SnAg2O3-Coated Adhesive Tape as a Recyclable Catalyst for Efficient Reduction of Methyl Orange
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Chemicals
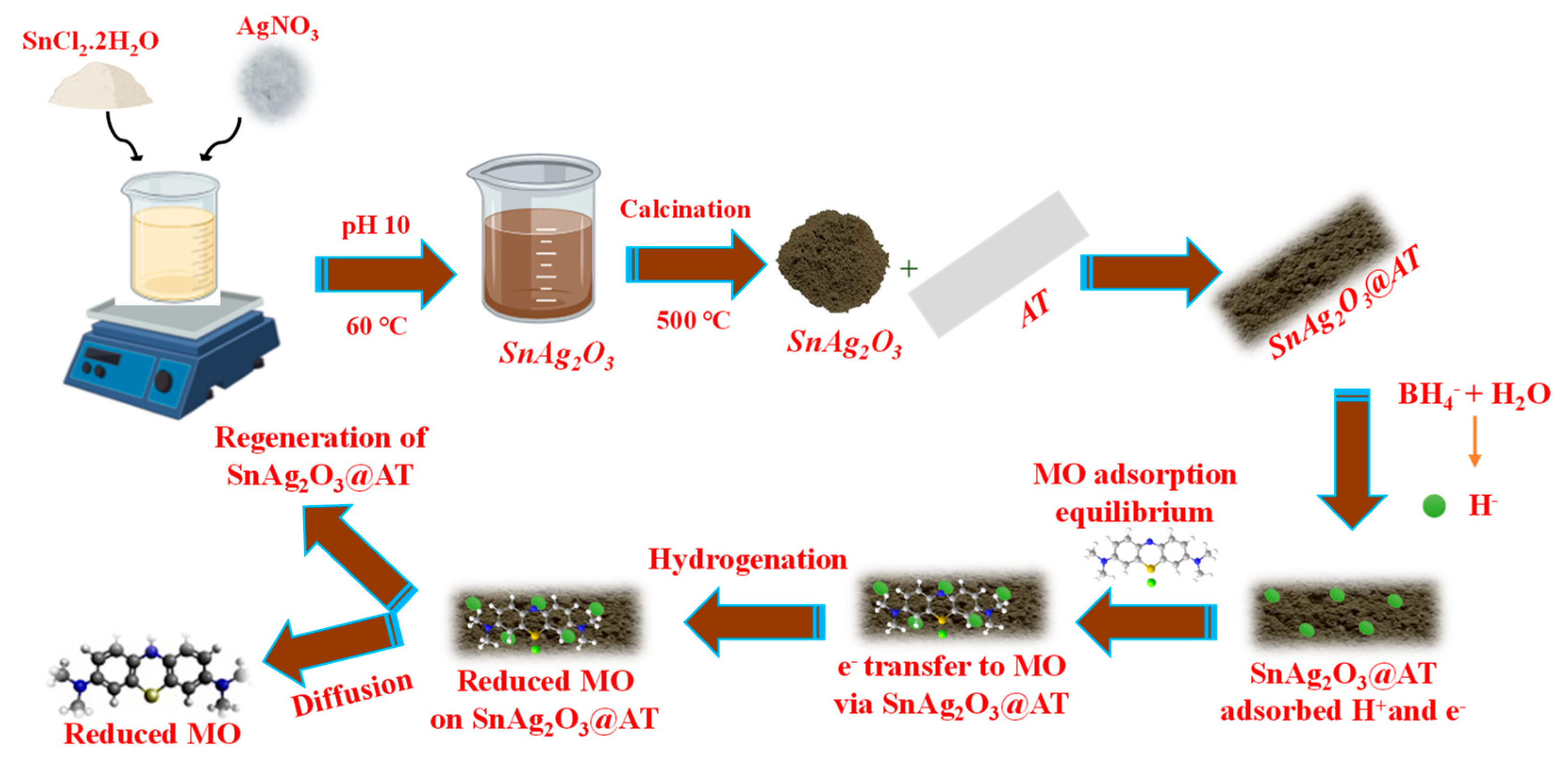
2.2. Preparation of SnAg2O3 and SnAg2O3@AT
2.3. Characterization of SnAg2O3
2.4. Catalytic Studies of SnAg2O3 and SnAg2O3@AT
3. Results and Discussion
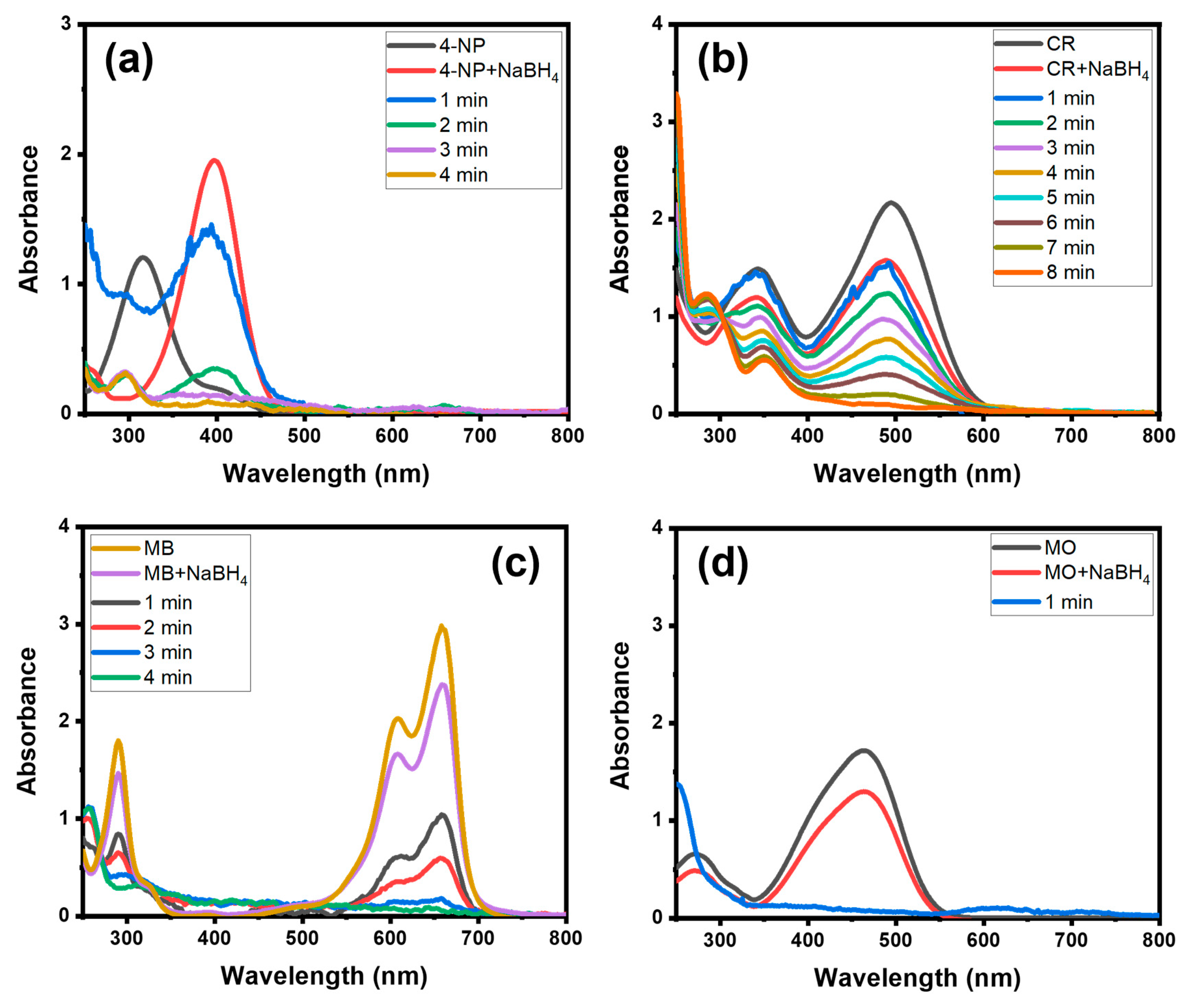
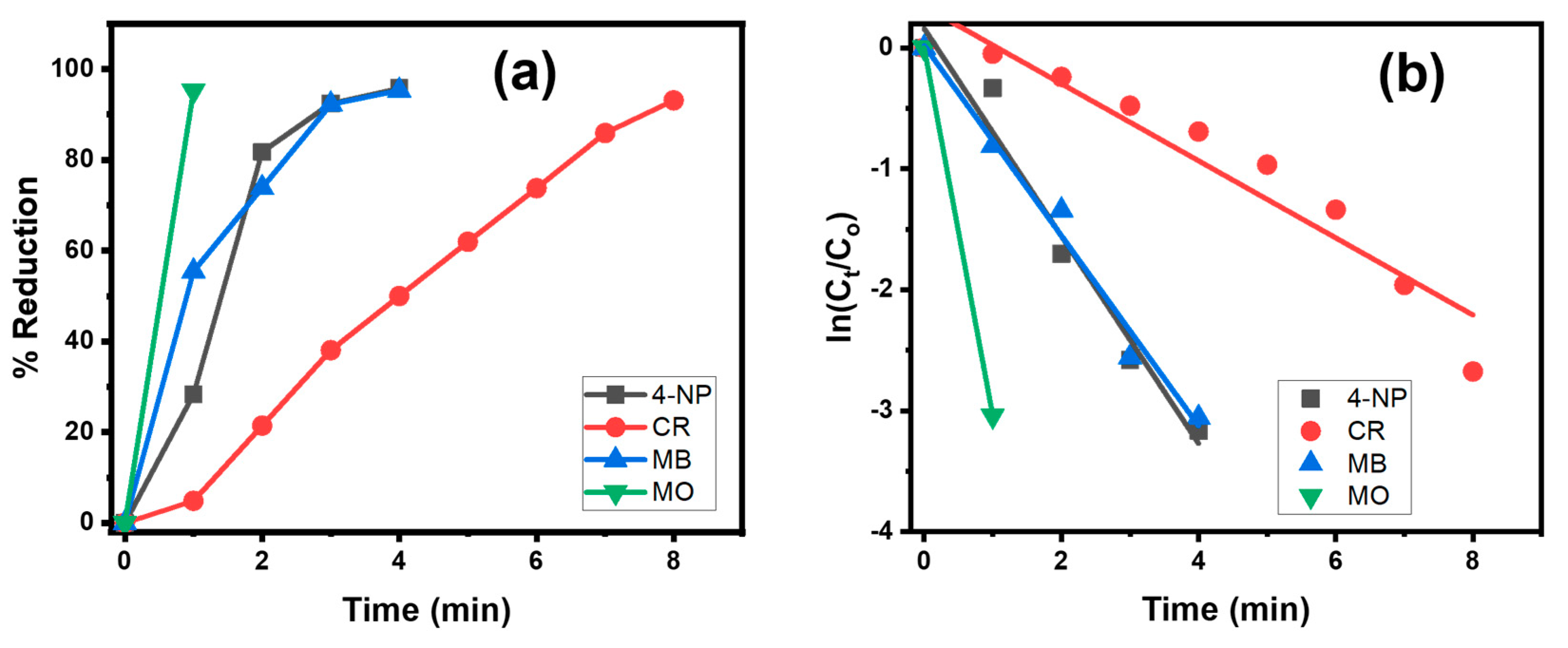
3.1. Catalyst Selection
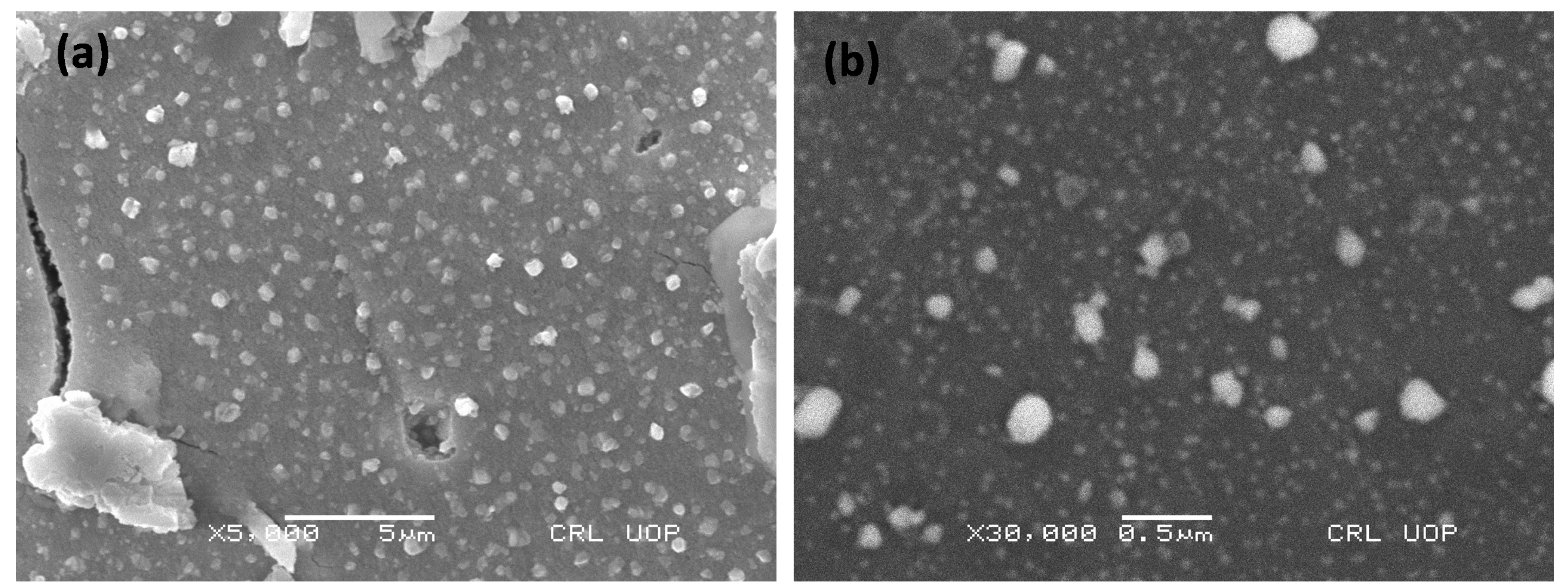
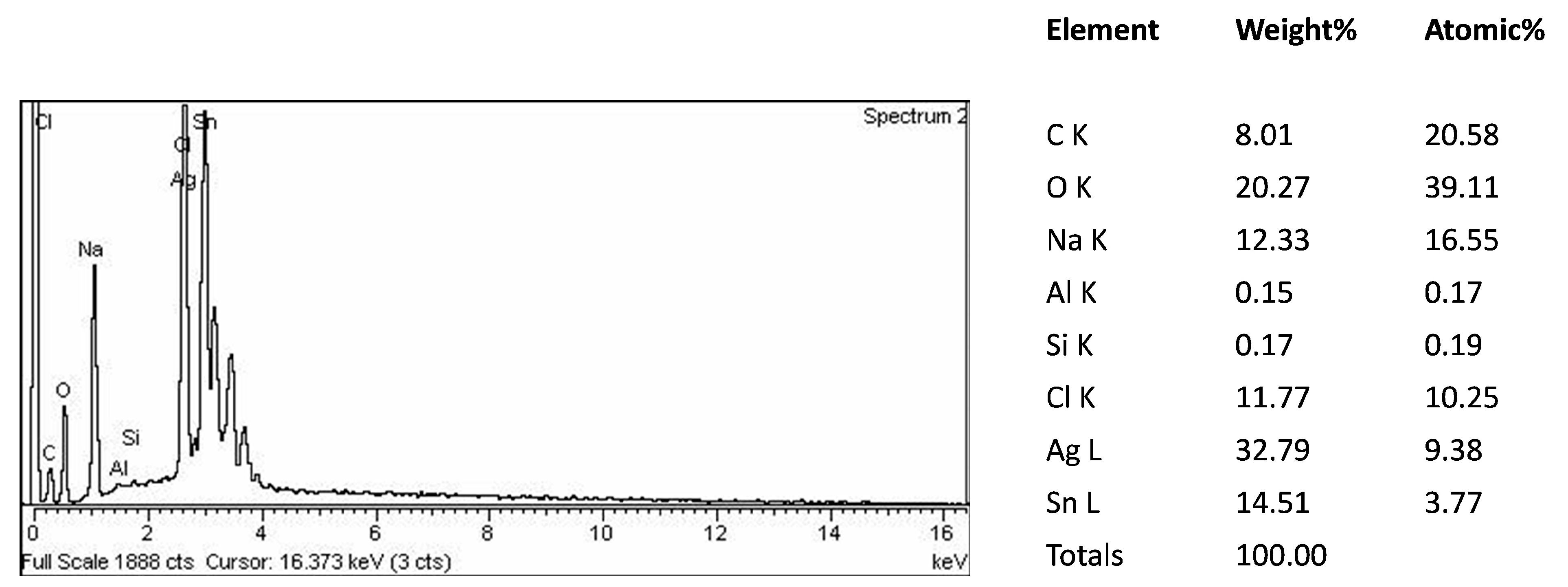
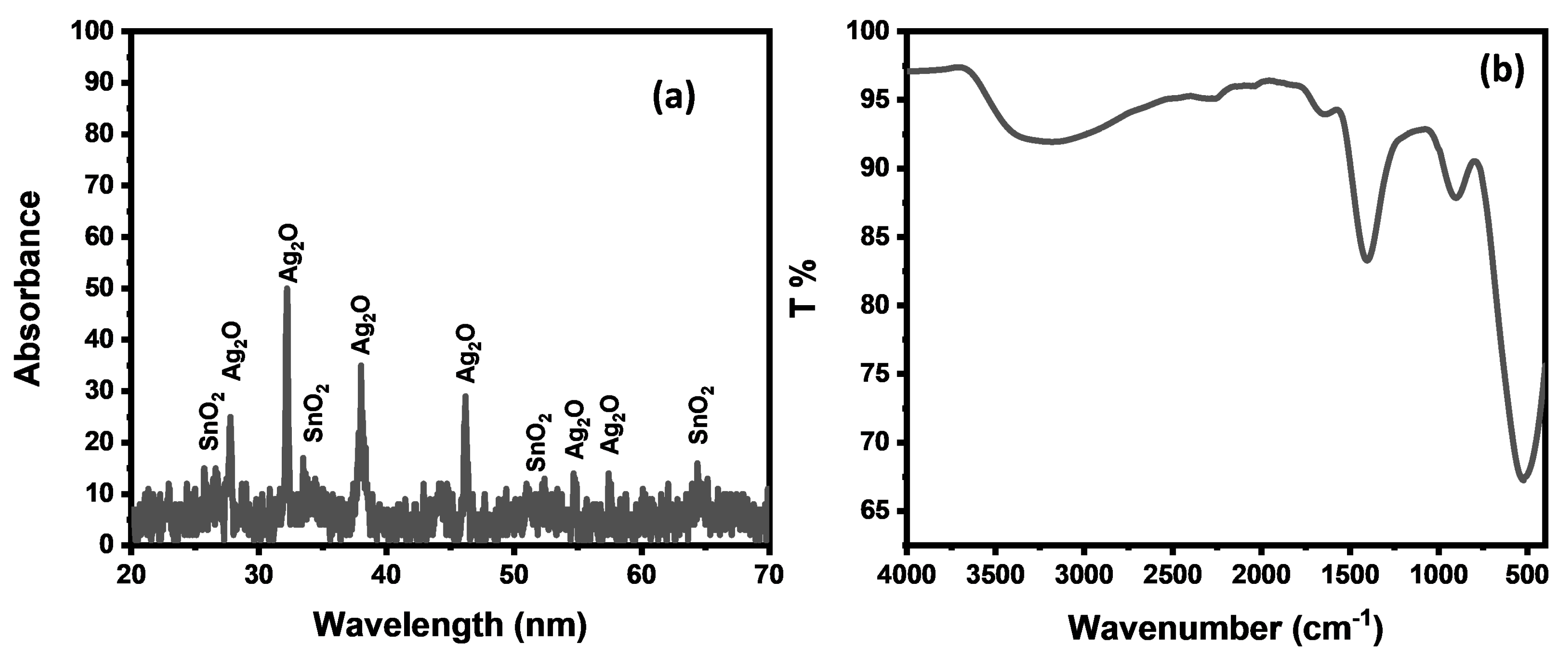
3.2. Physiochemical Characterization of SnAg2O3
4. Catalytic Properties of SnAg2O3
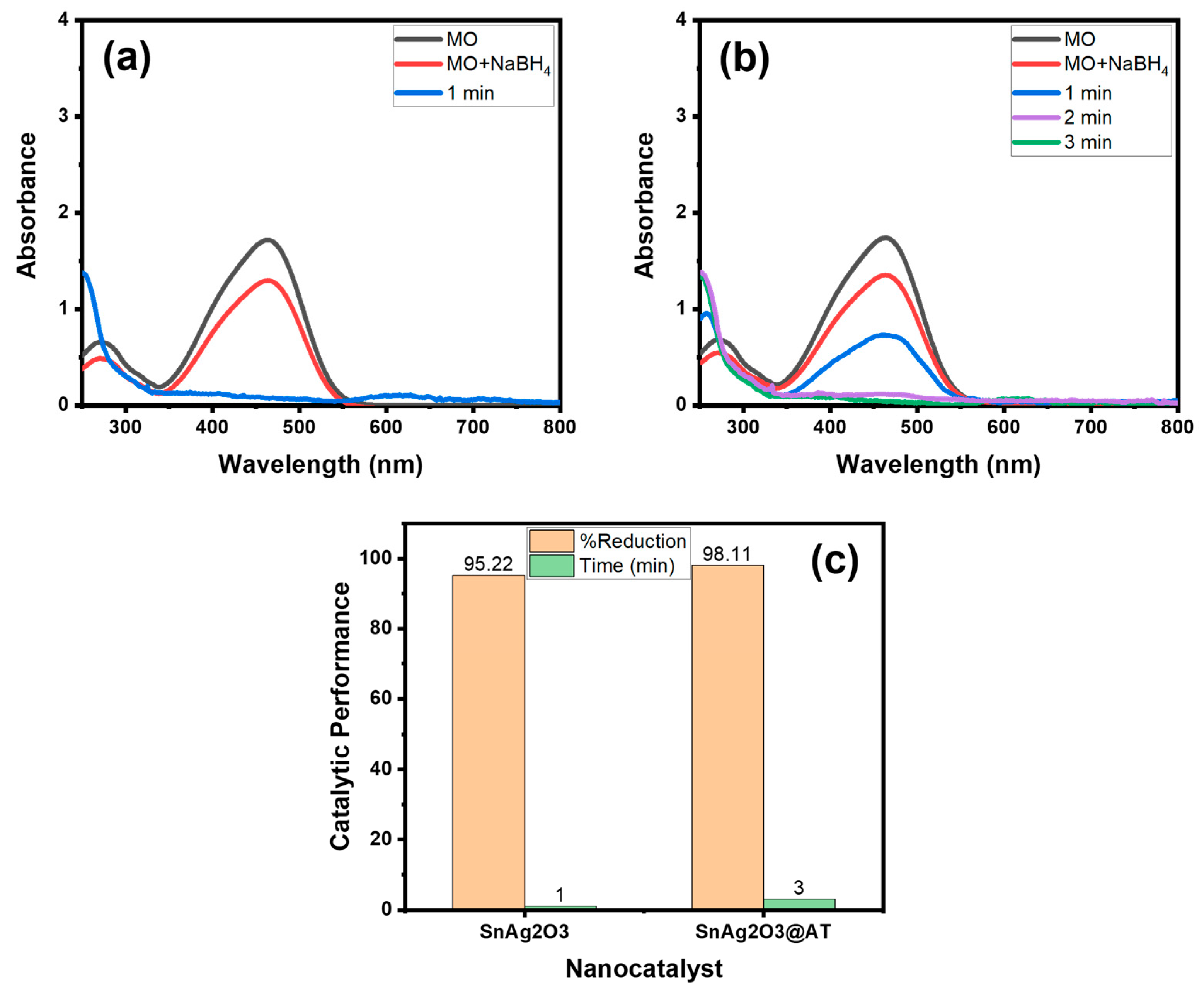
4.1. Catalytic Activity of SnAg2O3@AT toward MO
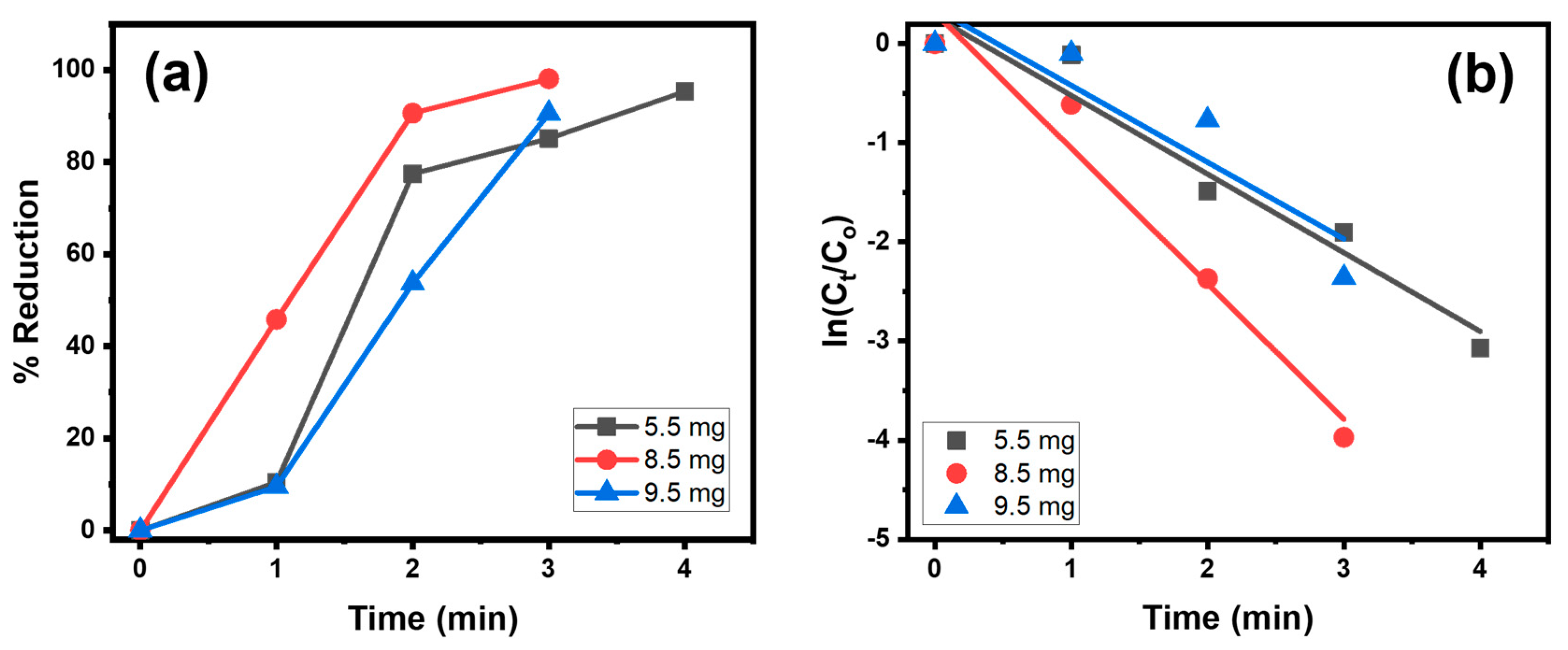
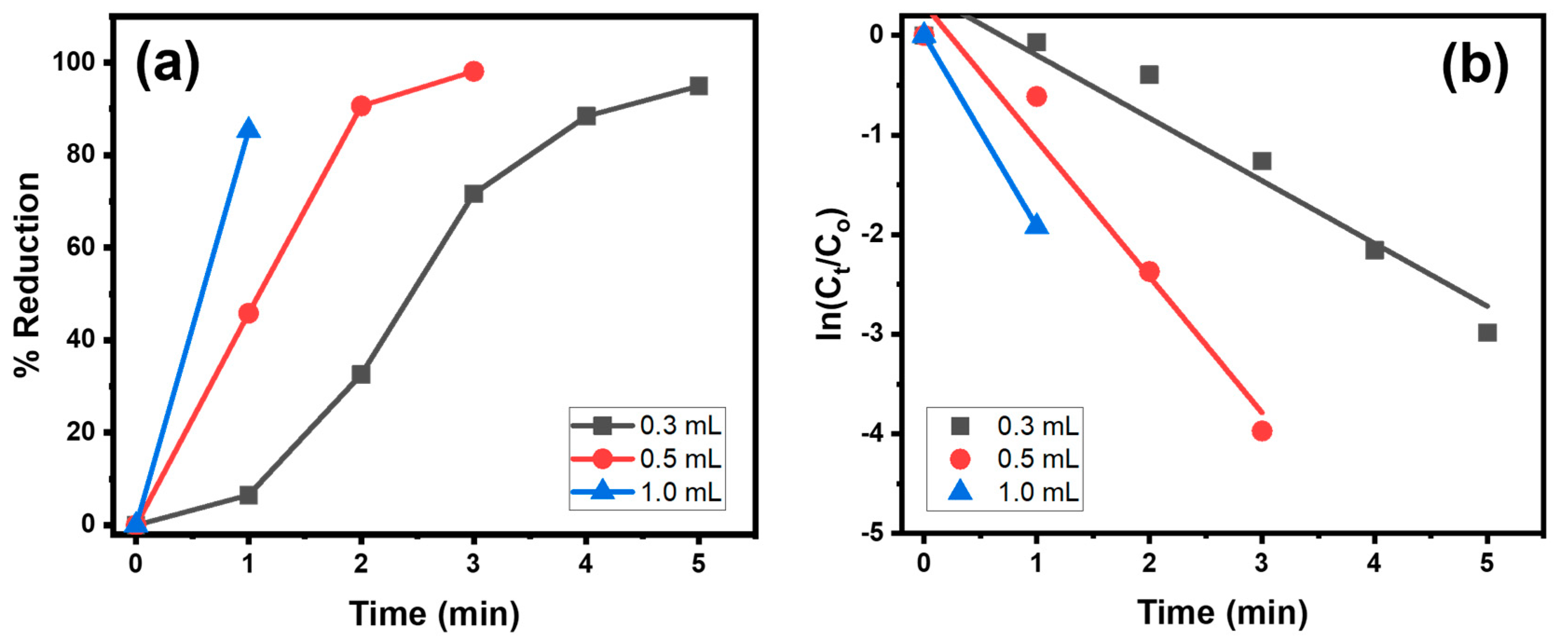
4.1.1. Effect of SnAg2O3@AT Dosage
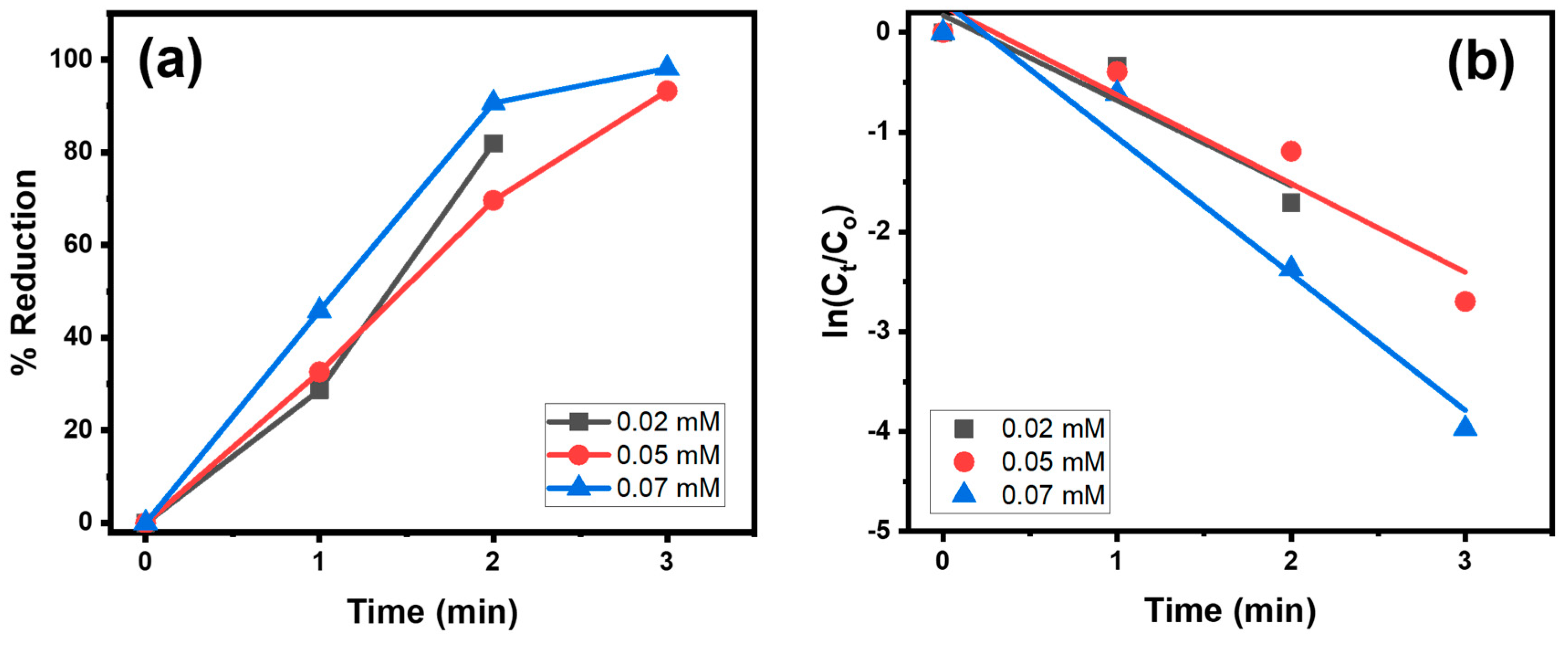
4.1.2. Effect of NaBH4 Concentration
4.1.3. Effect of MO Concentration
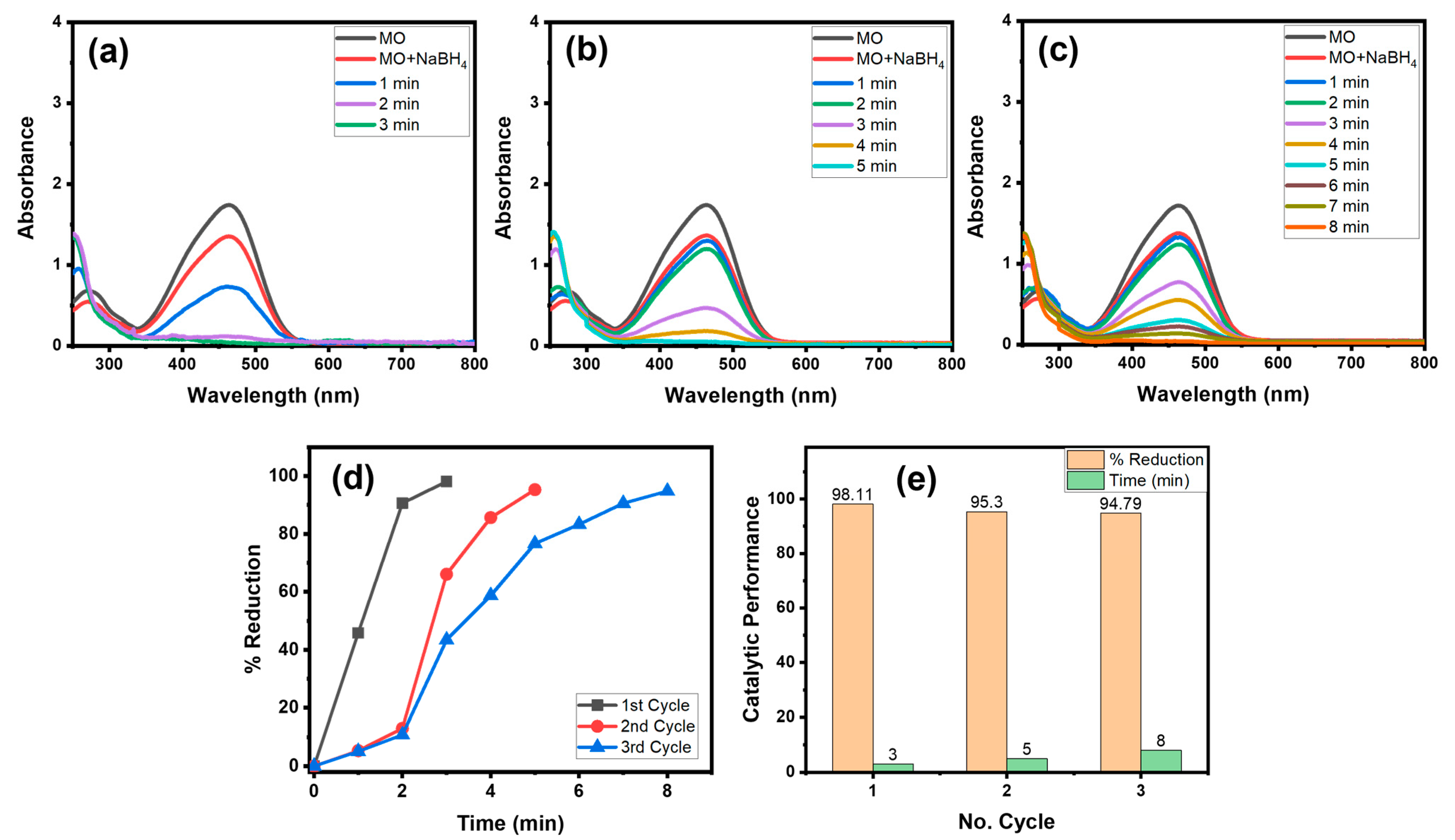
4.1.4. Recyclability
4.1.5. Mechanism of MO Reduction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mabate, T.P.; Maqunga, N.P.; Ntshibongo, S.; Maumela, M.; Bingwa, N. Metal oxides and their roles in heterogeneous catalysis: Special emphasis on synthesis protocols, intrinsic properties, and their influence in transfer hydrogenation reactions. SN Appl. Sci. 2023, 5, 196. [Google Scholar]
- Chen, F.; Jiang, X.; Zhang, L.; Lang, R.; Qiao, B. Single-atom catalysis: Bridging the homo- and heterogeneous catalysis. Chin. J. Catal. 2018, 39, 893–898. [Google Scholar] [CrossRef]
