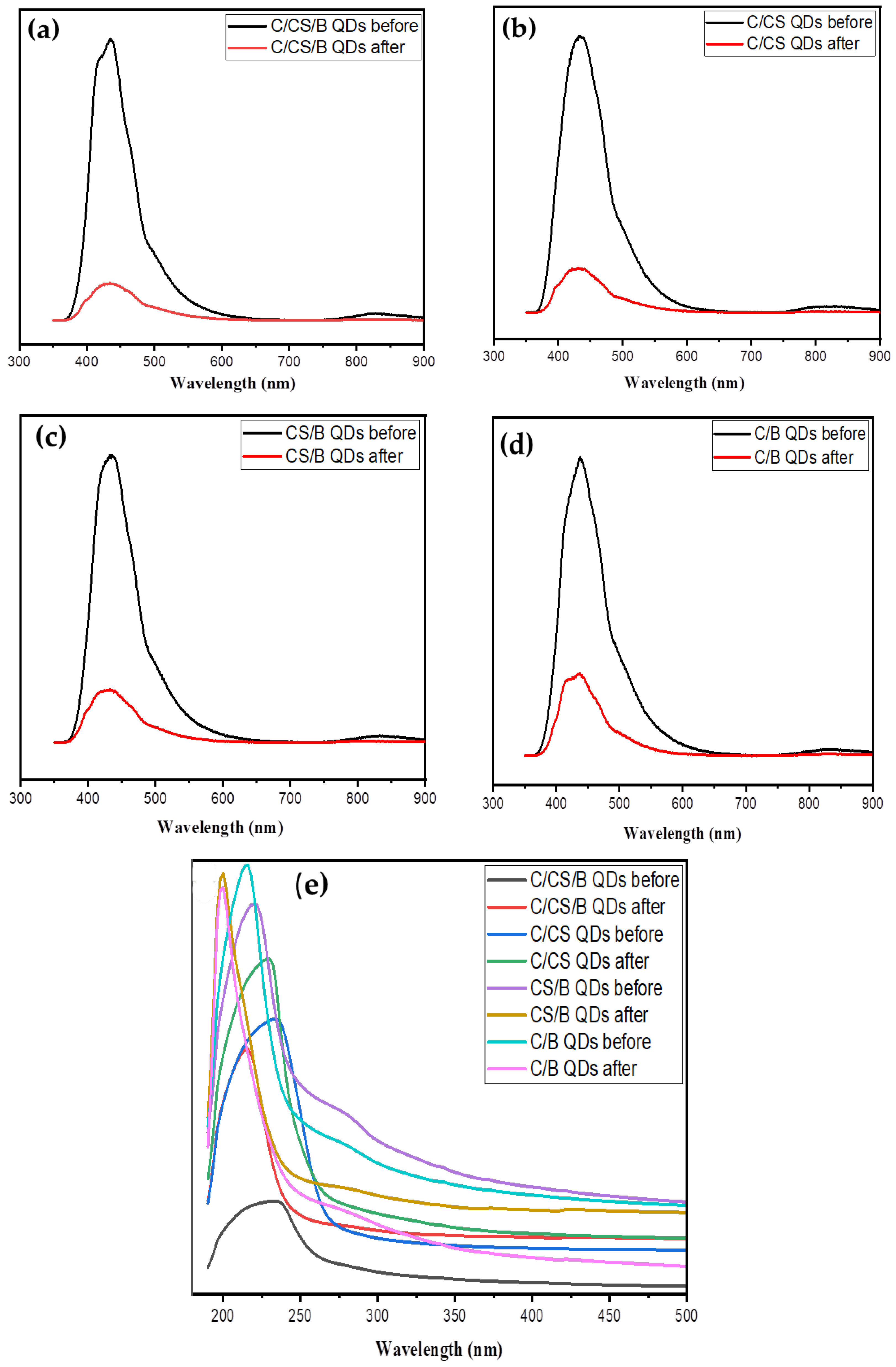
3.3. Application of Quantum Dots as Metal Sensor
3.4. Adsorption Comparsion Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tohamy, H.A.S.; El-Sakhawy, M.; Kamel, S. Eco-friendly Synthesis of Carbon Quantum Dots as an Effective Adsorbent. J. Fluoresc. 2022, 33, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, H.-A.S.; El-Sakhawy, M.; Kamel, S. Microwave-assisted synthesis of amphoteric fluorescence carbon quantum dots and their chromium adsorption from aqueous solution. Sci. Rep. 2023, 13, 11306. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, S.; Gupta, N.; Rawat, R.; Jain, B.; Solanki, A.; Panday, A.; Das, P.; Ganguly, S. Doped Carbon Quantum Dots Reinforced Hydrogels for Sustained Delivery of Molecular Cargo. J. Funct. Biomater. 2023, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Sadegh-Zadeh, F.; Seh-Bardan, B.J. Adsorption of As (III) and As (V) by Fe coated biochars and biochars produced from empty fruit bunch and rice husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar]
- Mészároš, L.; Šuránek, M.; Melichová, Z.; Frišták, V.; Ďuriška, L.; Kaňuchová, M.; Soja, G.; Pipíška, M. Green biochar-based adsorbent for radiocesium and Cu, Ni, and Pb removal. J. Radioanal. Nucl. Chem. 2023, 332, 4141–4155. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Zhang, P.; Shen, J.; Wang, S.; Li, Y. Development of iron-based biochar for enhancing nitrate adsorption: Effects of specific surface area, electrostatic force, and functional groups. Sci. Total Environ. 2023, 856, 159037. [Google Scholar] [CrossRef] [PubMed]
- Mon, P.P.; Cho, P.P.; Chandana, L.; Srikanth, V.; Madras, G.; Ch, S. Biowaste-derived Ni/NiO decorated-2D biochar for adsorption of methyl orange. J. Environ. Manag. 2023, 344, 118418. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, H.; Cai, M.; Li, Y.; Guo, C.; Han, Y.; Zhang, Y.; Song, B. Valorization of food waste digestate to ash and biochar composites for high performance adsorption of methylene blue. J. Clean. Prod. 2023, 397, 136612. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, X.; Zhang, S.; Luo, X.; Geng, H.; Liu, J.; Tong, X.; Zhang, Y.; Sun, P.; Zhao, L. Synergistic action of ferrate and biochar in the removal of trichloroethylene from water: Little biochar addition, large ferrate activity improvement. J. Environ. Chem. Eng. 2023, 11, 110165. [Google Scholar] [CrossRef]
- Li, S.; Chan, C.Y.; Sharbatmaleki, M.; Trejo, H.; Delagah, S. Engineered biochar production and its potential benefits in a closed-loop water-reuse agriculture system. Water 2020, 12, 2847. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Zaffiro, A.; Zimmerman, M.; Wendelken, S.; Smith, G.; Munch, D. METHOD 218.7: Determination of Hexavalent Chromium in Drinking Water by Ion Chromatography with Post-Column Derivatization and UV-Visible Spectroscopic Detection; United States Environmental Protection Agency: Cincinnati, OH, USA, 2011.
