Development of Mortars That Use Recycled Aggregates from a Sodium Silicate Process and the Influence of Graphene Oxide as a Nano-Addition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Design and Preparation of Mortars
2.3. Methods
2.3.1. Leaching Study
2.3.2. Physical Properties
2.3.3. Alkali–Silica Reaction Evaluation
2.3.4. Mechanical Properties
3. Results
3.1. Leaching Results
3.2. Physical Properties
3.3. Alkali–Silica Reaction
3.4. Mechanical Properties
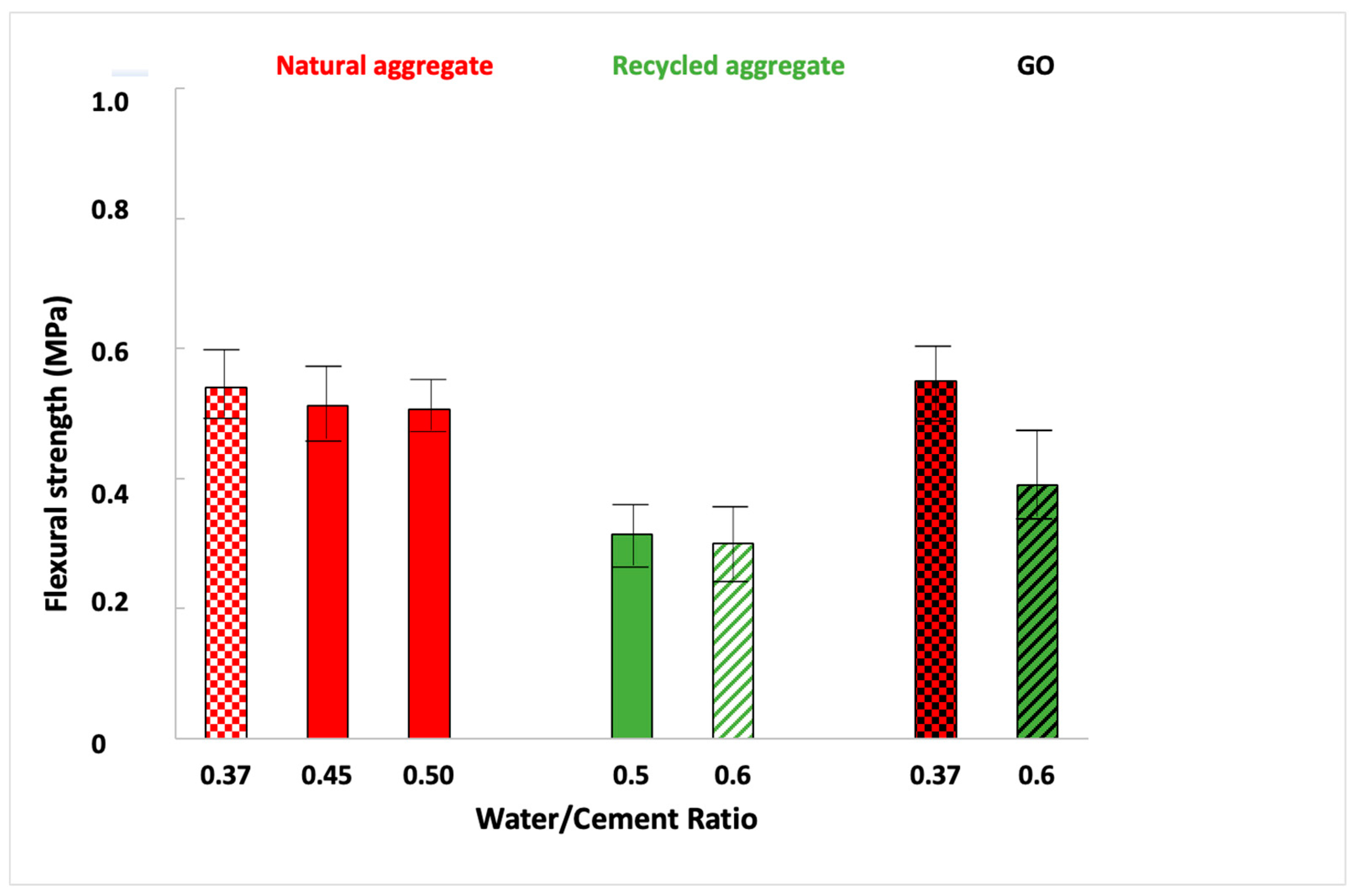
3.4.1. Flexural Strength
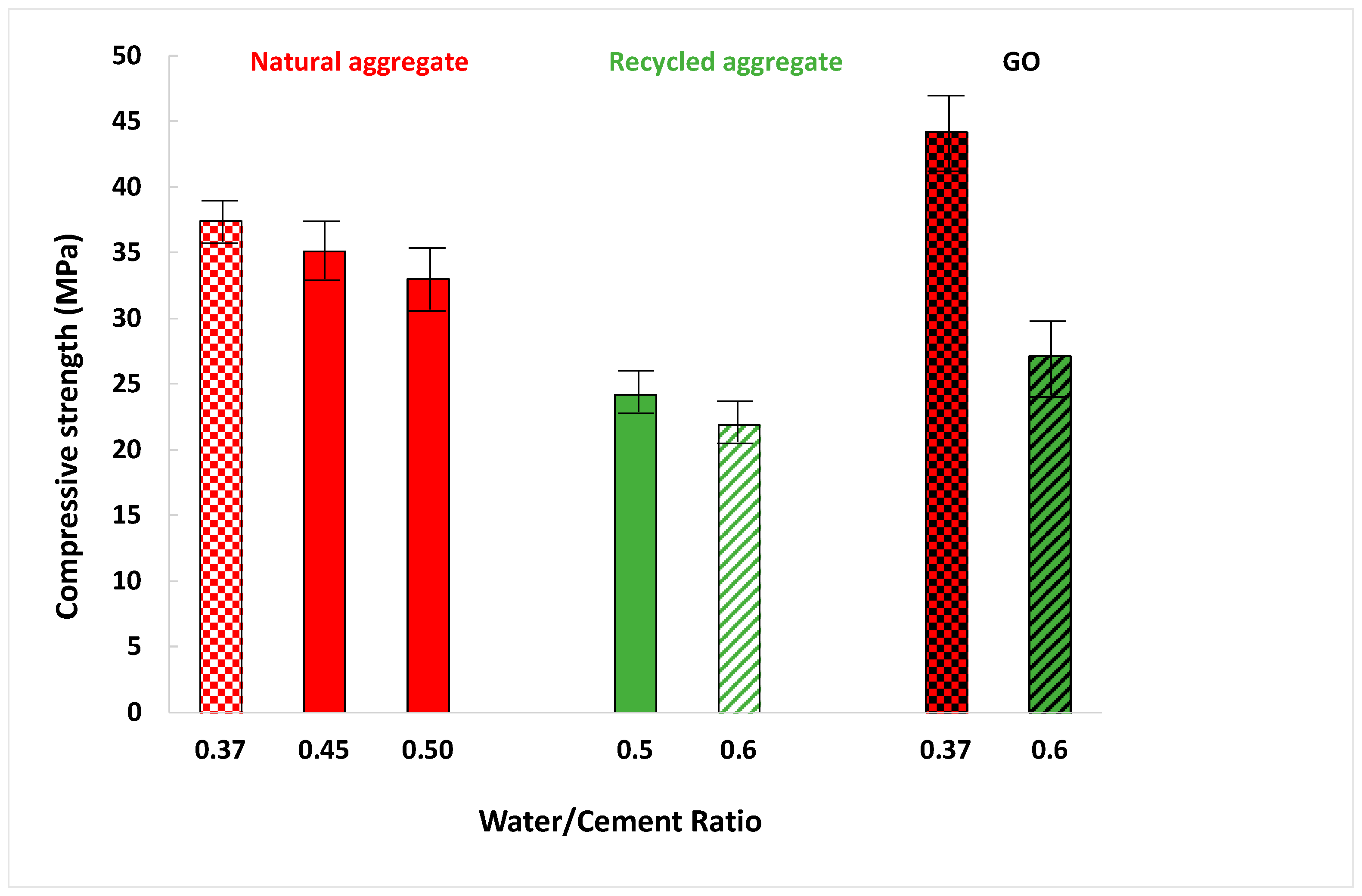
3.4.2. Compressive Strength
3.4.3. Acid Attack
4. Conclusions
- -
- It was confirmed that mortars manufactured with less water (lower water/cement ratio) have better physical and mechanical properties because it led to a lower-porosity mortar, and a diminution of 3% of water increased the mechanical properties by 10%.
- -
- From leaching behaviour, the waste can be classified as inert waste but presents a high Pb leaching, which prevents it from being used as a construction material according to some legislations for construction buildings (Italy and Cantabria).
- -
- Moreover, natural sand mortars are slightly better than recycled ones since, natural sand possesses fewer internal pores and, consequently, higher density (10%) and higher flexural (60%) and compressive strength (38%) were obtained for standard sand.
- -
- GO reduces the porosity of the mortar and it is an effective material in controlling the expansion of alkali–silica reaction, reducing the expansion by more than 80%. GO increases the mechanical properties (30% of the compressive strength of recycled mortars). GO improves the acid resistance, increasing the compressive strength after the acid attack compared to the values obtained before the attack.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Law, K.L.; Narayan, R. Reducing environmental plastic pollution by designing polymer materials for managed end-of-life. Nat. Rev. Mater 2022, 7, 104–116. [Google Scholar] [CrossRef]
- Baeza, F.; Payá, J.; Galao, O.; Saval, J.M.; Garcés, P. Blending of Industrial Waste from Different Sources as Partial Substitution of Portland Cement in Pastes and Mortars. Constr. Build. Mater. 2014, 66, 645–653. [Google Scholar] [CrossRef]
- Marinković, S.; Dragaš, J.; Ignjatović, I.; Tošić, N. Environmental Assessment of Green Concretes for Structural Use. J. Clean. Prod. 2017, 154, 633–649. [Google Scholar] [CrossRef]
- Pedro, D.; de Brito, J.; Evangelista, L. Influence of the Use of Recycled Concrete Aggregates from Different Sources on Structural Concrete. Constr. Build. Mater 2014, 71, 141–151. [Google Scholar] [CrossRef]
- Evangelista, L.; de Brito, J. Concrete with Fine Recycled Aggregates: A Review. Eur. J. Environ. Civ. Eng. 2013, 18, 129–172. [Google Scholar] [CrossRef]
