Synthesis and Luminescent Properties of Barium Molybdate Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Direct Mechanochemical Synthesis
2.2. Solid-State Reaction
2.3. Characterizations
3. Results and Discussion
3.1. XRD Analysis
3.2. Infrared Analysis
3.3. Ultraviolet–Visible Spectroscopy
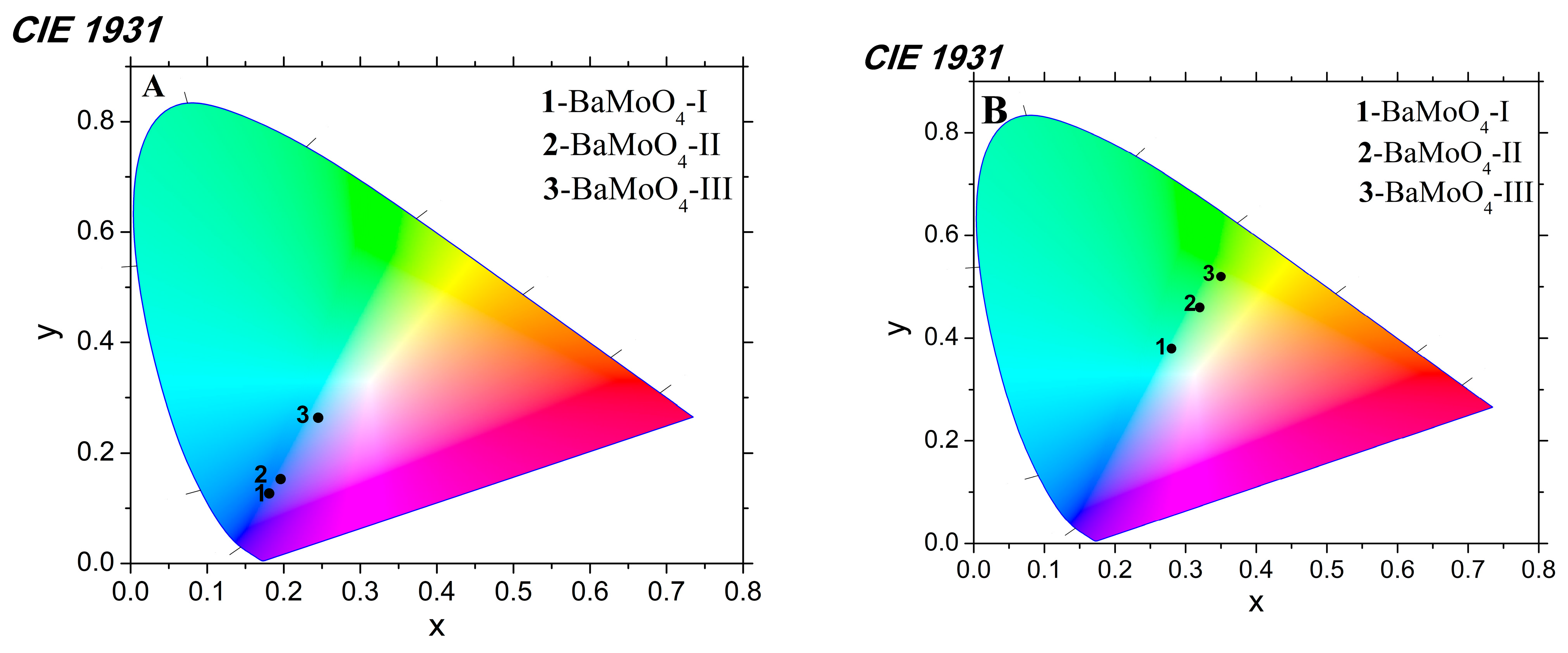
3.4. Photoluminescence (PL) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bazarganipou, M. Synthesis and characterization of BaMoO4 nanostructures prepared via a simple sonochemical method and their degradation ability of methylene blue. Ceram. Int. 2016, 42, 12617–12622. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, W.; Wu, J.; Jia, X. Preparation of flower-like BaMoO4 and application in rechargeable lithium and sodium ion batteries. Mater. Lett. 2017, 188, 248–251. [Google Scholar] [CrossRef]
- Marquesa, A.P.A.J.; Meloa, D.M.A.; Longo, E.; Paskocimasa, C.A.; Pizanic, P.S.; Leite, E.R. Photoluminescence properties of BaMoO4 amorphous thin films. J. Solid State Chem. 2005, 178, 2346–2353. [Google Scholar] [CrossRef]
- Künzel, R.; Latini, R.M.; Umisedo, N.K.; Yoshimura, E.M.; Okuno, E.; Courrol, L.C.; Marques, A.P.A. Effects of beta particles irradiation and thermal treatment on the traps levels structure and luminescent properties of BaMoO4 phosphor. Ceram. Int. 2019, 45, 7811–7820. [Google Scholar] [CrossRef]
- Wu, B.; Yang, W.; Liu, H.; Huang, L.; Zhao, B.; Wang, C.; Xu, G.; Lin, Y. Fluorescence spectra and crystal field analysis of BaMoO4: Eu3+ phosphors for white light-emitting diodes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 123, 12–17. [Google Scholar] [CrossRef]
- Jena, P.; Gupta, S.K.; Natarajan, V.; Sahu, M.; Satyanarayana, N.; Venkateswarlu, M. Structural characterization and photoluminescence properties of sol–gel derived nanocrystalline BaMoO4:Dy3+. J. Lum. 2015, 158, 203–210. [Google Scholar] [CrossRef]
- Sharath, R.A.; Rahulan, K.M.; Flower, N.A.L.; Sujatha, R.A.; Vinitha, G.; Rejeev, L.R.; Saha, T.; Prakashbabu, D. Third-order nonlinear optical characteristics of Er3+-doped BaMoO4 nanostructures. J. Mater. Sci. Mater. Electron. 2022, 33, 8542–8550. [Google Scholar] [CrossRef]
- Afanasiev, P. Molten salt synthesis of Barium molybdate and tungstate microcrystals. Mater. Lett. 2017, 61, 4622–4626. [Google Scholar] [CrossRef]
