Study of β-Ga2O3 Ceramics Synthesized under Powerful Electron Beam
Abstract
:1. Introduction
1.1. Main Properties and Application
1.2. Rare-Earth Doping β-Ga2O3
1.3. Methods of Synthesis
1.4. Electron-Beam Assisted Synthesis
2. Materials and Methods
3. Results
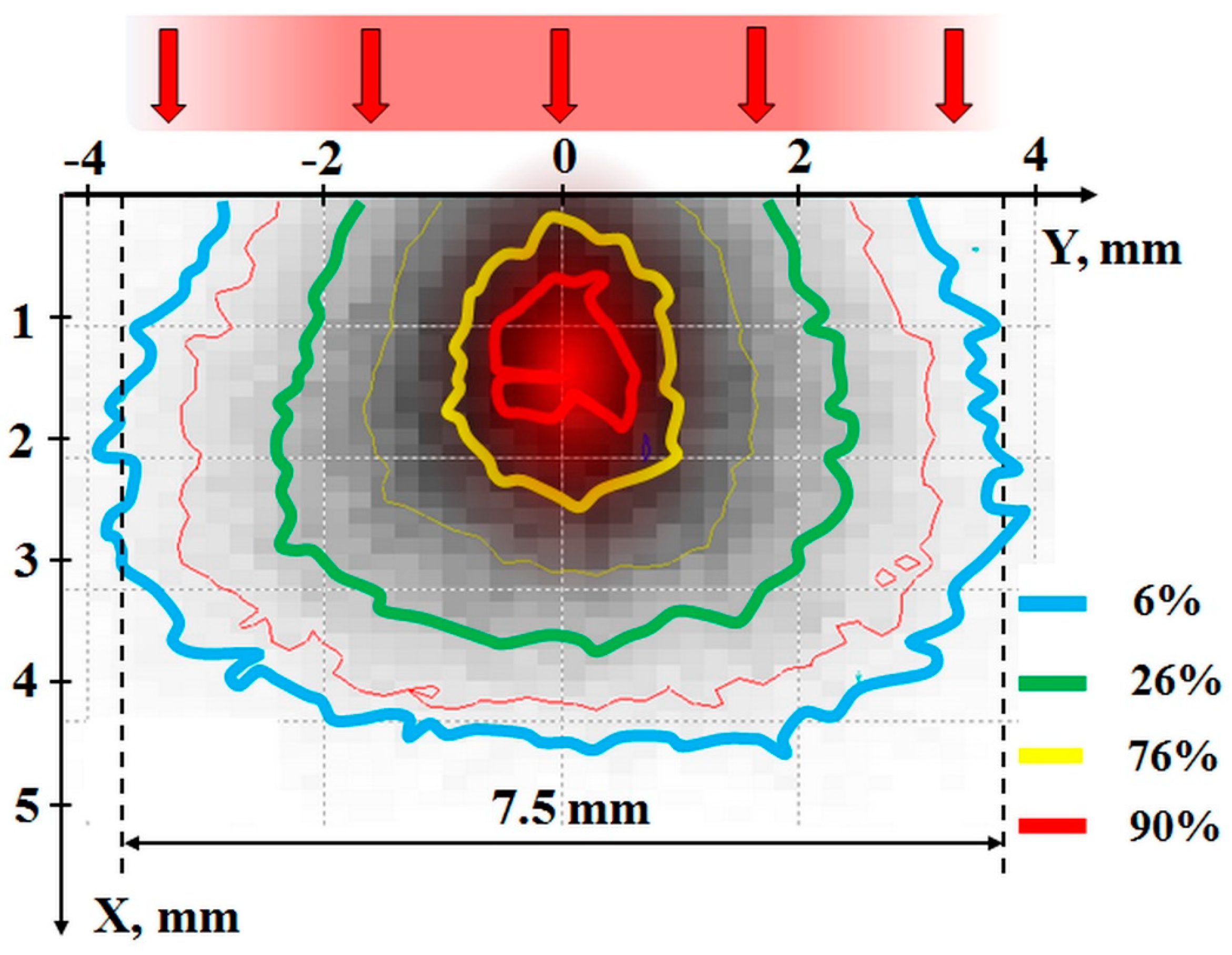
3.1. Assessment and Analysis of Electron Energy Loss Distribution in a Substance upon Irradiation
3.2. XRD Analysis
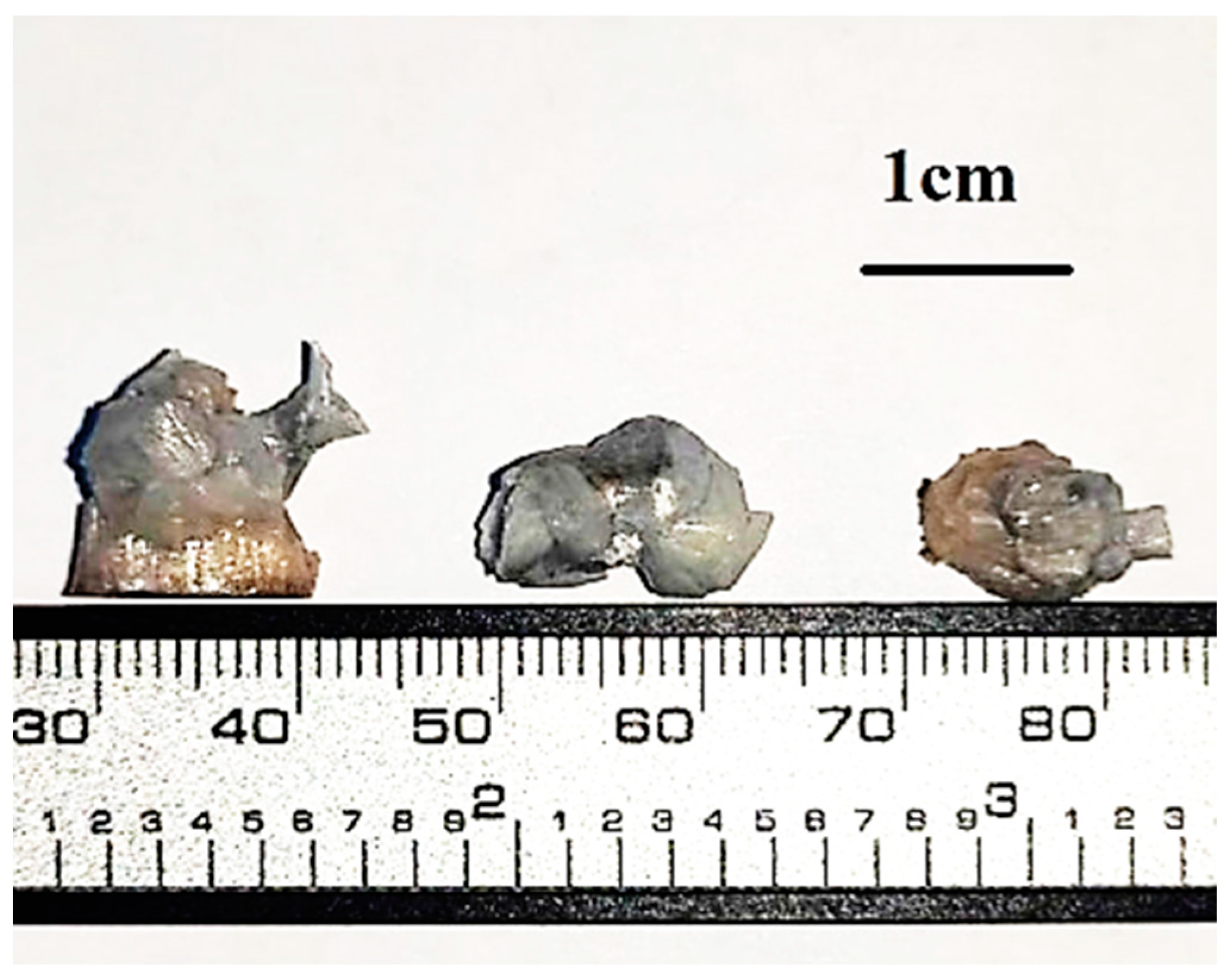
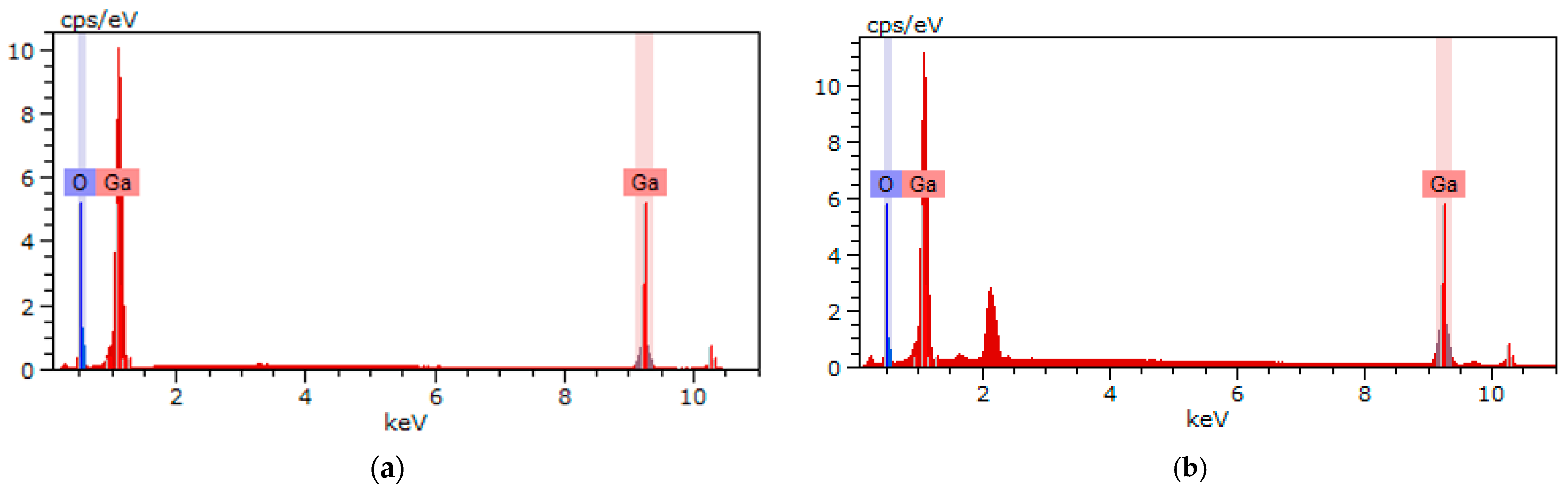
3.3. Surface Morphology and Elemental Composition
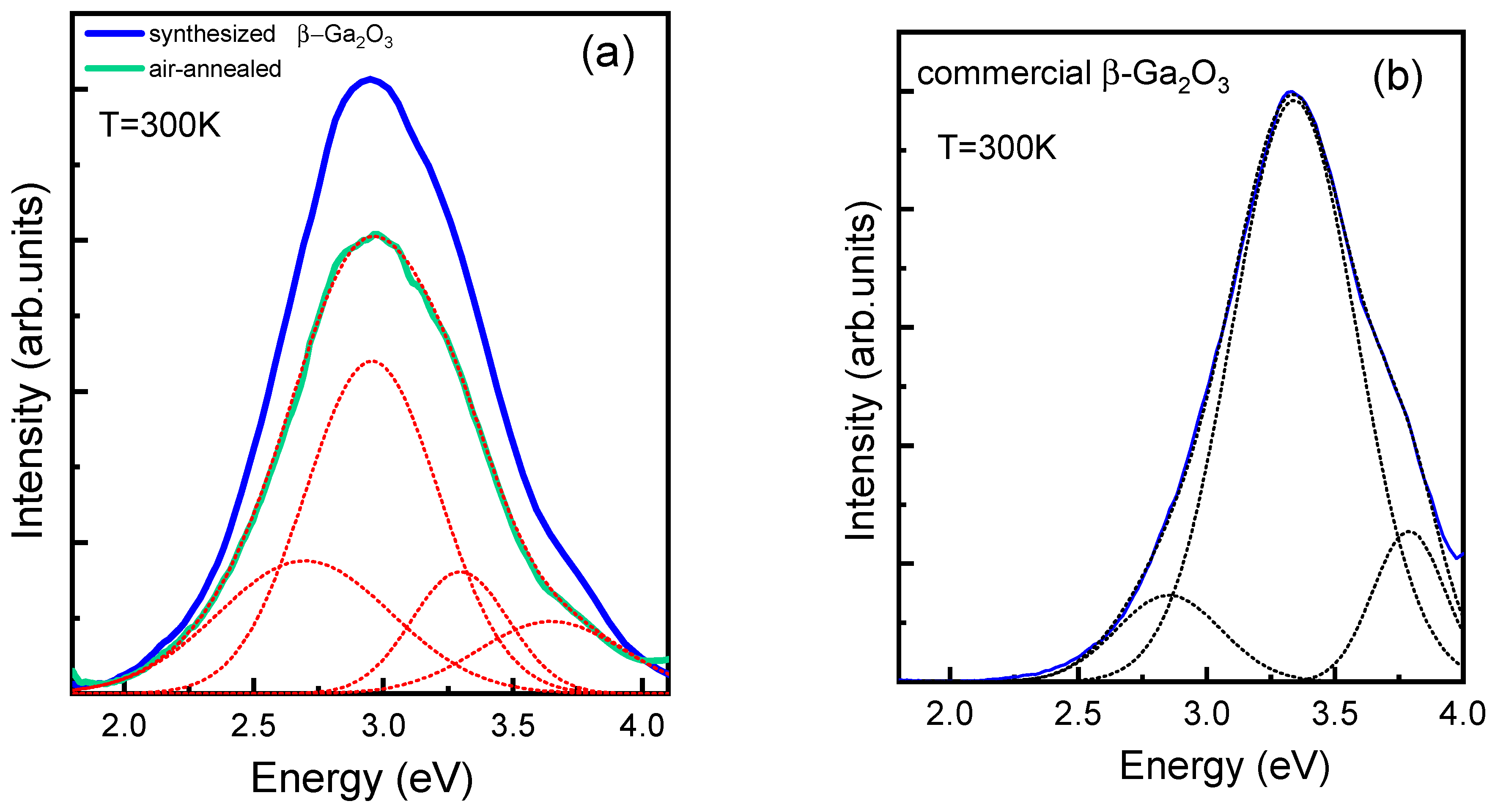
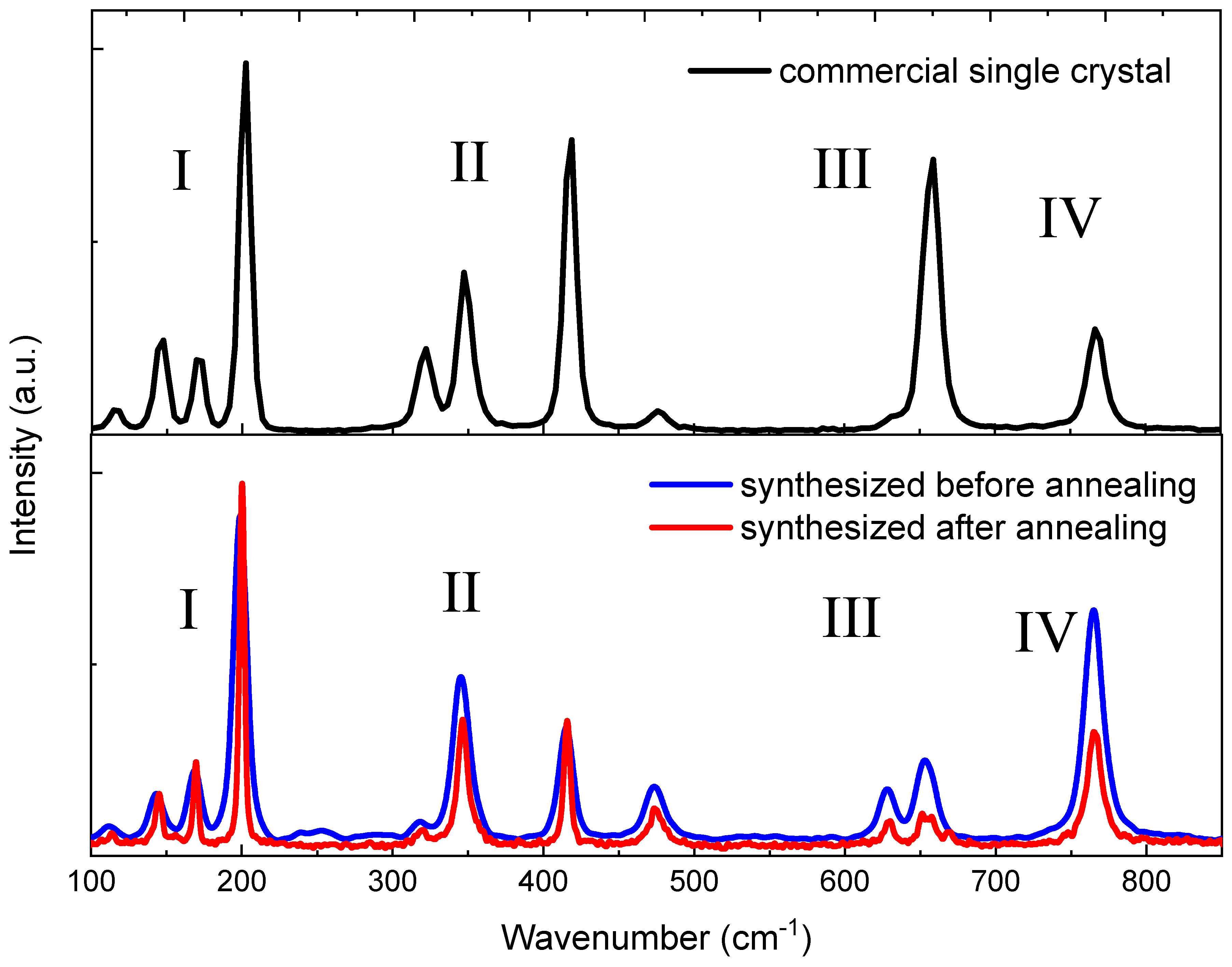
3.4. Luminescence Spectra of Sintered and Commercial β-Ga2O3 Ceramic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stepanov, S.I.; Nikolaev, V.I.; Bougrov, V.E.; Romanov, A.E. Gallium Oxide: Properties and Applications—A Review. Rev. Adv. Mater. Sci. 2016, 44, 63–86. [Google Scholar]
- Nikolaev, V.I.; Maslov, V.; Stepanov, S.I.; Pechnikov, A.I.; Krymov, V.; Nikitina, I.P.; Guzilova, L.I.; Bougrov, V.E.; Romanov, A.E. Growth and Characterization of β-Ga2O3 Crystals. J. Cryst. Growth 2017, 457, 132–136. [Google Scholar] [CrossRef]
- Mastro, M.A.; Kuramata, A.; Calkins, J.; Kim, J.; Ren, F.; Pearton, S.J. Perspective—Opportunities and Future Directions for Ga2O3. ECS J. Solid State Sci. Technol. 2017, 6, 356–359. [Google Scholar] [CrossRef]
- von Wenckstern, H. Group-III Sesquioxides: Growth, Physical Properties and Devices. Adv. Electron. Mater. 2017, 3, 1600350. [Google Scholar] [CrossRef]
- Orita, M.; Ohta, H.; Hirano, M.; Hosono, H. Deep-Ultraviolet Transparent Conductive β-Ga2O3 Thin Films. Appl. Phys. Lett. 2000, 77, 4166–4168. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Varley, J.B.; Schleife, A. Bethe–Salpeter Calculation of Optical-Absorption Spectra of In2O3 and Ga2O3. Semicond. Sci. Technol. 2015, 30, 024010. [Google Scholar] [CrossRef]
- Tadjer, M.J.; Mahadik, N.A.; Wheeler, V.D.; Glaser, E.R.; Ruppalt, L.; Koehler, A.D.; Hobart, K.D.; Eddy, C.R.; Kub, F.J. Editors’ Choice Communication—A (001) β-Ga2O3 MOSFET with +2.9 V Threshold Voltage and HfO2 Gate Dielectric. ECS J. Solid State Sci. Technol. 2016, 5, P468. [Google Scholar] [CrossRef]
