Surface Engineering of Bioactive Coatings for Improved Stent Hemocompatibility: A Comprehensive Review
Abstract
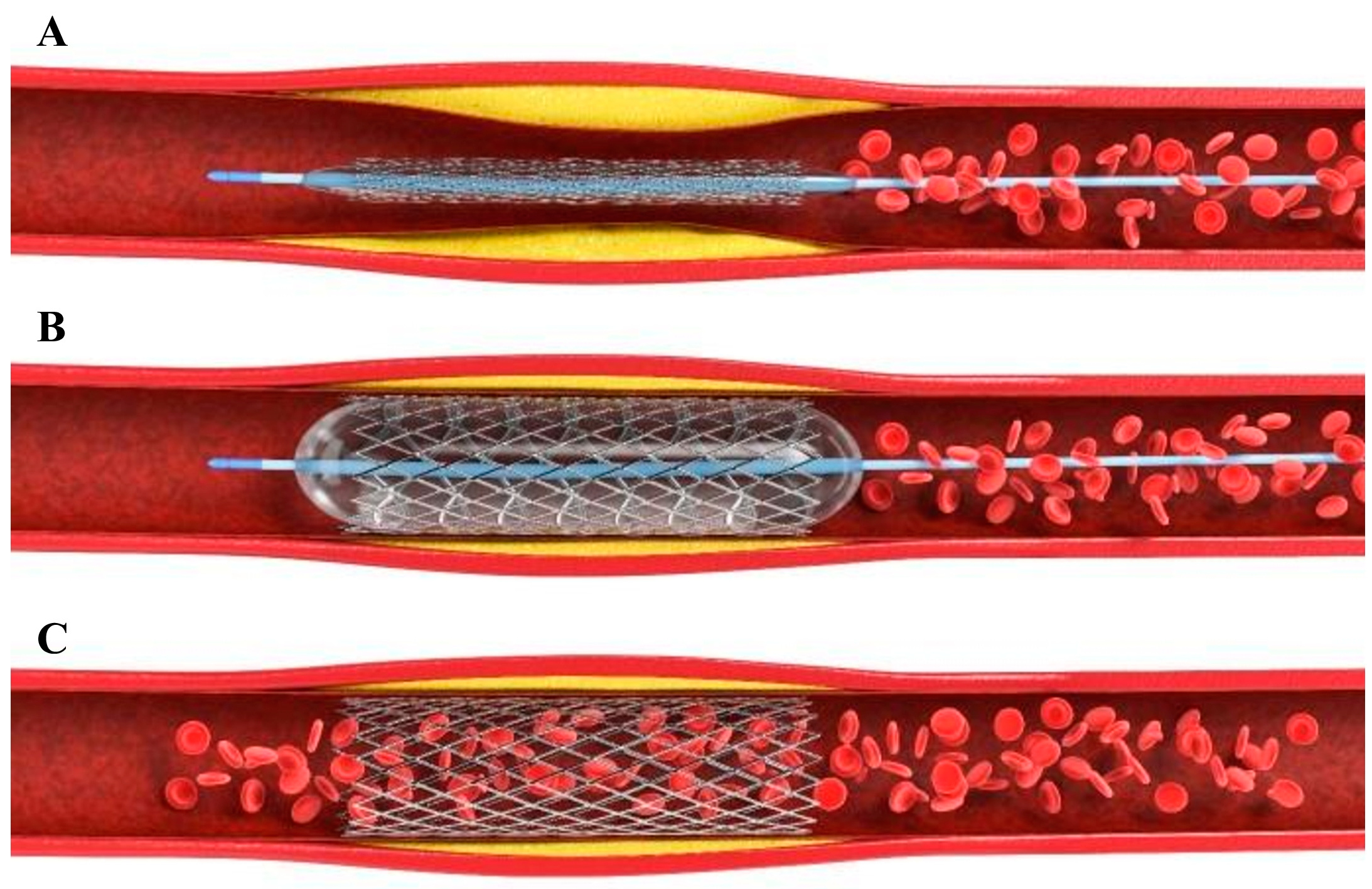
1. Introduction
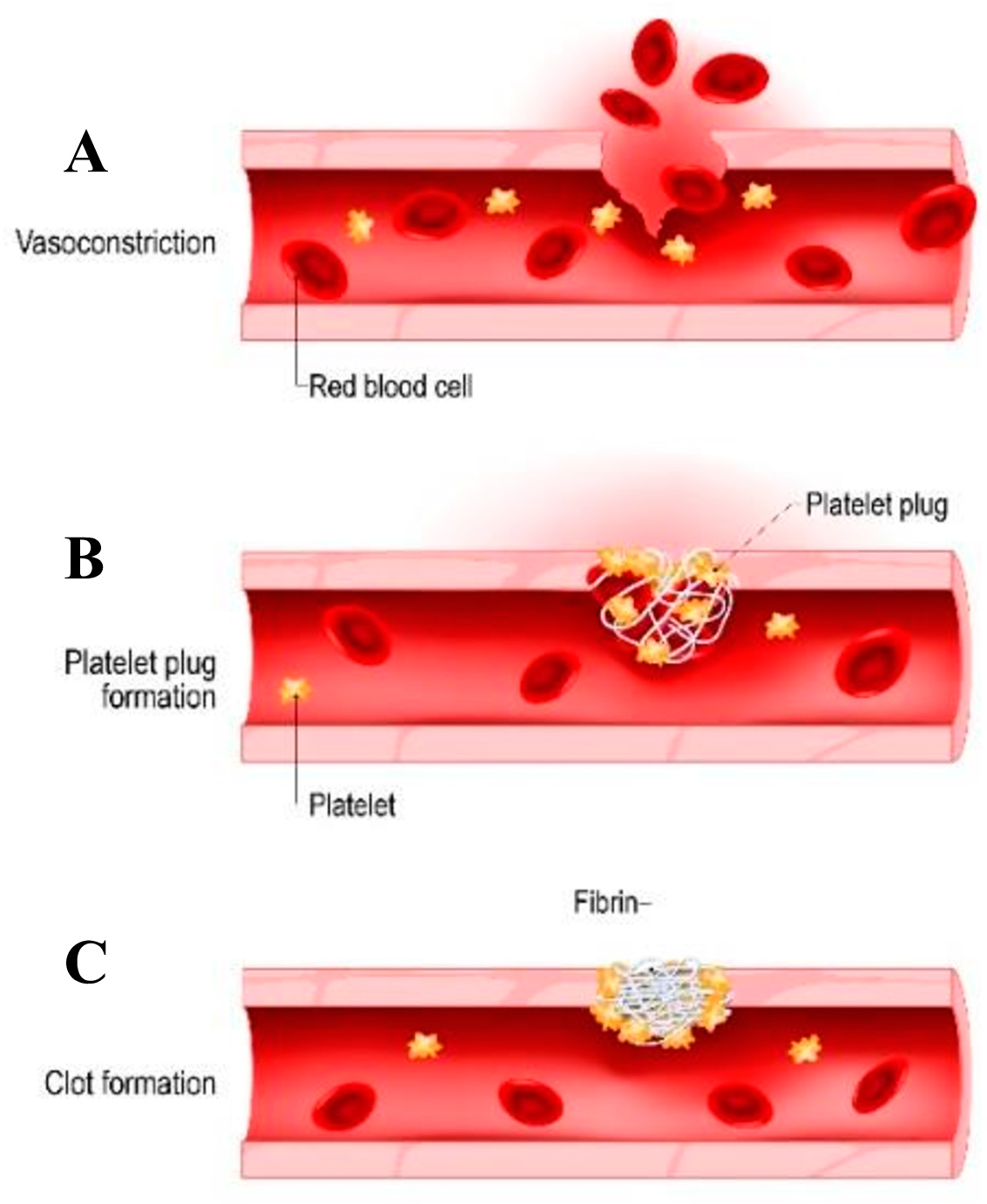
Physiological Process of Hemostasis and the Formation of Blood Clots
2. Bioactive Coating
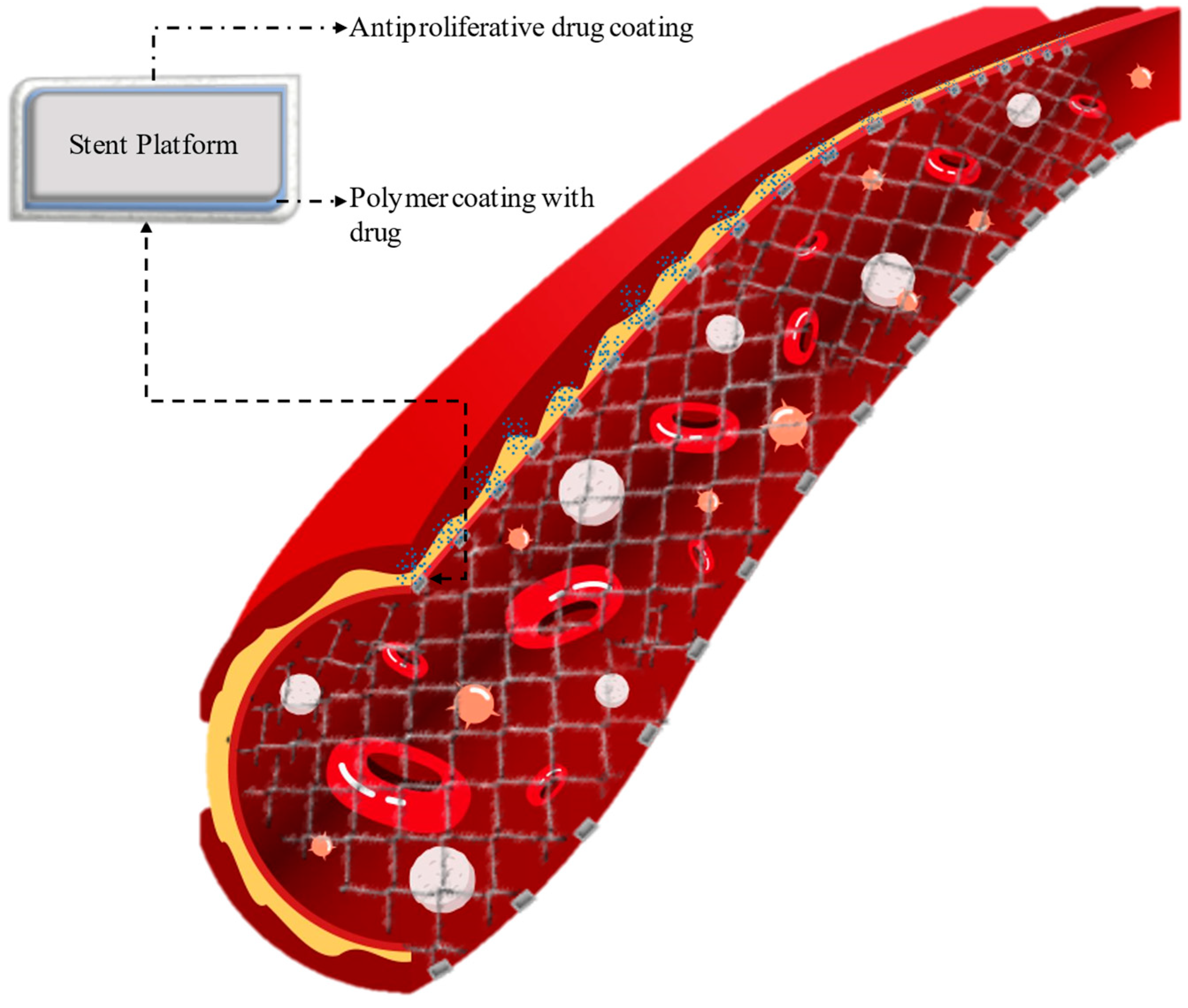
3. Drug-Eluting Stent (DES)
4. Inorganic Coatings
4.1. Gold Coating
4.2. Iridium Oxide
4.3. Silicon Carbide (SiC)
4.4. Carbon Coating
5. Biocompatible Polymer Stent Coatings
5.1. Biodegradable Polymers for Stents
5.2. Bioresorbable Cardiovascular Scaffolds (BRSs)
5.3. Nonbiodegradable Stents
6. Biomimetic Coatings
7. Surface Modification Techniques
7.1. Plasma Oxidation
7.2. Physical Vapor Deposition (PVD)
7.3. Chemical Vapor Deposition (CVD)
7.4. Electrodeposition
8. Evaluation Methods for Hemocompatibility and Thrombogenicity
- In vitro platelet adhesion and activation assays: Platelet adhesion and activation assays involve exposing stent coatings to platelet-rich plasma or whole blood in controlled laboratory settings. These assays measure the extent of platelet adhesion and activation on the coated surface using techniques such as scanning electron microscopy (SEM), flow cytometry, or immunofluorescence staining. By quantifying platelet attachment and activation markers, these assays provide valuable insights into the thrombogenic potential of stent coatings [81].
