Recycling of Silicomanganese Slag and Fly Ash for Preparation of Environment-Friendly Foamed Ceramics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
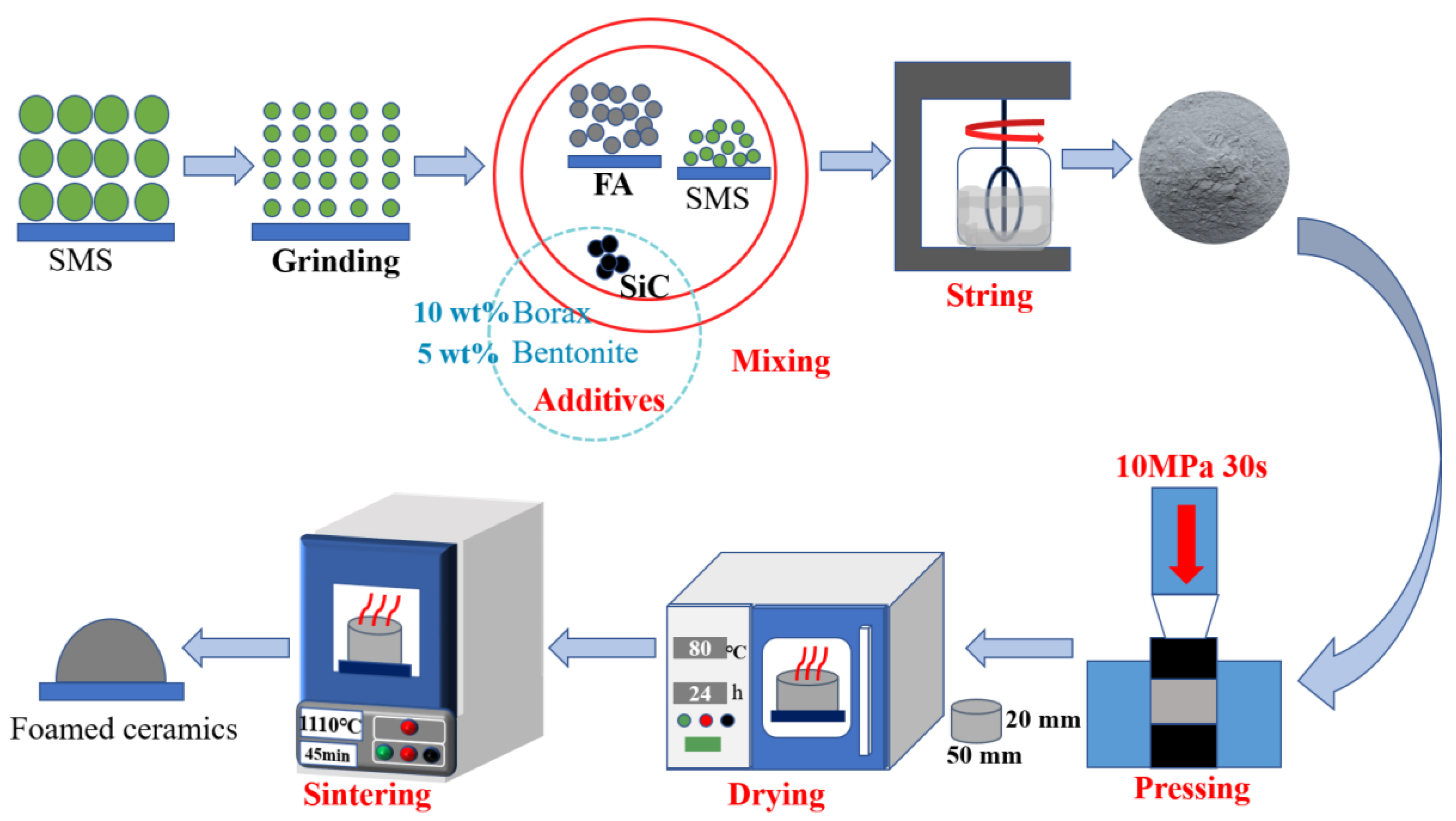
2.2. Material Treatment and Sample Preparation Processes
2.3. Characterization
2.3.1. X-ray Diffraction (XRD)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Pore Size Analysis
2.4. Physical and Mechanical Property Testing
3. Results and Discussion
3.1. The Influence of SMS Content on the Properties of Foamed Ceramics
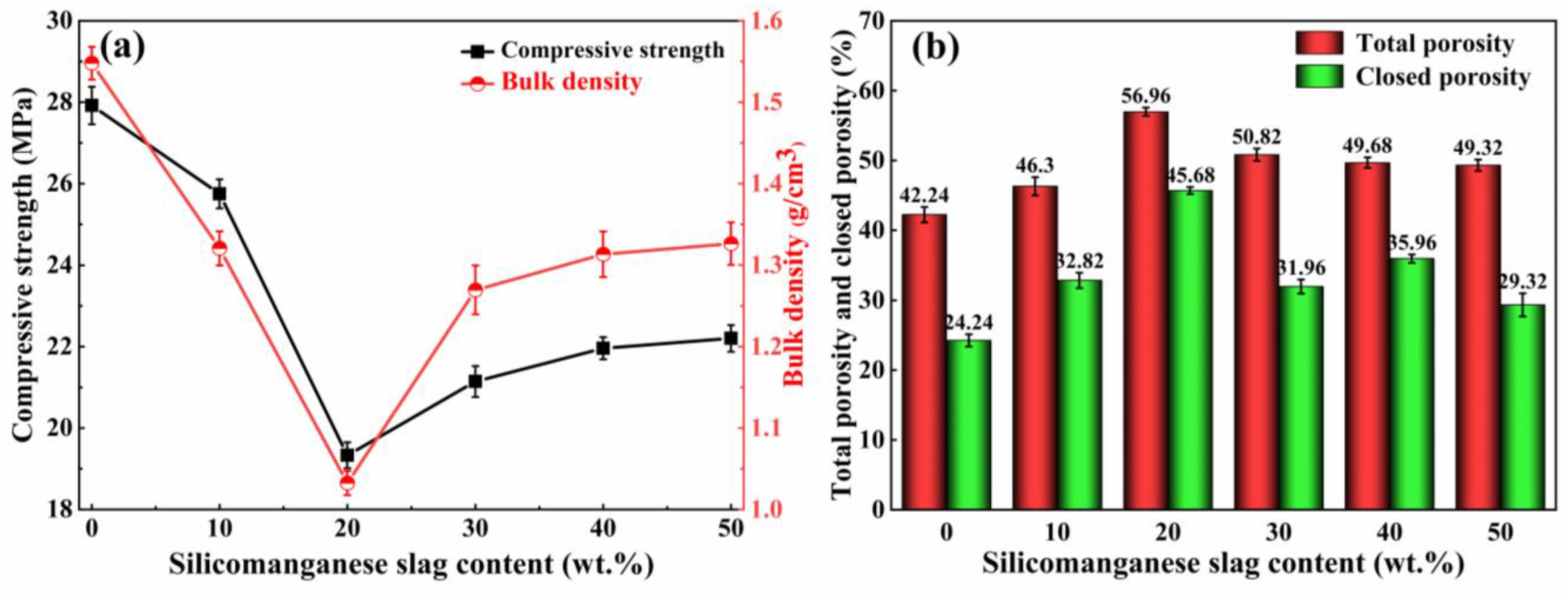
3.1.1. Effect of the SMS Content on the Physical and Mechanical Properties