- Li, P.; Gan, W.; Yao, G.; Huang, Q.; Zhao, R. Effect of Permeable Crystalline Materials on the Mechanical and Porosity Property of Recycled Aggregate and Recycled Aggregate Concrete. Materials 2023, 16, 4596. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Xie, X.; Zhuo, J.; He, Y.; Wang, K. Experimental Study on the Strength and Hydration Products of Cement Mortar with Hybrid Recycled Powders Based Industrial-Construction Residue Cement Stabilization of Crushed Aggregate. Materials 2023, 16, 4233. [Google Scholar] [CrossRef]
- Peceño, B.; Bakit, J.; Cortes, N.; Alonso-Fariñas, B.; Bonilla, E.; Leiva, C. Assessing Durability Properties and Economic Potential of Shellfish Aquaculture Waste in the Construction Industry: A Circular Economy Perspective. Sustainability 2022, 14, 8383. [Google Scholar] [CrossRef]
- Kanagaraj, B.; Anand, N.; Johnson Alengaram, U.; Samuvel Raj, R.; Kiran, T. Exemplification of Sustainable Sodium Silicate Waste Sediments as Coarse Aggregates in the Performance Evaluation of Geopolymer Concrete. Constr. Build. Mater 2022, 330, 127135. [Google Scholar] [CrossRef]
- Arenas, C.; Vilches, L.F.; Leiva, C.; Alonso-Fariñas, B.; Rodríguez-Galán, M. Recycling ceramic industry wastes in sound absorbing materials. Mater. Constr. 2016, 66, e106. [Google Scholar] [CrossRef]
- Luna-Galiano, Y.; Leiva Fernández, C.; Villegas Sánchez, R.; Fernández-Pereira, C. Development of Geopolymer Mortars Using Air-Cooled Blast Furnace Slag and Biomass Bottom Ashes as Fine Aggregates. Processes 2023, 11, 1597. [Google Scholar] [CrossRef]
- Arenas, C.; Ríos, J.D.; Cifuentes, H.; Vilches, L.F.; Leiva, C. Sound Absorbing Porous Concretes Composed of Different Solid Wastes. Eur. Eur. J. Environ. Civ. Eng. 2020, 26, 3805–3817. [Google Scholar] [CrossRef]
- Ríos, J.D.; Vahí, A.; Leiva, C.; Martínez-De la Concha, A.; Cifuentes, H. Analysis of the Utilization of Air-Cooled Blast Furnace Slag as Industrial Waste Aggregates in Self-Compacting Concrete. Sustainability 2019, 11, 1702. [Google Scholar] [CrossRef]
- Estanqueiro, B.; Dinis Silvestre, J.; de Brito, J.; Duarte Pinheiro, M. Environmental Life Cycle Assessment of Coarse Natural and Recycled Aggregates for Concrete. Eur. J. Environ. Civ. Eng. 2016, 22, 429–449. [Google Scholar] [CrossRef]
- Nedeljković, M.; Visser, J.; Šavija, B.; Valcke, S.; Schlangen, E. Use of Fine Recycled Concrete Aggregates in Concrete: A Critical Review. J. Build. Eng 2021, 38, 102196. [Google Scholar] [CrossRef]
- BellChem. Available online: https://www.bellchem.com/news/uses-of-sodium-silicate-also-known-as-water-glass (accessed on 31 August 2023).
- Producciones Nazarenas SL. Available online: http://www.silicatosodico.com/silicatoproductos.htm (accessed on 31 August 2023).
- Barreto Santos, M.; De Brito, J.; Santos Silva, A. A Review on Alkali-Silica Reaction Evolution in Recycled Aggregate Concrete. Materials 2020, 13, 2625. [Google Scholar] [CrossRef]