- Khalidi-Idrissi, A.; Madinzi, A.; Anouzla, A.; Pala, A.; Mouhir, L.; Kadmi, Y.; Souabi, S. Recent advances in the biological treatment of wastewater rich in emerging pollutants produced by pharmaceutical industrial discharges. Int. J. Environ. Sci. Technol. 2023, 20, 11719–11740. [Google Scholar] [CrossRef] [PubMed]
- Bani-Melhem, K.; Al-Kilani, M.R.; Tawalbeh, M. Evaluation of scrap metallic waste electrode materials for the application in electrocoagulation treatment of wastewater. Chemosphere 2023, 310, 136668. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Nguyen, T.M.; Chen, K.F.; Kao, C.M.; Surampalli, R.Y.; Zhang, T.C. Aerobic Biological Treatment of Microconstituents. In Microconstituents in the Environment: Occurrence, Fate, Removal and Management; Wiley: Hoboken, NJ, USA, 2023; pp. 405–425. [Google Scholar]
- Hasanin, M.; Abdelhameed, R.M.; Dacrory, S.; Abou-Yousef, H.; Kamel, S. Photocatalytic degradation of pesticide intermediate using green eco-friendly amino functionalized cellulose nanocomposites. Mater. Sci. Eng. B 2021, 270, 115231. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.A.; Elella, M.H.A.; Abdallah, H.M.; Mostafa, M.S.; Samy, M. Recent Advances in Various Starch Formulation for Wastewater Purification via Adsorption Technique: A Review. J. Polym. Environ. 2023, 31, 2792–2825. [Google Scholar] [CrossRef]
- Maftouh, A.; El Fatni, O.; El Hajjaji, S.; Jawish, M.W.; Sillanpää, M. Comparative review of different adsorption techniques used in heavy metals removal in water. Biointerface Res. Appl. Chem. 2023, 13, 397. [Google Scholar]
- Irshad, M.A.; Nawaz, R.; Wojciechowska, E.; Mohsin, M.; Nawrot, N.; Nasim, I.; Hussain, F. Application of nanomaterials for cadmium adsorption for sustainable treatment of wastewater: A review. Water Air Soil Pollut. 2023, 234, 54. [Google Scholar] [CrossRef]