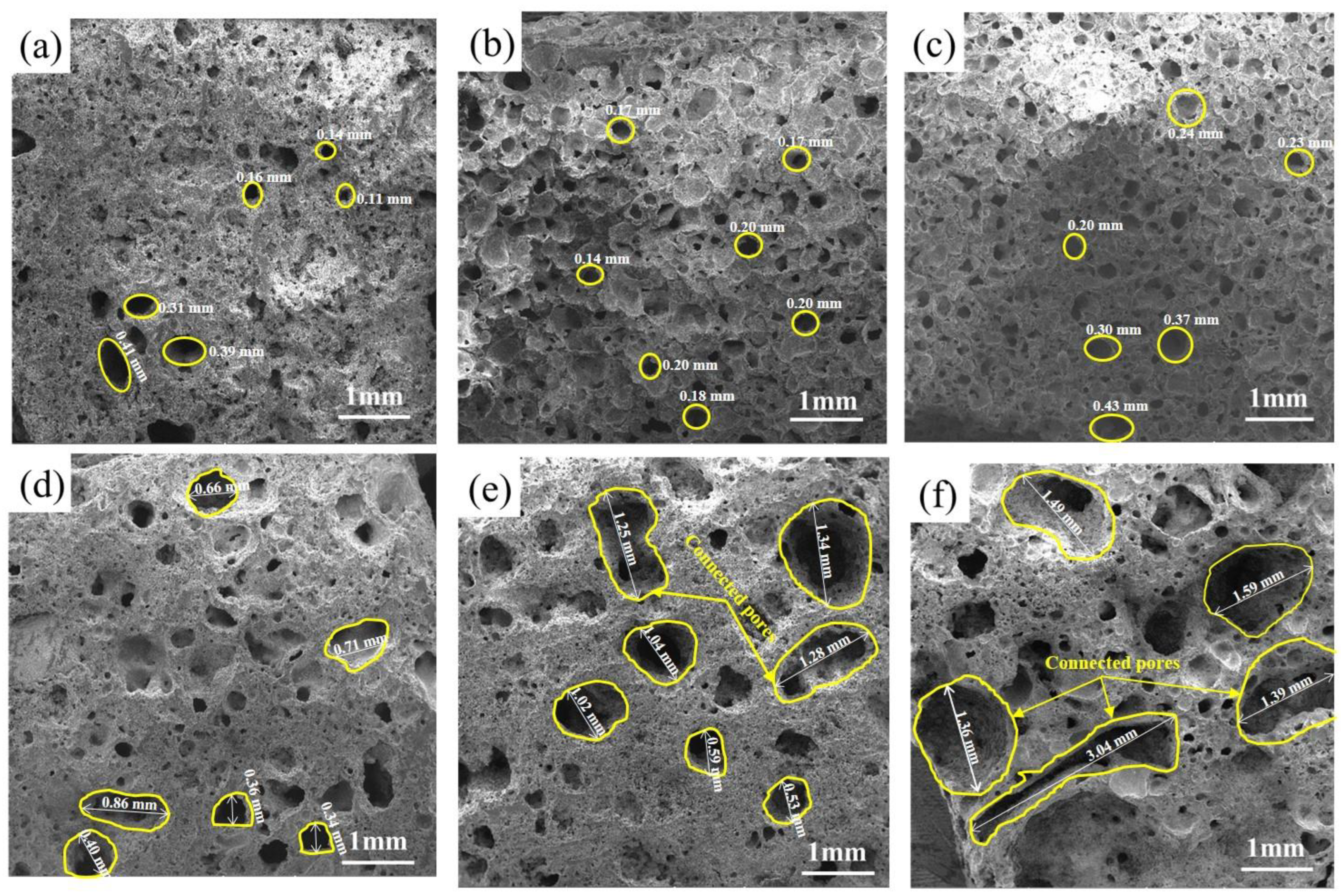
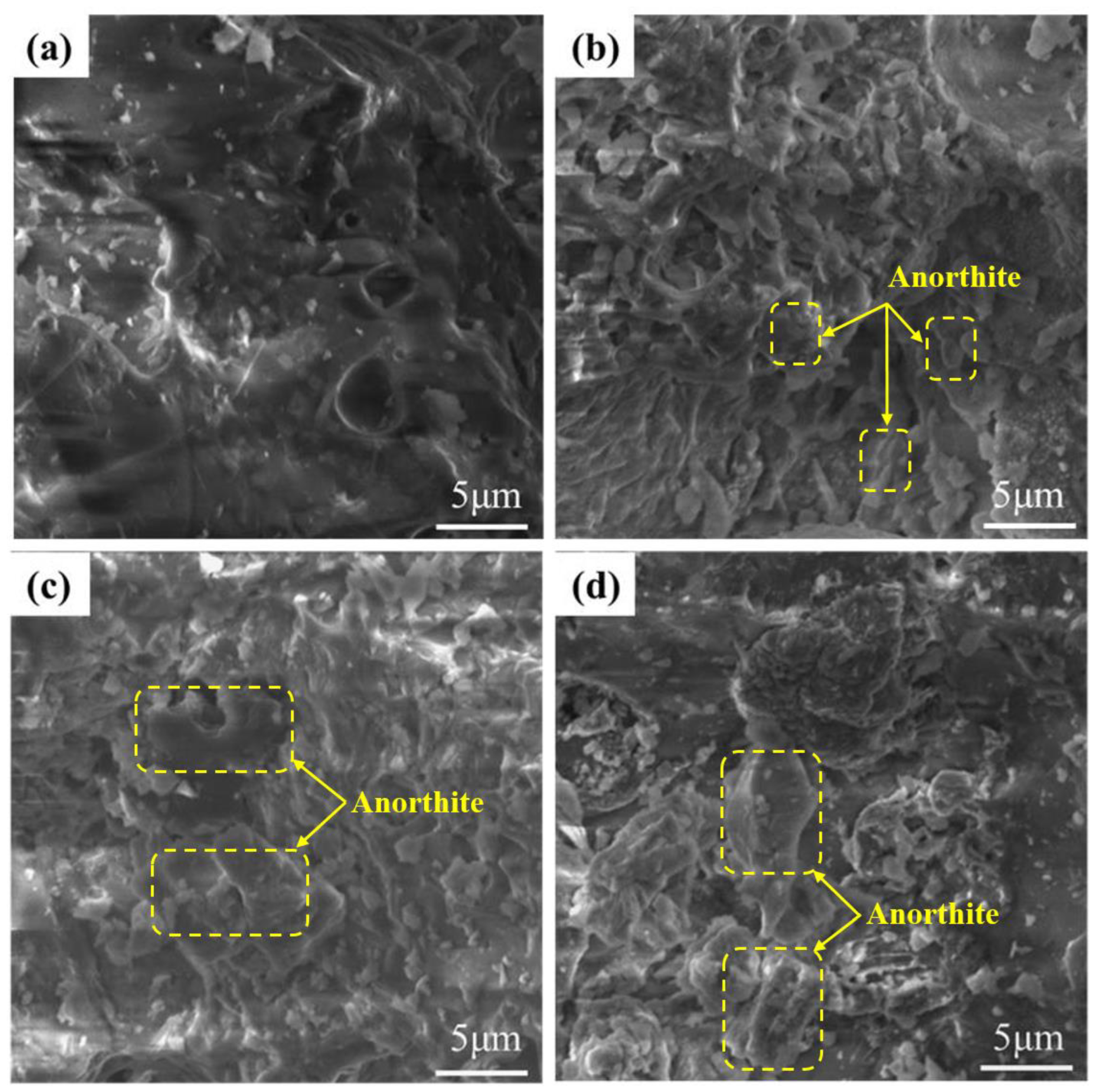
3.1.2. Effects of the SMS Content on the Pore Structure
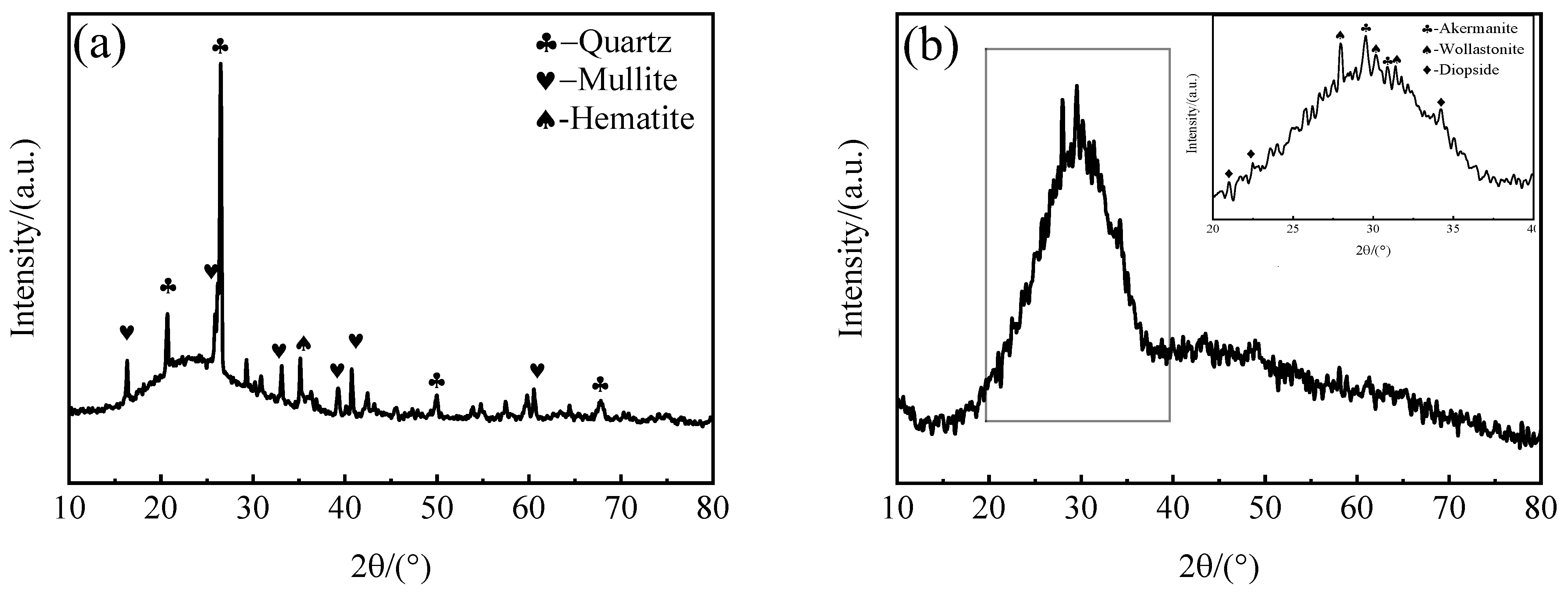
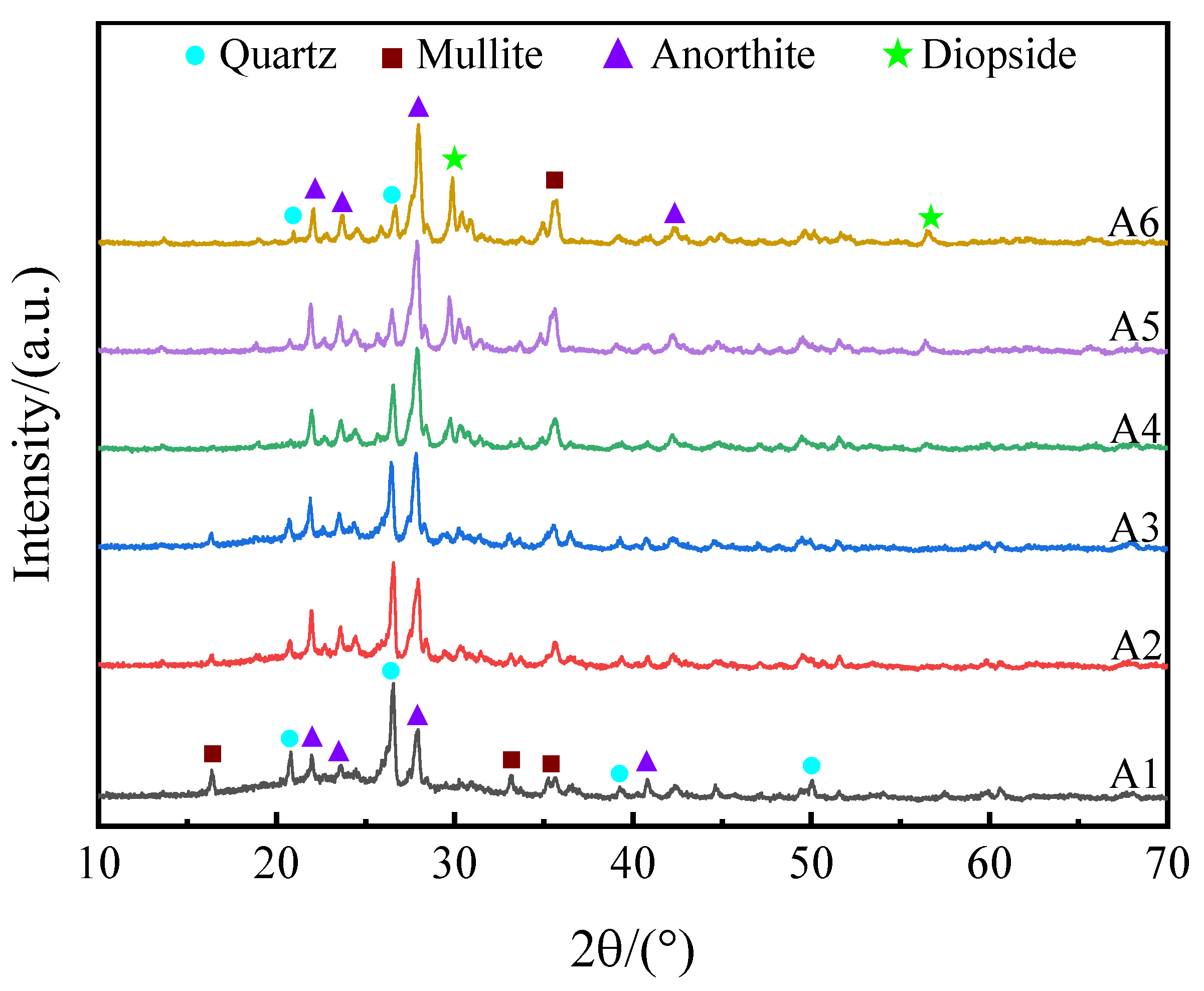
3.1.3. Effects of SMS on the Crystalline Phase
3.2. The Influence of Sintering Temperature on the Properties of Foamed Ceramics
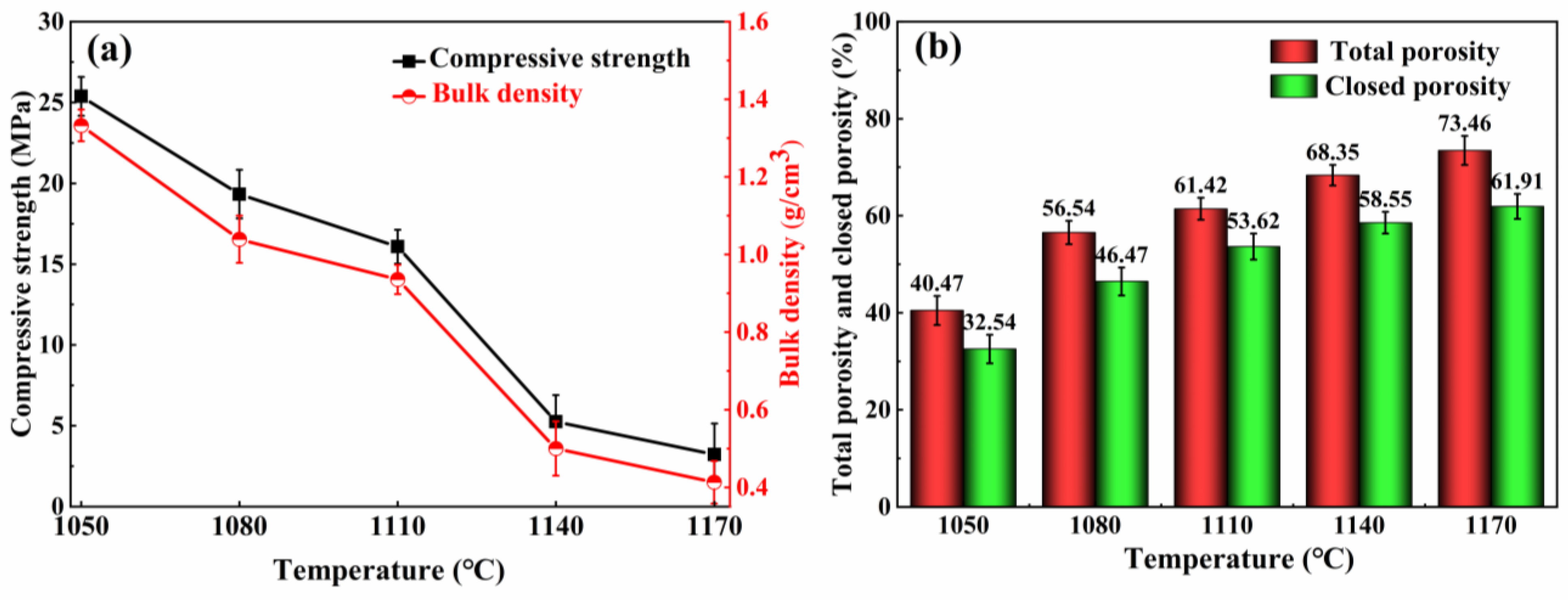
3.2.1. Effects of the Sintering Temperature on the Physical and Mechanical Properties
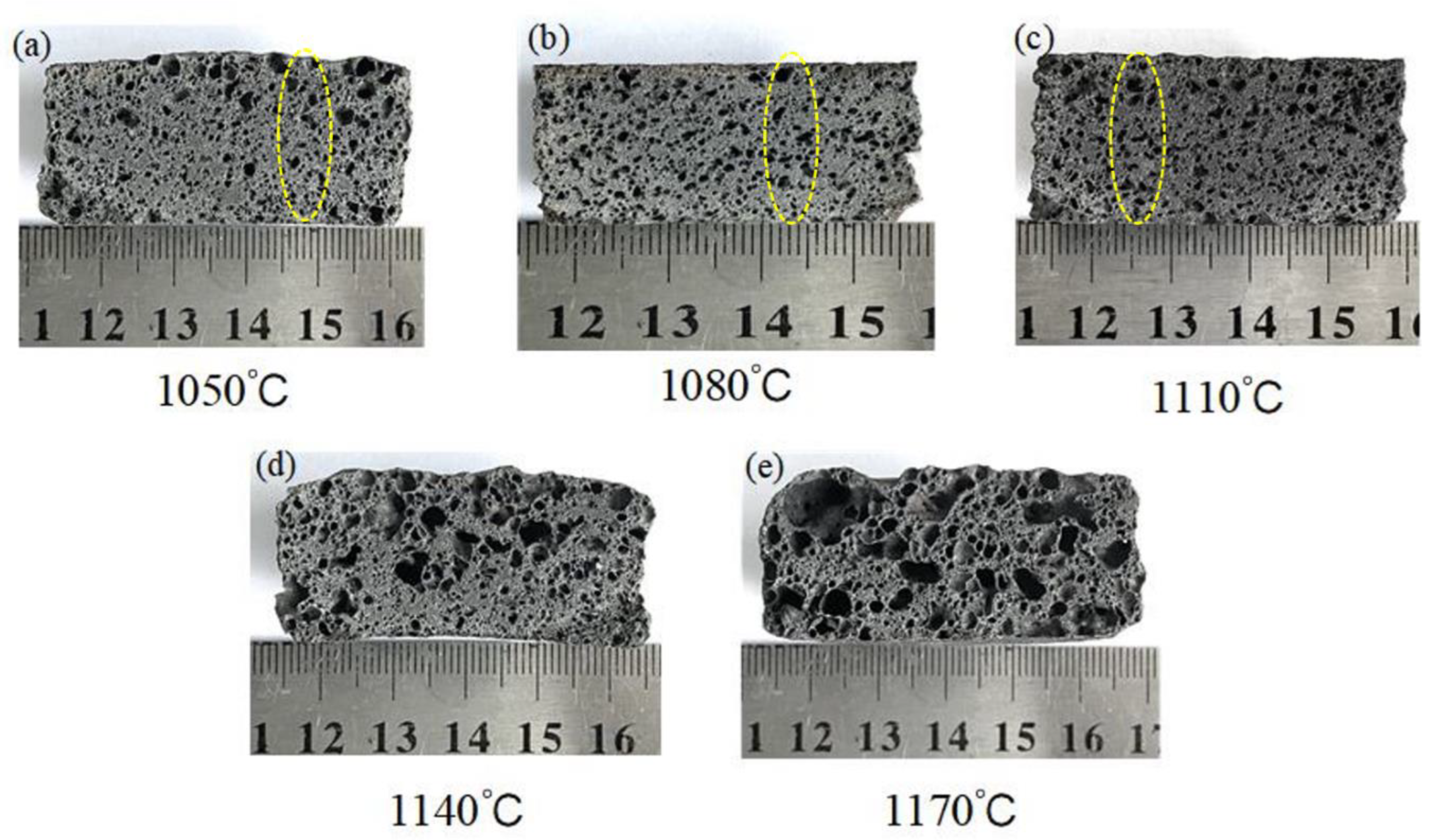
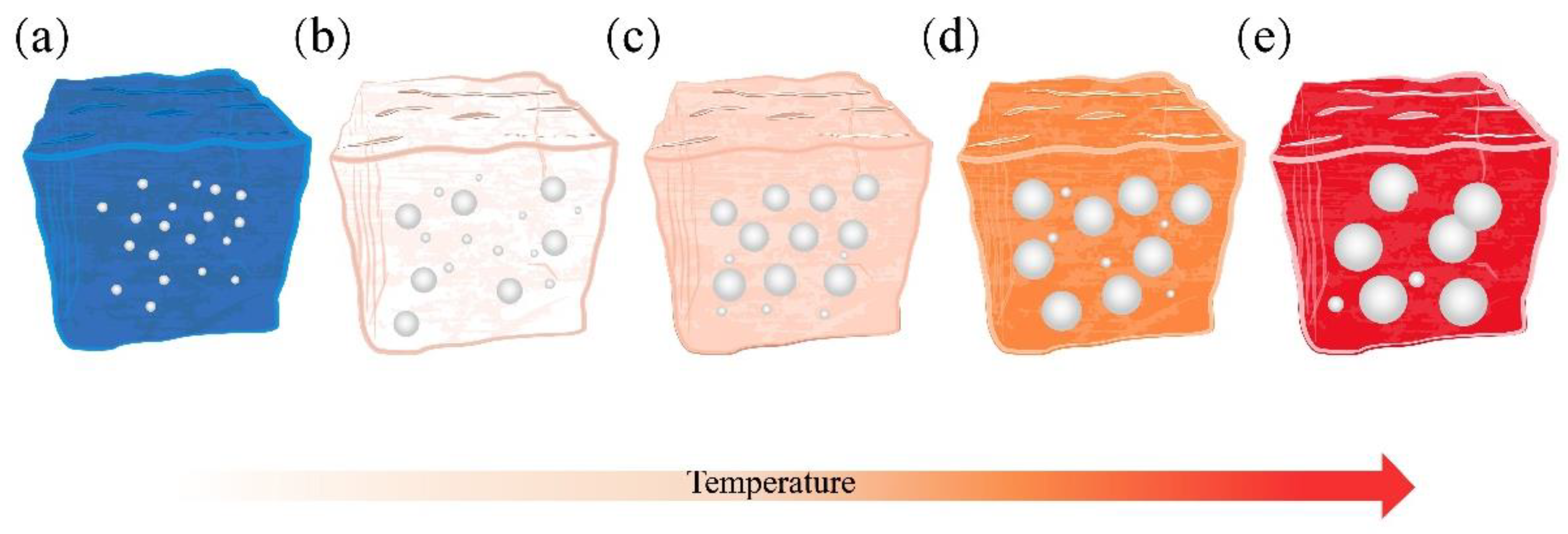
3.2.2. Effect of the Sintering Temperature on the Pore Structure
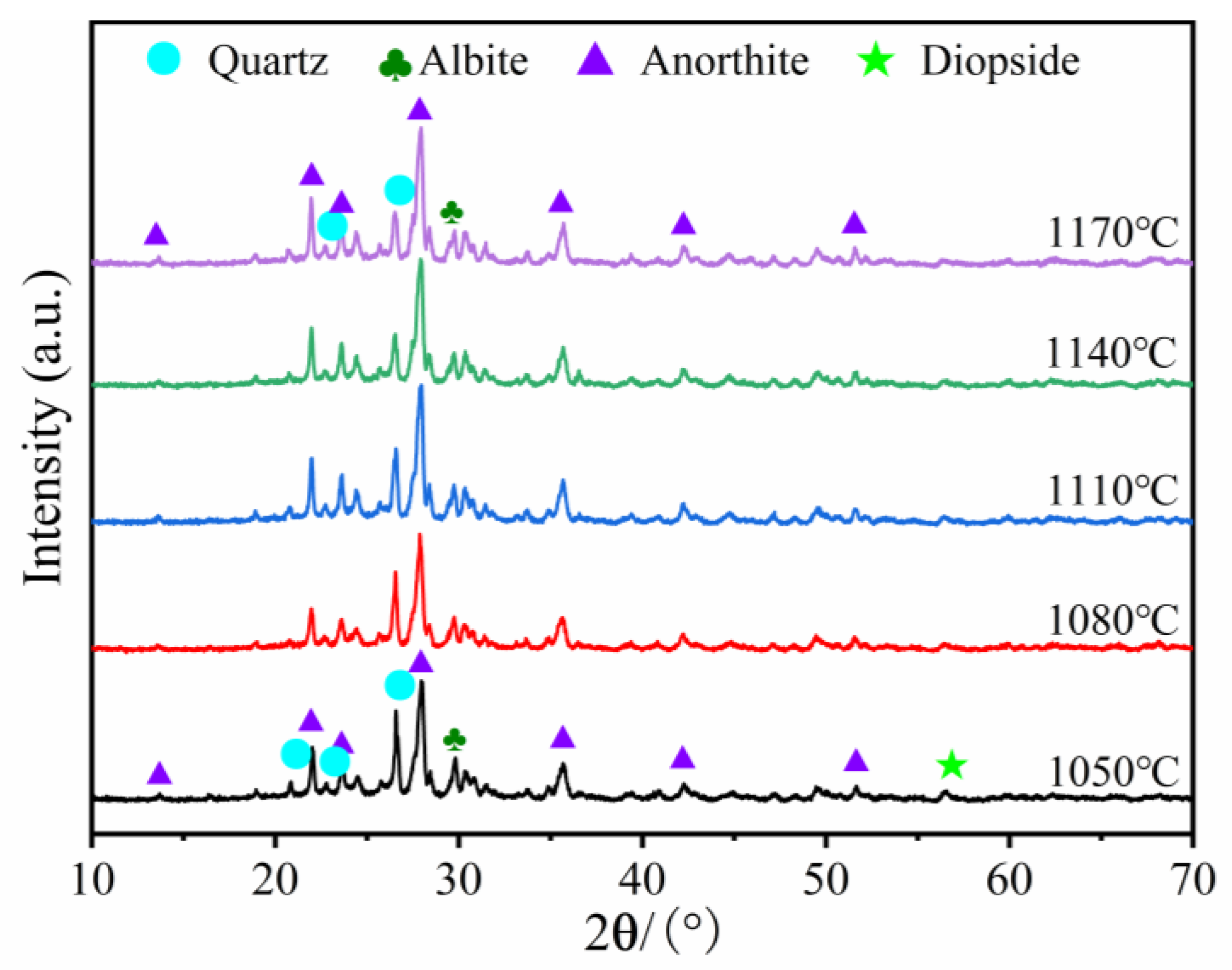
