Study on the Biodegradation of Poly(Butylene Succinate)/Wheat Bran Biocomposites
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
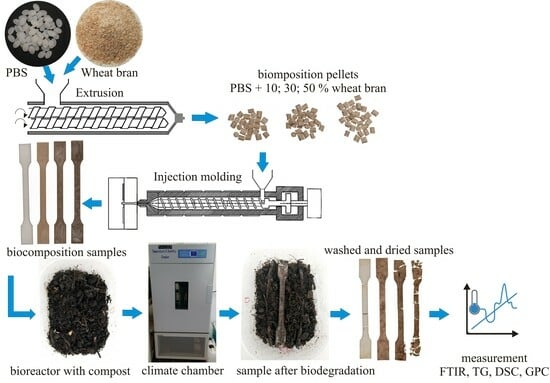
2.2. Procedure for the Production of Biocomposite Materials
2.3. Biodegradation Experiment
2.4. Materials Characterization
3. Results and Discussion
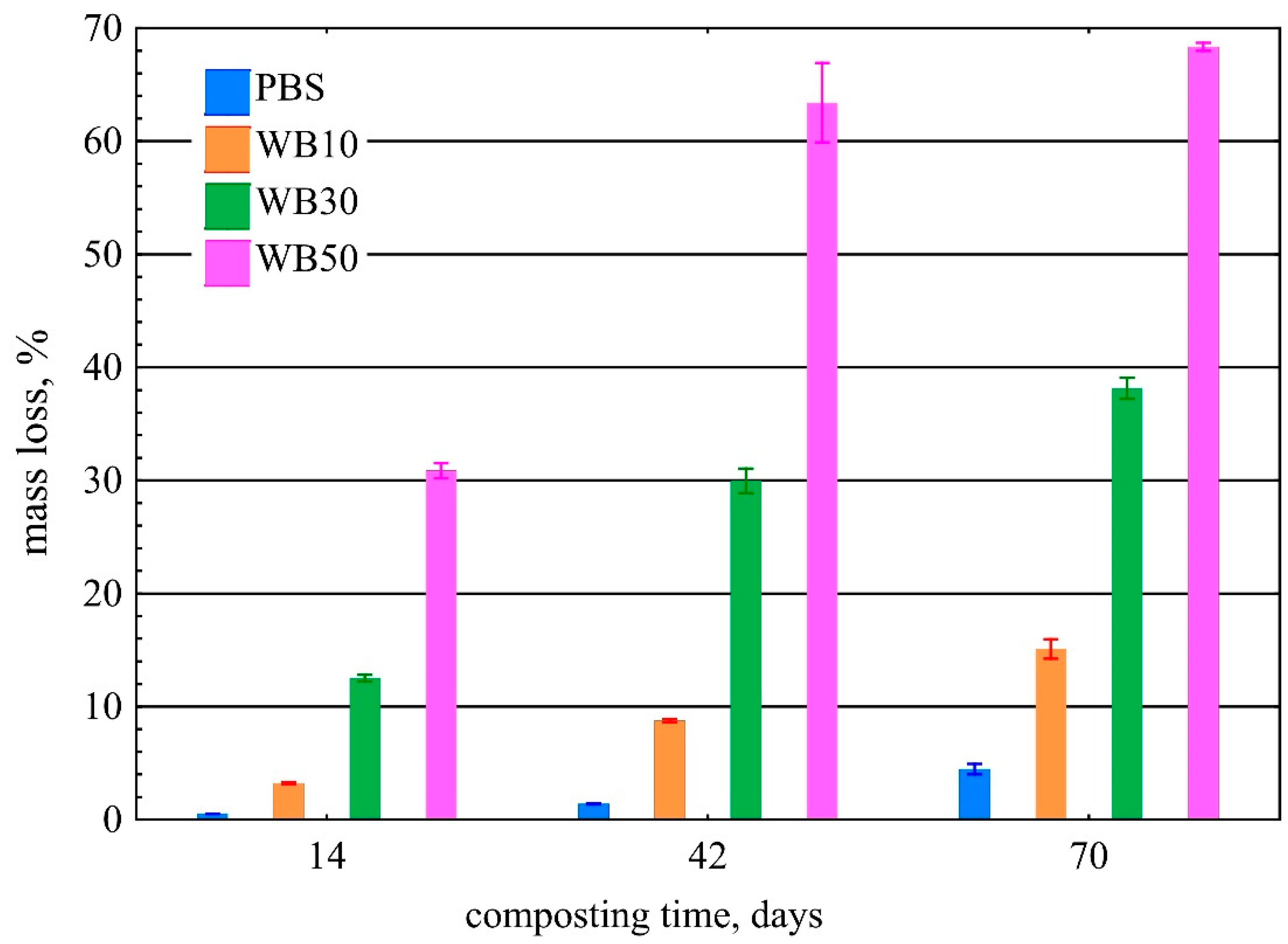
3.1. Mass Loss
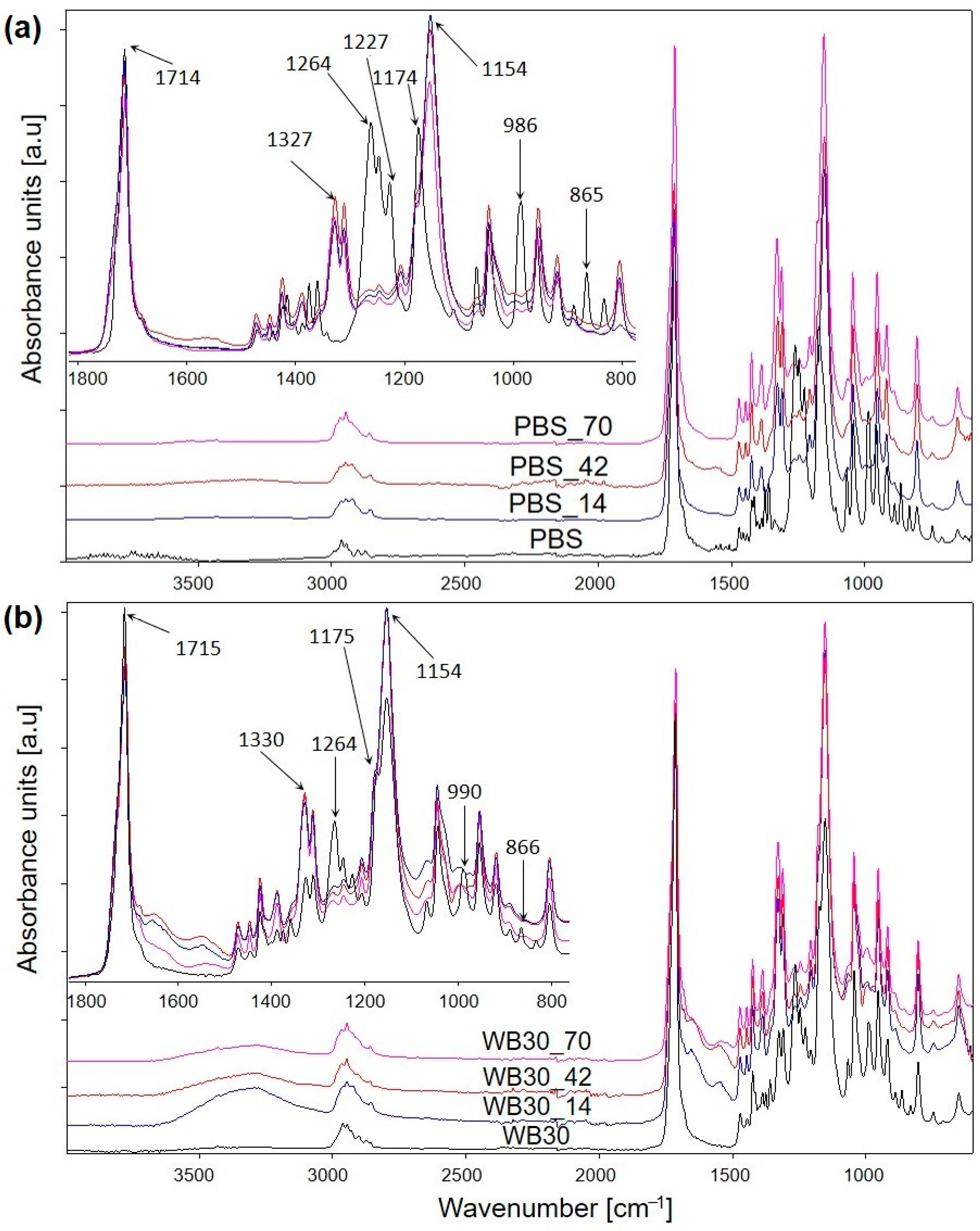
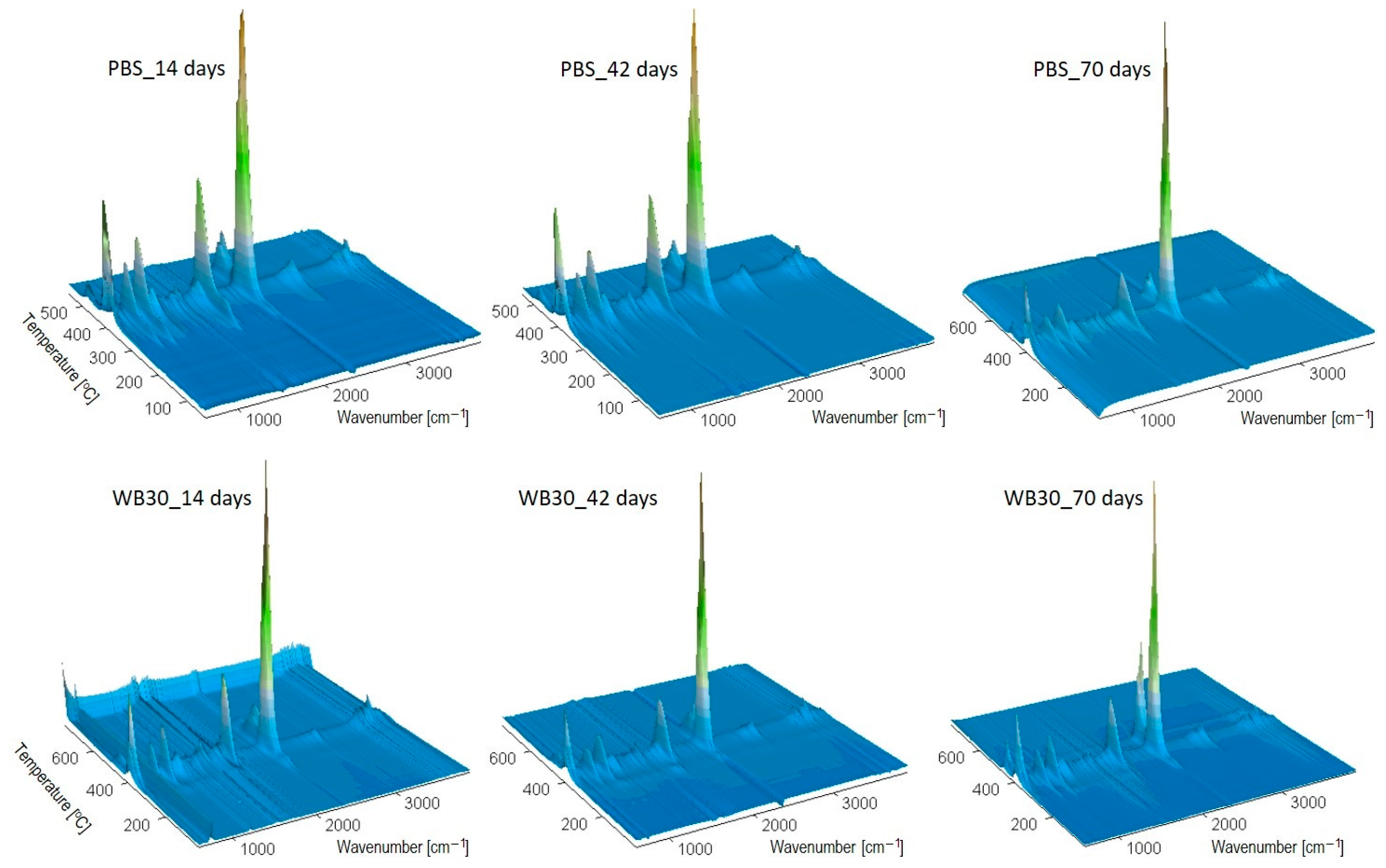
3.2. Chemical Structure
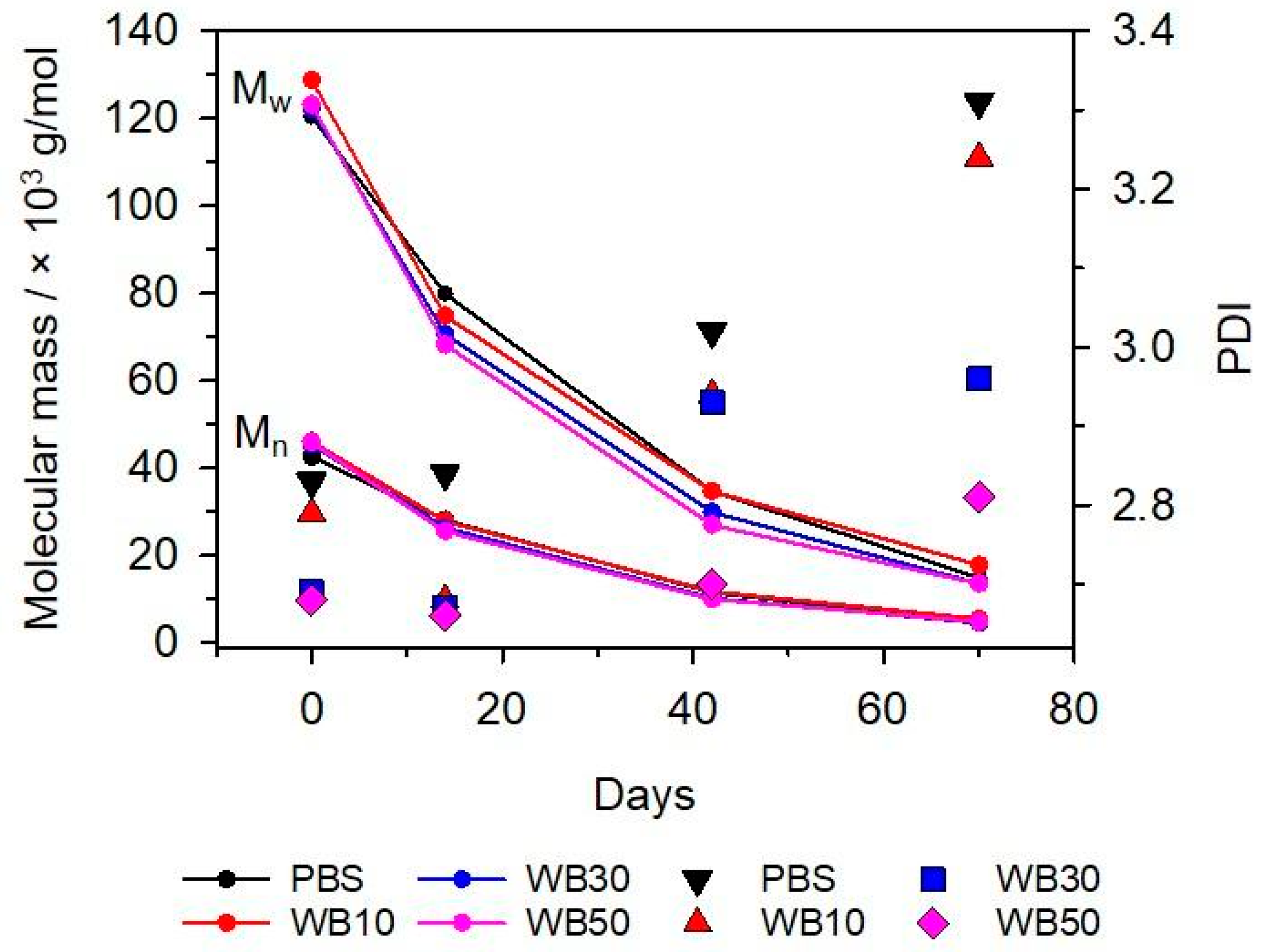
3.3. Molecular Mass
3.4. Morphology
3.5. Differential Scanning Calorimetry
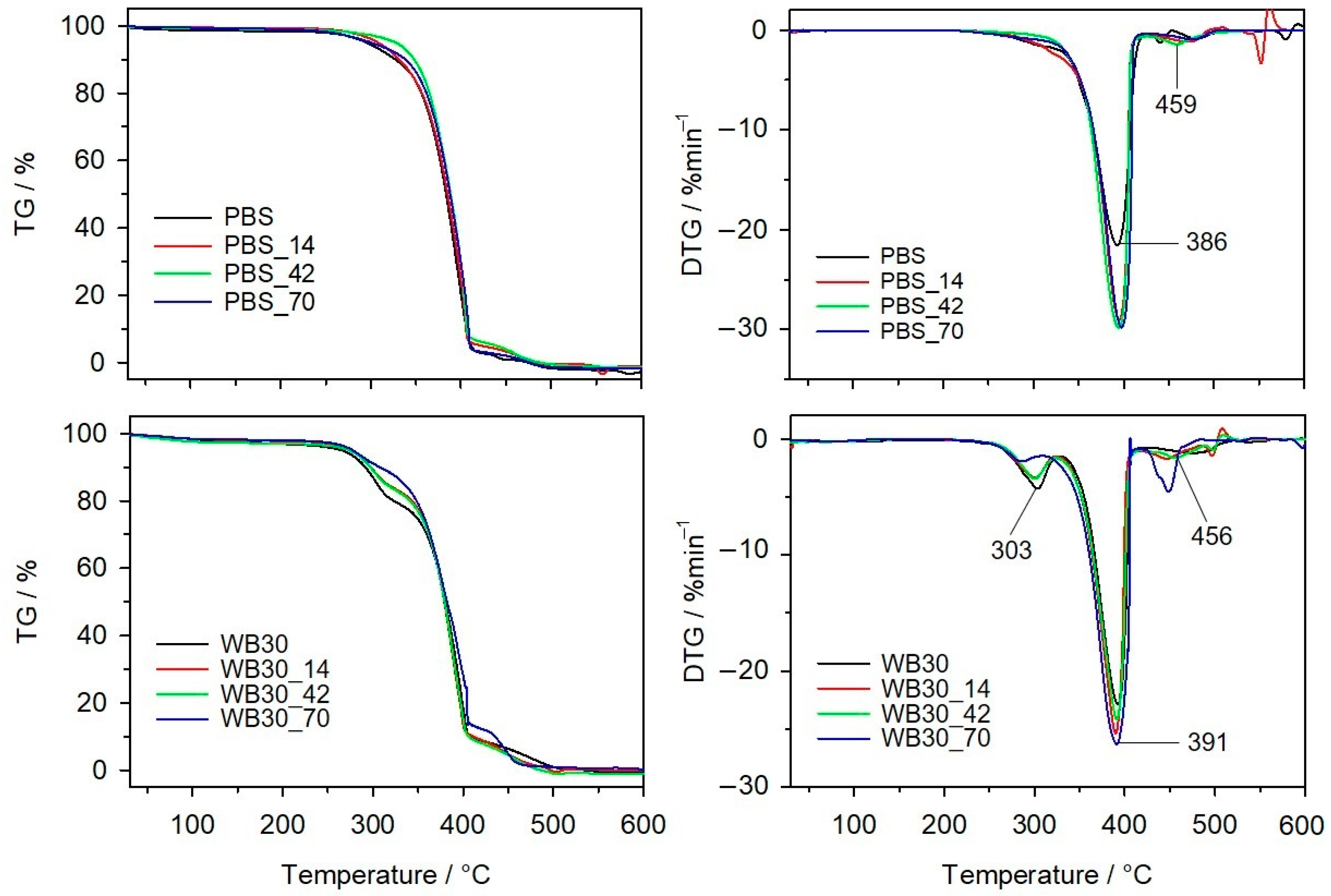
3.6. Thermal Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Oliveira, C.C.N.; Zotin, M.Z.; Rochedo, P.R.R.; Szklo, A. Achieving Negative Emissions in Plastics Life Cycles through the Conversion of Biomass Feedstock. Biofuels Bioprod. Biorefining 2021, 15, 430–453. [Google Scholar] [CrossRef]
- Ponis, S.; Aretoulaki, E.; Plakas, G.; Agalianos, K. Marine Plastic Littering: A Review of Socio Economic Impacts. J. Sustain. Sci. Manag. 2021, 16, 277–301. [Google Scholar] [CrossRef]
- Nielsen, T.D.; Hasselbalch, J.; Holmberg, K.; Stripple, J. Politics and the Plastic Crisis: A Review throughout the Plastic Life Cycle. WIREs Energy Environ. 2020, 9, e360. [Google Scholar] [CrossRef]
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; Organisation for Economic Co-operation and Development: Paris, France, 2022. [Google Scholar]
- Syberg, K.; Nielsen, M.B.; Westergaard Clausen, L.P.; van Calster, G.; van Wezel, A.; Rochman, C.; Koelmans, A.A.; Cronin, R.; Pahl, S.; Hansen, S.F. Regulation of Plastic from a Circular Economy Perspective. Curr. Opin. Green Sustain. Chem. 2021, 29, 100462. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Norton, M. Green Chemistry and the Plastic Pollution Challenge: Towards a Circular Economy. Green Chem. 2020, 22, 6310–6322. [Google Scholar] [CrossRef]
- Xanthos, D.; Walker, T.R. International Policies to Reduce Plastic Marine Pollution from Single-Use Plastics (Plastic Bags and Microbeads): A Review. Mar. Pollut. Bull. 2017, 118, 17–26. [Google Scholar] [CrossRef]
- Jiang, J.-Q. Occurrence of Microplastics and Its Pollution in the Environment: A Review. Sustain. Prod. Consum. 2018, 13, 16–23. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A.G. A Review on Grafting of Biofibers for Biocomposites. Materials 2016, 9, 303. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ko, Y.G.; Deri, F. Biodegradable Polymer Blends and Composites: An Overview. Polym. Sci. Ser. A 2014, 56, 812–829. [Google Scholar] [CrossRef]
- Pivsa-Art, W.; Chaiyasat, A.; Pivsa-Art, S.; Yamane, H.; Ohara, H. Preparation of Polymer Blends between Poly(Lactic Acid) and Poly(Butylene Adipate-Co-Terephthalate) and Biodegradable Polymers as Compatibilizers. Energy Procedia 2013, 34, 549–554. [Google Scholar] [CrossRef]