- Usui, Y.; Oya, T.; Okada, G.; Kawaguchi, N.; Yanagida, T. Ce-Doped Ga2O3 Single Crystalline Semiconductor Showing Scintillation Features. Optik 2017, 143, 150–157. [Google Scholar] [CrossRef]
- Drozdowski, W.; Makowski, M.; Witkowski, M.E.; Wojtowicz, A.J.; Galazka, Z.; Irmscher, K.; Schewski, R. β-Ga2O3:Ce as a Fast Scintillator: An Unclear Role of Cerium. Radiat. Meas. 2019, 121, 49–53. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Zhi, Y.; Zhang, X.; Chen, Z.; Chu, X.; Yang, H.; Wu, Z.; Tang, W. Fabrication of Cerium-Doped β-Ga2O3 Epitaxial Thin Films and Deep Ultraviolet Photodetectors. Appl. Opt. 2018, 57, 538. [Google Scholar] [CrossRef] [PubMed]
- Luchechko, A.; Vasyltsiv, V.; Zhydachevskyy, Y.; Kushlyk, M.; Ubizskii, S.; Suchocki, A. Luminescence Spectroscopy of Cr3+ Ions in Bulk Single Crystalline β-Ga2O3. J. Phys. D Appl. Phys. 2020, 53, 354001. [Google Scholar] [CrossRef]
- Blevins, J.; Yang, G. On Optical Properties and Scintillation Performance of Emerging Ga2O3: Crystal Growth, Emission Mechanisms and Doping Strategies. Mater. Res. Bull. 2021, 144, 111494. [Google Scholar] [CrossRef]
- Nakazawa, A.; Yasukawa, D.; Wakai, H.; Oda, H.; Yamanaka, A. Time-Resolved Spectroscopy of Luminescence in Cu- and Cr-Doped β-Ga2O3. Phys. Status Solidi 2013, 10, 1584–1587. [Google Scholar] [CrossRef]
- Galazka, Z. β-Ga2O3 for Wide-Bandgap Electronics and Optoelectronics. Semicond. Sci. Technol. 2018, 33, 113001. [Google Scholar] [CrossRef]
- Sawada, K.; Nakamura, T.; Adachi, S. Abnormal Photoluminescence Phenomena in (Tb3+, Eu3+) Codoped Ga2O3 Phosphor. J. Alloys Compd. 2016, 678, 448–455. [Google Scholar] [CrossRef]
- Chen, Z.; Saito, K.; Tanaka, T.; Nishio, M.; Arita, M.; Guo, Q. Low Temperature Growth of Europium Doped Ga2O3 Luminescent Films. J. Cryst. Growth 2015, 430, 28–33. [Google Scholar] [CrossRef]
- Li, W.; Peng, Y.; Wang, C.; Zhao, X.; Zhi, Y.; Yan, H.; Li, L.; Li, P.; Yang, H.; Wu, Z.; et al. Structural, Optical and Photoluminescence Properties of Pr-Doped β-Ga2O3 Thin Films. J. Alloys Compd. 2017, 697, 388–391. [Google Scholar] [CrossRef]
- Xia, N.; Liu, Y.; Wu, D.; Li, L.; Ma, K.; Wang, J.; Zhang, H.; Yang, D. β-Ga2O3 Bulk Single Crystals Grown by a Casting Method. J. Alloys Compd. 2023, 935, 168036. [Google Scholar] [CrossRef]