3.2.3. Effects of Sintering Temperature on the Crystalline Phase
3.3. The Influence of Foaming Agent Content on the Properties of Foamed Ceramics
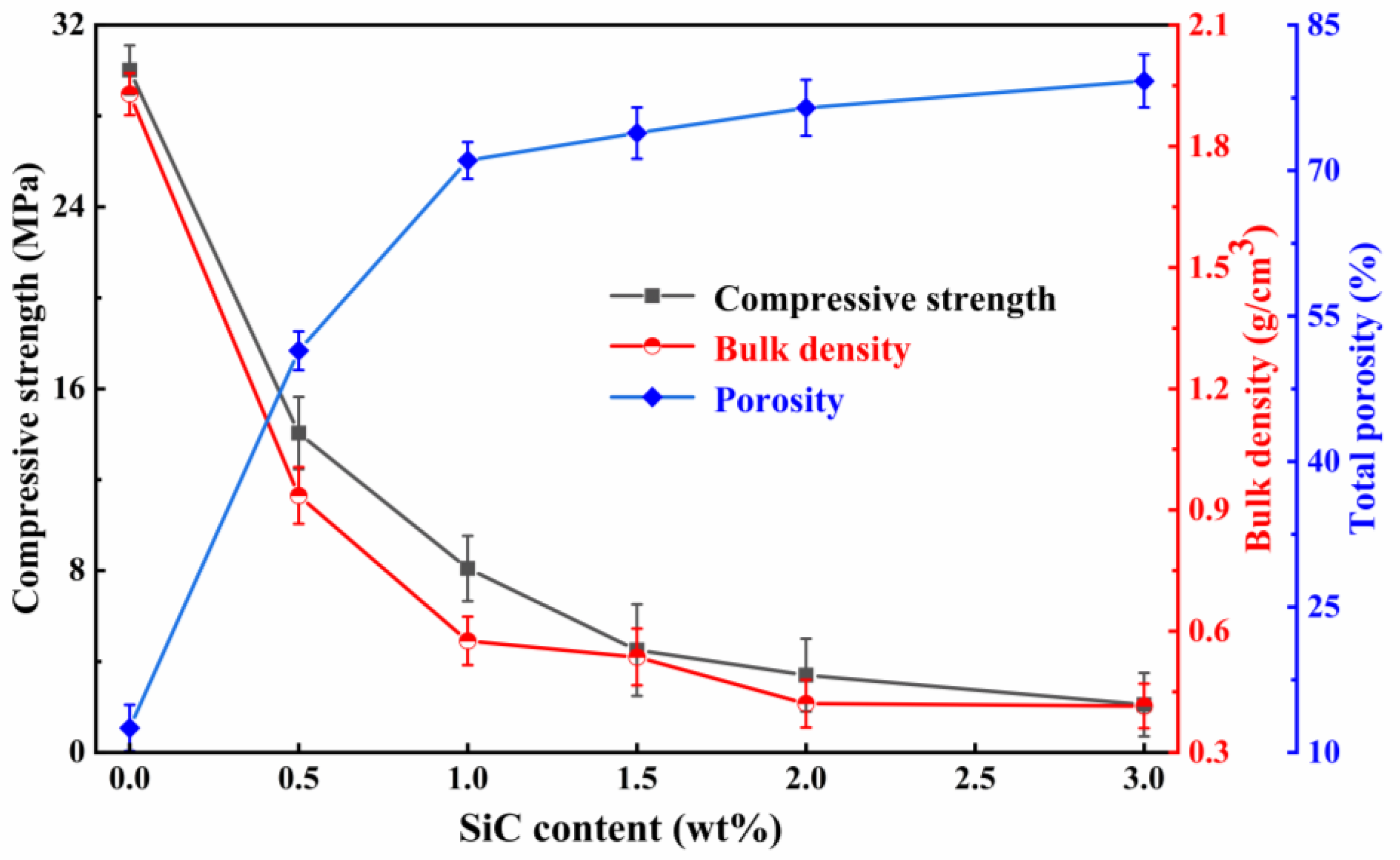
3.3.1. Effects of the Foaming Agent Content on the Physical and Mechanical Properties
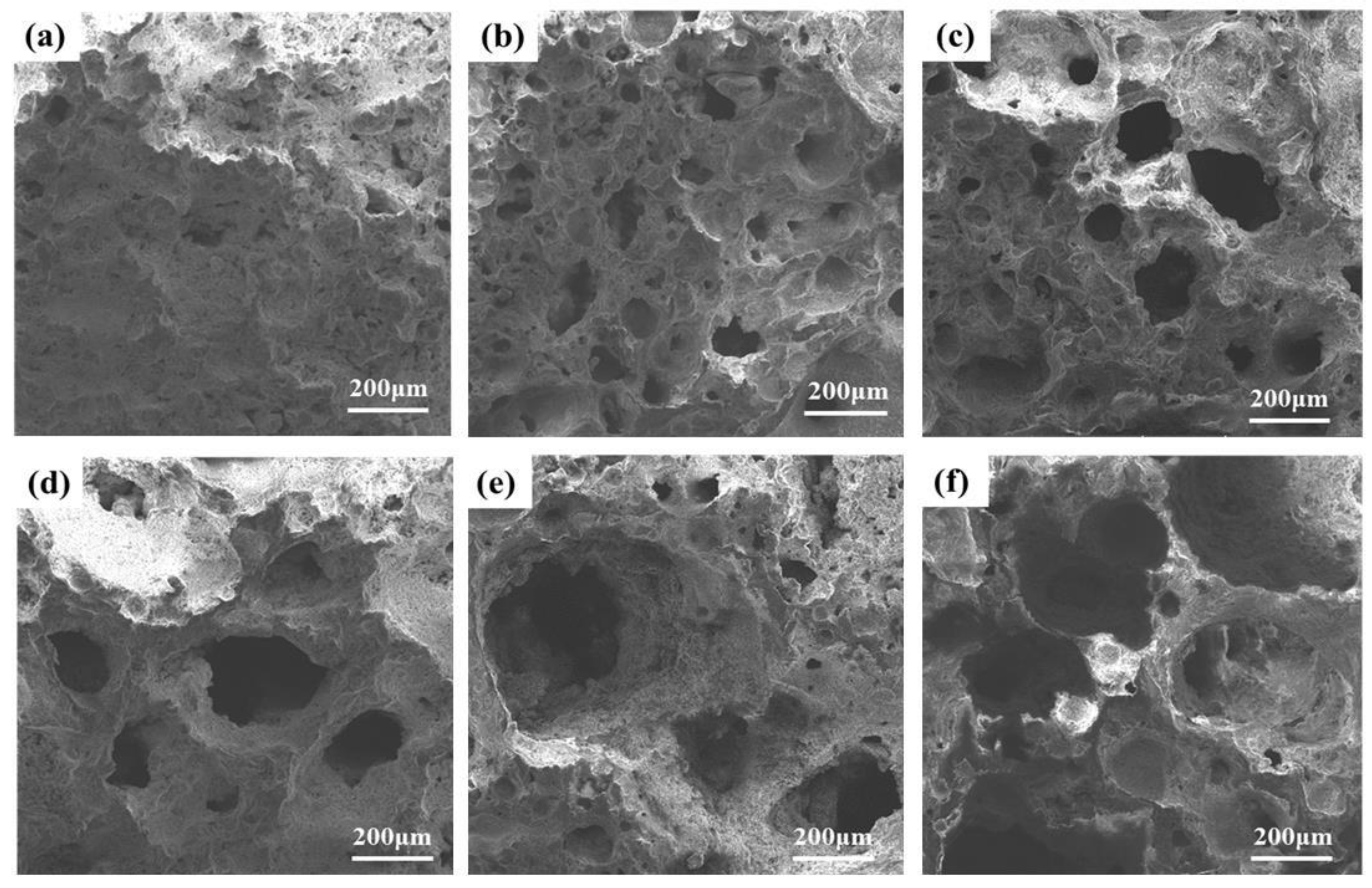
3.3.2. Effects of Foaming Agent Content on Pore Structure
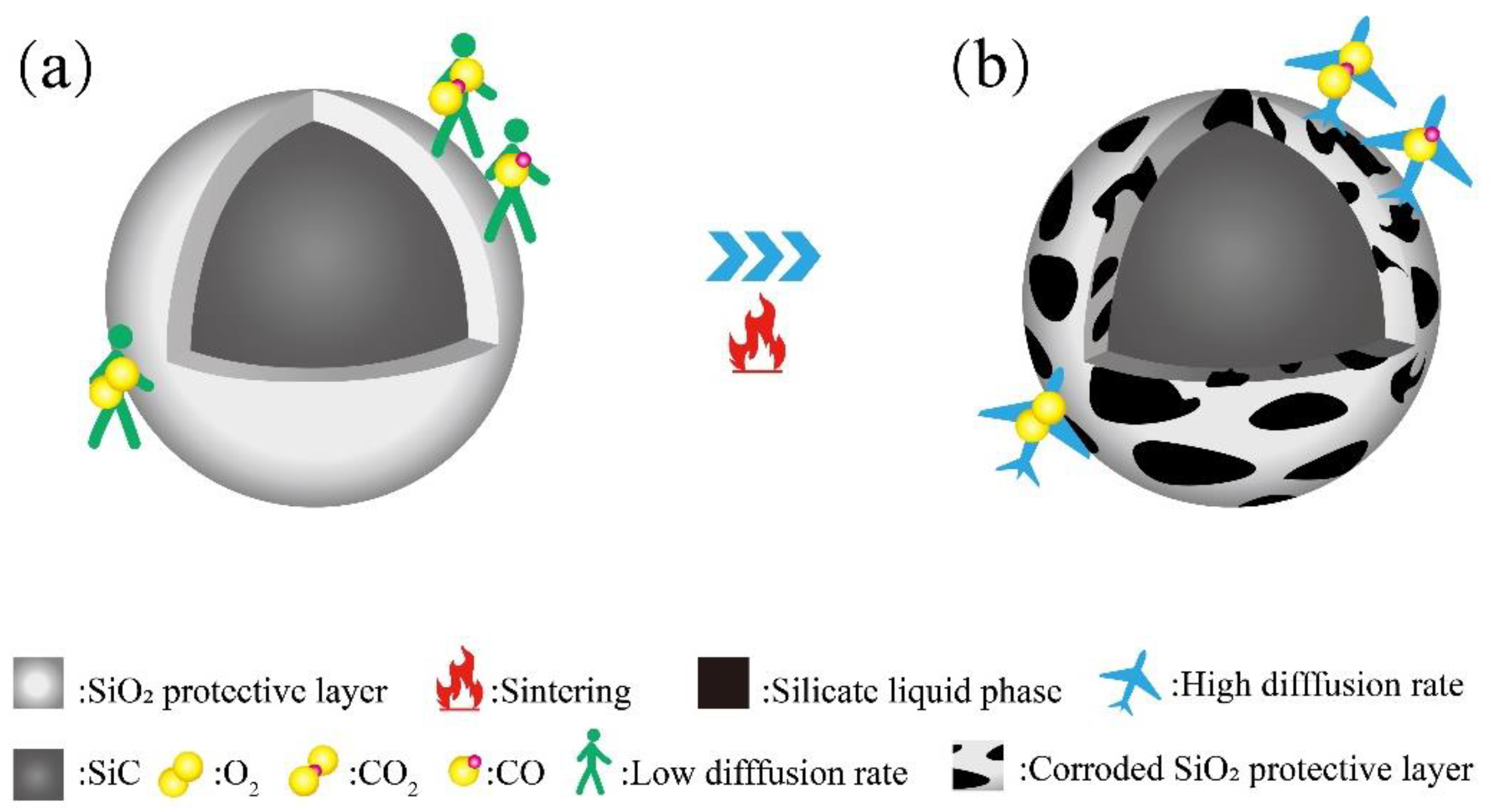
3.4. Foaming Mechanism
4. Conclusions
- At a 20% SMS content, both the total and closed porosity peaks of the foamed ceramics promoted the formation of the porous structure. Simultaneously, their compressive strength