- Bio-Degradable Polymers Market—Size, Share & Industry Trends. Available online: https://www.mordorintelligence.com/industry-reports/bio-degradable-polymers-market (accessed on 19 October 2023).
- Bher, A.; Uysal Unalan, I.; Auras, R.; Rubino, M.; Schvezov, C.E. Toughening of Poly(Lactic Acid) and Thermoplastic Cassava Starch Reactive Blends Using Graphene Nanoplatelets. Polymers 2018, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Acosta, D.; Rodriguez-Uribe, A.; Álvarez-Chávez, C.R.; Mohanty, A.K.; Misra, M.; López-Cervantes, J.; Madera-Santana, T.J. Physicochemical Characterization and Evaluation of Pecan Nutshell as Biofiller in a Matrix of Poly(Lactic Acid). J. Polym. Environ. 2019, 27, 521–532. [Google Scholar] [CrossRef]
- Väisänen, T.; Das, O.; Tomppo, L. A Review on New Bio-Based Constituents for Natural Fiber-Polymer Composites. J. Clean. Prod. 2017, 149, 582–596. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current Progress on Bio-Based Polymers and Their Future Trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Sustainable Green Composites: Value Addition to Agricultural Residues and Perennial Grasses. ACS Sustain. Chem. Eng. 2013, 1, 325–333. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(Butylene Succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.C.; Rodriguez-Uribe, A.; Thimmanagari, M.; Misra, M.; Mohanty, A.K. Sustainable Biocomposites from Poly(Butylene Succinate) and Apple Pomace: A Study on Compatibilization Performance. Waste Biomass Valorization 2020, 11, 3775–3787. [Google Scholar] [CrossRef]
- Meeks, D.; Hottle, T.; Bilec, M.M.; Landis, A.E. Compostable Biopolymer Use in the Real World: Stakeholder Interviews to Better Understand the Motivations and Realities of Use and Disposal in the US. Resour. Conserv. Recycl. 2015, 105, 134–142. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of Bioplastics: Opportunities and Challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Artham, T.; Doble, M. Biodegradation of Aliphatic and Aromatic Polycarbonates. Macromol. Biosci. 2008, 8, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent Advances in Biopolymers and Biopolymer-Based Nanocomposites for Food Packaging Materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef] [PubMed]
- Noorunnisa Khanam, P.; Abdul Khalil, H.P.S.; Ramachandra Reddy, G.; Venkata Naidu, S. Tensile, Flexural and Chemical Resistance Properties of Sisal Fibre Reinforced Polymer Composites: Effect of Fibre Surface Treatment. J. Polym. Environ. 2011, 19, 115–119. [Google Scholar] [CrossRef]
- Mohan, S.K.; Srivastava, T. Microbial Deterioration and Degradation of Polymeric Materials. J. Biochem. Technol. 2010, 2, 210–215. [Google Scholar]