- Alencar, L.D.S.; Mesquita, A.; Feitosa, C.A.C.; Balzer, R.; Probst, L.F.D.; Marcelo, D.C.B.; Rosmaninho, G.; Fajardo, H.V.; Bernardi, M.I.B. Preparation, characterization and catalytic application of Barium molybdate (BaMoO4) and Barium tungstate (BaWO4) in the gas-phase oxidation of toluene. Ceram. Int. 2017, 43, 4462–4469. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Gracia, L.; Nogueira, I.C.; Gurgel, M.F.C.; Mercury, J.M.R.; Longo, E.; Andres, J. On the morphology of BaMoO4 crystals: A theoretical and experimental approach. Cryst. Res. Technol. 2016, 51, 634–644. [Google Scholar] [CrossRef]
- Sczancoski, J.C.; Bomio, M.D.R.; Cavalcante, L.S.; Joya, M.R.; Pizani, P.S.; Varela, J.A.; Longo, E.; Siu, L.M.; Andres, J.A. Morphology and Blue Photoluminescence Emission of PbMoO4 Processed in Conventional Hydrothermal. J. Phys. Chem. 2009, C113, 5812–5822. [Google Scholar] [CrossRef]
- Marques, A.P.A.; Picon, F.C.; Melo, D.M.A.; Pizani, P.S.; Leite, E.R.; Varela, J.A.; Longo, E. Effect of the order and disorder of BaMoO4 powders in photoluminescent properties. J. Fluoresc. 2008, 18, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Phuruangat, A.; Thongtem, T.; Thongtem, S. Precipitate synthesis of BaMoO4 and BaWO4 nanoparticles at room temperature and their photolumincnescence properties. Superlattices Microstruct. 2012, 52, 78–83. [Google Scholar] [CrossRef]
- Lim, C.S. Microwave-assisted synthesis and photolumincescence of MMoO4 (M Ca, Ba) particles via a metathetic reaction. J. Lum. 2012, 132, 1774–1780. [Google Scholar] [CrossRef]
- Wu, X.; Du, J.; Li, H.; Zhang, M.; Xi, B.; Fan, H.; Zhu, Y.; Qian, Y. Aqueous mineralization process to synthesize uniform shuttle-like BaMoO4 microcrystals at room temperature. J. Solid State Chem. 2017, 180, 3288–3295. [Google Scholar] [CrossRef]
- Luo, Z.; Li, H.; Shu, H.; Wang, K.; Xia, J.; Yan, Y. Synthesis of BaMoO4 nestlike nanostructures under a new growth mechanism. Cryst Growth Des. 2008, 8, 2275–2281. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Zi, W.; Zou, L.; Gana, S.; Ji, G. Size-tailored synthesis and luminescent properties of three-dimensional BaMoO4, BaMoO4:Eu3+ micron-octahedrons and micron-flowers via sonochemical route. Luminescence 2015, 30, 280–289. [Google Scholar] [CrossRef]