- Moser, R.D.; Jayapalan, A.R.; Garas, V.Y.; Kurtis, K.E. Assessment of Binary and Ternary Blends of Metakaolin and Class C Fly Ash for Alkali-Silica Reaction Mitigation in Concrete. Cem. Concr. Res. 2010, 40, 1664–1672. [Google Scholar] [CrossRef]
- Bueno, E.T.; Paris, J.M.; Clavier, K.A.; Spreadbury, C.; Ferraro, C.C.; Townsend, T.G. A Review of Ground Waste Glass as a Supplementary Cementitious Material: A Focus on Alkali-Silica Reaction. J. Clean. Prod. 2020, 257, 120180. [Google Scholar] [CrossRef]
- Hong, X.; Lee, J.C.; Ng, J.L.; Md Yusof, Z.; He, Q.; Li, Q. Effect of Graphene Oxide on the Mechanical Properties and Durability of High-Strength Lightweight Concrete Containing Shale Ceramsite. Materials 2023, 16, 2756. [Google Scholar] [CrossRef]
- Raki, L.; Beaudoin, J.; Alizadeh, R.; Makar, J.; Sato, T. Cement and Concrete Nanoscience and Nanotechnology. Materials 2010, 3, 918–942. [Google Scholar] [CrossRef]
- Al-saffar, F.Y.; Wong, L.S.; Paul, S.C. An Elucidative Review of the Nanomaterial Effect on the Durability and Calcium-Silicate-Hydrate (C-S-H) Gel Development of Concrete. Gels 2023, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Dehghani, A.; Sherif, M.M. Carbon Nanotube Reinforced Cementitious Composites: A Comprehensive Review. Constr. Build Mater. 2022, 315, 125100. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Song, L.; Song, Y.; Dai, G.; Liu, J. An Intensive Review on the Role of Graphene Oxide in Cement-Based Materials. Constr. Build. Mater 2020, 241, 117939. [Google Scholar] [CrossRef]
- Huseien, G.F. A Review on Concrete Composites Modified with Nanoparticles. J. Compos. Sci. 2023, 7, 67. [Google Scholar] [CrossRef]
- Jalal, M.; Mansouri, E.; Sharifipour, M.; Pouladkhan, A.R. Mechanical, Rheological, Durability and Microstructural Properties of High Performance Self-Compacting Concrete Containing SiO2 Micro and Nanoparticles. Mater. Des. 2012, 34, 389–400. [Google Scholar] [CrossRef]
- Kawashima, S.; Hou, P.; Corr, D.J.; Shah, S.P. Modification of Cement-Based Materials with Nanoparticles. Cem. Concr. Compos. 2013, 36, 8–15. [Google Scholar] [CrossRef]
- Syamsunur, D.; Wei, L.; Ahmed Memon, Z.; Surol, S.; Md Yusoff, N.I. Concrete Performance Attenuation of Mix Nano-SiO2 and Nano-CaCO3 under High Temperature: A Comprehensive Review. Materials 2022, 15, 7073. [Google Scholar] [CrossRef] [PubMed]
- Chintalapudi, K.; Pannem, R.M. An Intense Review on the Performance of Graphene Oxide and Reduced Graphene Oxide in an Admixed Cement System. Constr. Build. Mater. 2020, 259, 120598. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, P.; Lu, Z. A Short Discussion on How to Effectively Use Graphene Oxide to Reinforce Cementitious Composites. Constr. Build. Mater. 2018, 189, 33–41. [Google Scholar] [CrossRef]
- Graphenea. Available online: https://www.graphenea.com/ (accessed on 31 August 2023).