- Loredo-Treviño, A.; Gutiérrez-Sánchez, G.; Rodríguez-Herrera, R.; Aguilar, C.N. Microbial Enzymes Involved in Polyurethane Biodegradation: A Review. J. Polym. Environ. 2012, 20, 258–265. [Google Scholar] [CrossRef]
- Pathak, V.M. Navneet Review on the Current Status of Polymer Degradation: A Microbial Approach. Bioresour. Bioprocess. 2017, 4, 15. [Google Scholar] [CrossRef]
- Jugé, A.; Moreno-Villafranca, J.; Perez-Puyana, V.M.; Jiménez-Rosado, M.; Sabino, M.; Capezza, A.J. Porous Thermoformed Protein Bioblends as Degradable Absorbent Alternatives in Sanitary Materials. ACS Appl. Polym. Mater. 2023, 5, 6976–6989. [Google Scholar] [CrossRef]
- Wolf, P.; Reimer, M.; Maier, M.; Zollfrank, C. Biodegradation of Polysaccharides, Polyesters and Proteins in Soil Based on the Determination of Produced Carbon Dioxide. Polym. Degrad. Stab. 2023, 217, 110538. [Google Scholar] [CrossRef]
- Brebu, M. Environmental Degradation of Plastic Composites with Natural Fillers—A Review. Polymers 2020, 12, 166. [Google Scholar] [CrossRef]
- Siracusa, V. Microbial Degradation of Synthetic Biopolymers Waste. Polymers 2019, 11, 1066. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological Degradation of Plastics: A Comprehensive Review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable Polymers for Food Packaging: A Review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Larché, J.-F.; Bussière, P.-O.; Thérias, S.; Gardette, J.-L. Photooxidation of Polymers: Relating Material Properties to Chemical Changes. Polym. Degrad. Stab. 2012, 97, 25–34. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic Implications of Plastic Degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Banerjee, A.; Chatterjee, K.; Madras, G. Enzymatic Degradation of Polymers: A Brief Review. Mater. Sci. Technol. 2014, 30, 567–573. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.; Kumosa, M. UV Degradation Model for Polymers and Polymer Matrix Composites. Polym. Degrad. Stab. 2018, 154, 203–210. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Kale, G.; Auras, R.; Singh, S.P.; Narayan, R. Biodegradability of Polylactide Bottles in Real and Simulated Composting Conditions. Polym. Test. 2007, 26, 1049–1061. [Google Scholar] [CrossRef]
- Pastorelli, G.; Cucci, C.; Garcia, O.; Piantanida, G.; Elnaggar, A.; Cassar, M.; Strlič, M. Environmentally Induced Colour Change during Natural Degradation of Selected Polymers. Polym. Degrad. Stab. 2014, 107, 198–209. [Google Scholar] [CrossRef]
- Sinyavsky, N.; Korneva, I. Study of Optical Properties of Polymeric Materials Subjected to Degradation. J. Polym. Environ. 2017, 25, 1280–1287. [Google Scholar] [CrossRef]