- Luo, Y.S.; Zhang, W.D.; Dai, X.J.; Yang, Y.; Fu, S.Y. Facile Synthesis and luminescent properties of novel flowerlike BaMoO4 nanostructures by a simple hydrothermal route. J. Phys. Chem. C 2009, 113, 4856–4861. [Google Scholar] [CrossRef]
- Ma, L.; Sun, Y.; Gao, P.; Yin, Y.; Qin, Z.; Zhou, B. Controlled synthesis and photoluminescence properties of hierarchical BaXO4 (X = Mo, W) nanostructures at room temperature. Matter. Lett. 2010, 64, 1235–1237. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Wang, S.; Fang, L.; Chen, X.; Yu, C.; Tang, S.; Liu, H.; Yi, Z.; Yang, H. Facile synthesis of BaMoO4 and BaMoO4/BaWO4 heterostructures with type -I band arrangement and enhanced photoluminescence properties. Adv. Powder Technol. 2021, 32, 4186–4197. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Sczancoski, J.C.; Tranquilin, R.L.; Joya, M.R.; Pizani, P.S.; Varela, J.A.; Longo, E. BaMoO4 powders processed in domestic microwave-hydrothermal: Synthesis, characterization and photoluminescence at room temperature. J. Phys. Chem. Solids 2008, 69, 2674–2680. [Google Scholar] [CrossRef]
- Sczancoski, J.C.; Cavalcante, L.S.; Marana, N.L.; Silva, R.O.; Tranquilin, R.L.; Joya, M.R.; Pizani, P.S.; Varela, J.A.; Sambrano, J.R.; Li, M.S.; et al. Electronic structure and optical properties of BaMoO4 powders. Curr. Appl. Phys. 2010, 10, 614–624. [Google Scholar] [CrossRef]
- Ryu, J.H.; Yoon, J.W.; Lim, C.S.; Shim, K.B. Microwave-assisted synthesis of barium molybdate by a citrate complex method and oriented aggregation. Mater. Res. Bull. 2005, 40, 1468–1476. [Google Scholar] [CrossRef]
- Marques, A.P.A.; Melo, D.M.A.; Longo, E.; Paskocimas, C.A.; Pizani, P.S.; Leite, E.R. Photoluminescent BaMoO4 nanopowders prepared by complex polymerization method (CPM). J. Solid State Chem. 2006, 179, 671–678. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, K.M.; Mhim, S.W.; Park, G.S.; Eun, J.W.; Shim, K.B.; Lim, C.S. Laser-induced synthesis of BaMoO4 nanocolloidal suspension and its optical properties. Appl. Phys. 2008, A92, 407–412. [Google Scholar] [CrossRef]
- Pereira, W.S.; Sczancoski, J.C.; Lango, E. Tailoring the photoluminescence of BaMoO4 and BaMoO4 heirararchical architectures via precipitation induced by a fast precursor injection. Mater. Lett. 2021, 293, 129681. [Google Scholar] [CrossRef]
- Baby, O.M.; Balamurugan, S.; Ashika, S.A.; Fathima, T.K.S. Synthesis and characterization of high NIR refecting ecofriendly BaMoO4 pigments in scheelite family. Emergent Mater. 2022, 5, 1213–1225. [Google Scholar] [CrossRef]