and bulk density reached 8.09 MPa and 0.57 g/cm3, near the foamed ceramic partition board (T/CBCSA 12-2019 [54]) 500-density requirement (0.48 g/cm3 ≤ Bulk density ≤ 0.54 g/cm3, compressive strength ≥ 7 MPa). Nevertheless, when the SMS content exceeded 20%, the porosity of the foamed ceramics decreased. This was primarily due to a reduced liquid phase content within the structure as the anorthite phase increased. The decrease in the liquid phase content constrained the foaming process and resulted in reduced porosity.
- In the 1050–1110 °C temperature range, the glassy liquid phase formed by quartz melting corroded the protective SiO2 layer on the SiC surface. This accelerated the reaction rate between O2 and SiC, trapping the resulting gas in the liquid phase. Consequently, this lowered the foamed ceramics’ bulk density and compressive strength while increasing their porosity. However, the liquid viscosity became insufficient when the firing temperature surpassed 1100 °C. This led to the merging and bridging of bubbles, an increase in the number of connecting holes, and the development of a porous collapsed structure. This was detrimental to the formation of the desired porous structure.
- At a concentration of SiC of 1.0 wt%, during high-temperature sintering, SiC oxidation released numerous gases that remained in the liquid glass phase, increasing porosity and facilitating foamed ceramics formation. However, when the SiC content exceeded 1.0 wt%, the increased SiC particles in the oxidation reaction generated more gas, leading to elevated pressure within the bubbles. When the surface tension was less than the pressure gradient inside of and exterior to the bubble, the bubble will rupture and fuse, forming defective pores and inhibiting the development of compressive strength. Therefore, considering pore structure, mechanical properties, and economic value, a SiC content of 1.0 wt% is optimal.
