Efficient Recovery of Vanadium and Titanium from Domestic Titanomagnetite Concentrate Using Molten Salt Roasting and Water Leaching
Abstract
:1. Introduction
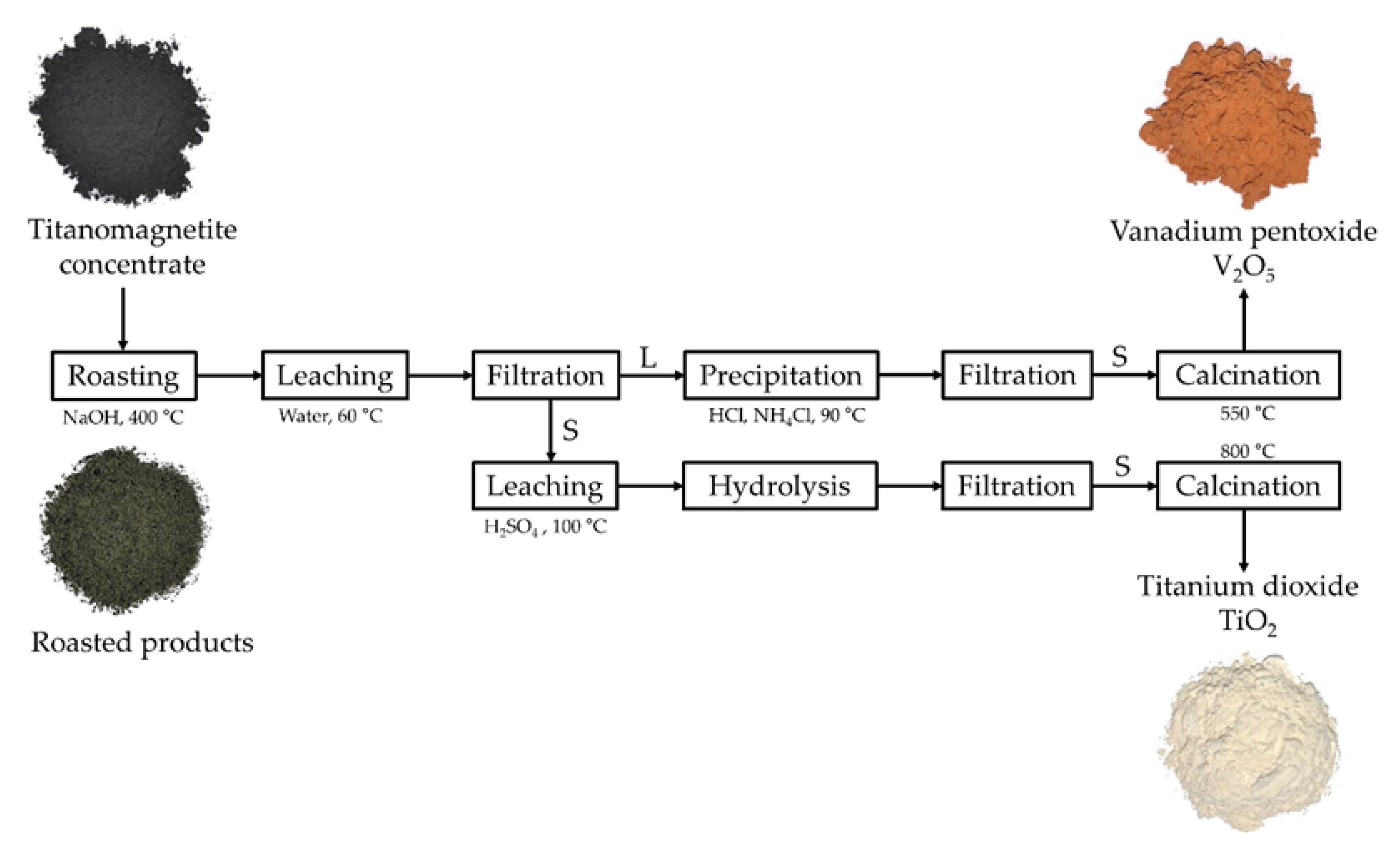
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. NaOH Roasting to Decompose the Vanadium Spinel in the Titanomagnetite Concentrate
3.1.1. Effect of Roasting Temperature and Time
3.1.2. Effect of NaOH Dosage
3.2. Water Leaching of Roasted Products
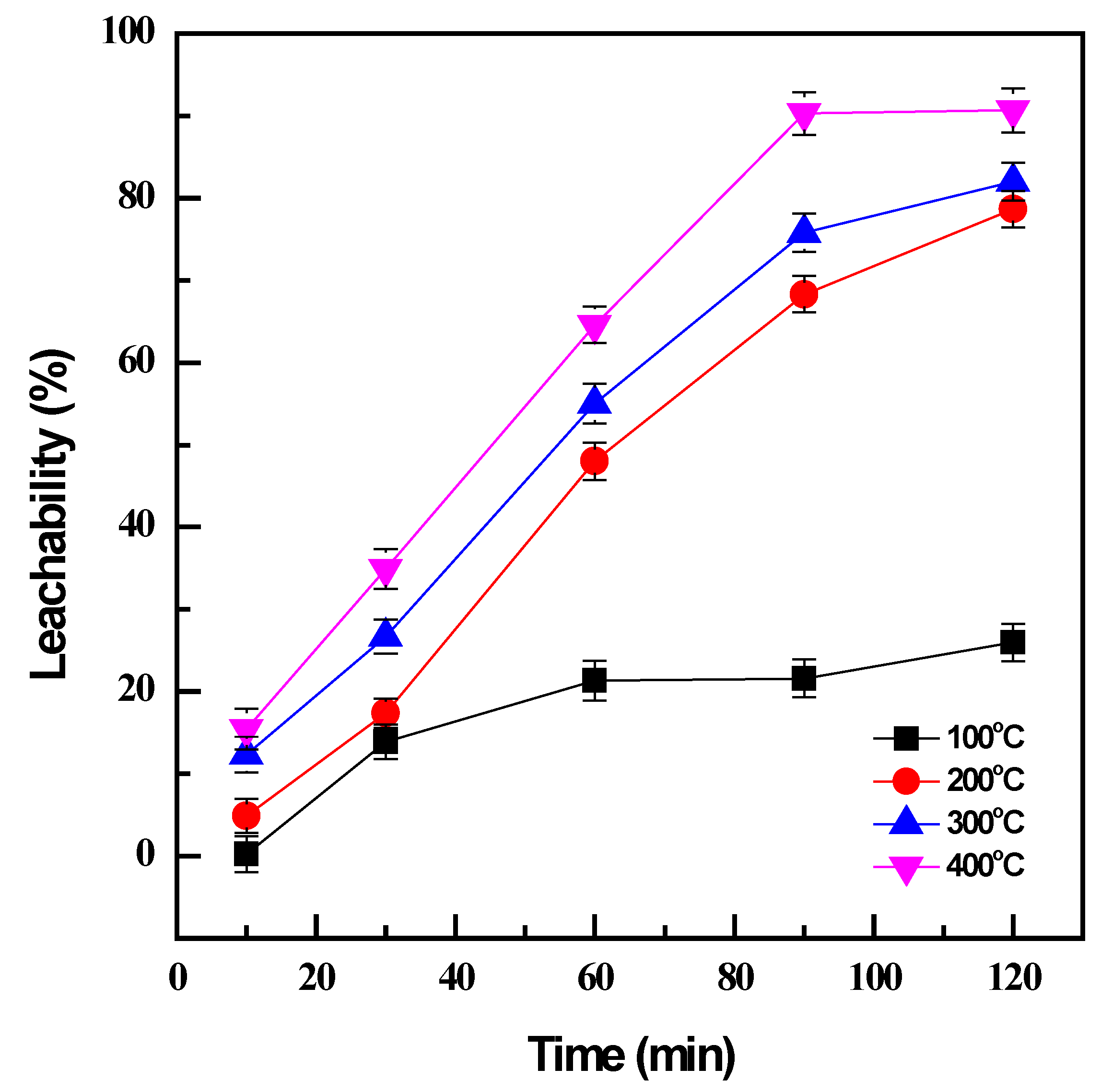
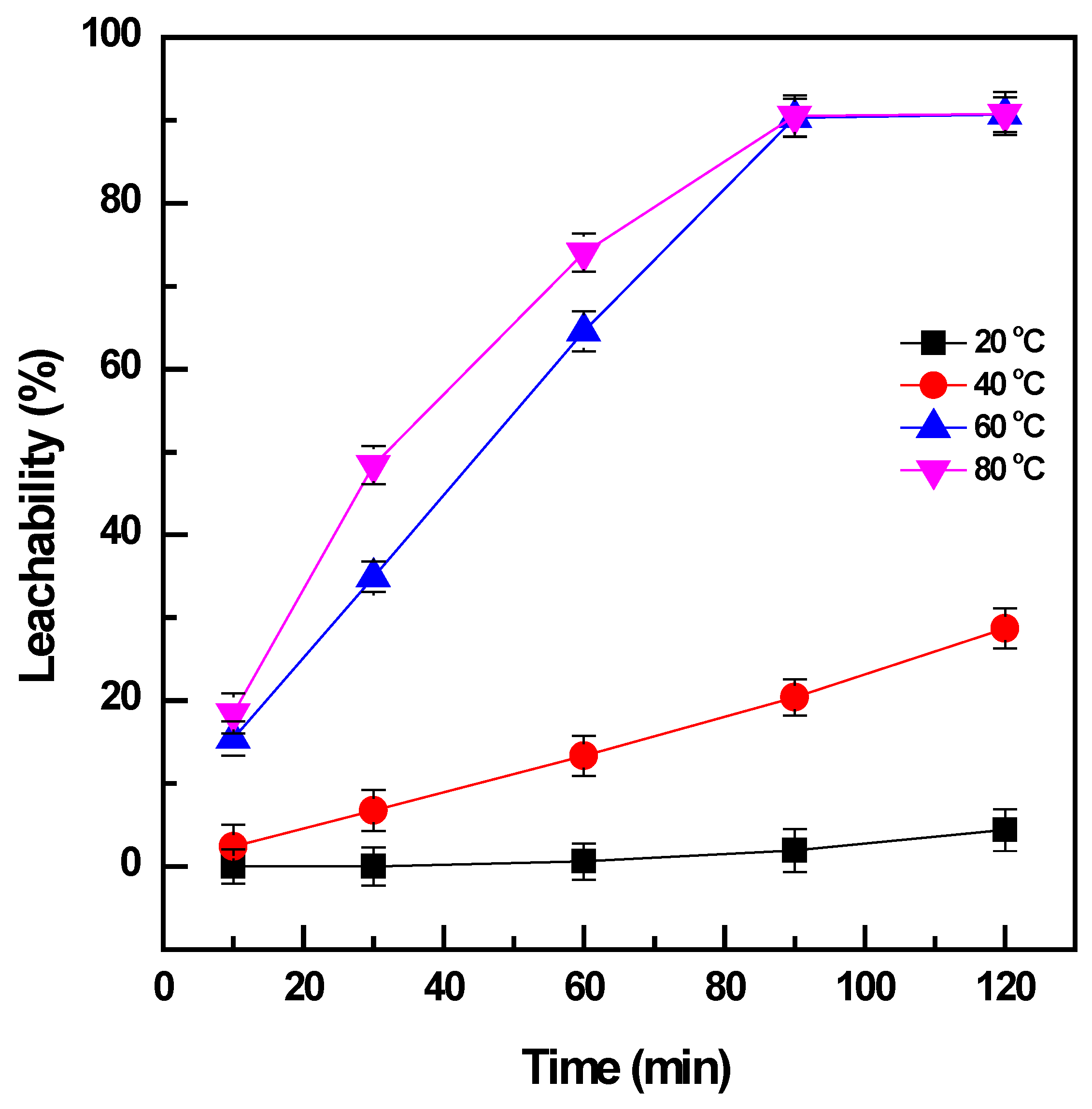
3.2.1. Effect of Leaching Temperature and Time
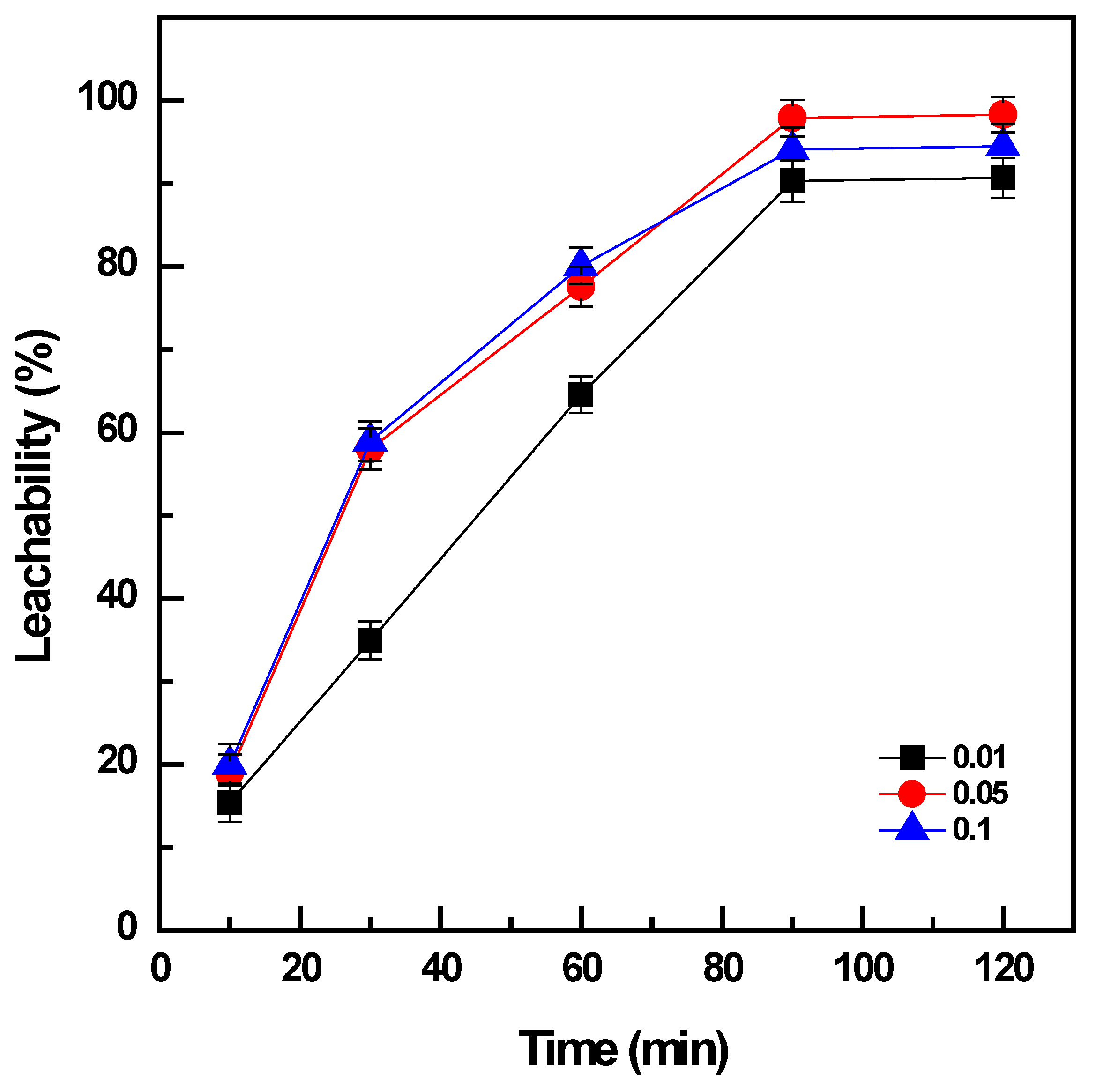
3.2.2. Effect of Pulp Density
3.3. Preparation and Characterization of the Final Products (V2O5 and TiO2)
3.4. Phase Transformation during the Recovery Process
3.5. Comparison between Modified and Conventional Roasting Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muroga, T.; Nagasaka, T.; Abe, K.; Chernov, V.M.; Matsui, H.; Smith, D.L.; Xu, Z.Y.; Zinkle, S.J. Vanadium alloys—Overview and recent results. J. Nucl. Mater. 2002, 307, 547–554. [Google Scholar] [CrossRef]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic applications of vanadium: A mechanistic perspective. Chem. Rev. 2018, 119, 2128–2191. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, R.; Nikoloski, A.N. The extraction of vanadium from titanomagnetites and other sources. Miner. Eng. 2020, 146, 106106. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, E.Y.; Chung, K.W.; Kim, R.; Jeon, H.S. A review on the metallurgical recycling of vanadium from slags: Towards a sustainable vanadium production. J. Mater. Res. Technol. 2021, 12, 343–364. [Google Scholar] [CrossRef]
- Peng, H. A literature review on leaching and recovery of vanadium. J. Environ. Chem. Eng. 2019, 7, 103313. [Google Scholar] [CrossRef]
- Barote, L.; Weissbach, R.; Teodorescu, R.; Marinescu, C.; Cirstea, M. Stand-alone wind system with vanadium redox battery energy storage. In Proceedings of the 2008 11th International Conference on Optimization of Electrical and Electronic Equipment, Brasov, Romania, 22–24 May 2008; pp. 407–412. [Google Scholar]
- Skyllas-Kazacos, M.; Kazacos, G.; Poon, G.; Verseema, H. Recent advances with UNSW vanadium-based redox flow batteries. Int. J. Energy Res. 2010, 34, 182–189. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.Y.; Kim, H.T. A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sustain. Energy Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Schindler, M.; Hawthorne, F.C.; Baur, W.H. A crystal-chemical approach to the composition and occurrence of vanadium minerals. Can. Mineral. 2000, 38, 1443–1456. [Google Scholar] [CrossRef]
- Chen, G.; Wang, X.; Wang, J.; Du, H.; Zhang, Y.; Zheng, S.L.; Zhang, Y. A new metallurgical process for the clean utilization of chromite ore. Int. J. Miner. Process. 2014, 131, 58–68. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, L.; Liu, Y.; Qi, T.; Wang, J.; Wang, L. A novel process for recovery of iron, titanium, and vanadium from titanomagnetite concentrates: NaOH molten salt roasting and water leaching processes. J. Hazard. Mater. 2013, 244, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.B.; Hao, D.U.; Wang, D.W.; Wang, S.N.; Zheng, S.L.; Zhang, Y. Kinetics analysis of decomposition of vanadium slag by KOH sub-molten salt method. Trans. Nonferrous Met. Soc. China 2013, 23, 1489–1500. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zheng, S.L.; Wang, S.N.; Biao, L.I.U.; Wang, D.W.; Hao, D.U.; Zhang, Y. Research and prospect on extraction of vanadium from vanadium slag by liquid oxidation technologies. Trans. Nonferrous Met. Soc. China 2014, 24, 1273–1288. [Google Scholar] [CrossRef]
- Teng, A.; Xue, X. A novel roasting process to extract vanadium and chromium from high chromium vanadium slag using a NaOH-NaNO3 binary system. J. Hazard. Mater. 2019, 379, 120805. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, H.Y.; Yin, X.C.; Yan, Z.M.; Yan, X.M.; Xie, B. Leaching kinetics of calcification roasted vanadium slag with high CaO content by sulfuric acid. Int. J. Miner. Process. 2014, 133, 105–111. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, C.J.; Yuan, Y.H.; Guo, Y.; Diao, J.; Xie, B. Magnesiation roasting-acid leaching: A zero-discharge method for vanadium extraction from vanadium slag. J. Clean. Prod. 2020, 260, 121091. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Yi, L.Y.; Wang, L.N.; Chen, D.S.; Wang, W.J.; Liu, Y.H.; Qi, T. A novel process for the recovery of iron, titanium, and vanadium from vanadium-bearing titanomagnetite: Sodium modification–direct reduction coupled process. Int. J. Miner. Metall. Mater. 2017, 24, 504–511. [Google Scholar] [CrossRef]
- Li, X.H.; Kou, J.; Sun, T.C.; Wu, S.C.; Zhao, Y.Q. Effects of calcium compounds on the carbothermic reduction of vanadium titanomagnetite concentrate. Int. J. Miner. Metall. Mater. 2020, 27, 301–309. [Google Scholar] [CrossRef]
- Li, W.; Fu, G.; Chu, M.; Zhu, M. An effective and cleaner process to recovery iron, titanium, vanadium, and chromium from Hongge vanadium titanomagnetite with hydrogen-rich gases. Ironmak. Steelmak. 2021, 48, 33–39. [Google Scholar] [CrossRef]
- Liu, S.; He, X.; Wang, Y.; Wang, L. Cleaner and effective extraction and separation of iron from vanadium slag by carbothermic reduction-chlorination-molten salt electrolysis. J. Clean. Prod. 2021, 284, 124674. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Habashi, F. Principles of Extractive Metallurgy; Gordon and Breach Science Publishers: London, UK, 1986. [Google Scholar]
- Xiong, P.; Zhang, Y.; Bao, S.; Huang, J. Precipitation of vanadium using ammonium salt in alkaline and acidic media and the effect of sodium and phosphorus. Hydrometallurgy 2018, 180, 113–120. [Google Scholar] [CrossRef]