- Chen, X.B.; Shao, Z.B.; Tian, Y.W. New method for preparation of luminescent lanthanide materials BaMoO4:Eu3+. Mater. Technol. 2011, 26, 67–70. [Google Scholar] [CrossRef]
- Rangappa, D.; Fujiwara, T.; Watanabe, T.; Yoshimura, M. Fabrication of AMoO4 (A = Ba, Sr) film on Mo substrate by solution reaction assisted ball-rotation. Mater. Res. Bull. 2008, 43, 3155–3163. [Google Scholar] [CrossRef]
- Xiao, E.C.; Li, J.; Wang, J.; Xing, C.; Guo, M.; Qiao, H.; Wang, Q.; Qi, Z.M.; Dou, G.; Shi, F. Phonon characteristics and dielectric properties of BaMoO4 ceramic. J. Mater. 2018, 4, 383–389. [Google Scholar] [CrossRef]
- Lima, R.C.; Anicete-Santos, M.; Orhan, E.; Maurera, M.A.M.A.; Souza, A.G.; Pizani, P.S.; Leite, E.R.; Varela, J.A.; Lango, E. Photoluminescent property of mechanically milled BaWO4 powder. J. Lim. 2007, 126, 741–746. [Google Scholar] [CrossRef]
- Dimitriev, Y.; Gancheva, M.; Iordanova, R. Effects of the mechanical activation of zinc carbonate hydroxide on the formation and properties of zinc oxides. J. Alloys Compd. 2012, 519, 161–166. [Google Scholar] [CrossRef]
- Gancheva, M.; Szafraniak-Wiza, I.; Iordanova, R.; Piroeva, I. Comparative analysis of nanocrystalline CuWO4 obtained by high-energy ball milling. J. Chem. Techn. Metall. 2019, 54, 1233–1239. Available online: https://journal.uctm.edu/node/j2019-6/14_18-220_p_1233-1239.pdf (accessed on 18 September 2023).
- Gancheva, M.; Iordanova, R.; Mihailov, L. Direct mechanochemical synthesis and characterization of SrWO4 nanoparticle. Bulg. Chem. Commun. 2022, 54, 337–342. Available online: http://bcc.bas.bg/BCC_Volumes/Volume_54_Number_4_2022/bcc-54-4-2022-337-342-gancheva-nc02.pdf (accessed on 18 September 2023).
- Black, D.; Mendenhall, M.; Henins, A.; Filliben, J.; Cline, J. The Certification of Standard Reference Material 660C for Powder Diffraction, Powder Diffraction. 2020. Available online: https://tsapps.nist.gov/srmext/certificates/660c.pdf (accessed on 2 November 2023).
- Iordanova, R.S.; Milanova, M.K.; Kostov, K.L. Glass formation in the MoO3–CuO system. Phys. Chem. Glas. Eur. J. Glass Sci. Technol. B. 2006, 47, 631–637. Available online: https://www.ingentaconnect.com/content/sgt/ejgst/2006/00000047/00000006/art00003 (accessed on 18 September 2023).