- Bond, T.; Ferrandiz-Mas, V.; Felipe-Sotelo, M.; van Sebille, E. The Occurrence and Degradation of Aquatic Plastic Litter Based on Polymer Physicochemical Properties: A Review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 685–722. [Google Scholar] [CrossRef]
- Salomez, M.; George, M.; Fabre, P.; Touchaleaume, F.; Cesar, G.; Lajarrige, A.; Gastaldi, E. A Comparative Study of Degradation Mechanisms of PHBV and PBSA under Laboratory-Scale Composting Conditions. Polym. Degrad. Stab. 2019, 167, 102–113. [Google Scholar] [CrossRef]
- Eubeler, J.P.; Bernhard, M.; Knepper, T.P. Environmental Biodegradation of Synthetic Polymers II. Biodegradation of Different Polymer Groups. TrAC Trends Anal. Chem. 2010, 29, 84–100. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, H.-J.; Lee, J.-W.; Choi, I.-G. Biodegradability of Bio-Flour Filled Biodegradable Poly(Butylene Succinate) Bio-Composites in Natural and Compost Soil. Polym. Degrad. Stab. 2006, 91, 1117–1127. [Google Scholar] [CrossRef]
- Lin, N.; Yu, J.; Chang, P.R.; Li, J.; Huang, J. Poly(Butylene Succinate)-Based Biocomposites Filled with Polysaccharide Nanocrystals: Structure and Properties. Polym. Compos. 2011, 32, 472–482. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Wan Yunus, W.M.Z. Effect of Organo-Modified Montmorillonite on Poly(Butylene Succinate)/Poly(Butylene Adipate-Co-Terephthalate) Nanocomposites. Express Polym. Lett. 2010, 4, 404–414. [Google Scholar] [CrossRef]
- Cai, Y.; Lv, J.; Feng, J. Spectral Characterization of Four Kinds of Biodegradable Plastics: Poly (Lactic Acid), Poly (Butylenes Adipate-Co-Terephthalate), Poly (Hydroxybutyrate-Co-Hydroxyvalerate) and Poly (Butylenes Succinate) with FTIR and Raman Spectroscopy. J. Polym. Environ. 2013, 21, 108–114. [Google Scholar] [CrossRef]
- Anstey, A.; Muniyasamy, S.; Reddy, M.M.; Misra, M.; Mohanty, A. Processability and Biodegradability Evaluation of Composites from Poly(Butylene Succinate) (PBS) Bioplastic and Biofuel Co-Products from Ontario. J. Polym. Environ. 2014, 22, 209–218. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, K.; Okamoto, M. Structure−Property Relationship in Biodegradable Poly(Butylene Succinate)/Layered Silicate Nanocomposites. Macromolecules 2003, 36, 2355–2367. [Google Scholar] [CrossRef]
- Puchalski, M.; Szparaga, G.; Biela, T.; Gutowska, A.; Sztajnowski, S.; Krucińska, I. Molecular and Supramolecular Changes in Polybutylene Succinate (PBS) and Polybutylene Succinate Adipate (PBSA) Copolymer during Degradation in Various Environmental Conditions. Polymers 2018, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Čolnik, M.; Knez-Hrnčič, M.; Škerget, M.; Knez, Ž. Biodegradable Polymers, Current Trends of Research and Their Applications, a Review. Chem. Ind. Chem. Eng. Q. 2020, 26, 401–418. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J. 7—Biodegradable and Biobased Polymers. In Applied Plastics Engineering Handbook, 2nd ed.; Kutz, M., Ed.; Plastics Design Library; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 127–143. ISBN 978-0-323-39040-8. [Google Scholar]