- Pittman, C.U., Jr.; Mohan, D.; Eseyin, A.; Li, Q.; Ingram, L.; Hassan, E.-B.M.; Mitchell, B.; Guo, H.; Steele, P.H. Characterization of bio-oils produced from fast pyrolysis of corn stalks in an auger reactor. Energy Fuels 2012, 26, 3816–3825. [Google Scholar] [CrossRef]
- Freeman, R.; Willner, I. Optical molecular sensing with semiconductor quantum dots (QDs). Chem. Soc. Rev. 2012, 41, 4067–4085. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.J.J.L.; Li, J.J.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.X.; Dong, L.; Yang, L.; Wang, J.; Li, S.J.; Lv, H.; Wang, S. Integration of Metal-Organic Frameworks with Bi-Nanoprobes as Dual-Emissive Ratiometric Sensors for Fast and Highly Sensitive Determination of Food Hazards. Molecules 2022, 27, 2356. [Google Scholar] [CrossRef] [PubMed]
- Nayl, A.A.; Abd-Elhamid, A.I.; Awwad, N.S.; Abdelgawad, M.A.; Wu, J.; Mo, X.; Gomha, S.M.; Aly, A.A.; Bräse, S. Review of the Recent Advances in Electrospun Nanofibers Applications in Water Purification. Polymers 2022, 14, 1594. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.; Healy, M.G.; Ryan, P.C.; Mellander, P.-E.; Morrison, L.; Harmon O’Driscoll, J.; Siggins, A. Batch adsorption of herbicides from aqueous solution onto diverse reusable materials and granulated activated carbon. J. Environ. Manag. 2022, 323, 116102. [Google Scholar] [CrossRef] [PubMed]
- Plappert, S.F.; Quraishi, S.; Pircher, N.; Mikkonen, K.S.; Veigel, S.; Klinger, K.M.; Potthast, A.; Rosenau, T.; Liebner, F.W. Transparent, Flexible, and Strong 2,3-Dialdehyde Cellulose Filmswith High Oxygen Barrier Properties. Biomacromolecules 2018, 19, 2969–2978. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, H.A.S.; El-Sakhawy, M.; Kamel, S. Development of magnetite/graphene oxide hydrogels from agricultural wastes for water treatment. J. Renew. Mater. 2022, 10, 1889. [Google Scholar] [CrossRef]
- Rajender, G.; Giri, P. Formation mechanism of graphene quantum dots and their edge state conversion probed by photoluminescence and Raman spectroscopy. J. Mater. Chem. C 2016, 4, 10852–10865. [Google Scholar] [CrossRef]