- Kim, S.; Trinh, H.B.; Lee, J. Titanium Dioxide Recovery from Soda-roasted Spent SCR Catalysts through Sulphuric Acid Leaching and Hydrolysis Precipitation. Resour. Recycl. 2020, 29, 48–54. [Google Scholar] [CrossRef]
- Rauf, S.B. Thermodynamics Made Simple for Energy Engineers; River Publishers: Copenhagen, Denmark, 2021. [Google Scholar]



| Metals | Concentration (wt.%) |
|---|---|
| ΣFe | 41.33 |
| TiO2 | 23.28 |
| V2O5 | 4.18 |
| SiO2 | 6.25 |
| Al2O3 | 4.35 |
| CaO | 2.54 |
| MgO | 4.37 |
| Na2O | 0.12 |
| K2O | 0.10 |
| MnO | 0.35 |
| P2O5 | 0.18 |
| Kinetic Model | Equation | Fitting Results | |
|---|---|---|---|
| Average Value of Regression Coefficient R2 | Active Energy Ea(leaching) (kJ/mol) | ||
| Film diffusion control a | 0.97 | 54.4 kJ/mol | |
| Ash diffusion control | 0.91 | 99.2 kJ/mol | |
| Chemical control | 0.98 | 69.0 kJ/mol | |
| Film diffusion control b | 0.98 | 61.1 kJ/mol | |
| Stage | Chemical Reactions | |
|---|---|---|
| NaOH roasting | 4FeV2O4 + 8 NaOH + 5O2 → 8 NaVO3 + 2Fe2O3 + 4H2O | (2) |
| 4FeV2O4 + 16 NaOH + 5O2 → 4Na4V2O7 + 2Fe2O3 + 8H2O | (3) | |
| SiO2 + 2NaOH → Na2SiO3 +H2O | (4) | |
| TiO2 + 2NaOH → Na2TiO3 +H2O | (5) | |
| Water leaching | NaVO3 + OH−→ HVO42−+ Na+ | (6) |
| Na4V2O7 + H2O → 2 HVO42−+ 4Na+ | (7) | |
| Na2TiO3 + H2O → TiO2 + 2NaOH | (8) | |
| Precipitation of vanadium | 3H2V10O284− + 10NH4+ + 2H+ → 5(NH4)2V6O16↓ + 4H2O | (9) |
| Hydrolysis of titanium | FeTiO3 + 2H2SO4 → FeSO4 + TiOSO4 +2H2O | (10) |