- Aida, H.; Nishiguchi, K.; Takeda, H.; Aota, N.; Sunakawa, K.; Yaguchi, Y. Growth of β-Ga2O3 Single Crystals by the Edge-Defined, Film Fed Growth Method. Jpn. J. Appl. Phys. 2008, 47, 8506–8509. [Google Scholar] [CrossRef]
- Galazka, Z.; Uecker, R.; Irmscher, K.; Albrecht, M.; Klimm, D.; Pietsch, M.; Brützam, M.; Bertram, R.; Ganschow, S.; Fornari, R. Czochralski Growth and Characterization of β-Ga2O3 Single Crystals. Cryst. Res. Technol. 2010, 45, 1229–1236. [Google Scholar] [CrossRef]
- Galazka, Z. Growth of Bulk β-Ga2O3 Single Crystals by the Czochralski Method. J. Appl. Phys. 2022, 131, 031103. [Google Scholar] [CrossRef]
- Hoshikawa, K.; Ohba, E.; Kobayashi, T.; Yanagisawa, J.; Miyagawa, C.; Nakamura, Y. Growth of β-Ga2O3 Single Crystals Using Vertical Bridgman Method in Ambient Air. J. Cryst. Growth 2016, 447, 36–41. [Google Scholar] [CrossRef]
- Wakabayashi, R.; Oshima, T.; Hattori, M.; Sasaki, K.; Masui, T.; Kuramata, A.; Yamakoshi, S.; Yoshimatsu, K.; Ohtomo, A. Oxygen-Radical-Assisted Pulsed-Laser Deposition of β-Ga2O3 and β-(AlxGa1−x)2O3 Films. J. Cryst. Growth 2015, 424, 77–79. [Google Scholar] [CrossRef]
- Kang, B.K.; Mang, S.R.; Lim, H.D.; Song, K.M.; Song, Y.H.; Go, D.H.; Jung, M.K.; Senthil, K.; Yoon, D.H. Synthesis, Morphology and Optical Properties of Pure and Eu3+ Doped β-Ga2O3 Hollow Nanostructures by Hydrothermal Method. Mater. Chem. Phys. 2014, 147, 178–183. [Google Scholar] [CrossRef]
- Fu, B.; Mu, W.; Li, Y.; Shi, Y.; Li, Y.; Jia, Z.; Tao, X. Investigation of the Blue Color Center in β-Ga2O3 Crystals by the EFG Method. CrystEngComm 2021, 23, 8360–8366. [Google Scholar] [CrossRef]
- Meng, L.; Feng, Z.; Bhuiyan, A.F.M.A.U.; Zhao, H. High-Mobility MOCVD β-Ga2O3 Epitaxy with Fast Growth Rate Using Trimethylgallium. Cryst. Growth Des. 2022, 22, 3896–3904. [Google Scholar] [CrossRef]
- Vogt, P.; Hensling, F.V.E.; Azizie, K.; Chang, C.S.; Turner, D.; Park, J.; McCandless, J.P.; Paik, H.; Bocklund, B.J.; Hoffman, G.; et al. Adsorption-Controlled Growth of Ga2O3 by Suboxide Molecular-Beam Epitaxy. APL Mater. 2021, 9, 31101. [Google Scholar] [CrossRef]
- Sasaki, K.; Kuramata, A.; Masui, T.; Víllora, E.G.; Shimamura, K.; Yamakoshi, S. Device-Quality β-Ga2O3 epitaxial Films Fabricated by Ozone Molecular Beam Epitaxy. Appl. Phys. Express 2012, 5, 035502. [Google Scholar] [CrossRef]