- Coagulation assays: Various coagulation assays can be used to assess the impact of stent coatings on the clotting cascade. Prothrombin time (PT) and activated partial thromboplastin time (aPTT) tests measure clotting time in the presence of the coating to evaluate the intrinsic and extrinsic coagulation pathways, respectively. Prothrombin time (PT) and activated partial thromboplastin time (aPTT) are critical blood tests that provide insights into distinct aspects of the coagulation or clotting process within the bloodstream. PT measures the time it takes for blood to clot through the extrinsic and common coagulation pathways, assessing factors like fibrinogen, prothrombin, and factors V, VII, and X. It is particularly useful for monitoring anticoagulant therapy, such as warfarin, and diagnosing conditions like liver disease and clotting disorders. On the other hand, aPTT assesses the clotting time via the intrinsic and common pathways, focusing on factors like VIII, IX, XI, and XII. It aids in diagnosing clotting disorders like hemophilia and monitoring heparin therapy. Thrombin generation assays can assess the effect of coatings on thrombin activity, while fibrinogen adsorption assays provide insights into the coating’s interaction with fibrinogen and its potential to initiate clot formation [82].
- Platelet function tests: Platelet function tests evaluate the functional response of platelets to stent coatings. Aggregometry measures the ability of platelets to aggregate when exposed to coating surfaces, indicating platelet activation and aggregation potential. Flow-based assays, such as microfluidic systems or perfusion chambers, mimic blood flow conditions and assess platelet adhesion, aggregation, and thrombus formation on stent coatings under shear stress conditions [83].
- Endothelial cell studies: Evaluating the interaction between stent coatings and endothelial cells is essential for assessing their hemocompatibility. Endothelial cell adhesion, proliferation, and morphology can be analyzed using techniques like cell viability assays, immunostaining, or scanning electron microscopy. In vitro studies can provide insights into the coating’s ability to promote endothelialization and prevent thrombus formation [84].
- Animal models and in vivo studies: Animal models are critical for assessing the hemocompatibility and thrombogenicity of stent coatings in a physiological context. Implanting coated stents in animal models allows for the evaluation of factors such as thrombus formation, neointimal hyperplasia (refers to the abnormal and excessive proliferation or growth of smooth muscle cells within the innermost layer of an artery, known as the intima), endothelialization, and inflammatory response. Various animal models, such as rats, rabbits, or pigs, are used to simulate human vascular environments and assess the safety and efficacy of stent coatings [85].
9. Recent Advances
10. Conclusions and Future Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malik, T.F.; Tivakaran, V.S. Percutaneous Transluminal Coronary Angioplasty (PTCA); StatPearls: Treasure Island, FL, USA, 2019. [Google Scholar]
- Claessen, B.E.; Henriques, J.P.; Jaffer, F.A.; Mehran, R.; Piek, J.J.; Dangas, G.D. Stent thrombosis: A clinical perspective. JACC Cardiovasc. Interv. 2014, 7, 1081–1092. [Google Scholar] [CrossRef]
- Alfonso, F.; Byrne, R.A.; Rivero, F.; Kastrati, A. Current treatment of in-stent restenosis. J. Am. Coll. Cardiol. 2014, 63, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Thierry, B.; Winnik, F.M.; Merhi, Y.; Silver, J.; Tabrizian, M. Bioactive coatings of endovascular stents based on polyelectrolyte multilayers. Biomacromolecules 2003, 4, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Wessely, R.; Schömig, A.; Kastrati, A. Sirolimus and paclitaxel on polymer-based drug-eluting stents: Similar but different. J. Am. Coll. Cardiol. 2006, 47, 708–714. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Cui, J.; Yu, F.; Liu, M.; Chen, Y.; Wu, J.; Sun, B.; Mo, X. Development of biodegradable polymeric stents for the treatment of cardiovascular diseases. Biomolecules 2022, 12, 1245. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Serruys, P. Biodegradable stents and non-biodegradable stents. Minerva Cardioangiol. 2009, 57, 537–565. [Google Scholar] [PubMed]
- Boon, G.D. An overview of hemostasis. Toxicol. Pathol. 1993, 21, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Greenberg, D.L.; Fujikawa, K.; Xu, W.; Chung, D.W.; Davie, E.W. Protease-activated receptor 1 is the primary mediator of thrombin-stimulated platelet procoagulant activity. Proc. Natl. Acad. Sci. USA 1999, 96, 11189–11193. [Google Scholar] [CrossRef]
- Mohammad, S.F.; Anderson, W.H.; Smith, J.B.; Chuang, H.Y.; Mason, R.G. Effects of heparin on platelet aggregation and release and thromboxane A2 production. Am. J. Pathol. 1981, 104, 132. [Google Scholar]
- Steinhubl, S.R.; Moliterno, D.J. The role of the platelet in the pathogenesis of atherothrombosis. Am. J. Cardiovasc. Drugs 2005, 5, 399–408. [Google Scholar]