- Qiu, L.; Yang, X.; Gou, X.; Yang, W.; Ma, Z.-F.; Wallace, G.G.; Li, D. Dispersing Carbon Nanotubes with Graphene Oxide in Water and Synergistic Effects between Graphene Derivatives. Chem. Eur. J. 2010, 16, 10653–10658. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable Aqueous Dispersions of Graphitic Nanoplatelets via the Reduction of Exfoliated Graphite Oxide in the Presence of Poly(Sodium 4-Styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Liu, S.; Lu, F.; Chen, Y.; Dong, B.; Du, H.; Li, X. Efficient Use of Graphene Oxide in Layered Cement Mortar. Materials 2022, 15, 2181. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Liu, Y.; Chen, S.J.; Wang, C.M.; Sanjayan, J.G.; Duan, W.H. Improvement of Mechanical Properties by Incorporating Graphene Oxide into Cement Mortar. Mech. Adv. Mater. Struct. 2017, 25, 1313–1322. [Google Scholar] [CrossRef]
- Peng, H.; Ge, Y.; Cai, C.S.; Zhang, Y.; Liu, Z. Mechanical Properties and Microstructure of Graphene Oxide Cement-Based Composites. Constr. Build. Mater. 2019, 194, 102–109. [Google Scholar] [CrossRef]
- Lv, S.; Liu, J.; Sun, T.; Ma, Y.; Zhou, Q. Effect of GO nanosheets on shapes of cement hydration crystals and their formation process. Constr. Build Mater. 2014, 64, 231–239. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Chen, S.J.; Liu, Y.M.; Duan, W.H.; Shah, S.P. Effects of graphene oxide on early-age hydration and electrical resistivity of Portland cement paste. Constr. Build Mater. 2017, 136, 506–514. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Liu, Y.; Li, W.; Dong, B.; Duan, W.H. Dispersion of graphene oxide agglomerates in cement paste and its effects on electrical resistivity and flexural strength. Cem. Concr. Compos. 2018, 92, 145–154. [Google Scholar] [CrossRef]
- EN 197-1; Cement—Part 1: Composition, Specifications, and Conformity Criteria for Common Cements. European Committee for Standardization (CEN): Brussels, Belgium, 2011.
- EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization (CEN): Brussels, Belgium, 2018.
- EN 13139; Aggregates for Mortars. European Committee for Standardization (CEN): Brussels, Belgium, 2003.
- Leiva, C.; Arroyo-Torralvo, F.; Luna-Galiano, Y.; Villegas, R.; Vilches, L.F.; Fernández Pereira, C. Valorization of Bayer Red Mud in a Circular Economy Process: Valuable Metals Recovery and Further Brick Manufacture. Processes 2022, 10, 2367. [Google Scholar] [CrossRef]
- EN 12390-2; Testing Hardened Concrete—Part 2: Making and Curing Specimens for Strength Tests. European Committee for Standardization (CEN): Brussels, Belgium, 2020.
- Ministero dell’Ambiente e della Tutela Del Territorio. Decreto 5 Aprile 2006, n 186. Regolamento Recante Modifiche al Decreto Ministeriale 5 Febbraio 1998 «Individuazione Dei Rifiuti Non Pericolosi Sottoposti Alle Procedure Semplificate di Recupero, ai Sensi Degli Articoli 31 e 33 Del Decreto Legislativo 5 Febbraio 1997, n. 22». Gazzeta Ufficiale, GU Serie Generale n.115 del 19-05-2006, Italia (Roma). 2006. Available online: https://www.gazzettaufficiale.it/eli/id/2006/05/19/006G0202/sg (accessed on 12 November 2023).
- DL 183/2009; Waste Disposal at Landfills. Transposition to the Portuguese law of Council. Directive 1999/31/CE, April 26. Portuguese Official Journal. Portuguese Mint and Official Printing Office: Lisbon, Portugal, 2009.
- Decreto 104/2006; de 19 de Octubre, de Valorización de Escorias En La Comunidad Autónoma de Cantabria. Boletín official de Cantabria. 2006. Available online: https://boc.cantabria.es/boces/verAnuncioAction.do?idAnuBlob=333876 (accessed on 12 November 2023).
- EN 12457-4; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 4: One Stage Batch Test at a Liquid to Solid Ratio of 10 l/kg for Materials with Particle Size Below 10 mm (without or with Size Reduction). European Committee for Standardization (CEN): Brussels, Belgium, 2003.
- EN 196-3; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. European Committee for Standardization (CEN): Brussels, Belgium, 2017.
- ASTM C 1260; Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar Bar Method). American Society for Testing and Materials: West Conshohocken, PA, USA, 2001.
- EN 1015-11; Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar. European Committee for Standardization (CEN): Brussels, Belgium, 2000.
- Peceño, B.; Hurtado-Bermudez, S.; Alonso-Fariñas, B.; Villa-Alfageme, M.; Más, J.L.; Leiva, C. Recycling Bio-Based Wastes into Road-Base Binder: Mechanical, Leaching, and Radiological Implications. Appl. Sci. 2023, 13, 1644. [Google Scholar] [CrossRef]
- Council Directive 1999/31/EC of 26 April 1999 on the landfill of waste. Official Journal L 182, 16/07/1999 P. 0001–0019. European Commission, 1999. Available online: http://data.europa.eu/eli/dir/1999/31/oj (accessed on 12 November 2023).