- Ramana, C.V.; Roy, S.; Zade, V.; Battu, A.K.; Makeswaran, N.; Shutthanandan, V. Electronic Structure and Chemical Bonding in Transition-Metal-Mixed Gallium Oxide (Ga2O3) Compounds. J. Phys. Chem. Solids 2021, 157, 110174. [Google Scholar] [CrossRef]
- Gopal, R.; Goyal, A.; Saini, A.; Nagar, M.; Sharma, N.; Gupta, D.K.; Dhayal, V. Sol- Gel Synthesis of Ga2O3 Nanorods and Effect of Precursor Chemistry on Their Structural and Morphological Properties. Ceram. Int. 2018, 44, 19099–19105. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, G.; Carloni, D.; Wu, Y. Fabrication, Microstructure and Optical Properties of Ga2O3 Transparent Ceramics. Ceram. Int. 2020, 46, 21757–21761. [Google Scholar] [CrossRef]
- Alpyssova, G.; Lisitsyn, V.; Golkovski, M.; Mussakhanov, D.; Karipbayev, Z.; Grechkina, T.; Karabekova, D.; Kozlovskiy, A. Luminescence Efficiency of Cerium-Doped Yttrium Aluminum Garnet Ceramics Formed by Radiation Assisted Synthesis. East.-Eur. J. Enterp. Technol. 2021, 6, 49–57. [Google Scholar] [CrossRef]
- Karipbayev, Z.T.; Lisitsyn, V.M.; Mussakhanov, D.A.; Alpyssova, G.K.; Popov, A.I.; Polisadova, E.F.; Elsts, E.; Akilbekov, A.T.; Kukenova, A.B.; Kemere, M.; et al. Time-Resolved Luminescence of YAG:Ce and YAGG:Ce Ceramics Prepared by Electron Beam Assisted Synthesis. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 479, 222–228. [Google Scholar] [CrossRef]
- Lisitsyna, L.A.; Popov, A.I.; Karipbayev, Z.T.; Mussakhanov, D.A.; Feldbach, E. Luminescence of MgF2-WO3 Ceramics Synthesized in the Flux of 1.5 MeV Electron Beam. Opt. Mater. 2022, 133, 112999. [Google Scholar] [CrossRef]
- Avilov, M.; Fadeev, S.; Fernandes, S.; Golkovsky, M.; Mittig, W.; Pellemoine, F.; Schein, M. A 50-KW Prototype of the High-Power Production Target for the FRIB. J. Radioanal. Nucl. Chem. 2015, 305, 817–823. [Google Scholar] [CrossRef]
- Novel Crystal Technology, Inc. Creating Gallium Oxide Will Create a New Futuristic Way of Living. Available online: https://www.novelcrystal.co.jp/eng/ (accessed on 5 September 2022).
- Drouin, D.; Couture, A.R.; Joly, D.; Tastet, X.; Aimez, V.; Gauvin, R. CASINO V2.42—A Fast and Easy-to-Use Modeling Tool for Scanning Electron Microscopy and Microanalysis Users. Scanning 2007, 29, 92–101. [Google Scholar] [CrossRef]