- Wang, W.; Pan, Y.; Zhang, W.; Liu, X.; Li, L. The size effect to O2−—Ce4+ charge transfer emission and band gap structure of Sr2CeO4. Luminescence 2018, 33, 907–912. [Google Scholar] [CrossRef]
- Bandi, S.; Vidyasagar, D.; Adil, S.; Singhd, M.K.; Basud, J.; Srivastav, A.K. Crystallite size induced bandgap tuning in WO3 derived from nanocrystalline tungsten. Scr. Mater. 2020, 176, 47–52. [Google Scholar] [CrossRef]
- Spassky, D.A.; Ivanov, S.N.; Kolobanov, V.N.; Mikhailin, V.V.; Zemskov, V.N.; Zadneprovski, B.I.; Potkin, L.I. Optical and luminescent properties of the lead and barium molybdates. Radiat. Meas. 2004, 38, 607–610. [Google Scholar] [CrossRef]
- Tauc, J. Absorption edge and internal electric fields in amorphous semiconductors. Mater. Res. Bull. 1970, 5, 721–729. [Google Scholar] [CrossRef]
- Klinbumrung, A.; Phuruangrat, A.; Thongtem, T.; Thongtem, S. Synthesis, Characterization and optical properties of BaMoO4 synthesized by microwave plasma method. Russ. J. Inorg. Chem. 2018, 63, 725–731. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Sczancoski, J.C.; Espinosa, J.W.M.; Varela, J.A.; Pizani, P.S.; Longo, E. Photoluminescent behavior of BaWO4 powders processed in microwave-hydrothermal. J. Alloys Compd. 2009, 474, 195–200. [Google Scholar] [CrossRef]
- Raghunath, S.; Balan, R. Solvent assisted synthesis and characterization of AMoO4 (A = Ca, Sr & Ba) nanomaterials. Mater. Today Proc. 2021, 46, 2930–2933. [Google Scholar]
- Benchikhi, M.; Ouatib, R.E.; Guillement-Fritsch, S.; Er-Rakho, L.; Durand, B. Characterization and photoluminescence properties of ultrafine copper molybdate (α-CuMoO4) powders via a combustion—Like process. Int. J. Mener. Metall. Mater. 2016, 23, 13401345. [Google Scholar] [CrossRef]