- Saha, A.K.; Sarker, P.K. Expansion Due to Alkali-Silica Reaction of Ferronickel Slag Fine Aggregate in OPC and Blended Cement Mortars. Constr. Build. Mater. 2016, 123, 135–142. [Google Scholar] [CrossRef]
- Leiva, C.; Arenas, C.; Cifuentes, H.; Vilches, L.F.; Rios, J.D. Radiological, Leaching, and Mechanical Properties of Combustion Fly Ash in Cements. J. Hazard. Toxic Radioact. Waste 2017, 21, 04017011. [Google Scholar] [CrossRef]
- Ramezani, M.; Kim, Y.H.; Sun, Z.; Sherif, M.M. Influence of Carbon Nanotubes on Properties of Cement Mortars Subjected to Alkali-Silica Reaction. Cem. Concr. Compos. 2022, 131, 104596. [Google Scholar] [CrossRef]
- Ríos, J.D.; Cifuentes, H.; Leiva, C.; Seitl, S. Analysis of the Mechanical and Fracture Behavior of Heated Ultra-High-Performance Fiber-Reinforced Concrete by X-Ray Computed Tomography. Cem. Concr. Res. 2019, 119, 77–88. [Google Scholar] [CrossRef]
- Ramezani, M.; Kim, Y.H.; Sun, Z. Elastic Modulus Formulation of Cementitious Materials Incorporating Carbon Nanotubes: Probabilistic Approach. Constr. Build Mater. 2021, 274, 122092. [Google Scholar] [CrossRef]
- Kaish, A.B.M.A.; Odimegwu, T.C.; Zakaria, I.; Abood, M.M. Effects of Different Industrial Waste Materials as Partial Replacement of Fine Aggregate on Strength and Microstructure Properties of Concrete. J. Build. Eng. 2021, 35, 102092. [Google Scholar] [CrossRef]