- Karipbaeyev, Z.; Polisadova, E.; Ermolaev, A.; Lisitsyn, V.; Alpyssova, G.; Mussakhanov, D.; Kukenova, A.; Abil, Z. Dependence of the Efficiency Electron Beam Assisted Synthesis of YAG:Ce Ceramics on the Power Density of the Electron Flow. In Proceedings of the 2020 7th International Congress on Energy Fluxes and Radiation Effects (EFRE), Tomsk, Russia, 14–26 September 2020. [Google Scholar] [CrossRef]
- Ahman, J.; Svensson, G.; Albertsson, J. A Reinvestigation of β-Gallium Oxide. Acta Crystallogr. Sect. C 1996, 52, 1336–1338. [Google Scholar] [CrossRef]
- Cui, H.; Sai, Q.; Qi, H.; Zhao, J.; Si, J.; Pan, M. Analysis on the Electronic Trap of β-Ga2O3 Single Crystal. J. Mater. Sci. 2019, 54, 12643–12649. [Google Scholar] [CrossRef]
- Alhalaili, B.; Bunk, R.J.; Mao, H.; Cansizoglu, H.; Vidu, R.; Woodall, J.; Islam, M.S. Gallium Oxide Nanowires for UV Detection with Enhanced Growth and Material Properties. Sci. Rep. 2020, 10, 21434. [Google Scholar] [CrossRef]
- Varley, J.B.; Weber, J.R.; Janotti, A.; Walle, C.G. Van De Oxygen Vacancies and Donor Impurities in B-Ga2O3. Appl. Phys. Lett. 2010, 97, 142106. [Google Scholar] [CrossRef]
- McCluskey, M.D. Point Defects in Ga2O3. J. Appl. Phys. 2020, 127, 101101. [Google Scholar] [CrossRef]
- Harwig, T.; Kellendonk, F.; Slappendel, S. The Ultraviolet Luminescence of β-Galliumsesquioxide. J. Phys. Chem. Solids 1978, 39, 675–680. [Google Scholar] [CrossRef]
- Harwig, T.; Kellendonk, F. Some Observations on the Photoluminescence of Doped β-Galliumsesquioxide. J. Solid State Chem. 1978, 24, 255–263. [Google Scholar] [CrossRef]
- Binet, L.; Gourier, D. Origin of the Blue Luminescence of β-Ga2O3. J. Phys. Chem. Solids 1998, 59, 1241–1249. [Google Scholar] [CrossRef]
- Binet, L.; Gourier, D.; Minot, C. Relation between Electron Band Structure and Magnetic Bistability of Conduction Electrons in β-Ga2O3. J. Solid State Chem. 1994, 113, 420–433. [Google Scholar] [CrossRef]
- Onuma, T.; Saito, S.; Sasaki, K.; Goto, K.; Masui, T.; Yamaguchi, T.; Honda, T.; Kuramata, A.; Higashiwaki, M. Temperature-Dependent Exciton Resonance Energies and Their Correlation with IR-Active Optical Phonon Modes in β-Ga2O3 Single Crystals. Appl. Phys. Lett. 2016, 108, 101904. [Google Scholar] [CrossRef]