- The best overall performance was achieved using 20 wt% SMS and 80 wt% FA as raw materials, with SiC addition of 1.0 wt% and a sintering temperature of 1100 °C. Under these conditions, the compressive strength, bulk density, and total porosity of the product were 8.09 MPa, 0.57 g/cm3, and 71.04%, respectively. Its excellent porous structure and mechanical properties make it suitable for use as an insulating material or as a decorative material for building partitions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, W.; Cheng, X.; Gong, L.; Li, Y.; Zhang, R.; Zhang, H. Thermal conductivity of highly porous ceramic foams with different agar concentrations. Mater. Lett. 2015, 139, 66–69. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.; Li, Y.; Xu, N. Novel applications of waste ceramics on the fabrication of foamed materials for exterior building walls insulation. Constr. Build. Mater. 2018, 180, 291–297. [Google Scholar] [CrossRef]
- Zhou, L.Z.; Wang, C.A.; Huang, Y. Effects of the Starting Powders on Microstructure and Properties of Porous Silica Ceramics. Key Eng. Mater. 2010, 434–435, 674–677. [Google Scholar] [CrossRef]
- Chi, Y.Z.; Yu, A.M.; Shen, G.Y. The influence of forming pressure and firing temperature on producing closed porosity ceramics using solid residue and tailings. China Ceram. 2009, 45, 69–71. (In Chinese) [Google Scholar] [CrossRef]
- Yu, K.M.; Jiang, C.; Yu, S.H. The preparation of alumina foaming porous ceramics. China Ceram. 2014, 50, 51–53. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.; Gong, Y.F.; Wang, L. The preparation of alumina foaming porous ceramics. Bull. Chin. Ceram. Soc. 2014, 33, 1636–1639. (In Chinese) [Google Scholar] [CrossRef]
- García-Ten, J.; Saburit, A.; Bernardo, E.; Colombo, P. Development of lightweight porcelain stoneware tiles using foaming agents. J. Eur. Ceram. Soc. 2012, 32, 745–752. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Chen, Z.; Ji, R.; Liu, L.; Wang, X. Fabrication and characterization of porous cordierite ceramics prepared from fly ash and natural minerals. Ceram. Int. 2019, 45, 18306–18314. [Google Scholar] [CrossRef]
- Sumi, K.; Kobayashi, Y.; Kato, E. Preparation of Dense Cordierite Ceramics from Ultrafine Particles of Magnesium Hydroxide and Kaolin. J. Ceram. Soc. Jpn. 1998, 106, 693–697. [Google Scholar] [CrossRef]
- Goren, R.; Gocmez, H.; Ozgur, C. Synthesis of cordierite powder from talc, diatomite and alumina. Ceram. Int. 2006, 32, 407–409. [Google Scholar] [CrossRef]
- Banjuraizah, J.; Mohamad, H.; Ahmad, Z.A. Effect of impurities content from minerals on phase transformation, densification and crystallization of α-cordierite glass-ceramic. J. Alloys Compd. 2011, 509, 7645–7651. [Google Scholar] [CrossRef]
- Wang, W.; Shi, Z.; Wang, X.; Fan, W. The phase transformation and thermal expansion properties of cordierite ceramics prepared using drift sands to replace pure quartz. Ceram. Int. 2016, 42, 4477–4485. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Tan, K.; Zhang, L.; Tang, K.; Shi, X. Fabrication and characterization of porous cordierite ceramics prepared from ferrochromium slag. Ceram. Int. 2016, 42, 734–742. [Google Scholar] [CrossRef]
- Yio, M.H.N.; Xiao, Y.; Ji, R.; Russell, M.; Cheeseman, C. Production of foamed glass-ceramics using furnace bottom ash and glass. Ceram. Int. 2021, 47, 8697–8706. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, Z. Preparation and performance of lightweight porous ceramics using metallurgical steel slag. Ceram. Int. 2021, 47, 25169–25176. [Google Scholar] [CrossRef]
- Dong, Y.; Hampshire, S.; Zhou, J.; Ji, Z.; Wang, J.; Meng, G. Sintering and characterization of flyash-based mullite with MgO addition. J. Eur. Ceram. Soc. 2011, 31, 687–695. [Google Scholar] [CrossRef]
- Yang, L.; Li, D.; Zhang, L.; Yan, X.; Ran, J.; Wang, Y.; Zhang, H. On the utilization of waste fried oil as flotation collector to remove carbon from coal fly ash. Waste Manag. 2020, 113, 62–69. [Google Scholar] [CrossRef]
- Shao, H.; Liang, K.; Zhou, F.; Wang, G.; Peng, F. Characterization of cordierite-based glass-ceramics produced from fly ash. J. Non-Cryst. Solids 2004, 337, 157–160. [Google Scholar] [CrossRef]
- Vasilopoulos, K.C.; Tulyaganov, D.U.; Agathopoulos, S.; Karakassides, M.A.; Ferreira, J.M.F.; Tsipas, D. Bulk nucleated fine grained mono-mineral glass-ceramics from low-silica fly ash. Ceram. Int. 2009, 35, 555–558. [Google Scholar] [CrossRef]
- Liu, W.; Liang, J.; Fu, C.; Zeng, B.; Huang, M.; Yang, G.; Luo, X.; An, D.; Wei, S.; Xie, Z.; et al. Waste recycling of coal fly ash: A novel approach to prepare hierarchically porous coal fly ash/Al2O3 ceramic composite with high porosity and high strength templated by emulsion-assisted self-assembly. Ceram. Int. 2022, 48, 18588–18595. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, M.; Li, H.; Zhao, M.; Xu, P.; Deng, L. Preparation of ceramic foams from ceramic tile polishing waste and fly ash without added foaming agent. Ceram. Int. 2021, 47, 23338–23349. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Liu, L.; Ji, R.; Wang, X. Integrated utilization of fly ash and waste glass for synthesis of foam/dense bi-layered insulation ceramic tile. Energy Build. 2018, 168, 67–75. [Google Scholar] [CrossRef]
- Huo, W.; Zhang, X.; Chen, Y.; Lu, Y.; Liu, J.; Yan, S.; Wu, J.-M.; Yang, J. Novel mullite ceramic foams with high porosity and strength using only fly ash hollow spheres as raw material. J. Eur. Ceram. Soc. 2018, 38, 2035–2042. [Google Scholar] [CrossRef]
- Yin, Y.; Ma, B.; Hu, C.; Liu, G.; Li, H.; Su, C.; Ren, X.; Yu, J.; Zhang, Y.; Yu, J. Preparation and properties of porous SiC–Al2O3 ceramics using coal ash. Int. J. Appl. Ceram. Technol. 2018, 16, 23–31. [Google Scholar] [CrossRef]
- Miao, X.W.; Bai, Z.T.; Lu, G.H. Review of comprehensive utilization of typical ferroalloy slags. Chin. J. Eng. 2020, 42, 663–679. (In Chinese) [Google Scholar] [CrossRef]
- Tang, Z.; Liang, J.; Jiang, W.; Liu, J.; Jiang, F.; Feng, G.; Lao, X.; Tang, H.; Wang, T. Preparation of high strength foam ceramics from sand shale and steel slag. Ceram. Int. 2020, 46, 9256–9262. [Google Scholar] [CrossRef]
- Zhou, M.; Ge, X.; Wang, H.; Chen, L.; Chen, X. Effect of the CaO content and decomposition of calcium-containing minerals on properties and microstructure of ceramic foams from fly ash. Ceram. Int. 2017, 43, 9451–9457. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Evaluation of the suitability of ground granulated silico-manganese slag in Portland slag cement. Constr. Build. Mater. 2016, 125, 127–134. [Google Scholar] [CrossRef]
- Allahverdi, A.; Ahmadnezhad, S. Mechanical activation of silicomanganese slag and its influence on the properties of Portland slag cement. Powder Technol. 2014, 251, 41–51. [Google Scholar] [CrossRef]
- Navarro, R.; Alcocel, E.G.; Sánchez, I.; Garcés, P.; Zornoza, E. Mechanical properties of alkali activated ground SiMn slag mortars with different types of aggregates. Constr. Build. Mater. 2018, 186, 79–89. [Google Scholar] [CrossRef]
- Shareef, U.; Cheela VR, S.; Raju, S.G. Study on physical and mechanical properties of quartzite and silico-manganese slag as alternative material for coarse aggregate. Int. J. Sci. Res. Dev. 2015, 3, 72–74. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Reaction kinetics, microstructure and strength behavior of alkali activated silico-manganese (SiMn) slag—Fly ash blends. Constr. Build. Mater. 2017, 147, 371–379. [Google Scholar] [CrossRef]
- Navarro, R.; Zornoza, E.; Garcés, P.; Sánchez, I.; Alcocel, E.G. Optimization of the alkali activation conditions of ground granulated SiMn slag. Constr. Build. Mater. 2017, 150, 781–791. [Google Scholar] [CrossRef]
- GB/T 1964–1996; Test Method for Crushing Strength of Porous Ceramic. China Standard Press: Beijing, China, 1996.