- Liu, L.; Yu, J.; Cheng, L.; Yang, X. Biodegradability of Poly(Butylene Succinate) (PBS) Composite Reinforced with Jute Fibre. Polym. Degrad. Stab. 2009, 94, 90–94. [Google Scholar] [CrossRef]
- Ok Han, S.; Muk Lee, S.; Ho Park, W.; Cho, D. Mechanical and Thermal Properties of Waste Silk Fiber-Reinforced Poly(Butylene Succinate) Biocomposites. J. Appl. Polym. Sci. 2006, 100, 4972–4980. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Li, Y.-J.; Xu, B.-P.; Zhang, D.-W.; Qu, J.-P.; He, H.-Z. Effect of Fiber Morphology on Rheological Properties of Plant Fiber Reinforced Poly(Butylene Succinate) Composites. Compos. Part B Eng. 2013, 44, 193–199. [Google Scholar] [CrossRef]
- Liang, Z.; Pan, P.; Zhu, B.; Dong, T.; Inoue, Y. Mechanical and Thermal Properties of Poly(Butylene Succinate)/Plant Fiber Biodegradable Composite. J. Appl. Polym. Sci. 2010, 115, 3559–3567. [Google Scholar] [CrossRef]
- Nie, S.; Liu, X.; Dai, G.; Yuan, S.; Cai, F.; Li, B.; Hu, Y. Investigation on Flame Retardancy and Thermal Degradation of Flame Retardant Poly(Butylene Succinate)/Bamboo Fiber Biocomposites. J. Appl. Polym. Sci. 2012, 125, E485–E489. [Google Scholar] [CrossRef]
- Kim, H.-S.; Yang, H.-S.; Kim, H.-J. Biodegradability and Mechanical Properties of Agro-Flour–Filled Polybutylene Succinate Biocomposites. J. Appl. Polym. Sci. 2005, 97, 1513–1521. [Google Scholar] [CrossRef]
- Yen, F.-S.; Liao, H.-T.; Wu, C.-S. Characterization and Biodegradability of Agricultural Residue-Filled Polyester Ecocomposites. Polym. Bull. 2013, 70, 1613–1629. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Efficiency of Twin-Screw Extrusion of Biodegradable Poly (Butylene Succinate)-Wheat Bran Blend. Materials 2021, 14, 424. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Analysis of Selected Properties of Injection Moulded Sustainable Biocomposites from Poly(Butylene Succinate) and Wheat Bran. Materials 2021, 14, 7049. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Artificial Ageing, Chemical Resistance, and Biodegradation of Biocomposites from Poly(Butylene Succinate) and Wheat Bran. Materials 2021, 14, 7580. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Barkane, A.; Gaidukova, G.; Grase, L.; Thakur, V.K.; Filipova, I.; Fridrihsone, V.; Skute, M.; Laka, M. Highly Loaded Cellulose/Poly (Butylene Succinate) Sustainable Composites for Woody-Like Advanced Materials Application. Molecules 2020, 25, 121. [Google Scholar] [CrossRef] [PubMed]