- Vasil’tsiv, V.I.; Zakharko, Y.M.; Prim, Y.I. Nature of the Blue and Green Luminescence Bands of β-Gallium Sesquioxide. Ukr. Fiz. Zhurnal 1988, 33, 1320. [Google Scholar]
- Usseinov, A.; Koishybayeva, Z.; Platonenko, A.; Pankratov, V.; Suchikova, Y.; Akilbekov, A.; Zdorovets, M.; Purans, J.; Popov, A.I. Vacancy Defects in Ga2O3: First-Principles Calculations of Electronic Structure. Materials 2021, 14, 7384. [Google Scholar] [CrossRef]
- Usseinov, A.; Koishybayeva, Z.; Platonenko, A.; Akilbekov, A.; Purans, J.; Pankratov, V.; Suchikova, Y.; Popov, A.I. Ab-Initio Calculations of Oxygen Vacancy in Ga2O3 Crystals. Latv. J. Phys. Tech. Sci. 2021, 58, 3–10. [Google Scholar] [CrossRef]
- Usseinov, A.; Platonenko, A.; Koishybayeva, Z.; Akilbekov, A.; Zdorovets, M.; Popov, A.I. Pair Vacancy Defects in β-Ga2O3 Crystal: Ab Initio Study. Opt. Mater. X 2022, 16, 100200. [Google Scholar] [CrossRef]
- Kranert, C.; Sturm, C.; Schmidt-Grund, R.; Grundmann, M. Raman Tensor Elements of β-Ga2O3. Sci. Rep. 2016, 6, 35964. [Google Scholar] [CrossRef]
- Dohy, D.; Lucazeau, G.; Revcolevschi, A. Raman Spectra and Valence Force Field of Single-Crystalline β Ga2O3. J. Solid State Chem. 1982, 45, 180–192. [Google Scholar] [CrossRef]
- Machon, D.; McMillan, P.F.; Xu, B.; Dong, J. High-Pressure Study of the β -to- α Transition in Ga2O3. Phys. Rev. B-Condens. Matter Mater. Phys. 2006, 73, 094125. [Google Scholar] [CrossRef]
- Onuma, T.; Fujioka, S.; Yamaguchi, T.; Itoh, Y.; Higashiwaki, M.; Sasaki, K.; Masui, T.; Honda, T. Polarized Raman Spectra in β-Ga2O3 Single Crystals. J. Cryst. Growth 2014, 401, 330–333. [Google Scholar] [CrossRef]
- Galván, C.; Galván, M.; Arias-Cerón, J.S.; López-Luna, E.; Vilchis, H.; Sánchez-R, V.M. Structural and Raman Studies of Ga2O3 Obtained on GaAs Substrate. Mater. Sci. Semicond. Process. 2016, 41, 513–518. [Google Scholar] [CrossRef]
- Rusevich, L.L.; Kotomin, E.A.; Zvejnieks, G.; Popov, A.I. Ab Initio Calculations of Structural, Electronic and Vibrational Properties of BaTiO3 and SrTiO3 Perovskite Crystals with Oxygen Vacancies. Low Temp. Phys. 2020, 46, 1185–1195. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Piskunov, S.; Isakoviča, I.; Makarenko, I.; Karbovnyk, I.; Popov, A.I. Raman Spectra of Vacancy-Containing LiF: Predictions from First Principles. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 480, 33–37. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Zaki, S.E.; Tihtih, M.; Boukhoubza, I.; En-nadir, R.; Derkaoui, I.; Attia, G.F.; Ateş, Ş.; Eker, Y.R. Self-Powered UV Photodetector Utilizing Plasmonic Hot Carriers in 2D α-MoO3/Ir/Si Schottky Heterojunction Devices. Phys. Status Solidi–Rapid Res. Lett. 2023, 2300175. [Google Scholar] [CrossRef]


| a (Ǻ) | b (Ǻ) | c (Ǻ) | β (deg) | V (Ǻ3) | Density (g/cm3) | Crystallites Size (nm) | Crystallinity (%) | |
|---|---|---|---|---|---|---|---|---|
| Pristine Ga2O3 powder | 12.17376 | 3.04365 | 5.77367 | 103.291 | 208.20 | 5.4874 | 22.34 | 76.1 |
| Before annealing Ga2O3 | 12.08948 | 3.00727 | 5.76147 | 103.651 | 203.55 | 5.6128 | 141.06 | 79.9 |
| After annealing Ga2O3 | 12.23419 | 3.04665 | 5.81359 | 103.922 | 210.33 | 5.4318 | 34.82 | 90.5 |
| PDF-01-0871901 | 12.2140 | 3.0371 | 5.7981 | 103.830 | 208.846 | 5.4704 | – |
| 2θ (Degree) | h k l | |||
|---|---|---|---|---|
| Reference | Pristine Powder | Before Annealing | After Annealing | |
| 30.36 | 30.22 | 29.97 | 1 1 0 | |
| 31.76 | 31.74 | 31.93 | 31.56 | −2 0 2 |
| 33.49 | 33.57 | −1 1 1 | ||
| 35.22 | 35.22 | 1 1 1 | ||
| 37.48 | 37.48 | 37.27 | 4 0 1 | |
| 38.42 | 38.44 | 38.76 | 38.42 | −4 0 2 |
| 43.10 | 43.06 | −1 1 2 | ||
| 45.84 | 45.86 | 45.98 | 45.69 | 1 1 2 |
| 48.67 | 48.67 | 48.55 | 5 1 0 | |
| 49.66 | 49.62 | 49.48 | 4 0 2 | |
| 50.95 | 50.76 | 51.2 | 50.77 | −4 0 3 |
| 54.51 | 54.55 | 54.49 | 5 1 1 | |
| 56.53 | 56.48 | −1 1 3 | ||
| 57.66 | 57.65 | 57.44 | 57.46 | −3 1 3 |
| 59.19 | 59.08 | 59.44 | 59.14 | −6 0 3 |
| 59.97 | 59.915 | 59.6 | 59.84 | 1 1 3 |
| 60.96 | 60.96 | 60.43 | 0 2 0 | |
| 62.75 | 62.69 | 62.66 | 7 1 0 | |
| 64.20 | 64.21 | 64 | −2 0 4 | |
| 64.71 | 64.69 | 64.73 | 64.61 | −7 1 2 |
| 69.49 | 69.4 | 69.12 | 4 2 0 | |
| 70.39 | 70.27 | 0 2 2 | ||
| 72.47 | 72.35 | 72.64 | 73.41 | −6 0 4 |
| 73.80 | 73.78 | 4 2 1 | ||
| 82.38 | 82.35 | 82.48 | −8 0 4 | |
| 84.44 | 84.38 | 84.41 | 84.31 | −7 1 4 |
| 89.13 | 89.23 | 90.07 | −6 0 5 | |
| 91.38 | 91.51 | 91.31 | −8 2 1 | |
| 93.14 | 93.06 | 92.93 | 8 2 0 | |
| 94.56 | 94.46 | 94.39 | −2 2 4 | |
| 96.23 | 96.28 | 96.48 | 5 1 4 | |
| Atom | Synthesized β-Ga2O3 | Pristine Powder β-Ga2O3 | |
|---|---|---|---|