- Sabri, N.S.; Yahya, A.K.; Talari, M.K. Emission properties of Mn doped ZnO nanoparticles prepared by mechanochemical processing. J. Lim. 2012, 132, 1735–1739. [Google Scholar] [CrossRef]

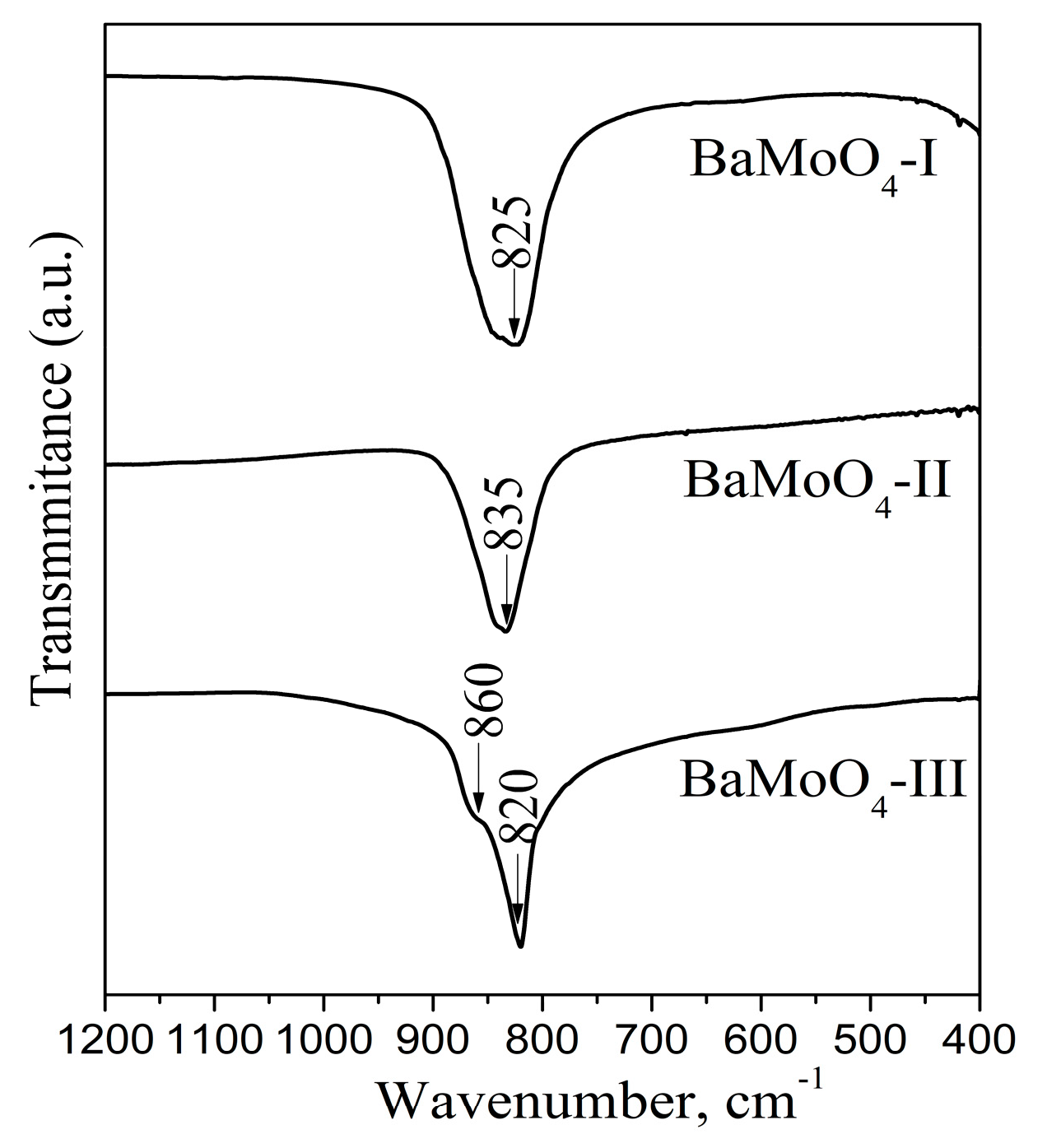
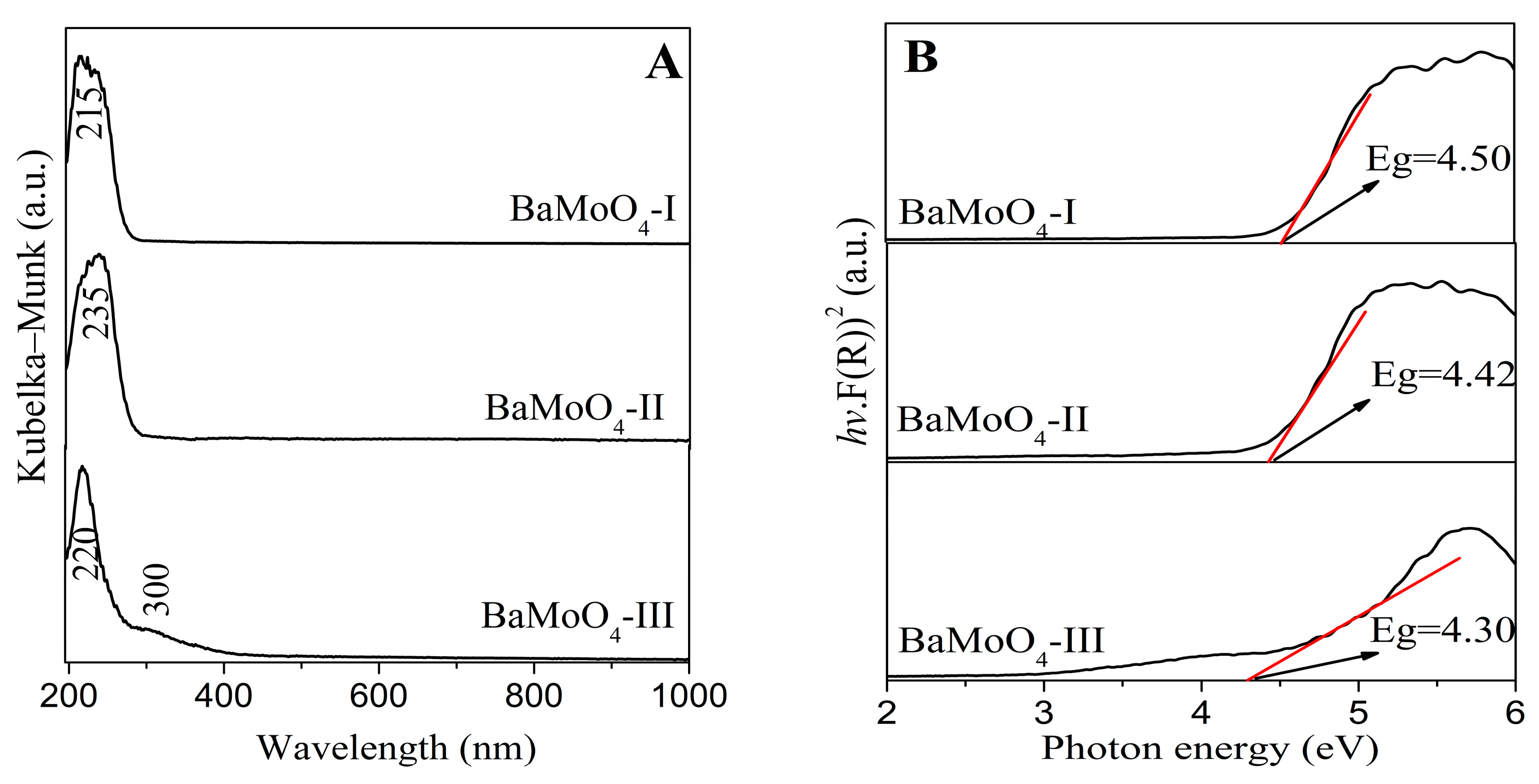
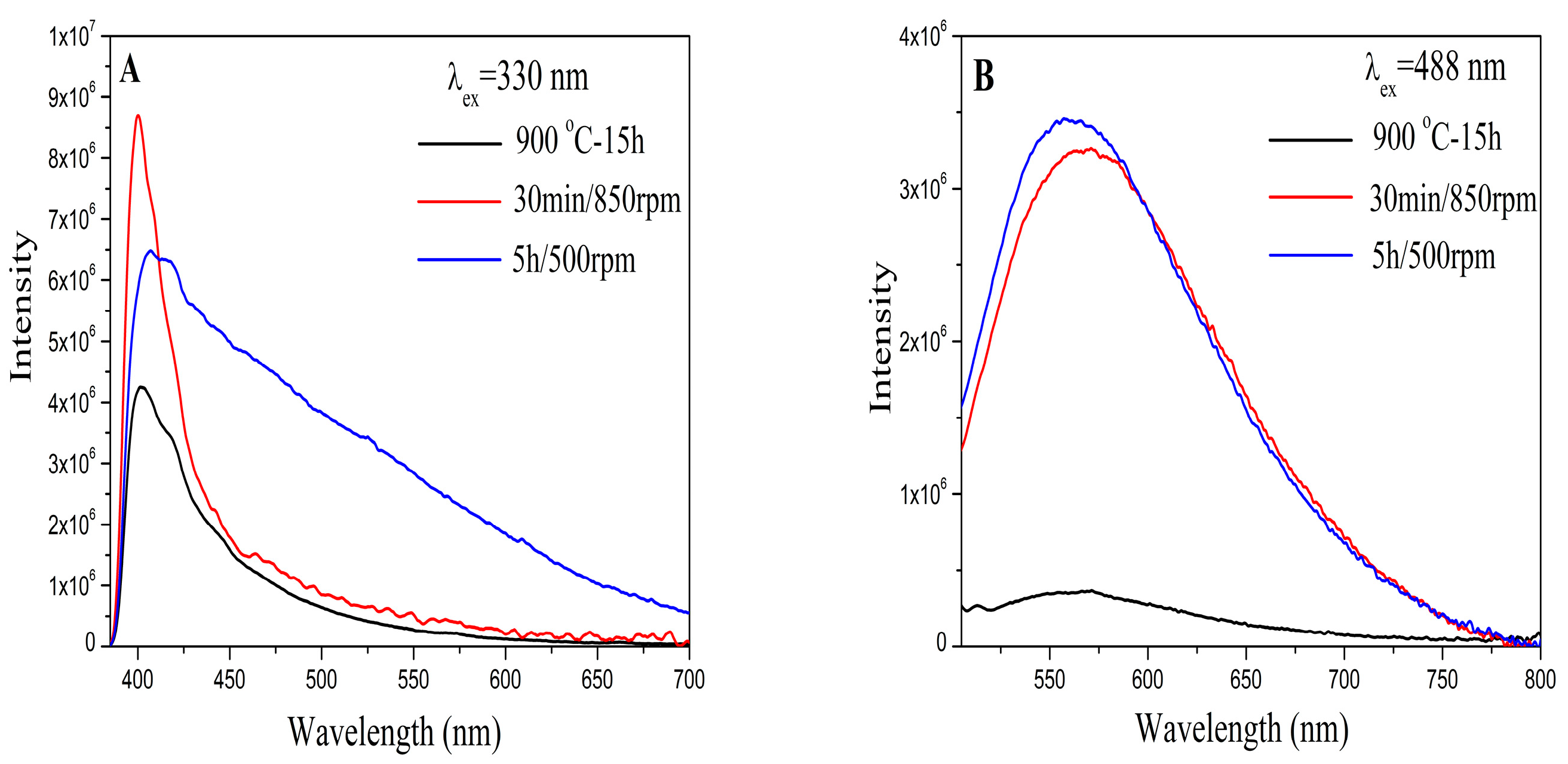
| Samples | a = b (Å) | c (Å) | Unit Cell Volume (Å) | Average Crystallite Size (Å) |
|---|---|---|---|---|
| BaMoO4-I 5 h/500 rpm | 5.5718 (9) | 12.800 (3) | 397.3864 | 240 (5) |
| BaMoO4-II 30 min/850 rpm | 5.5811 (4) | 12.819 (1) | 399.2755 | 270 (5) |
| BaMoO4-III 900 °C-15 h | 5.6083 (2) | 12.7019 (5) | 399.5213 | 1540 (4) |
| PDF-BaMoO4 | 5.58 | 12.82 | 399.17 | - |
| Samples | x, y (exc. 330 nm) | x, y (exc. 488 nm) |
|---|---|---|
| BaMoO4-I | 0.18, 0.12 | 0.28, 0.38 |
| BaMoO4-II | 0.20, 0.15 | 0.32, 0.46 |
| BaMoO4-III | 0.25, 0.26 | 0.35, 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gancheva, M.; Iordanova, R.; Koseva, I.; Avdeev, G.; Burdina, G.; Ivanov, P. Synthesis and Luminescent Properties of Barium Molybdate Nanoparticles. Materials 2023, 16, 7025. https://doi.org/10.3390/ma16217025
Gancheva M, Iordanova R, Koseva I, Avdeev G, Burdina G, Ivanov P. Synthesis and Luminescent Properties of Barium Molybdate Nanoparticles. Materials. 2023; 16(21):7025. https://doi.org/10.3390/ma16217025
Chicago/Turabian StyleGancheva, Maria, Reni Iordanova, Iovka Koseva, Georgi Avdeev, Gergana Burdina, and Petar Ivanov. 2023. "Synthesis and Luminescent Properties of Barium Molybdate Nanoparticles" Materials 16, no. 21: 7025. https://doi.org/10.3390/ma16217025