- ASTM C 270–02; Standard Specification for Mortar for Unit Masonry. American Society for Testing and Materials: West Conshohocken, PA, USA, 2017.
- Tatineni, Y.S.; Putta, J. Enhanced Strength, Durability, and Microstructural Attributes of Graphene Oxide-Modified Ultrafine Slag Cement Mortar. Buildings 2022, 12, 2199. [Google Scholar] [CrossRef]
- Gladwin Alex, A.; Kedir, A.; Gebrehiwet Tewele, T. Review on Effects of Graphene Oxide on Mechanical and Microstructure of Cement-Based Materials. Constr. Build. Mater. 2022, 360, 129609. [Google Scholar] [CrossRef]
- Chintalapudi, K.; Pannem, R.M. Enhanced Chemical Resistance to Sulphuric Acid Attack by Reinforcing Graphene Oxide in Ordinary and Portland Pozzolana Cement Mortars. Case Stud. Constr. Mater. 2022, 17, e01452. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Wu, Y.-Y.; Deng, X.; Zheng, Z. The Effect of Graphene Oxide on the Mechanical Properties, Impermeability and Corrosion Resistance of Cement Mortar Containing Mineral Admixtures. Constr. Build. Mater. 2021, 288, 123059. [Google Scholar] [CrossRef]

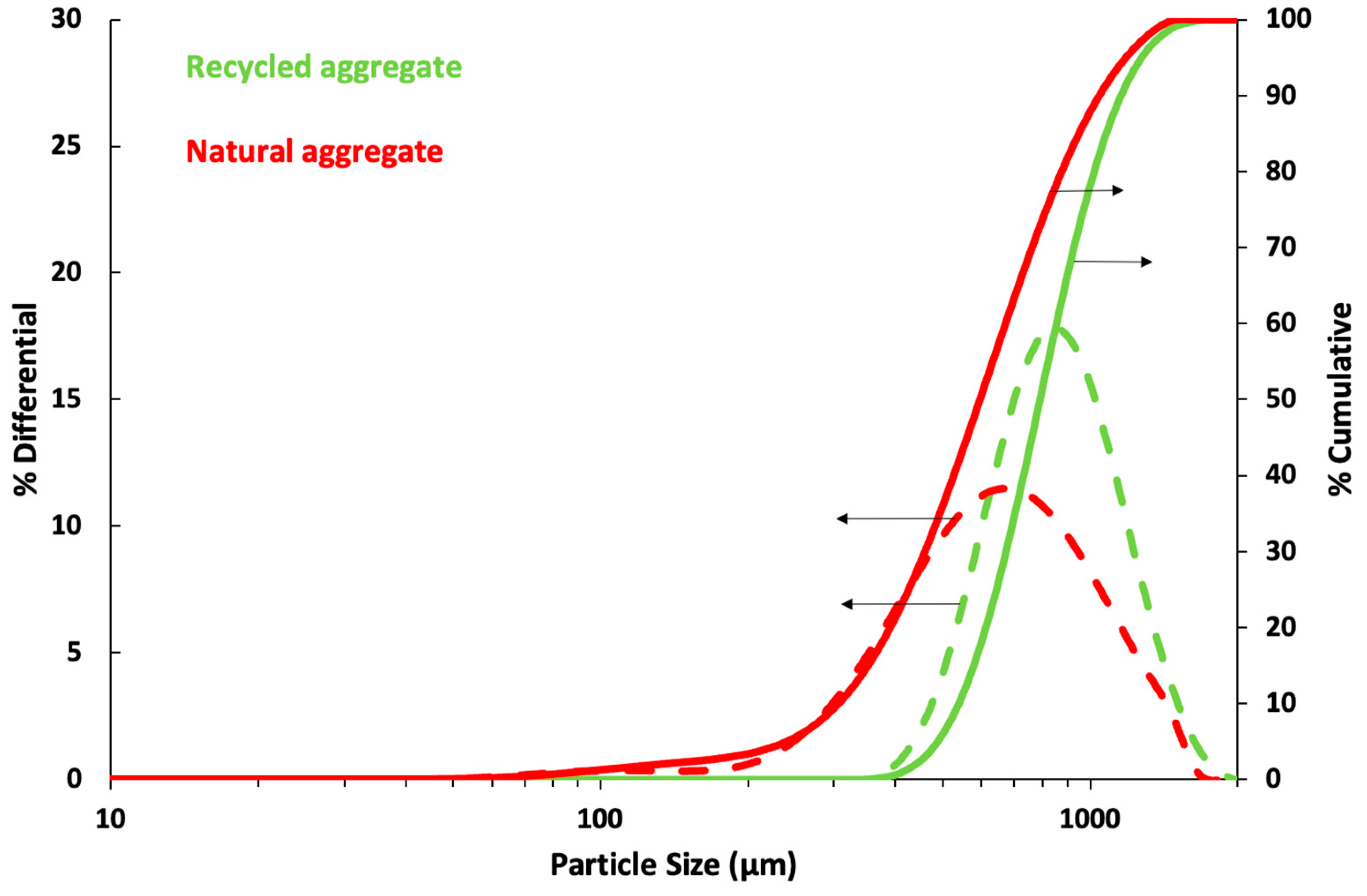