| Before Annealing | After Annealing | ||
| Ga | 39.31 | 33.17 | 33.40 |
| O | 60.69 | 66.83 | 66.60 |
| O/Ga ratio | 1.54 | 2.0 | 1.99 |
| Commercial Single Crystal | Ceramic Sample before Annealing | Ceramic Sample after Annealing | [54] | [55] | [56] | [57] | [54] | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ν, cm−1 | FWHM, cm−1 | ν, cm−1 | FWHM, cm−1 | ν, cm−1 | FWHM, cm−1 | Experiment | Theory | |||
| ν, cm−1 | ||||||||||
| 115.7 | 8.1 | 111.4 | 0.4 | 113.9 | 3.6 | 114.8 | 114 | 113.6 | 115 | 118.6 |
| 146.3 | 9.5 | 143.1 | 0.4 | 145.0 | 5.3 | 144.8 | 147 | 144.7 | 149 | 145.6 |
| 171.1 | 8.3 | 168.7 | 8.4 | 169.1 | 0.4 | 169.9 | 169 | 169.2 | 173 | 176.4 |
| 201.7 | 9.0 | 199.0 | 9.3 | 200.2 | 3.8 | 200.2 | 199 | 200.4 | 205 | 199.1 |
| 321.5 | 12.9 | 318.1 | 0.5 | 319.6 | 8.5 | 320.0 | 318 | 318.6 | 322 | 318.5 |
| 347.9 | 11.9 | 345.6 | 13.2 | 346.7 | 9.4 | 346.6 | 346 | 346.4 | 350 | 342.5 |
| 417.7 | 9.9 | 414.4 | 10.6 | 415.8 | 5.2 | 416.2 | 415 | 415.7 | 421 | 432.0 |
| 476.6 | 10.9 | 475.2 | 14.9 | 474.8 | 11.3 | 474.9 | 475 | n.o. | 479 | 472.8 |
| 633.3 | 5.4 | 628.9 | 12.3 | 629.1 | 6.8 | 630.0 | 628 | 628.7 | 635 | 624.4 |
| 657.7 | 14.3 | 654.2 | 14.3 | 654.5 | 12.9 | 658.3 | 657 | n.o. | 663 | 655.8 |
| 767.2 | 13.8 | 765.5 | 15.5 | 765.5 | 13.3 | 766.7 | 763 | 763.9 | 772 | 767.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usseinov, A.B.; Karipbayev, Z.T.; Purans, J.; Kakimov, A.B.; Bakytkyzy, A.; Zhunusbekov, A.M.; Koketai, T.A.; Kozlovskyi, A.L.; Suchikova, Y.; Popov, A.I. Study of β-Ga2O3 Ceramics Synthesized under Powerful Electron Beam. Materials 2023, 16, 6997. https://doi.org/10.3390/ma16216997
Usseinov AB, Karipbayev ZT, Purans J, Kakimov AB, Bakytkyzy A, Zhunusbekov AM, Koketai TA, Kozlovskyi AL, Suchikova Y, Popov AI. Study of β-Ga2O3 Ceramics Synthesized under Powerful Electron Beam. Materials. 2023; 16(21):6997. https://doi.org/10.3390/ma16216997
Chicago/Turabian StyleUsseinov, Abay B., Zhakyp T. Karipbayev, Juris Purans, Askhat B. Kakimov, Aizat Bakytkyzy, Amangeldy M. Zhunusbekov, Temirgali A. Koketai, Artem L. Kozlovskyi, Yana Suchikova, and Anatoli I. Popov. 2023. "Study of β-Ga2O3 Ceramics Synthesized under Powerful Electron Beam" Materials 16, no. 21: 6997. https://doi.org/10.3390/ma16216997