- Kalafatis, M.; Egan, J.O.; van’t Veer, C.; Cawthern, K.M.; Mann, K.G. The regulation of clotting factors. Crit. Rev. Eukaryot. Gene Expr. 1997, 7, 241–280. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.S. The nomenclature of blood clotting factors. Thromb. Haemost. 1962, 7, 381–388. [Google Scholar] [CrossRef]
- Eilertsen, K.E.; Østerud, B. Tissue factor:(patho) physiology and cellular biology. Blood Coagul. Fibrinolysis 2004, 15, 521–538. [Google Scholar] [PubMed]
- Lüscher, T.F.; Steffel, J.; Eberli, F.R.; Joner, M.; Nakazawa, G.; Tanner, F.C.; Virmani, R. Drug-eluting stent and coronary thrombosis: Biological mechanisms and clinical implications. Circulation 2007, 115, 1051–1058. [Google Scholar] [CrossRef]
- Mackman, N. Triggers, targets and treatments for thrombosis. Nature 2008, 451, 914–918. [Google Scholar] [CrossRef]
- Inoue, T.; Croce, K.; Morooka, T.; Sakuma, M.; Node, K.; Simon, D.I. Vascular inflammation and repair: Implications for re-endothelialization, restenosis, and stent thrombosis. JACC Cardiovasc. Interv. 2011, 4, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Gori, T.; Polimeni, A.; Indolfi, C.; Räber, L.; Adriaenssens, T.; Münzel, T. Predictors of stent thrombosis and their implications for clinical practice. Nat. Rev. Cardiol. 2019, 16, 243–256. [Google Scholar] [CrossRef]
- Nishi, S.; Nakayama, Y.; Ishibashi-Ueda, H.; Okamoto, Y.; Yoshida, M. Development of microporous self-expanding stent grafts for treating cerebral aneurysms: Designing micropores to control intimal hyperplasia. J. Artif. Organs 2011, 14, 348–356. [Google Scholar] [CrossRef]
- McFadden, E.P.; Stabile, E.; Regar, E.; Cheneau, E.; Ong, A.T.; Kinnaird, T.; Suddath, W.O.; Weissman, N.J.; Torguson, R.; Kent, K.M.; et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004, 364, 1519–1521. [Google Scholar] [CrossRef]
- Feres, F.; Costa, J.R., Jr.; Abizaid, A. Very late thrombosis after drug-eluting stents. Catheter. Cardiovasc. Interv. 2006, 68, 83–88. [Google Scholar] [CrossRef]
- Roy, P.; Waksman, R. Intravascular ultrasound guidance in drug-eluting stent deployment. Minerva Cardioangiol. 2008, 56, 67–77. [Google Scholar] [PubMed]
- Chen, J.L.; Li, Q.L.; Chen, J.Y.; Chen, C.; Huang, N. Improving blood-compatibility of titanium by coating collagen–heparin multilayers. Appl. Surf. Sci. 2009, 255, 6894–6900. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Jing, Q.M.; Wang, X.Z.; Ma, Y.Y.; Wang, G.; Xu, B.; Gao, R.L.; Han, Y.L.; CREATE Investigators. Dual Antiplatelet Therapy Over 6 Months Increases the Risk of Bleeding after Biodegradable Polymer-Coated Sirolimus Eluting Stents Implantation: Insights from the CREATE Study. J. Interv. Cardiol. 2014, 27, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Li, J.Y.; Chen, R.J.; Chen, T.Y.; Hsu, S.H.; Wang, H.H.; Peng, H.Y.; Sun, Y.Y.; Lu, W.J. Paclitaxel exerts antiplatelet and antithrombotic activities: Additional benefit from use of paclitaxel-coated balloons and-eluting stents in coronary revascularization and prevention of in-stent restenosis. Thromb. Res. 2023, 225, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, A.J.; Stoler, R.; Feldman, R.; Neumann, F.J.; Boutis, L.; Tahirkheli, N.; Toelg, R.; Othman, I.; Stein, B.; Choi, J.W.; et al. Primary results of the EVOLVE short DAPT study: Evaluation of 3-month dual antiplatelet therapy in high bleeding risk patients treated with a bioabsorbable polymer-coated everolimus-eluting stent. Circ. Cardiovasc. Interv. 2021, 14, e010144. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Kirtane, A.J.; Windecker, S.; Latib, A.; Kedhi, E.; Mehran, R.; Price, M.J.; Abizaid, A.; Simon, D.I.; Worthley, S.G.; et al. One-month dual antiplatelet therapy following percutaneous coronary intervention with zotarolimus-eluting stents in high-bleeding-risk patients. Circ. Cardiovasc. Interv. 2020, 13, e009565. [Google Scholar] [CrossRef]
- Bartorelli, A.L.; Tamburino, C.; Trabattoni, D.; Galassi, A.; Serdoz, R.; Sheiban, I.; Piovaccari, G.; Zimarino, M.; Benassi, A.; Di Mario, C.; et al. Comparison of two antiplatelet regimens (aspirin alone versus aspirin+ ticlopidine or clopidogrel) after intracoronary implantation of a carbofilm-coated stent. Am. J. Cardiol. 2007, 99, 1062–1066. [Google Scholar] [CrossRef]
- Bakola, V.; Karagkiozaki, V.; Tsiapla, A.R.; Pappa, F.; Moutsios, I.; Pavlidou, E.; Logothetidis, S. Dipyridamole-loaded biodegradable PLA nanoplatforms as coatings for cardiovascular stents. Nanotechnology 2018, 29, 275101. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, J.F.; Llanos, G.; Holmer, E. Heparin-coated stents. J. Long-Term Eff. Med. Implant. 2000, 10, 19–45. [Google Scholar] [CrossRef]
- Alt, E.; Seliger, C. Antithrombotic stent coatings: Hirudin/iloprost combination. Semin. Interv. Cardiol. SIIC 1998, 3, 177–183. [Google Scholar]