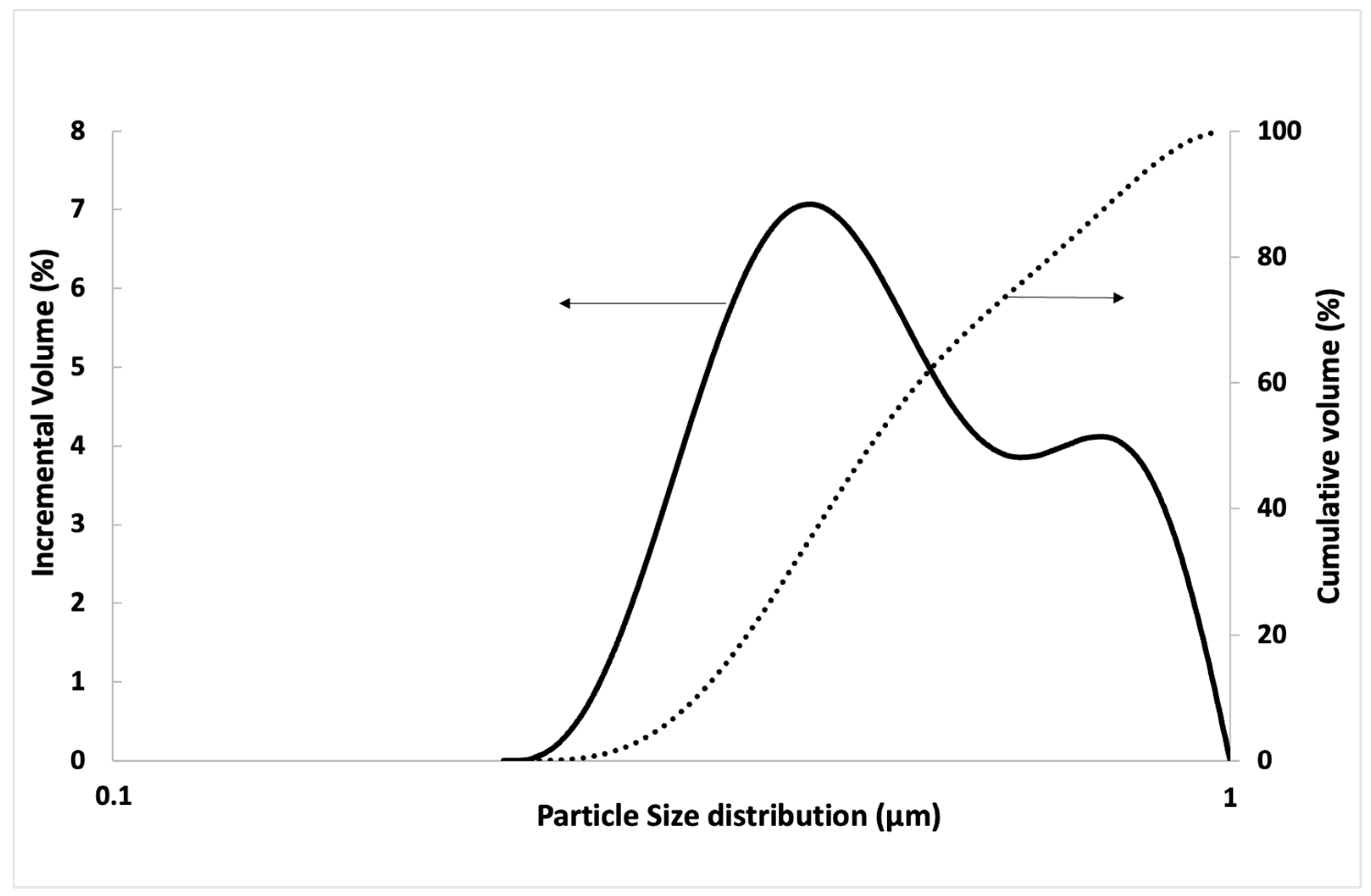

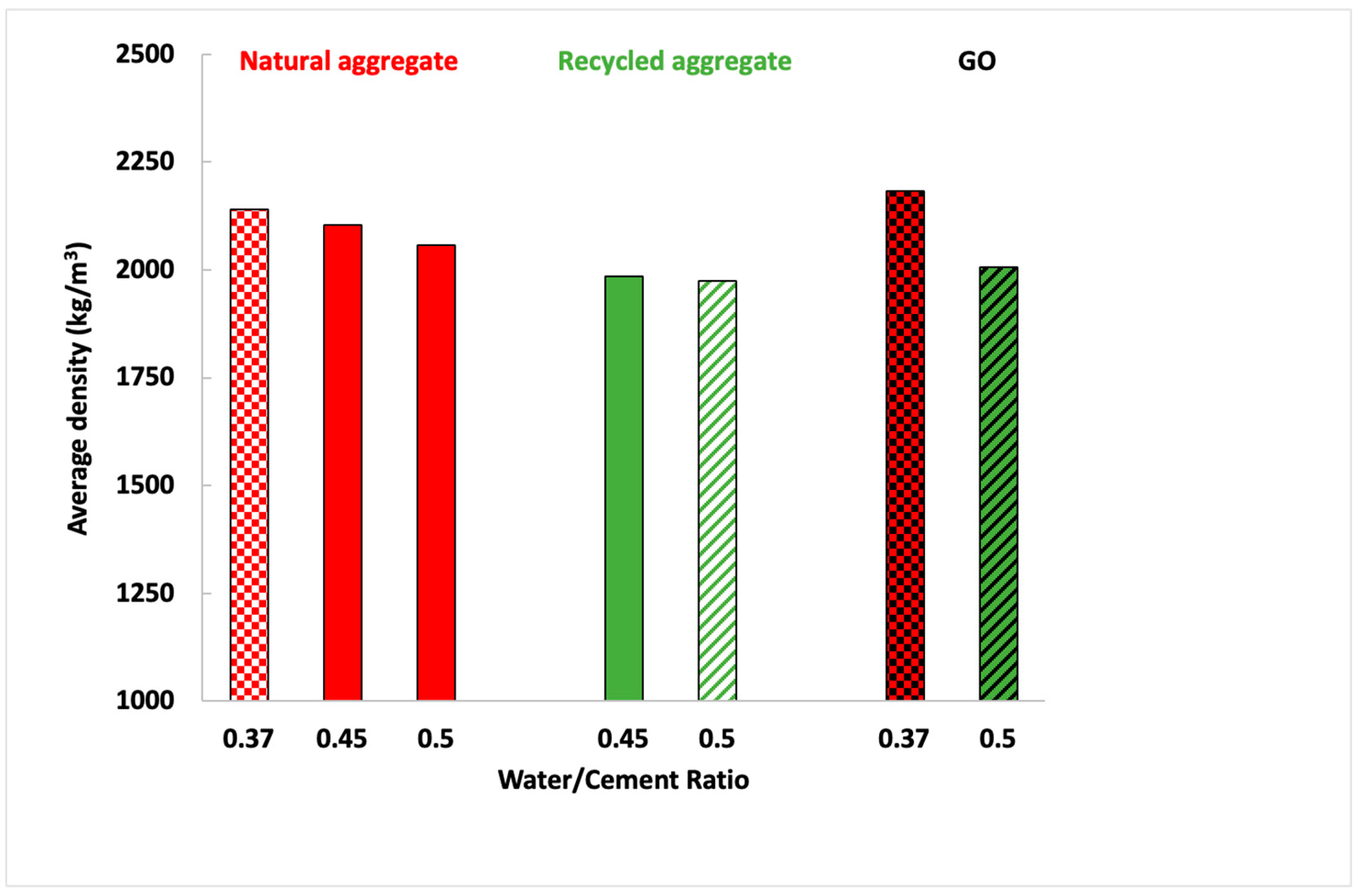


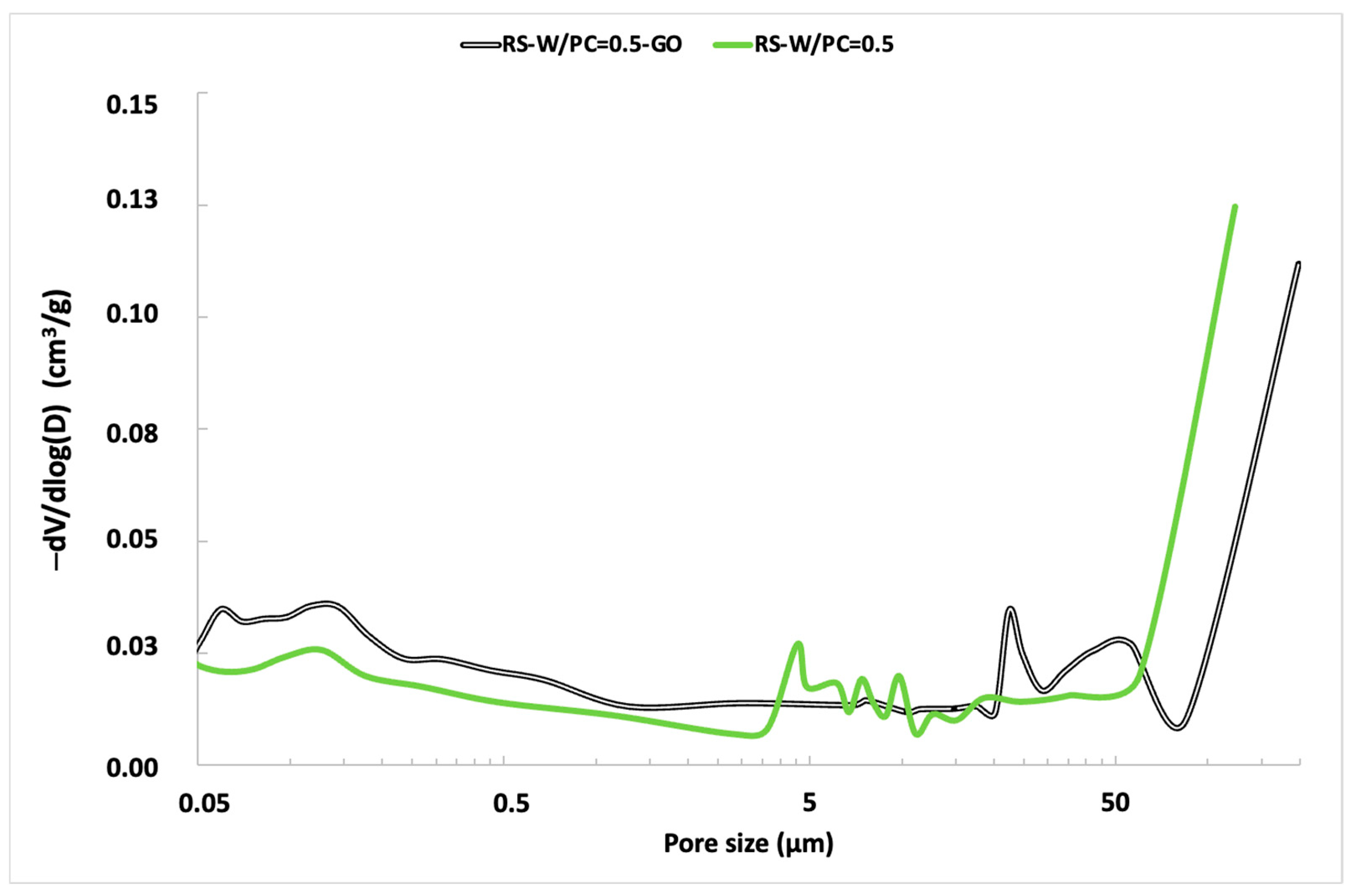



| Component (%) | Recycled Aggregate | Natural Aggregate | Portland Cement |
|---|---|---|---|
| Al2O3 | - | 0.76 | 5.74 |
| CaO | - | 0.13 | 60.89 |
| Fe2O3 | - | 0.22 | 2.46 |
| K2O | - | 0.30 | 0.73 |
| Na2O | 4.93 | 0.05 | 0.36 |
| SO3 | - | 0.02 | 1.11 |
| SiO2 | 92.18 | 96.21 | 20.96 |
| TiO2 | - | 0.12 | 0.28 |
| Loss on ignition | 2.46 | 0.31 | 5.20 |
| Specific gravity (g/cm3) | 1.73 | 2.62 | 3.15 |
| Ba | Cr | Ga | La | Mn | Mo | Nb | Ni |
| 12.5 | 39.5 | 1.2 | 7.6 | 39.0 | 1.1 | 1.0 | 5.6 |
| P | Pb | Sr | Ta | V | Y | Zr | F |
| 38.3 | 4.8 | 5.5 | 2.9 | 3.2 | 4.4 | 349 | 336 |
| Material Mix Design | Cement (kg) | Natural Aggregate (kg) | Recycled Aggregate (kg) | Superplasticiser (kg) | Water (L) | Graphene Oxide (kg) |
|---|---|---|---|---|---|---|
| NA-0.37 | 333.3 | 999.9 | - | 42 | 123.3 | - |
| NA-0.45 | 42 | 150.0 | ||||
| NA-0.5 | 42 | 166.7 | ||||
| NA-0.37-GO | 42 | 123.3 | 0.10 | |||
| RA-0.45 | - | 999.9 | 42 | 150.0 | - | |
| RA-0.5 | 42 | 166.7 | ||||
| RA-0.5-GO | 42 | 0.10 |
| Recycled Sand (mg/kg) | Standard Sand (mg/kg) | Inert Waste [54] and Portuguese Limit [47] | Non-Hazardous Waste [54] | Italian Limit [46] | Cantabria Limits [48] | |