- Guan, Y.; Chaffart, D.; Liu, G.; Tan, Z.; Zhang, D.; Wang, Y.; Li, J.; Ricardez-Sandoval, L. Machine learning in solid heterogeneous catalysis: Recent developments, challenges and perspectives. Chem. Eng. Sci. 2022, 248, 117224. [Google Scholar]
- Melián-Cabrera, I. Catalytic materials: Concepts to understand the pathway to implementation. Ind. Eng. Chem. Res. 2021, 60, 18545–18559. [Google Scholar]
- Pan, Y.; Shen, X.; Yao, L.; Bentalib, A.; Peng, Z. Active sites in heterogeneous catalytic reaction on metal and metal oxide: Theory and Practice. Catalysts 2018, 8, 478. [Google Scholar] [CrossRef]
- Kumar, A.; Belwal, M.; Maurya, R.R.; Mohan, V.; Vishwanathan, V. Heterogeneous catalytic reduction of anthropogenic pollutant, 4-nitrophenol by Au/AC nanocatalysts. Mater. Sci. Energy Technol. 2019, 2, 526–531. [Google Scholar] [CrossRef]
- García-Serna, J.; Piñero-Hernanz, R.; Durán-Martín, D. Inspirational perspectives and principles on the use of catalysts to create sustainability. Catal. Today 2022, 387, 237–243. [Google Scholar] [CrossRef]
- Sulman, E.M.; Matveeva, V.G.; Bronstein, L.M. Design of biocatalysts for efficient catalytic processes. Curr. Opin. Chem. Eng. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Li, Z.; Hong, R.; Zhang, Z.; Wang, H.; Wu, X.; Wu, Z. Single-atom catalysts in environmental engineering: Progress, outlook and challenges. Molecules 2023, 28, 3865. [Google Scholar]
- SKhan, A.; Khan, S.B.; Asiri, A.M. Layered double hydroxide of Cd-Al/C for the mineralization and de-coloration of dyes in solar and visible light exposure. Sci. Rep. 2016, 6, 35107. [Google Scholar]