- Kruse, K.R.; Crowley, J.J.; Tanguay, J.F.; Santos, R.M.; Millare, D.S.; Phillips, H.R.; Zidar, J.P.; Stack, R.S. Local drug delivery of Argatroban from a polymeric-metallic composite stent reduces platelet deposition in a swine coronary model. Catheter. Cardiovasc. Interv. 1999, 46, 503–507. [Google Scholar] [CrossRef]
- Yang, Z.; Tu, Q.; Maitz, M.F.; Zhou, S.; Wang, J.; Huang, N. Direct thrombin inhibitor-bivalirudin functionalized plasma polymerized allylamine coating for improved biocompatibility of vascular devices. Biomaterials 2012, 33, 7959–7971. [Google Scholar] [CrossRef]
- Ham, H.O.; Haller, C.A.; Su, G.; Dai, E.; Patel, M.S.; Liu, D.R.; Liu, J.; Chaikof, E.L. A rechargeable anti-thrombotic coating for blood-contacting devices. Biomaterials 2021, 276, 121011. [Google Scholar] [CrossRef]
- Goh, E.T.; Wong, E.; Farhatnia, Y.; Tan, A.; Seifalian, A.M. Accelerating in situ endothelialisation of cardiovascular bypass grafts. Int. J. Mol. Sci. 2014, 16, 597–627. [Google Scholar] [CrossRef]
- Morra, M. Biomolecular modification of implant surfaces. Expert Rev. Med. Devices 2007, 4, 361–372. [Google Scholar] [CrossRef]
- Ceylan, H.; Tekinay, A.B.; Guler, M.O. Selective adhesion and growth of vascular endothelial cells on bioactive peptide nanofiber functionalized stainless steel surface. Biomaterials 2011, 32, 8797–8805. [Google Scholar] [CrossRef]
- Paul, A.; Shao, W.; Shum-Tim, D.; Prakash, S. The attenuation of restenosis following arterial gene transfer using carbon nanotube coated stent incorporating TAT/DNAAng1+ Vegf nanoparticles. Biomaterials 2012, 33, 7655–7664. [Google Scholar] [CrossRef]
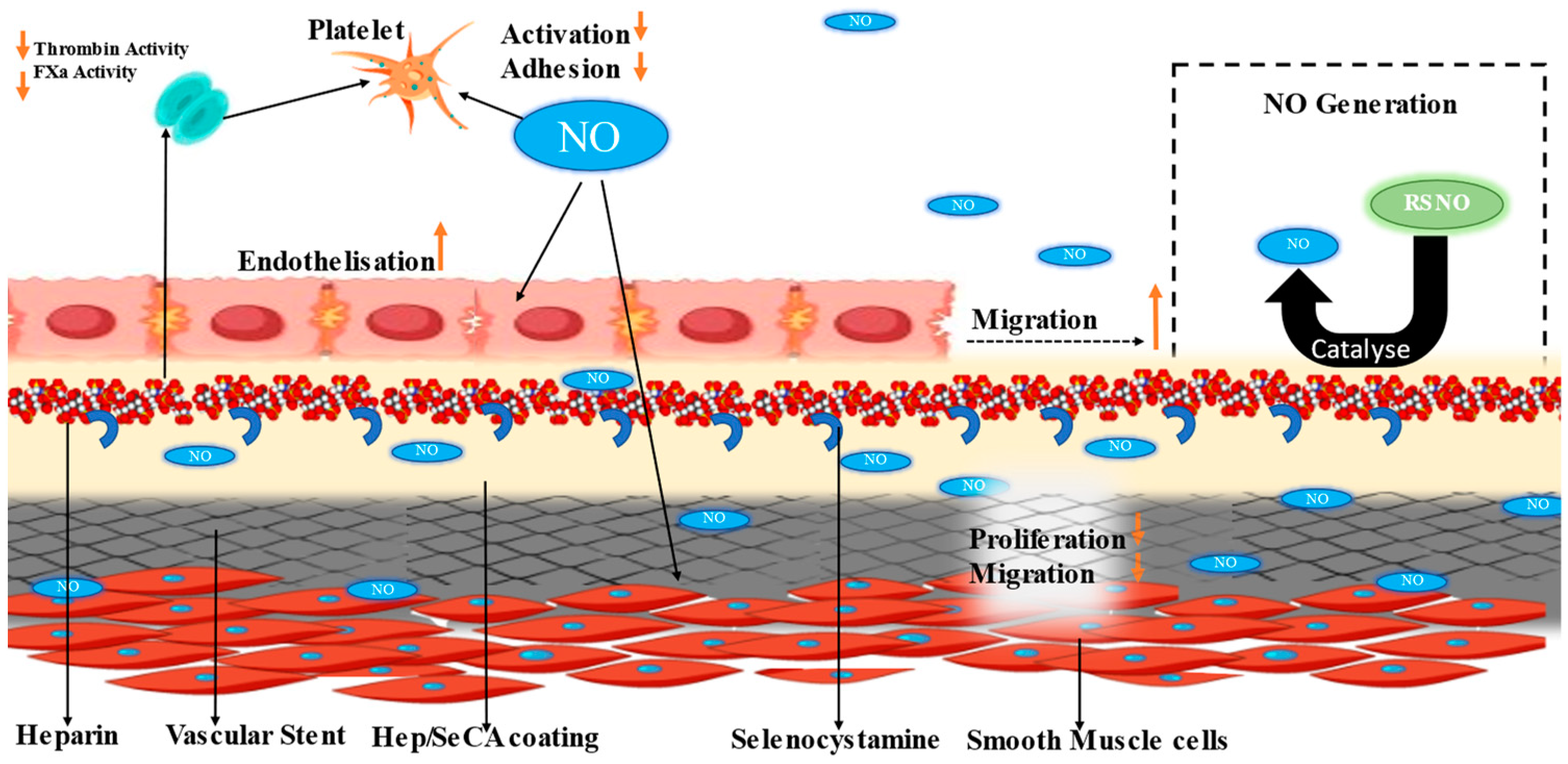
- Yang, Z.; Yang, Y.; Xiong, K.; Li, X.; Qi, P.; Tu, Q.; Jing, F.; Weng, Y.; Wang, J.; Huang, N. Nitric oxide producing coating mimicking endothelium function for multifunctional vascular stents. Biomaterials 2015, 63, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; De Scheerder, I.; Desmet, W. Dexamethasone-eluting stent: An anti-inflammatory approach to inhibit coronary restenosis. Expert Rev. Cardiovasc. Ther. 2004, 2, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, O.; Popescu-Pelin, G.; Florian, P.; Icriverzi, M.; Roseanu, A.; Mitran, V.; Cimpean, A.; Socol, G. Bioactive ibuprofen-loaded PLGA coatings for multifunctional surface modification of medical devices. Polymers 2021, 13, 1413. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, H.; Lu, L.; He, Q.; Xi, B.; Yu, H.; Luo, R.; Wang, Y.; Zhang, X. A tailored extracellular matrix (ECM)-Mimetic coating for cardiovascular stents by stepwise assembly of hyaluronic acid and recombinant human type III collagen. Biomaterials 2021, 276, 121055. [Google Scholar] [CrossRef] [PubMed]
- Kamann, S.; Haase, T.; Stolzenburg, N.; Löchel, M.; Peters, D.; Schnorr, J. Resveratrol-coated balloon catheters in porcine coronary and peripheral arteries. Int. J. Mol. Sci. 2019, 20, 2285. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Hashemzaei, M.; Sahebkar, A. The regulatory role of curcumin on platelet functions. J. Cell. Biochem. 2018, 119, 8713–8722. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, M.M.; De, S.; Trigwell, S.; Ali, N.; Mazumder, M.K.; Mehta, J.L. Corrosion resistance of polyurethane-coated nitinol cardiovascular stents. J. Biomater. Sci. Polym. Ed. 2003, 14, 1351–1362. [Google Scholar] [CrossRef]
- Mikhalovska, L.; Chorna, N.; Lazarenko, O.; Haworth, P.; Sudre, A.; Mikhalovsky, S. Inorganic coatings for cardiovascular stents: In vitro and in vivo studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96, 333–341. [Google Scholar] [CrossRef]
- Qiu, H.; Qi, P.; Liu, J.; Yang, Y.; Tan, X.; Xiao, Y.; Maitz, M.F.; Huang, N.; Yang, Z. Biomimetic engineering endothelium-like coating on cardiovascular stent through heparin and nitric oxide-generating compound synergistic modification strategy. Biomaterials 2019, 207, 10–22. [Google Scholar] [CrossRef]
- Gu, X.; Mao, Z.; Ye, S.H.; Koo, Y.; Yun, Y.; Tiasha, T.R.; Shanov, V.; Wagner, W.R. Biodegradable, elastomeric coatings with controlled anti-proliferative agent release for magnesium-based cardiovascular stents. Colloids Surf. B Biointerfaces 2016, 144, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Tahir, H.; Awan, G.M.; Omar, B. Angioplasty Beginnings. 40 Years Later. Cardiofel. Newslet. 2018, 1, 17–18. [Google Scholar]
- Senst, B.; Goyal, A.; Basit, H.; Borger, J. Drug Eluting Stent Compounds; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Omar, W.A.; Kumbhani, D.J. The current literature on bioabsorbable stents: A review. Curr. Atheroscler. Rep. 2019, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Joner, M.; Kastrati, A. Stent thrombosis and restenosis: What have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur. Heart J. 2015, 36, 3320–3331. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Jurewitz, D.; Aragon, J.; Forrester, J.; Makkar, R.R.; Kar, S. Stent fracture associated with drug-eluting stents: Clinical characteristics and implications. Catheter. Cardiovasc. Interv. 2007, 69, 387–394. [Google Scholar] [CrossRef]