- Lota, G.; Krawczyk, P.; Lota, K.; Sierczyńska, A.; Kolanowski, Ł.; Baraniak, M.; Buchwald, T. The application of activated carbon modified by ozone treatment for energy storage. J. Solid State Electrochem. 2016, 20, 2857–2864. [Google Scholar] [CrossRef]
- Rossi, B.L.; Andrade, C.; Therézio, E.M.; Ramos, R.J.; Vasconcelos, L.G.; Terezo, A.J.; De Siqueira, A.B. Carbon quantum dots: An environmentally friendly and valued approach to sludge disposal. Front. Chem. 2022, 10, 858323. [Google Scholar] [CrossRef]
- Dong, X.; Ma, L.Q.; Li, Y. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 2011, 190, 909–915. [Google Scholar] [CrossRef]
- Hassan, A.; Kaewsichan, L. Removal of Pb(II) from Aqueous Solutions Using Mixtures of Bamboo Biochar and Calcium Sulphate, and Hydroxyapatite and Calcium Sulphate. Environ. Asia 2016, 9, 37–44. [Google Scholar]
- Saleh, M.E.; El-Refaey, A.A.; Mahmou, A.H. Effectiveness of sunflower seed husk biochar for removing copper ions from wastewater: A comparative study. Soil Water Res. 2016, 11, 53–63. [Google Scholar] [CrossRef]
- Kokab, T.; Ashraf, H.S.; Shakoor, M.B.; Jilani, A.; Ahmad, S.R.; Majid, M.; Hakeem, K.R. Effective removal of Cr (VI) from wastewater using biochar derived from walnut shell. Int. J. Environ. Res. Public Health 2021, 18, 9670. [Google Scholar] [CrossRef]
- Fseha, Y.H.; Sizirici, B.; Yildiz, I. Manganese and nitrate removal from groundwater using date palm biochar: Application for drinking water. Environ. Adv. 2022, 8, 100237. [Google Scholar] [CrossRef]

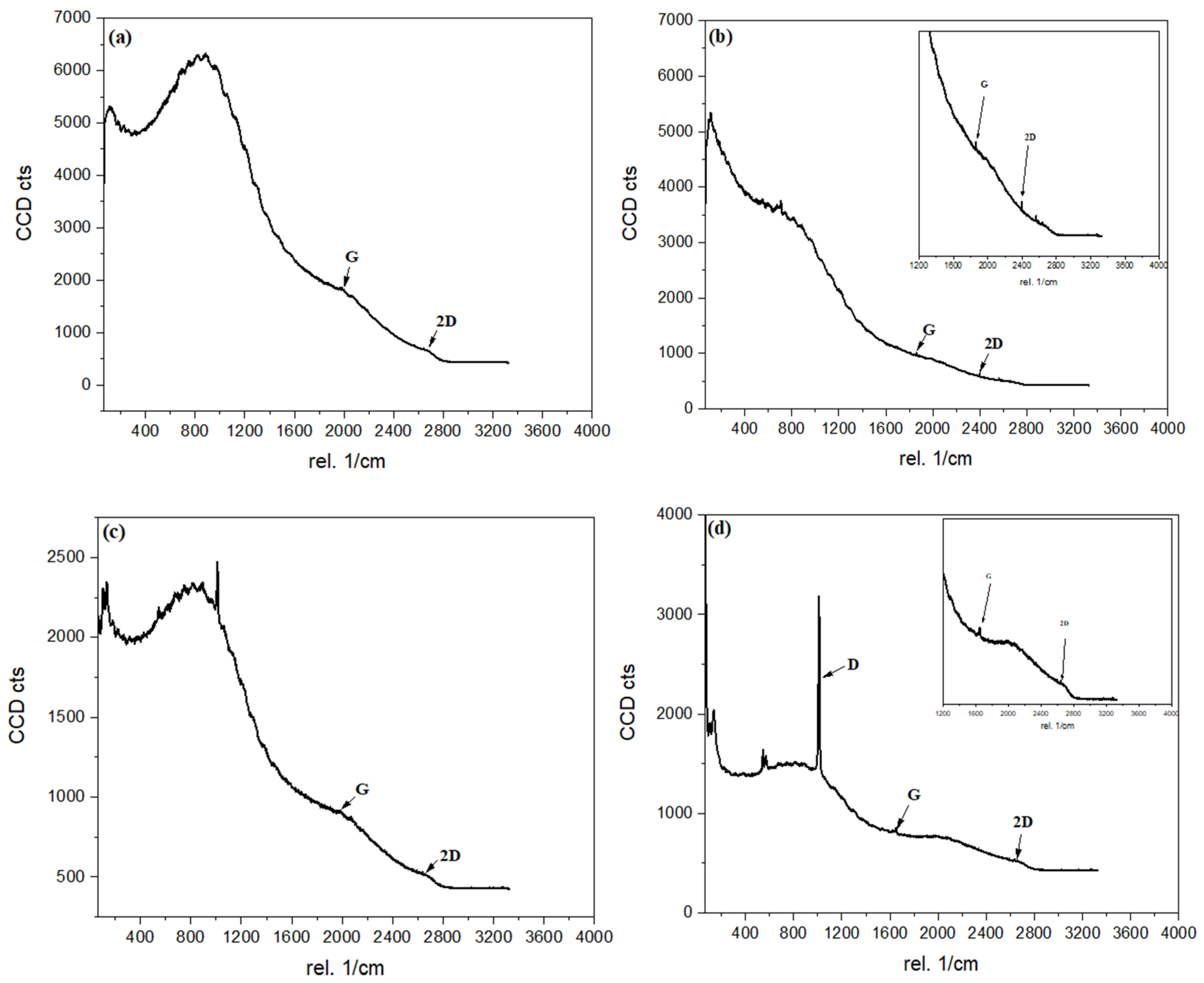
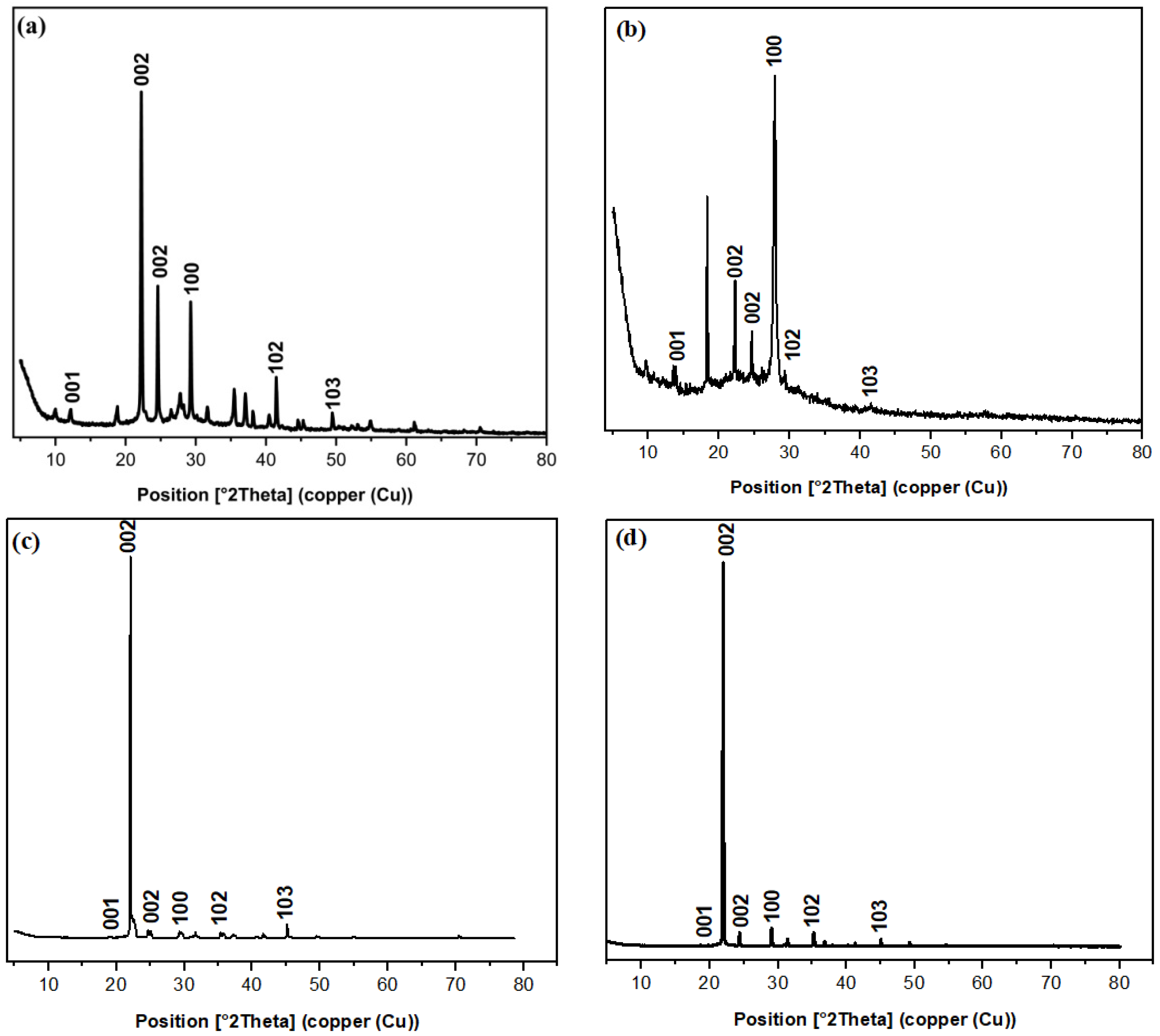
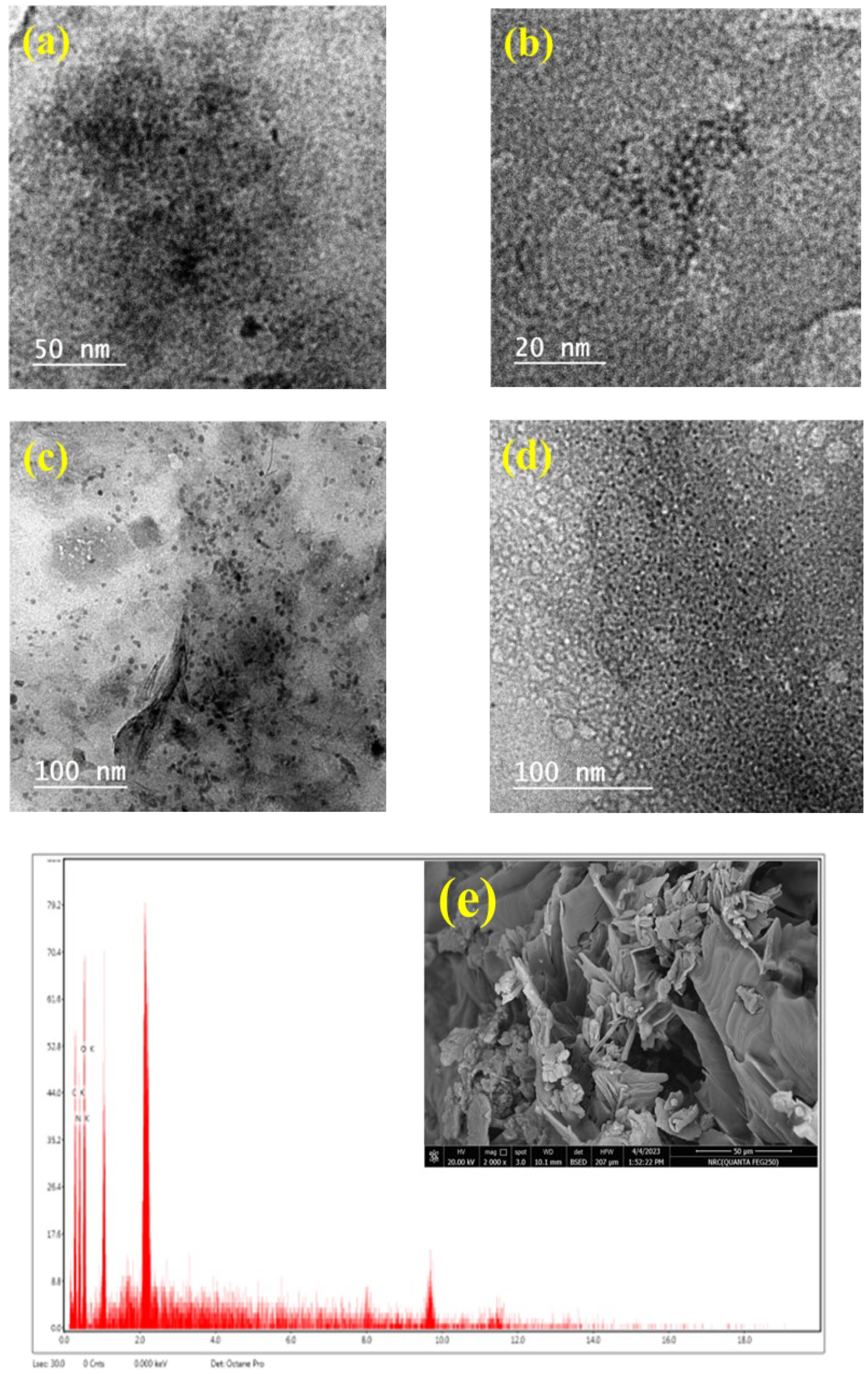
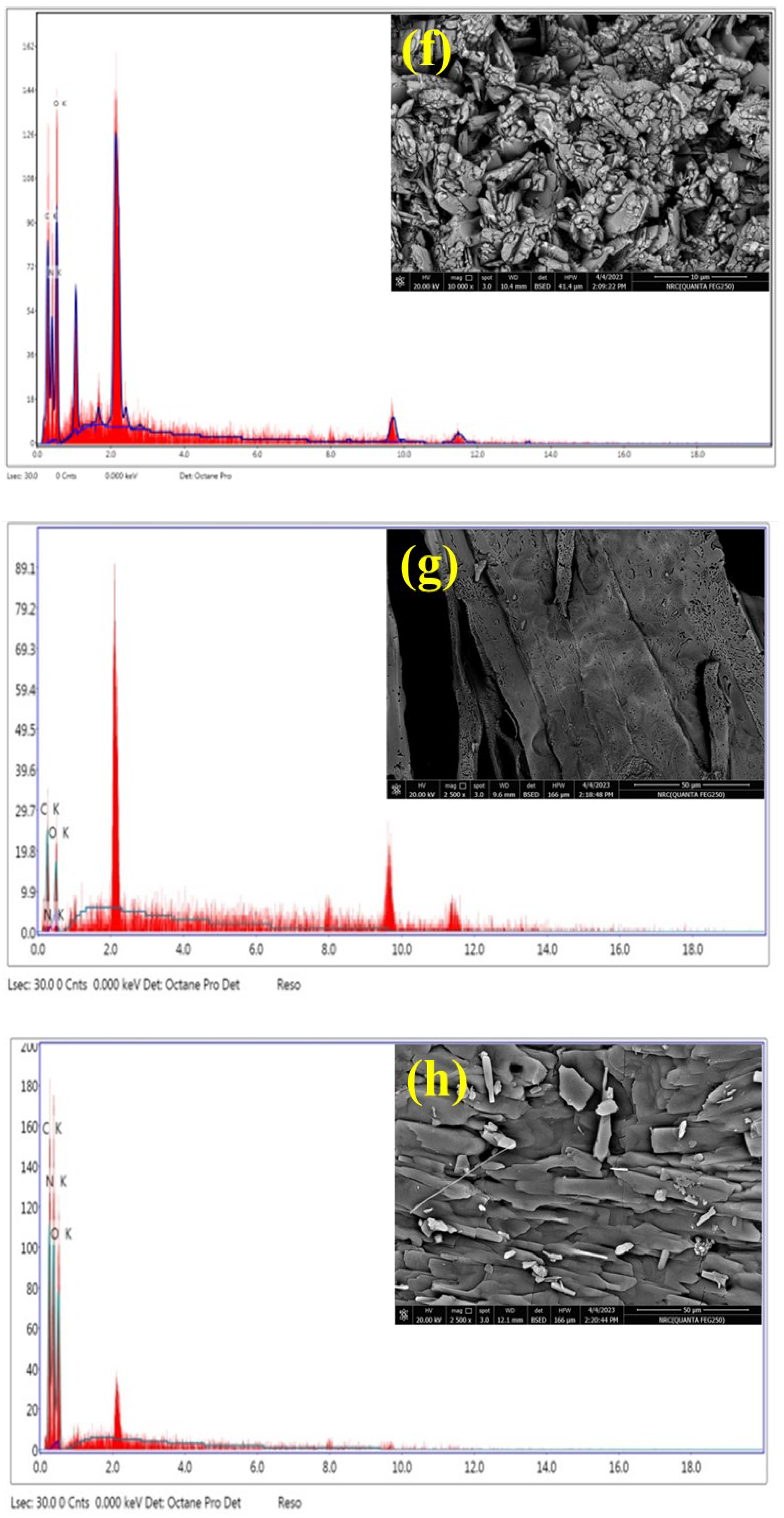



| Parameter | C/CS/B QDs | C/CS QDs | CS/B QDs | C/B QDs |
|---|---|---|---|---|
| D band (cm−1) | – | – | 1000 | 1014 |
| G band (cm−1) | 1982 | 1860 | 1982 | 1650 |
| 2D band (cm−1) | 2660 | 2398 | 2674 | 2660 |
| ID/IG | – | – | – | 2.02 |