- Rahman, M.M.; Khan, S.B.; Asiri, A.M.; Alamry, K.A.; Khan, A.A.P.; Khan, A.; Rub, M.A.; Azum, N. Acetone sensor based on solvothermally prepared ZnO doped with Co3O4 nanorods. Microchim. Acta 2013, 180, 675–685. [Google Scholar]
- Védrine, J.C. Importance, features and uses of metal oxide catalysts in heterogeneous catalysis. Chin. J. Catal. 2019, 40, 1627–1636. [Google Scholar]
- Védrine, J.C. Heterogeneous catalysis on metal oxides. Catalysts 2017, 7, 341. [Google Scholar]
- Sohni, S.; Norulaini, N.A.N.; Hashim, R.; Khan, S.B.; Fadhullah, W.; Omar, A.K.M. Physicochemical characterization of Malaysian crop and agro-industrial biomass residues as renewable energy resources. Ind. Crops Prod. 2018, 111, 642–650. [Google Scholar]
- Sharma, R.K.; Bandichhor, R.; Mishra, V.; Sharma, S.; Yadav, S.; Mehta, S.; Arora, B.; Rana, P.; Dutta, S.; Solanki, K. Advanced metal oxide-based nanocatalysts for the oxidative synthesis of fine chemicals. Mater. Adv. 2023, 4, 1795–1830. [Google Scholar]
- Manjunathan, P.; Marakatti, V.S.; Chandra, P.; Kulal, A.B.; Umbarkar, S.B.; Ravishankar, R.; Shanbhag, G.V. Mesoporous tin oxide: An efficient catalyst with versatile applications in acid and oxidation catalysis. Catal. Today 2018, 309, 61–76. [Google Scholar]
- Bakhsh, E.M.; Akhtar, K.; Fagieh, T.M.; Asiri, A.M.; Khan, S.B. Sodium alginate nanocomposite based efficient system for the removal of organic and inorganic pollutants from wastewater. Int. J. Biol. Macromol. 2021, 191, 243–254. [Google Scholar]
- Bakhsh, E.M.; Akhtar, K.; Fagieh, T.M.; Khan, S.B.; Asiri, A.M. Development of alginate@tin oxide–cobalt oxide nanocomposite based catalyst for the treatment of wastewater. Int. J. Biol. Macromol. 2021, 187, 386–398. [Google Scholar]