- Edelman, E.R.; Seifert, P.; Groothuis, A.; Morss, A.; Bornstein, D.; Rogers, C. Gold-coated NIR stents in porcine coronary arteries. Circulation 2001, 103, 429–434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- ürgen vom Dahl, J.; Haager, P.K.; Grube, E.; Gross, M.; Beythien, C.; Kromer, E.P.; Cattelaens, N.; Hamm, C.W.; Hoffmann, R.; Reineke, T.; et al. Effects of gold coating of coronary stents on neointimal proliferation following stent implantation. Am. J. Cardiol. 2002, 89, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Mani, G.; Feldman, M.D.; Patel, D.; Agrawal, C.M. Coronary stents: A materials perspective. Biomaterials 2007, 28, 1689–1710. [Google Scholar] [CrossRef]
- Danzi, G.; Capuano, C.; Sesana, M.; Blasi, A.D.; Predolini, S.; Antoniucci, D. Patterns of in-stent restenosis after placement of NIR goldcoated stents in unselected patients. Catheter Cardiovasc. Interv. 2002, 55, 157–162. [Google Scholar] [CrossRef]
- Atanasoska, L.; Gupta, P.; Deng, C.; Warner, R.; Larson, S.; Thompson, J. XPS, AES, and electrochemical study of iridium oxide coating materials for cardiovascular stent application. ECS Trans. 2009, 16, 37. [Google Scholar] [CrossRef]
- Guildford, A.; Santin, M.; Phillips, G.J. Cardiovascular stents. In Biomaterials and Devices for the Circulatory System; Woodhead Publishing: Sawston, UK, 2010; pp. 173–216. [Google Scholar]
- Tanajura, L.F.L.; Sousa, J.; Sousa, A.G.; Abizaid, A.; Paula, J.E.T.; Albertal, M.; Feres, F.; Mattos, L.A.P.; Staico, R.; Pinto, I.M. Randomized intravascular ultrasound comparison between endoprostheses with and without amorphous silicon-carbide. Arq. Bras. De Cardiol. 2004, 83, 59–63. [Google Scholar]
- Antoniucci, D.; Bartorelli, A.; Valenti, R.; Montorsi, P.; Santoro, G.M.; Fabbiocchi, F.; Bolognese, L.; Loaldi, A.; Trapani, M.; Trabattoni, D.; et al. Clinical and angiographic outcome after coronary arterial stenting with the carbostent. Am. J. Cardiol. 2000, 85, 821–825. [Google Scholar] [CrossRef]
- Szycher, M.; Armini, A.; Bajgar, C.; Lucas, A. Drug-Eluting Stents to Prevent Coronary Restenosis [Online]. Retrieved from the Internet 2002, 1–10. Available online: http://www.implantsciences.com/pdf/IMXpaperv2-rev2.pdf (accessed on 1 October 2023).
- Guo, Q.; Knight, P.T.; Mather, P.T. Tailored drug release from biodegradable stent coatings based on hybrid polyurethanes. J. Control. Release 2009, 137, 224–233. [Google Scholar] [CrossRef]
- Pinchuk, L.; Boden, M.; Bluestein, D. The use of poly (styrene-block-isobutylene-block-styrene) and analogs for long-term implant applications. In Macromolecular Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 211–235. [Google Scholar]
- Falotico, R.; Zhao, J. Polymers and drug-eluting stents. In Textbook of Interventional Cardiovascular Pharmacology; CRC Press: Boca Raton, FL, USA, 2007; pp. 289–297. [Google Scholar]
- Rankin, S. A mini case study of product strategy: The Cypher stent from Cordis (a Johnson & Johnson company). Mark. Rev. 2004, 4, 211–224. [Google Scholar]
- Kamath, K.R.; Barry, J.J.; Miller, K.M. The Taxus™ drug-eluting stent: A new paradigm in controlled drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Kim, J.I.; Kang, H.J.; Lee, B.; Lee, K.W.; Kim, M.S. Biodegradable stent. J. Biomed. Sci. Eng. 2012, 5, 18516. [Google Scholar] [CrossRef]
- Rykowska, I.; Nowak, I.; Nowak, R. Drug-eluting stents and balloons—Materials 2012, structure designs, and coating techniques: A review. Molecules 2020, 25, 4624. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Okura, H.; Kume, T.; Fukuhara, K.; Koyama, T.; Higa, T.; Neishi, Y.; Yoshida, K.; Uemura, S. Impact of stent platform on longitudinal stent deformation: An in vivo frequency domain optical coherence tomography study. Cardiovasc. Interv. Ther. 2017, 32, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.I.; Pacetti, S.D.; Tang, F.W.; Gada, M.; Roorda, W. XIENCE V™ stent design and rationale. J. Interv. Cardiol. 2009, 22, S18–S27. [Google Scholar] [CrossRef]
- Han, Y.; Xu, B.; Jing, Q.; Lu, S.; Yang, L.; Xu, K.; Li, Y.; Li, J.; Guan, C.; Kirtane, A.J.; et al. A randomized comparison of novel biodegradable polymer-and durable polymer–coated cobalt-chromium sirolimus-eluting stents. JACC: Cardiovasc. Interv. 2014, 7, 1352–1360. [Google Scholar] [CrossRef][Green Version]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. 3D-printed PCL/PLA composite stents: Towards a new solution to cardiovascular problems. Materials 2018, 11, 1679. [Google Scholar] [CrossRef]
- Yao, S.; Cui, J.; Chen, S.; Zhou, X.; Li, J.; Zhang, K. Extracellular Matrix Coatings on Cardiovascular Materials—A Review. Coatings 2022, 12, 1039. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Chen, H.; Liu, T.; Yang, P.; Zhao, Y.; Huang, N. A novel coating of type IV collagen and hyaluronic acid on stent material-titanium for promoting smooth muscle cell contractile phenotype. Mater. Sci. Eng. C 2014, 38, 235–243. [Google Scholar] [CrossRef]