| I2D/IG | 0.37 | 0.57 | 0.58 | 0.63 |
| Sample | Stage | Temp. (°C) | Max. Temp. (°C) | ML (%) | R2 | N | A (s−1) | ΔH (kj.mol−1) | ΔG (kj.mol−1) | Δs (kj.mol−1) | Ea (kj.mol−1) | SE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C/CS/B QDs | 1st | 41.82–264.60 | 214.05 | 37.51 | 0.912 | 2.0 | 0.11 | 66.72 | 196.75 | –0.26 | 70.76 | 58 × 10−1 |
| 2nd | 266.27–357.27 | 331.02 | 13.29 | 0.984 | 2.0 | 0.24 | 14.47 | 173.06 | –0.26 | 19.49 | 15 × 10−2 | |
| 357.40–978.72 | 397.27 | 25.86 RW = 23.34% | 0.996 | 2.0 | 0.24 | 12.43 | 188.95 | –0.26 | 18.00 ∑E = 37.49 | 16 × 10−2 | ||
| C/CS QDs | 1st | 41.70–276.06 | 215.76 | 35.80 | 0.952 | 3.0 | 0.04 | 133.58 | 268.25 | –0.27 | 137.64 | 12 × 10−1 |
| 2nd | 277.83–361.05 | 330.05 | 10.78 | 0.988 | 2.0 | 0.24 | 14.32 | 172.55 | –0.26 | 19.34 | 11 × 10−2 | |
| 362.71–979.81 | 400.36 | 26.59 RW = 26.83% | 0.994 | 2.0 | 0.24 | 12.87 | 190.15 | –0.26 | 18.47 ∑E = 37.81 | 20 × 10−2 | ||
| CS/B QDs | 1st | 42.15–270.61 | 216.53 | 14.76 | 0.936 | 2.0 | 0.14 | 57.33 | 187.05 | –0.26 | 61.40 | 41 × 10−1 |
| 2nd | 279.27–379.82 | 342.99 | 18.56 | 0.978 | 2.0 | 0.21 | 32.64 | 195.13 | –0.26 | 37.76 | 39 × 10−2 | |
| 379.90–452.90 | 403.60 | 8.18 | 0.987 | 2.0 | 0.23 | 24.05 | 202.61 | –0.26 | 29.67 | 11 × 10−2 | ||
| 3rd | 598.23–759.90 | 663.30 | 13.16 RW = 58.50% | 0.999 | 3.0 | 0.20 | 14.03 | 264.68 | –0.26 | 21.81 ∑E = 89.24 | 33 × 10−3 | |
| C/B QDs | 1st | 45.26–119.92 | 96.38 | 2.55 | 0.835 | 2.0 | 0.007 | 88.00 | 187.17 | –0.26 | 91.07 | 60 × 10−1 |
| 2nd | 151.98–269.50 | 217.19 | 32.58 | 0.972 | 2.0 | 0.17 | 44.63 | 173.92 | –0.26 | 48.71 | 10 × 10−1 | |
| 3rd | 282.05–371.05 | 340.47 | 6.18 | 0.972 | 2.0 | 0.24 | 17.11 | 178.24 | –0.26 | 22.21 | 20 × 10−2 | |
| 372.16–979.23 | 402.43 | 9.81 RW = 48.88% | 0.994 | 2.0 | 0.25 | 13.10 | 190.92 | –0.26 | 18.72 ∑E = 89.64 | 21 × 10−2 |
| Kinetic Model | Metal Ion | Parameter | C/CS/B QDs | C/CS QDs | CS/B QDs | C/B QDs |
|---|---|---|---|---|---|---|