- Maurya, A.; Yadav, M. Sphere shaped Mo-doped transition metal oxide as electrocatalyst for oxygen evolution reaction in alkaline medium. J. Alloys Compd. 2023, 956, 170208. [Google Scholar]
- Wang, L.; Zhou, S.; You, M.; Yu, D.; Zhang, C.; Gao, S.; Yu, X.; Zhao, Z. Research progress on metal oxides for the selective catalytic reduction of NOx with ammonia. Catalysts 2023, 13, 1086. [Google Scholar]
- Lavrynenko, O.M.; Zahornyi, M.M.; Vember, V.V.; Pavlenko, O.Y.; Lobunets, T.F.; Kolomys, O.F.; Povnitsa, O.Y.; Artiukh, L.O.; Naumenko, K.S.; Zahorodnia, S.D.; et al. Nanocomposites based on cerium, lanthanum, and titanium oxides doped with silver for biomedical application. Condens. Matter 2022, 7, 45. [Google Scholar] [CrossRef]
- Choudhari, U.; Jagtap, S. Lanthanum doped tin oxide: Synthesis, characterization and application. Mater. Today Proc. 2022, 49, 3325–3330. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Akbari, R.; Sakhaei, S.; Nezafat, Z.; Banazadeh, S.; Orooji, Y.; Hegde, G. Polymer supported copper complexes/nanoparticles for treatment of environmental contaminants. J. Mol. Liq. 2021, 330, 115668. [Google Scholar]
- Sarkar, S.; Guibal, E.; Quignard, F.; SenGupta, A.K. Polymer-supported metals and metal oxide nanoparticles: Synthesis, characterization, and applications. J. Nanoparticle Res. 2012, 14, 715. [Google Scholar]
- Khan, S.A.; Bakhsh, E.M.; Akhtar, K.; Khan, S.B. A template of cellulose acetate polymer-ZnAl/C layered double hydroxide composite fabricated with Ni NPs: Applications in the hydrogenation of nitrophenols and dyes degradation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118671. [Google Scholar] [CrossRef]