- Hui, T.; Sun, H.; Peng, T.; Liu, L.; Ding, W.; Liu, B.; Wang, C. Recycling of extracted titanium slag and gold tailings for preparation of self-glazed ceramic foams. Ceram. Int. 2022, 48, 23415–23427. [Google Scholar] [CrossRef]
- Wang, Z.; Lyu, X.; Yao, G.; Wu, P.; Wang, J.; Wei, J. Preparation of Ca–Si–Al–Mg porous ceramics by Co-operation of Ca&Mg-contained soda residue and altered rock gold tailings. J. Clean. Prod. 2020, 262, 121345. [Google Scholar] [CrossRef]
- Ge, X.; Zhou, M.; Wang, H.; Chen, L.; Li, X.; Chen, X. Effects of flux components on the properties and pore structure of ceramic foams produced from coal bottom ash. Ceram. Int. 2019, 45, 12528–12534. [Google Scholar] [CrossRef]
- Ge, X.X. Study on composition design, pore structure and mechanical properties of foamed ceramic from coal bottom ash. Wuhan Wuhan Univ. Technol. 2020. (In Chinese) [Google Scholar] [CrossRef]
- Ge, X.; Zhou, M.; Fan, C.; Zhang, Y.; Zhang, X. Investigation on strength and failure behavior of ceramic foams prepared from silicoaluminous industrial waste under uniaxial compression. Constr. Build. Mater. 2022, 317, 125912. [Google Scholar] [CrossRef]
- Moesgaard, M.; Yue, Y. Compositional dependence of fragility and glass forming ability of calcium aluminosilicate melts. J. Non-Cryst. Solids 2009, 355, 867–873. [Google Scholar] [CrossRef]
- Hasheminia, S.; Nemati, A.; Yekta, B.E.; Alizadeh, P. Preparation and characterisation of diopside-based glass–ceramic foams. Ceram. Int. 2012, 38, 2005–2010. [Google Scholar] [CrossRef]
- Gao, H.T.; Liu, X.H.; Chen, J.Q.; Qi, J.L.; Wang, Y.B.; Ai, Z.R. Preparation of glass-ceramics with low density and high strength using blast furnace slag, glass fiber and water glass. Ceram. Int. 2018, 44, 6044–6053. [Google Scholar] [CrossRef]
- Xi, X.; Xu, L.; Shui, A.; Wang, Y.; Naito, M. Effect of silicon carbide particle size and CaO content on foaming properties during firing and microstructure of porcelain ceramics. Ceram. Int. 2014, 40, 12931–12938. [Google Scholar] [CrossRef]
- Xi, X.; Shui, A.; Li, Y.; Wang, Y.; Abe, H.; Naito, M. Effects of magnesium oxychloride and silicon carbide additives on the foaming property during firing for porcelain ceramics and their microstructure. J. Eur. Ceram. Soc. 2012, 32, 3035–3041. [Google Scholar] [CrossRef]
- Zhu, J.P.; Le, H.Z.; Bai, R.; Zhu, J.G.; Li, H.D. Research progress on raw materials and technology of porous ceramics. Bull. Chin. Ceram. Soc. 2021, 40, 2989–2997. (In Chinese) [Google Scholar] [CrossRef]
- Li, L.; Jiang, T.; Zhou, M.; Chen, B.; Chen, C. The influence of temperature and SiC content on the recycling of iron ore tailings for the preparation of value-added foam ceramics. J. Mater. Cycles Waste Manag. 2020, 23, 330–340. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Yue, Y. Influence of the glass–calcium carbonate mixture’s characteristics on the foaming process and the properties of the foam glass. J. Eur. Ceram. Soc. 2014, 34, 1591–1598. [Google Scholar] [CrossRef]
- Li, L.; Jiang, T.; Chen, B.; Zhou, M.; Chen, C. Overall utilization of vanadium–titanium magnetite tailings to prepare lightweight foam ceramics. Process Saf. Environ. Prot. 2020, 139, 305–314. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Ji, R.; Liu, L.; Wang, X. Integrated utilization of high alumina fly ash for synthesis of foam glass ceramic. Ceram. Int. 2018, 44, 13681–13688. [Google Scholar] [CrossRef]
- Avinash, G.; Harika, V.; Sandeepika, C.; Gupta, N. Pore Size Control in Aluminium Foam by Standardizing Bubble Rise Velocity and Melt Viscosity. IOP Conf. Ser. Mater. Sci. Eng. 2018, 338, 012010. [Google Scholar] [CrossRef]
- Vijayan, S.; Wilson, P.; Prabhakaran, K. Porosity and cell size control in alumina foam preparation by thermo-foaming of powder dispersions in molten sucrose. J. Asian Ceram. Soc. 2016, 4, 344–350. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Chen, Z.; Liu, L.; Ji, R.; Wang, X. Synthesis of a foam ceramic based on ceramic tile polishing waste using SiC as foaming agent. Ceram. Int. 2018, 44, 10078–10086. [Google Scholar] [CrossRef]
- Shui, A.; Xi, X.; Wang, Y.; Cheng, X. Effect of silicon carbide additive on microstructure and properties of porcelain ceramics. Ceram. Int. 2011, 37, 1557–1562. [Google Scholar] [CrossRef]
- T/CBCSA 12-2019; Cellular Ceramic Partition Slab. China Building Material Industry Press: Beijing, China, 2019.

| Raw Materials | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | Na2O | K2O | MnO | SO3 | TiO2 | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 57.01 | 30.57 | 2.36 | 1.44 | 4.36 | 1.71 | 0.38 | 0.04 | 0.57 | 0.79 | 0.67 |
| SMS | 42.02 | 13.49 | 26.38 | 5.00 | 0.48 | 0.74 | 1.85 | 7.53 | 0.75 | 0.87 | 0.89 |
| Sample | Temperature (°C) | FA/% | SMS/% | SiC/% | Na2B4O5(OH)·10H2O/% | Bentonite/% |
|---|---|---|---|---|---|---|
| A1 | 1080 °C | 100 | 0 | 0.5 | 10 | 5 |
| A2 | 1080 °C | 90 | 10 | 0.5 | 10 | 5 |
| A3 | 1080 °C | 80 | 20 | 0.5 | 10 | 5 |
| A4 | 1080 °C | 70 | 30 | 0.5 | 10 | 5 |
| A5 | 1080 °C | 60 | 40 | 0.5 | 10 | 5 |
| A6 | 1080 °C | 50 | 50 | 0.5 | 10 | 5 |
| A3-1 | 1050 °C | 80 | 20 | 0.5 | 10 | 5 |
| A3-2 | 1080 °C | 80 | 20 | 0.5 | 10 | 5 |
| A3-3 | 1110 °C | 80 | 20 | 0.5 | 10 | 5 |
| A3-4 | 1140 °C | 80 | 20 | 0.5 | 10 | 5 |
| A3-5 | 1170 °C | 80 | 20 | 0.5 | 10 | 5 |
| A3-3-1 | 1110 °C | 80 | 20 | 0 | 10 | 5 |
| A3-3-2 | 1110 °C | 80 | 20 | 1 | 10 | 5 |
| A3-3-3 | 1110 °C | 80 | 20 | 1.5 | 10 | 5 |
| A3-3-4 | 1110 °C | 80 | 20 | 2 | 10 | 5 |
| A3-3-5 | 1110 °C | 80 | 20 | 3 | 10 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Gao, W.; Yao, Y.; Zhang, T.; Fu, Y.; Kong, X. Recycling of Silicomanganese Slag and Fly Ash for Preparation of Environment-Friendly Foamed Ceramics. Materials 2023, 16, 6724. https://doi.org/10.3390/ma16206724
Yu G, Gao W, Yao Y, Zhang T, Fu Y, Kong X. Recycling of Silicomanganese Slag and Fly Ash for Preparation of Environment-Friendly Foamed Ceramics. Materials. 2023; 16(20):6724. https://doi.org/10.3390/ma16206724
Chicago/Turabian StyleYu, Guihang, Wei Gao, Yanbin Yao, Tingting Zhang, Ying Fu, and Xiangqing Kong. 2023. "Recycling of Silicomanganese Slag and Fly Ash for Preparation of Environment-Friendly Foamed Ceramics" Materials 16, no. 20: 6724. https://doi.org/10.3390/ma16206724
APA StyleYu, G., Gao, W., Yao, Y., Zhang, T., Fu, Y., & Kong, X. (2023). Recycling of Silicomanganese Slag and Fly Ash for Preparation of Environment-Friendly Foamed Ceramics. Materials, 16(20), 6724. https://doi.org/10.3390/ma16206724