| Pseudo-first order | Pb(II) | qexp. (mg/g) | 99.75 | 96.75 | 92.50 | 97.25 |
| qCalc. (mg/g) | 99.09 | 93.07 | 90.24 | 93.95 | ||
| k1 | 35 × 10−4 | 16 × 10−4 | 62 × 10−6 | 74 × 10−6 | ||
| R2 | 0.722 | 0.821 | 0.858 | 0.769 | ||
| Cu(II) | qexp. (mg/g) | 99.75 | 98.50 | 97.25 | 98.50 | |
| qCalc. (mg/g) | 94.08 | 96.18 | 95.96 | 97.47 | ||
| k1 | 22 × 10−4 | 73 × 10−6 | 26 × 10−6 | 37 × 10−6 | ||
| R2 | 0.881 | 0.608 | 0.845 | 0.947 | ||
| Cr(VI) | qexp. (mg/g) | 99.97 | 99.97 | 99.97 | 99.75 | |
| qCalc. (mg/g) | 99.73 | 99.77 | 98.61 | 100.79 | ||
| k1 | 78 × 10−5 | 99 × 10−5 | 36 × 10−4 | 23 × 10−4 | ||
| R2 | 0.904 | 0.675 | 0.842 | 0.865 | ||
| Mn(II) | qexp. (mg/g) | 99.95 | 98.00 | 98.00 | 97.25 | |
| qCalc. (mg/g) | 96.02 | 96.62 | 92.50 | 96.19 | ||
| k1 | 85 × 10−4 | 45 × 10−4 | 31 × 10−4 | 34 × 10−4 | ||
| R2 | 0.818 | 0.937 | 0.920 | 0.767 | ||
| Pseudo-second order | Pb(II) | qCalc. (mg/g) | 5.10 | 3.44 | 9.55 | 3.52 |
| k2 | 11 × 10−2 | 37 × 10−1 | 14 × 10−1 | 22 × 10−1 | ||
| R2 | 0.993 | 0.855 | 0.994 | 0.963 | ||
| Cu(II) | qCalc. (mg/g) | 1.91 | 1.79 | 3.94 | 1.33 | |
| k2 | 11 × 10−2 | 94 × 10−1 | 96 × 10−2 | 19 × 10−1 | ||
| R2 | 0.972 | 0.824 | 0.982 | 0.974 | ||
| Cr(VI) | qCalc. (mg/g) | 0.23 | 0.031 | 0.031 | 0.037 | |
| k2 | 39 × 10−2 | 18 × 10−1 | 11 × 10−1 | 36 × 10−2 | ||
| R2 | 0.958 | 0.917 | 0.973 | 0.592 | ||
| Mn(II) | qCalc. (mg/g) | 2.39 | 1.94 | 7.45 | 2.11 | |
| k2 | 10 × 10−1 | 43 × 10−2 | 31 × 10−2 | 18 × 10−1 | ||
| R2 | 0.986 | 0.931 | 0.937 | 0.984 | ||
| Intra-particle diffusion | Pb(II) | kp (mg·g−1·min−1(0.5)) | 1.53 | 30 × 10−1 | 25 × 10−1 | 26 × 10−1 |
| C(mg/g) | 21.35 | 8.14 | 11.46 | 7.17 | ||
| R2 | 0.788 | 0.834 | 0.906 | 0.865 | ||
| Cu(II) | kp (mg·g−1·min−1(0.5)) | 8 × 10−2 | 6 × 10−2 | 2 × 10−2 | 1 × 10−2 | |
| C(mg/g) | 3.23 | 0.76 | 4.19 | 0.196 | ||
| R2 | 0.846 | 0.852 | 0.954 | 0.791 | ||
| Cr(VI) | kp (mg·g−1·min−1(0.5)) | 23 × 10−2 | 21 × 10−2 | 17 × 10−2 | 29 × 10−2 | |