- Das, T.K.; Jesionek, M.; Çelik, Y.; Poater, A. Catalytic polymer nanocomposites for environmental remediation of wastewater. Sci. Total Environ. 2023, 901, 165772. [Google Scholar]
- Benali, F.; Boukoussa, B.; Benkhedouda, N.-E.-H.; Cheddad, A.; Issam, I.; Iqbal, J.; Hachemaoui, M.; Abboud, M.; Mokhtar, A. Catalytic Reduction of Dyes and Antibacterial Activity of AgNPs@Zn@Alginate Composite Aerogel Beads. Polymers 2022, 14, 4829. [Google Scholar]
- Mallakpour, S.; Azadi, E.; Dinari, M. Mesoporous Ca-alginate/melamine-rich covalent organic polymer/cupric oxide-based microgel beads as heterogeneous catalyst for efficient catalytic reduction of hazardous water pollutants. J. Environ. Chem. Eng. 2023, 11, 109294. [Google Scholar]
- Meena, P.L.; Saini, J.K. Synthesis of polymer-metal oxide (PANI/ZnO/MnO2) ternary nanocomposite for effective removal of water pollutants. Results Chem. 2023, 5, 100764. [Google Scholar]
- Naghash-Hamed, S.; Arsalani, N.; Mousavi, S.B. The catalytic reduction of nitroanilines using synthesized CuFe2O4 nanoparticles in an aqueous medium. ChemistryOpen 2022, 11, e202200156. [Google Scholar] [CrossRef]
- Nagaraj, K.; Thangamuniyandi, P.; Kamalesu, S.; Dixitkumar, M.; Saini, A.K.; Sharma, S.K.; Naman, J.; Priyanshi, J.; Uthra, C.; Lokhandwala, S.; et al. Silver nanoparticles using Cassia Alata and its catalytic reduction activities of rhodamine6G, methyl orange and methylene blue dyes. Inorg. Chem. Commun. 2023, 155, 110985. [Google Scholar] [CrossRef]
- Naz, M.; Rafiq, A.; Ikram, M.; Haider, A.; Ahmad, S.O.A.; Haider, J.; Naz, S. Elimination of dyes by catalytic reduction in the absence of light: A review. J. Mater. Sci. 2021, 56, 15572–15608. [Google Scholar] [CrossRef]