- Tong, P.; Chen, L.; Sun, X.; Li, H.; Feng, Y.; Li, J.; Guan, S. Surface modification of biodegradable magnesium alloy with poly (L-lactic acid) and sulfonated hyaluronic acid nanoparticles for cardiovascular application. Int. J. Biol. Macromol. 2023, 237, 124191. [Google Scholar] [CrossRef] [PubMed]
- Dini, J.W. Properties of coatings: Comparisons of electroplated, physical vapor deposited, chemical vapor deposited, and plasma sprayed coatings. Mater. Manuf. Process 1997, 12, 437–472. [Google Scholar] [CrossRef]
- Huan, Z.; Fratila-Apachitei, L.E.; Apachitei, I.; Duszczyk, J. Characterization of porous TiO2 surfaces formed on 316L stainless steel by plasma electrolytic oxidation for stent applications. J. Funct. Biomater. 2012, 3, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.J.; Chou, H.H.; Ou, K.L.; Sugiatno, E.; Ruslin, M.; Abd Waris, R.; Huang, C.F.; Liu, C.M.; Peng, P.W. Evaluation of surface characteristics and hemocompatibility on the oxygen plasma-modified biomedical titanium. Metals 2018, 8, 513. [Google Scholar] [CrossRef]
- Subramanian, B.; Muraleedharan, C.V.; Ananthakumar, R.; Jayachandran, M. A comparative study of titanium nitride (TiN), titanium oxy nitride (TiON) and titanium aluminum nitride (TiAlN), as surface coatings for bio implants. Surf. Coat. Technol. 2011, 205, 5014–5020. [Google Scholar] [CrossRef]
- Motlagh, D.; Yang, J.; Lui, K.Y.; Webb, A.R.; Ameer, G.A. Hemocompatibility evaluation of poly (glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials 2006, 27, 4315–4324. [Google Scholar] [CrossRef] [PubMed]
- Balan, V.; Verestiuc, L. Strategies to improve chitosan hemocompatibility: A review. Eur. Polym. J. 2014, 53, 171–188. [Google Scholar] [CrossRef]
- Breet, N.J.; van Werkum, J.W.; Bouman, H.J.; Kelder, J.C.; Ruven, H.J.; Bal, E.T.; Deneer, V.H.; Harmsze, A.M.; van der Heyden, J.A.; Rensing, B.J.; et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. Jama 2010, 303, 754–762. [Google Scholar] [CrossRef]
- Yeh, H.I.; Lu, S.K.; Tian, T.Y.; Hong, R.C.; Lee, W.H.; Tsai, C.H. Comparison of endothelial cells grown on different stent materials. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. 2006, 76, 835–841. [Google Scholar] [CrossRef]
- Iqbal, J.; Chamberlain, J.; Francis, S.E.; Gunn, J. Role of animal models in coronary stenting. Ann. Biomed. Eng. 2016, 44, 453–465. [Google Scholar] [CrossRef]
- Stefanini, G.G.; Byrne, R.A.; Serruys, P.W.; de Waha, A.; Meier, B.; Massberg, S.; Jüni, P.; Schömig, A.; Windecker, S.; Kastrati, A. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: A pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur. Heart J. 2012, 33, 1214–1222. [Google Scholar]
- Piccolo, R.; Pilgrim, T. The impact of thin-strut, biodegradable polymer stent designs. Card. Interv. Today 2017, 11, 43–46. [Google Scholar]
- Hamon, M.; Niculescu, R.; Deleanu, D.; Dorobantu, M.; Weissman, N.J.; Waksman, R. Clinical and angiographic experience with a third-generation drug-eluting Orsiro stent in the treatment of single de novo coronary artery lesions (BIOFLOW-I): A prospective, first-in-man study. EuroIntervention 2013, 8, 1006–1011. [Google Scholar] [CrossRef]
- Verheye, S.; Abizaid, A.; Botelho, R.; Costa, R.; Tanajura, L.F.; Waseda, K.; Morrison, L.; Toyloy, S.; Fitzgerald, P.J.; Schofer, J. TCT-460 Multi-Center, Randomized Evaluation of the Elixir DESyne® Novolimus Eluting Coronary Stent System with Biodegradable Polymer Compared to a Zotarolimus-Eluting Coronary Stent System: Final 5-Year Results from the EXCELLA BD Study. J. Am. Coll. Cardiol. 2016, 68, B185. [Google Scholar] [CrossRef]
- Muramatsu, T.; Onuma, Y.; Zhang, Y.J.; Bourantas, C.V.; Kharlamov, A.; Diletti, R.; Farooq, V.; Gogas, B.D.; Garg, S.; García-García, H.M.; et al. Progress in treatment by percutaneous coronary intervention: The stent of the future. Rev. Española De Cardiol. (Engl. Ed.) 2013, 66, 483–496. [Google Scholar] [CrossRef]
- Chiarito, M.; Sardella, G.; Colombo, A.; Briguori, C.; Testa, L.; Bedogni, F.; Fabbiocchi, F.; Paggi, A.; Palloshi, A.; Tamburino, C.; et al. Safety and efficacy of polymer-free drug-eluting stents: Amphilimus-eluting Cre8 versus biolimus-eluting BioFreedom stents. Circ. Cardiovasc. Interv. 2019, 12, e007311. [Google Scholar] [CrossRef]
- Vishnu, J.; Calin, M.; Pilz, S.; Gebert, A.; Kaczmarek, B.; Michalska-Sionkowska, M.; Hoffmann, V.; Manivasagam, G. Superhydrophilic nanostructured surfaces of beta Ti29Nb alloy for cardiovascular stent applications. Surf. Coat. Technol. 2020, 396, 125965. [Google Scholar] [CrossRef]
- Park, K.S.; Kang, S.N.; Kim, D.H.; Kim, H.B.; Im, K.S.; Park, W.; Hong, Y.J.; Han, D.K.; Joung, Y.K. Late endothelial progenitor cell-capture stents with CD146 antibody and nanostructure reduce in-stent restenosis and thrombosis. Acta Biomater. 2020, 111, 91–101. [Google Scholar] [CrossRef]
- Mohan, C.C.; Cherian, A.M.; Kurup, S.; Joseph, J.; Nair, M.B.; Vijayakumar, M.; Nair, S.V.; Menon, D. Stable titania nanostructures on stainless steel coronary stent surface for enhanced corrosion resistance and endothelialization. Adv. Healthc. Mater. 2017, 6, 1601353. [Google Scholar] [CrossRef]