- Gowman, A.; Wang, T.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M. Bio-Poly(Butylene Succinate) and Its Composites with Grape Pomace: Mechanical Performance and Thermal Properties. ACS Omega 2018, 3, 15205–15216. [Google Scholar] [CrossRef]
- Tolga, S.; Kabasci, S.; Duhme, M. Progress of Disintegration of Polylactide (PLA)/Poly(Butylene Succinate) (PBS) Blends Containing Talc and Chalk Inorganic Fillers under Industrial Composting Conditions. Polymers 2021, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Phua, Y.J.; Chow, W.S.; Mohd Ishak, Z.A. The Hydrolytic Effect of Moisture and Hygrothermal Aging on Poly(Butylene Succinate)/Organo-Montmorillonite Nanocomposites. Polym. Degrad. Stab. 2011, 96, 1194–1203. [Google Scholar] [CrossRef]
- Phua, Y.J.; Lau, N.S.; Sudesh, K.; Chow, W.S.; Mohd Ishak, Z.A. Biodegradability Studies of Poly(Butylene Succinate)/Organo-Montmorillonite Nanocomposites under Controlled Compost Soil Conditions: Effects of Clay Loading and Compatibiliser. Polym. Degrad. Stab. 2012, 97, 1345–1354. [Google Scholar] [CrossRef]
- Nakasaki, K.; Matsuura, H.; Tanaka, H.; Sakai, T. Synergy of Two Thermophiles Enables Decomposition of Poly-ε-Caprolactone under Composting Conditions. FEMS Microbiol. Ecol. 2006, 58, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Tabasi, R.Y.; Ajji, A. Selective Degradation of Biodegradable Blends in Simulated Laboratory Composting. Polym. Degrad. Stab. 2015, 120, 435–442. [Google Scholar] [CrossRef]
- Mihai, M.; Legros, N.; Alemdar, A. Formulation-Properties Versatility of Wood Fiber Biocomposites Based on Polylactide and Polylactide/Thermoplastic Starch Blends. Polym. Eng. Sci. 2014, 54, 1325–1340. [Google Scholar] [CrossRef]
- Kale, A.G.; Deshmukh, M.; Dudhare, V. Patil Microbial Degradation Of Plastic—A Review. IJPR 2015, 6, 952–961. [Google Scholar] [CrossRef]
- Sirichalarmkul, A.; Kaewpirom, S. Enhanced Biodegradation and Processability of Biodegradable Package from Poly(Lactic Acid)/Poly(Butylene Succinate)/Rice-Husk Green Composites. J. Appl. Polym. Sci. 2021, 138, 50652. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł. Biodegradable Polymer Composition. Polish Patent pat.239238, 15 November 2021. [Google Scholar]
- Soccio, M.; Dominici, F.; Quattrosoldi, S.; Luzi, F.; Munari, A.; Torre, L.; Lotti, N.; Puglia, D. PBS-Based Green Copolymer as an Efficient Compatibilizer in Thermoplastic Inedible Wheat Flour/Poly(Butylene Succinate) Blends. Biomacromolecules 2020, 21, 3254–3269. [Google Scholar] [CrossRef] [PubMed]
- Strangis, G.; Rossi, D.; Cinelli, P.; Seggiani, M. Seawater Biodegradable Poly(Butylene Succinate-Co-Adipate)—Wheat Bran Biocomposites. Materials 2023, 16, 2593. [Google Scholar] [CrossRef]
- BioPBS FZ91PB—Technical Data Sheet. Available online: Https://Www.Pttmcc.Com/File_upload/Tds/TDS-FZ91PM_PB.Pdf (accessed on 22 September 2022).