- Dhoondia, Z.H.; Chakraborty, H. Lactobacillus mediated synthesis of silver oxide nanoparticles. Nanomater. Nanotechnol. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Li, J.; Li, S.; Dong, C. Synthesis of tin-glycerate and it conversion into SnO2 spheres for highly sensitive low-ppm-level acetone detection. J. Mater. Sci. Mater. Electron. 2020, 31, 16539–16547. [Google Scholar] [CrossRef]
- Pervez, M.N.; He, W.; Zarra, T.; Naddeo, V.; Zhao, Y. New sustainable approach for the production of Fe3O4/graphene oxide-activated persulfate system for dye removal in real wastewater. Water 2020, 12, 733. [Google Scholar] [CrossRef]
- Heck, K.N.; Wang, Y.; Wu, G.; Wang, F.; Tsai, A.-L.; Adamson, D.T.; Wong, M.S. Effectiveness of metal oxide catalysts for the degradation of 1,4-dioxane. RSC Adv. 2019, 9, 27042–27049. [Google Scholar] [CrossRef]
- Hou, J.; Si, L.; Shi, Z.; Miao, C.; Zhao, Y.; Ji, X.; Hou, Q.; Ai, S. Effective adsorption and catalytic reduction of nitrophenols by amino-rich Cu(I)–I coordination polymer. Chemosphere 2023, 311, 136903. [Google Scholar] [CrossRef]
- Umamaheswari, C.; Lakshmanan, A.; Nagarajan, N.S. Green synthesis, characterization and catalytic degradation studies of gold nanoparticles against congo red and methyl orange. J. Photochem. Photobiol. B Biol. 2018, 178, 33–39. [Google Scholar] [CrossRef]
- Narasaiah, B.P.; Mandal, B.K. Remediation of azo-dyes based toxicity by agro-waste cotton boll peels mediated palladium nanoparticles. J. Saudi Chem. Soc. 2020, 24, 267–281. [Google Scholar] [CrossRef]