| TiO2 + H2SO4→ TiOSO4 + H2O | (11) | |
| TiOSO4 + 2H2O → H2TiO3↓ + H2SO4 | (12) | |
| Calcination | (NH4)2V6O16 → 3V2O5 + 2 NH3 + H2O | (13) |
| H2TiO3 → TiO2 +H2O | (14) | |
| Additives | Roasting Temperature (°C) | Specific Heat (J/mol.K) | Heat of Fusion (kJ/mol) | Q (kJ) | Heat for Melting (kJ) | Total | Gas/ Volume (L) |
|---|---|---|---|---|---|---|---|
| NaOH | 400 | 59.5 | 8.4 | 557.8 | 210.0 | 767.8 | None |
| NaCl | 850 | 50.0 | 27.9 | 705.1 | 476.9 | 1182.0 | Cl2/191.5 |
| Na2CO3 | 1000 | 112.3 | 29.7 | 1032.9 | 280.2 | 1313.1 | CO2/210.5 |
| Na2SO4 | 1250 | 128.2 | 200.8 | 1105.9 | 1414.1 | 2520.0 | SO2/156.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, H.B.; Kim, S.; Lee, J.; Oh, S. Efficient Recovery of Vanadium and Titanium from Domestic Titanomagnetite Concentrate Using Molten Salt Roasting and Water Leaching. Materials 2023, 16, 6918. https://doi.org/10.3390/ma16216918
Trinh HB, Kim S, Lee J, Oh S. Efficient Recovery of Vanadium and Titanium from Domestic Titanomagnetite Concentrate Using Molten Salt Roasting and Water Leaching. Materials. 2023; 16(21):6918. https://doi.org/10.3390/ma16216918
Chicago/Turabian StyleTrinh, Ha Bich, Seunghyun Kim, Jaeryeong Lee, and Seokhoon Oh. 2023. "Efficient Recovery of Vanadium and Titanium from Domestic Titanomagnetite Concentrate Using Molten Salt Roasting and Water Leaching" Materials 16, no. 21: 6918. https://doi.org/10.3390/ma16216918