- ISO 294-1:2017; Plastics—Injection Moulding of Test Specimens of Thermoplastic Materials—Part 1: General Principles, and Moulding of Multipurpose and Bar Test Specimens. ISO: Geneva, Switzerland, 2017.
- ISO 20200:2015; Plastics—Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test. ISO: Geneva, Switzerland, 2015.
- ISO 11357-1:2016; Plastics—Differential Scanning Calorimetry (DSC)—Part 1: General Principles. ISO: Geneva, Switzerland, 2016.
- Huang, Z.; Qian, L.; Yin, Q.; Yu, N.; Liu, T.; Tian, D. Biodegradability Studies of Poly(Butylene Succinate) Composites Filled with Sugarcane Rind Fiber. Polym. Test. 2018, 66, 319–326. [Google Scholar] [CrossRef]
- Vu, V.; Farkas, C.; Riyad, O.; Bujna, E.; Kilin, A.; Sipiczki, G.; Sharma, M.; Usmani, Z.; Gupta, V.K.; Nguyen, Q.D. Enhancement of the Enzymatic Hydrolysis Efficiency of Wheat Bran Using the Bacillus Strains and Their Consortium. Bioresour. Technol. 2022, 343, 126092. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Lærke, H.N.; Knudsen, K.-E.B.; Lampi, A.-M.; Piironen, V.; Adlercreutz, H.; Katina, K.; Poutanen, K.; Åman, P. Physical, Microscopic and Chemical Characterisation of Industrial Rye and Wheat Brans from the Nordic Countries. Food Nutr. Res. 2009, 53, 1912. [Google Scholar] [CrossRef]
- Greffeuille, V.; Abecassis, J.; Lapierre, C.; Lullien-Pellerin, V. Bran Size Distribution at Milling and Mechanical and Biochemical Characterization of Common Wheat Grain Outer Layers: A Relationship Assessment. Cereal Chem. 2006, 83, 641–646. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons LTD: Chichester, UK, 2001. [Google Scholar]
- Zhang, X.; Zhang, Y. Reinforcement Effect of Poly(Butylene Succinate) (PBS)-Grafted Cellulose Nanocrystal on Toughened PBS/Polylactic Acid Blends. Carbohydr. Polym. 2016, 140, 374–382. [Google Scholar] [CrossRef]
- Mili, M.; Gupta, A.; Monika; Katiyar, V. Designing of Poly(l-Lactide)–Nicotine Conjugates: Mechanistic and Kinetic Studies and Thermal Release Behavior of Nicotine. ACS Omega 2017, 2, 6131–6142. [Google Scholar] [CrossRef]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Mokhena, T.C. A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS). Polymers 2021, 13, 1200. [Google Scholar] [CrossRef] [PubMed]
- Pantaloni, D.; Shah, D.; Baley, C.; Bourmaud, A. Monitoring of Mechanical Performances of Flax Non-Woven Biocomposites during a Home Compost Degradation. Polym. Degrad. Stab. 2020, 177, 109166. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Guo, B. Photodegradation Behavior of Poly(Butylene Succinate-Co-Butylene Adipate)/ZnO Nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 173–181. [Google Scholar] [CrossRef]
- Terzopoulou, Z.N.; Papageorgiou, G.Z.; Papadopoulou, E.; Athanassiadou, E.; Reinders, M.; Bikiaris, D.N. Development and Study of Fully Biodegradable Composite Materials Based on Poly(Butylene Succinate) and Hemp Fibers or Hemp Shives. Polym. Compos. 2016, 37, 407–421. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook, Standard Reference Database Number 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 4 April 2023). [CrossRef]