- Sherin, L.; Sohail, A.; Amjad, U.-E.-S.; Mustafa, M.; Jabeen, R.; Ul-Hamid, A. Facile green synthesis of silver nanoparticles using Terminalia bellerica kernel extract for catalytic reduction of anthropogenic water pollutants. Colloid Interface Sci. Commun. 2020, 37, 100276. [Google Scholar] [CrossRef]
- Ali, H.S.H.M.; Khan, S.A. Stabilization of various zero-valent metal nanoparticles on a superabsorbent polymer for the removal of dyes, nitrophenol, and pathogenic bacteria. ACS Omega 2020, 5, 7379–7391. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Abdeta, A.B.; Kuo, D.-H.; Wu, Q.; Guo, Y.; Zelekew, O.A.; Yuan, Z.; Lin, J.; Chen, X. Activated carbon supported CuSnOS catalyst with an efficient catalytic reduction of pollutants under dark condition. J. Mol. Liq. 2021, 334, 116079. [Google Scholar] [CrossRef]
- Fagieh, T.M.; Bakhsh, E.M.; Khan, S.B.; Akhtar, K.; Asiri, A.M. Alginate/banana waste beads supported metal nanoparticles for efficient water remediation. Polymers 2021, 13, 4054. [Google Scholar] [CrossRef] [PubMed]
- Naseem, K.; Ali, F.; Tahir, M.H.; Afaq, M.; Yasir, H.M.; Ahmed, K.; Aljuwayid, A.M.; Habila, M.A. Investigation of catalytic potential of sodium dodecyl sulfate stabilized silver nanoparticles for the degradation of methyl orange dye. J. Mol. Struct. 2022, 1262, 132996. [Google Scholar] [CrossRef]
- Jones, C.W. On the stability and recyclability of supported metal–ligand complex catalysts: Myths, misconceptions and critical research needs. Top. Catal. 2010, 53, 942–952. [Google Scholar] [CrossRef]