| C(mg/g) | 0.39 | 0.34 | 2.54 | 7.66 | ||
| R2 | 0.948 | 0.793 | 0.559 | 0.740 | ||
| Mn(II) | kp (mg·g−1·min−1(0.5)) | 44 × 10−1 | 35 × 10−1 | 1 × 10−2 | 29 × 10−1 | |
| C(mg/g) | 8.38 | 8.84 | 0.45 | 7.66 | ||
| R2 | 0.537 | 0.853 | 0.905 | 0.811 |
| Kinetic Model | Metal Ion | Parameter | C/CS/B QDs | C/CS QDs | CS/B QDs | C/B QDs |
|---|---|---|---|---|---|---|
| Langmuir isotherm | Pb(II) | qm (mg/g) | 99.70 | 96.99 | 92.45 | 97.20 |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | ||
| Cu(II) | qm (mg/g) | 97.94 | 98.16 | 96.44 | 98.44 | |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | ||
| Cr(VI) | qm (mg/g) | 91.51 | 90.39 | 89.81 | 89.08 | |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | ||
| Mn(II) | qm (mg/g) | 97.47 | 97.97 | 88.48 | 99.89 | |
| R2 | 0.448 | 0.999 | 0.999 | 0.999 | ||
| Freundlich isotherm | Pb(II) | Kf (mg(1−1/n) g−1 L1/n) | 7.37 | 7.31 | 7.24 | 7.32 |
| R2 | 0.994 | 0.998 | 0.994 | 0.994 | ||
| Cu(II) | Kf (mg(1−1/n) g−1 L1/n) | 7.33 | 7.34 | 7.31 | 7.34 | |
| R2 | 0.959 | 0.993 | 0.982 | 0.947 | ||
| Cr(VI) | Kf (mg(1−1/n) g−1 L1/n) | 7.21 | 7.20 | 7.19 | 7.42 | |
| R2 | 0.979 | 0.957 | 0.978 | 0.951 | ||
| Mn(II) | Kf (mg(1−1/n) g−1 L1/n) | 7.32 | 7.33 | 7.15 | 7.38 | |
| R2 | 0.976 | 0.914 | 0.991 | 0.991 |
| Kinetic Model | Metal Ion | C/CS/B QDs | C/CS QDs | CS/B QDs | C/B QDs | |
|---|---|---|---|---|---|---|
| Δs (kJ/mole) | Pb(II) | 83 × 10−6 | 66 × 10−6 | 41 × 10−6 | −69 × 10−6 | |
| Cu(II) | 68 × 10−6 | 75 × 10−6 | 65 × 10−6 | 76 × 10−6 | ||
| Cr(VI) | 15 × 10−4 | 15 × 10−4 | 14 × 10−4 | 13 × 10−4 | ||
| Mn(II) | 70 × 10−5 | 73 × 10−6 | 14 × 10−6 | 84 × 10−6 | ||
| ΔH (kJ/mole) | Pb(II) | −50 × 10−2 | −82 × 10−1 | −20 | −69 × 10−1 | |
| Cu(II) | −49 × 10−1 | −42 × 10−1 | −89 × 10−1 | −36 × 10−1 | ||
| Cr(VI) | 22 | 27 | 30 | 18 | ||
| Mn(II) | −63 × 10−1 | −5 × 10−1 | −32 | 11 × 10−1 | ||
| ΔG (kJ/mole) | 298 K | Pb(II) | −25 × 10−2 | −25 × 10−2 | −25 × 10−2 | −25 × 10−2 |