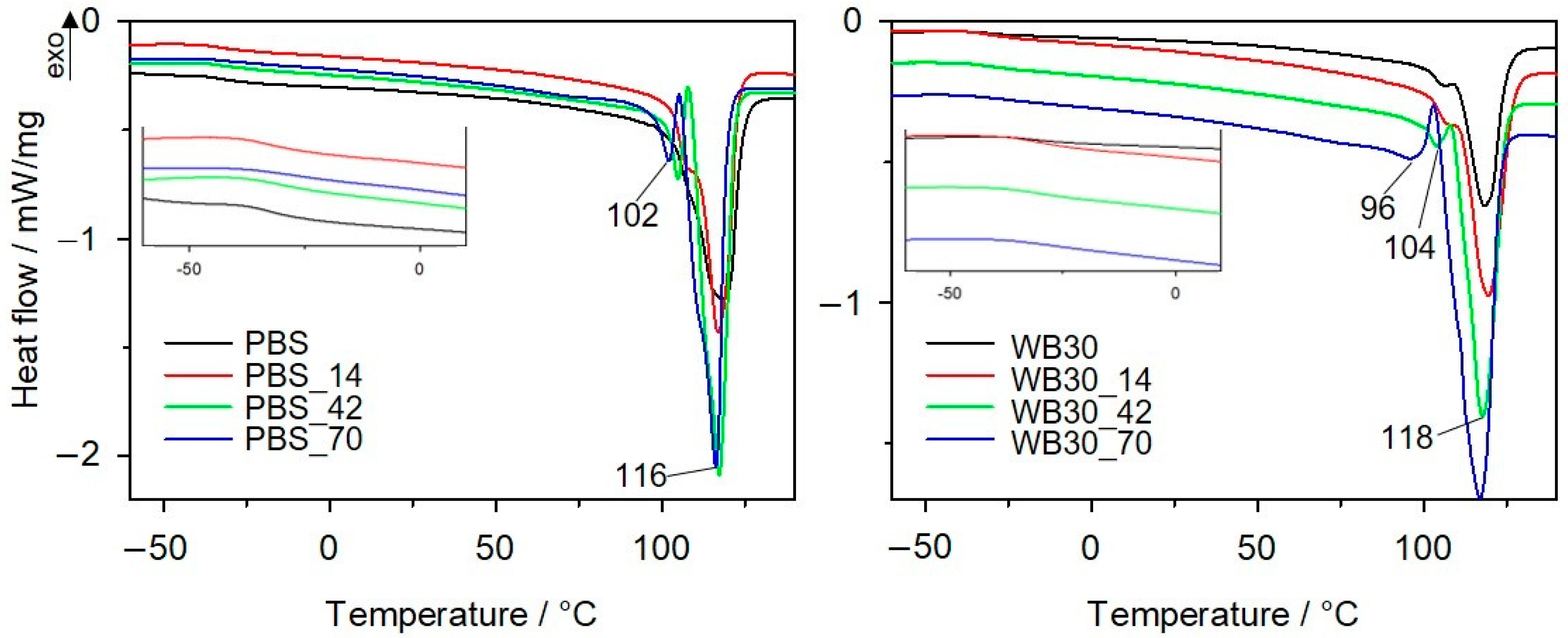
| Sample | Cooling | Heating II | |||
|---|---|---|---|---|---|
| Tc | Tg | Tm1/Tm2 | ΔHm | Xc | |
| (°C) | (°C) | (°C) | (J/g) | (%) | |
| PBS | 86.2 | −31.7 | 118 | 66.7 | 60.5 |
| PBS_14 | 88.2 | −32.5 | 107/117 | 70.9 | 64.3 |
| PBS_42 | 86.4 | −32.8 | 105/117 | 84.8 | 76.9 |
| PBS_70 | 84.3 | −34.5 | 102/116 | 85.2 | 77.2 |
| WB10 | 86.3 | −33.0 | 107/116 | 55.3 | 55.7 |
| WB10_14 | 86.4 | −33.1 | 107/119 | 67.5 | 67.6 |
| WB10_42 | 87.9 | −33.9 | 107/117 | 82.8 | 82.5 |
| WB10_70 | 88.3 | −34.1 | 104/115 | 85.2 | 83.1 |
| WB30 | 79.6 | −32 | 107/118 | 38.8 | 50.3 |
| WB30_14 | 83.9 | −32.8 | 106/119 | 47.6 | 57.5 |
| WB30_42 | 83.7 | −33.4 | 104/118 | 62.3 | 74.3 |
| WB30_70 | 79.3 | −35.4 | 96/117 | 93.5 | 96.3 |
| WB50 | 83.3 | −31.4 | 106/117 | 30.6 | 54.5 |
| WB50_14 | 84.1 | −31.9 | 108/121 | 44.3 | 73 |
| WB50_42 | 83.5 | −32.6 | 107/119 | 60.8 | 76.6 |
| WB50_70 | 79.6 | −33.6 | 103/120 | 68.6 | 79.7 |
| Sample | T5% | T50% | Tmax1 | Δm1 | Tmax2 | Δm2 | Tmax3 | Δm3 |
|---|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (%) | (°C) | (%) | (°C) | (%) | |
| bran | 201 | 303 | 296 | 68.0 | - | - | 459 | 29.7 |
| PBS | 308 | 393 | - | - | 386 | 97.9 | 463 | 2.1 |
| PBS_14 | 306 | 395 | - | - | 385 | 94.5 | 471 | 5.5 |
| PBS_42 | 305 | 395 | - | - | 388 | 95.3 | 459 | 4.7 |
| PBS_70 | 308 | 398 | - | - | 388 | 96.4 | 475 | 3.6 |
| WB10 | 299 | 383 | 305 | 8.5 | 387 | 84.3 | 478 | 7.2 |
| WB10_14 | 312 | 387 | 304 | 8.1 | 395 | 87.8 | 468 | 6.2 |
| WB10_42 | 298 | 385 | 303 | 7.5 | 394 | 85.9 | 479 | 5.9 |
| WB10_70 | 296 | 381 | 286 | 6.3 | 391 | 82.5 | 468 | 7.4 |
| WB30 | 274 | 381 | 303 | 19.8 | 391 | 71.1 | 476 | 9.1 |
| WB30_14 | 276 | 379 | 301 | 16.8 | 390 | 73.6 | 446 | 9.6 |
| WB30_42 | 277 | 379 | 300 | 16.1 | 391 | 74.2 | 456 | 9 |
| WB30_70 | 279 | 382 | 297 | 13.2 | 391 | 73.9 | 449 | 12.9 |
| WB50 | 231 | 372 | 300 | 32.4 | 386 | 53.5 | 462 | 14.1 |
| WB50_14 | 233 | 372 | 297 | 29.5 | 386 | 57.6 | 443 | 12.9 |
| WB50_42 | 265 | 377 | 288 | 18.2 | 386 | 66.9 | 417 | 14.9 |
| WB50_70 | 271 | 371 | 285 | 14.1 | 375 | 66 | 410 | 19.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Study on the Biodegradation of Poly(Butylene Succinate)/Wheat Bran Biocomposites. Materials 2023, 16, 6843. https://doi.org/10.3390/ma16216843
Sasimowski E, Majewski Ł, Grochowicz M. Study on the Biodegradation of Poly(Butylene Succinate)/Wheat Bran Biocomposites. Materials. 2023; 16(21):6843. https://doi.org/10.3390/ma16216843
Chicago/Turabian StyleSasimowski, Emil, Łukasz Majewski, and Marta Grochowicz. 2023. "Study on the Biodegradation of Poly(Butylene Succinate)/Wheat Bran Biocomposites" Materials 16, no. 21: 6843. https://doi.org/10.3390/ma16216843
APA StyleSasimowski, E., Majewski, Ł., & Grochowicz, M. (2023). Study on the Biodegradation of Poly(Butylene Succinate)/Wheat Bran Biocomposites. Materials, 16(21), 6843. https://doi.org/10.3390/ma16216843