- Shende, V.S.; Saptal, V.B.; Bhanage, B.M. Recent advances utilized in the recycling of homogeneous catalysis. Chem. Rec. 2019, 19, 2022–2043. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.; Frontera, P.; Macario, A.; Malara, A. Recovery/reuse of heterogeneous supported spent catalysts. Catalysts 2021, 11, 591. [Google Scholar] [CrossRef]
| Catalyst | Rate Constant (min−1) | Reference |
|---|---|---|
| Alg-tin oxide–cobalt oxide | 1.2775 | [18] |
| AuNPs | 0.1020 | [38] |
| Pd NPs | 0.0909 | [39] |
| K-AgNPs | 0.0286 | [40] |
| WBs loaded with Ni NPs | 0.1500 | [41] |
| CuSnOS/AC | 1.1480 | [42] |
| Cu@Alg/BP | 0.2702 | [43] |
| Sd@Ag-NPs | 0.3850 | [44] |
| SnAg2O3 | 3.0412 | This Study |
| SnAg2O3@AT | 1.3669 | This Study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, K.; Alhaj, A.A.; Bakhsh, E.M.; Khan, S.B.; Fagieh, T.M. SnAg2O3-Coated Adhesive Tape as a Recyclable Catalyst for Efficient Reduction of Methyl Orange. Materials 2023, 16, 6978. https://doi.org/10.3390/ma16216978
Akhtar K, Alhaj AA, Bakhsh EM, Khan SB, Fagieh TM. SnAg2O3-Coated Adhesive Tape as a Recyclable Catalyst for Efficient Reduction of Methyl Orange. Materials. 2023; 16(21):6978. https://doi.org/10.3390/ma16216978
Chicago/Turabian StyleAkhtar, Kalsoom, Asma A. Alhaj, Esraa M. Bakhsh, Sher Bahadar Khan, and Taghreed M. Fagieh. 2023. "SnAg2O3-Coated Adhesive Tape as a Recyclable Catalyst for Efficient Reduction of Methyl Orange" Materials 16, no. 21: 6978. https://doi.org/10.3390/ma16216978
APA StyleAkhtar, K., Alhaj, A. A., Bakhsh, E. M., Khan, S. B., & Fagieh, T. M. (2023). SnAg2O3-Coated Adhesive Tape as a Recyclable Catalyst for Efficient Reduction of Methyl Orange. Materials, 16(21), 6978. https://doi.org/10.3390/ma16216978