| 308 K | −25 × 10−2 | −25 × 10−2 | −25 × 10−2 | −25 × 10−2 | ||
| 318 K | −26 × 10−2 | −26 × 10−2 | −26 × 10−2 | −26 × 10−2 | ||
| 328 K | −27 × 10−2 | −27 × 10−2 | −27 × 10−2 | −27 × 10−2 | ||
| 338 K | −28 × 10−2 | −28 × 10−2 | −28 × 10−2 | −28 × 10−2 | ||
| 298 K | Cu(II) | −25 × 10−2 | −25 × 10−2 | −25 × 10−2 | −25 × 10−2 | |
| 308 K | −25 × 10−2 | −25 × 10−2 | −25 × 10−2 | −25 × 10−2 | ||
| 318 K | −26 × 10−2 | −26 × 10−2 | −26 × 10−2 | −26 × 10−2 | ||
| 328 K | −27 × 10−2 | −27 × 10−2 | −27 × 10−2 | −27 × 10−2 | ||
| 338 K | −28 × 10−2 | −28 × 10−2 | −28 × 10−2 | −28 × 10−2 | ||
| 298 K | Cr(VI) | −24 × 10−2 | −24 × 10−2 | −24 × 10−2 | −26 × 10−2 | |
| 308 K | −27 × 10−2 | −27 × 10−2 | −28 × 10−2 | −26 × 10−2 | ||
| 318 K | −29 × 10−2 | −29 × 10−2 | −29 × 10−2 | −29 × 10−2 | ||
| 328 K | −29 × 10−2 | −31 × 10−2 | −30 × 10−2 | −30 × 10−2 | ||
| 338 K | −31 × 10−3 | −31 × 10−3 | −30 × 10−3 | −30 × 10−3 | ||
| 298 K | Mn(II) | −25 × 10−3 | −25 × 10−2 | −28 × 10−2 | −24 × 10−2 | |
| 308 K | −25 × 10−2 | −25 × 10−2 | −25 × 10−2 | −25 × 10−2 | ||
| 318 K | −26 × 10−2 | −26 × 10−2 | −26 × 10−2 | −26 × 10−2 | ||
| 328 K | −27 × 10−2 | −27 × 10−2 | −27 × 10−2 | −27 × 10−2 | ||
| 338 K | −28 × 10−2 | −28 × 10−2 | −28 × 10−2 | −28 × 10−2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohamy, H.-A.S.; El-Sakhawy, M.; Hassan, E.B.; Kamel, S. Microwave-Prepared Quantum Dots and Their Potential Applications as Adsorbents and Chemosensors. Materials 2023, 16, 6722. https://doi.org/10.3390/ma16206722
Tohamy H-AS, El-Sakhawy M, Hassan EB, Kamel S. Microwave-Prepared Quantum Dots and Their Potential Applications as Adsorbents and Chemosensors. Materials. 2023; 16(20):6722. https://doi.org/10.3390/ma16206722
Chicago/Turabian StyleTohamy, Hebat-Allah S., Mohamed El-Sakhawy, El Barbary Hassan, and Samir Kamel. 2023. "Microwave-Prepared Quantum Dots and Their Potential Applications as Adsorbents and Chemosensors" Materials 16, no. 20: 6722. https://doi.org/10.3390/ma16206722