| Clotting Factor | Clotting Factor Name | Function | Plasma Half-Life (Hours) | Plasma Concentration (mg/L) |
|---|---|---|---|---|
| Factor I | Fibrinogen | Converts to fibrin, forms clot mesh | 4 | 1.5–4.0 |
| Factor II | Prothrombin | Converts to thrombin, activates clotting cascade | 60–72 | 0.12–0.16 |
| Factor III | Tissue factor | Initiates extrinsic pathway of coagulation | N/A | Trace amounts |
| Factor IV | Calcium | Cofactor in multiple coagulation reactions | N/A | 2.2–2.7 |
| Factor V | Labile factor | Cofactor in prothrombinase complex | 12–18 | 8–20 |
| Factor VII | Stable factor | Initiates extrinsic pathway of coagulation | 2–6 | 0.5–2.0 |
| Factor VIII | Antihemophilic factor A | Cofactor in intrinsic pathway, enhances factor IX | 8–12 | 0.02–0.20 |
| Factor IX | Christmas factor | Activates factor X in intrinsic pathway | 18–24 | 0.1–0.2 |
| Factor X | Stuart–Prower factor | Prothrombin is transformed into thrombin | 24–48 | 5–15 |
| Factor XI | Plasma thromboplastin antecedent (PTA) | In the intrinsic pathway, it triggers the activation of factor IX | 40–60 | 0.05–0.15 |
| Factor XII | Hageman factor | It initiates the coagulation intrinsic pathway | 48–72 | 0.03–0.08 |
| Factor XIII | Fibrin-stabilizing factor | It forms cross-links within fibrin, providing stability to the clot | 10–14 | 0.02–0.05 |
| Factor Leading to Thrombosis of Stent Surface | Mechanism | Prevention Measures | Ref. |
|---|---|---|---|
| Activation of Platelets |
|
| [16] |
| Inflammation and Endothelial Dysfunction |
|
| [17] |
| Disruption of Blood Flow |
|
| [18] |
| Stent Surface Characteristics |
|
| [19] |
| Delayed Endothelialization |
|
| [20] |
| Drug-Eluting Stents |
|
| [21] |
| Stent Under-expansion or Malposition |
|
| [22] |
| Stent | Manufacturer | Polymer Material | Biodegradation | Coating Location and Thickness | Drug Release |
|---|---|---|---|---|---|
| Synergy | Boston Scientific | PLGA | 3–4 months | Located on the outer surface, at a distance of 4 μm. | 50% for 30 days |
| Orsiro | Biotronik | PLLA | 1–2 years | Spread evenly around the circumference, spanning 7 μm. | 50% for 30 days |
| DESyne BD | Elixir Medical | PDLLA | 6–9 months | Evenly distributed around the circumference, measuring less than 3 μm. | 90% for 90 days |
| Combo | OrbusNeich | PDLLA, PLGA | 3 months | Situated on the outer side, extending to a distance of 5 μm. | 95% for 30 days |
| MiStent | Micell | PLGA | 2–3 months | Evenly distributed around the circumference, the presence of crystalline sirolimus. | DESSOLVE-I (n = 30) |
| Ultimaster | Terumo | Poly (D, L-lactide) and poly(ε-caprolactone) | 3–4 months | Positioned on the outer side, extending to a distance of 15 μm. | 90% for 90 days |
| Type | Producer | Material | Biodegradation Time | Coating Material | Drug Used | Drug Release Period |
|---|---|---|---|---|---|---|
| BVS V.1.0 and BVS V.1.1 | Abbott | Poly-L-lactic acid | 2 years | PDLLA | Everolimus | Over a 30-day period, there was an 80% occurrence rate |
| DESolve | Elixir Medical | Poly-L-lactic acid | 1–2 years | PLLA | Myolimus | Over a 30-day period, there was an 80% occurrence rate |
| ReZolve and REVA Gen | REVA Medical | PC | 2 years | - | Sirolimus and paclitaxel | - |
| IDEAL | Xenogenics | Poly-anhydride ester | 200 | 9–12 months | Salicylate | Sirolimus |
| ART 18Z | Arterial Remodeling Technology | Poly-D,L-lactic acid | 18 months | - | - | - |
| Xinsorb BRS | Huaan Biotechnology | PLLA, PCL, PLGA | - | - | Sirolimus | - |
| Amaranth BRS | Amaranth Medical | PLLA | 1 year | - | - | - |
| AMS-1 and AMS-2 | Biotronik | Mg alloy | <4 months and >4 months resp. | - | - | - |
| DREAMS-1 and DREAMS-2 | Biotronik | Mg alloy | 9 months | PLGA and poly-lactic acid | Paclitaxel and sirolimus | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raikar, A.S.; Priya, S.; Bhilegaonkar, S.P.; Somnache, S.N.; Kalaskar, D.M. Surface Engineering of Bioactive Coatings for Improved Stent Hemocompatibility: A Comprehensive Review. Materials 2023, 16, 6940. https://doi.org/10.3390/ma16216940
Raikar AS, Priya S, Bhilegaonkar SP, Somnache SN, Kalaskar DM. Surface Engineering of Bioactive Coatings for Improved Stent Hemocompatibility: A Comprehensive Review. Materials. 2023; 16(21):6940. https://doi.org/10.3390/ma16216940
Chicago/Turabian StyleRaikar, Amisha S., Sushma Priya, Shilpa P. Bhilegaonkar, Sandesh N. Somnache, and Deepak M. Kalaskar. 2023. "Surface Engineering of Bioactive Coatings for Improved Stent Hemocompatibility: A Comprehensive Review" Materials 16, no. 21: 6940. https://doi.org/10.3390/ma16216940
APA StyleRaikar, A. S., Priya, S., Bhilegaonkar, S. P., Somnache, S. N., & Kalaskar, D. M. (2023). Surface Engineering of Bioactive Coatings for Improved Stent Hemocompatibility: A Comprehensive Review. Materials, 16(21), 6940. https://doi.org/10.3390/ma16216940