|---|---|---|---|---|---|---|
| As | <0.01 | ≤0.01 | 0.5 | 2 | 0.5 | 0.5 |
| Ba | 0.25 | 0.82 | 20 | 100 | 10 | 20 |
| Cd | <0.01 | ≤0.01 | 0.04 | 1 | 0.05 | 0.04 |
| Co | <0.01 | ≤0.01 | - | - | 2.5 | - |
| Cr | 0.176 | ≤0.02 | 0.5 | 10 | 0.5 | 0.5 |
| Cu | <0.1 | ≤0.1 | 2 | 50 | 0.5 | 2 |
| Hg | ≤0.005 | ≤0.005 | 0.01 | 0.2 | 0.01 | 0.01 |
| Mo | <0.05 | ≤0.2 | 0.5 | 10 | - | 0.5 |
| Ni | 0.21 | ≤0.01 | 0.4 | 10 | 0.1 | 0.4 |
| Pb | 1.84 | ≤0.25 | 0.5 | 10 | 0.5 | 0.5 |
| Sb | ≤0.02 | ≤0.02 | 0.06 | 0.7 | - | 0.06 |
| Se | <0.01 | ≤0.025 | 0.1 | 0.5 | 0.1 | 0.1 |
| Sr | 0.65 | - | - | - | - | - |
| V | <0.1 | ≤0.1 | - | - | 2.5 | - |
| Zn | <0.01 | 0.067 | 4 | 50 | 0.03 | 4 |
| Mortar | Volume Stability (mm) | Water Absorption Capacity (%) | Open Void Porosity (%) |
|---|---|---|---|
| NA-0.37 | <2 | 8.3 ± 0.3 | 27.1 ± 0.9 |
| NA-0.45 | <2 | 10.1 ± 0.4 | 30.3 ± 1.2 |
| NA-0.5 | <2 | 10.8 ± 0.4 | 32.8 ± 1.2 |
| NA-0.37-GO | <2 | 4.94 ± 0.6 | 12.9 ± 0.6 |
| RA-0.45 | <2 | 12.5 ± 0.7 | 33.3 ± 1.2 |
| RA-0.5 | <2 | 15.9 ± 0.9 | 33.9 ± 0.5 |
| RA-0.5-GO | <2 | 6.8 ± 0.2 | 14.2 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz Martinez, J.D.; Cifuentes, H.; Rios, J.D.; Ariza, P.; Leiva, C. Development of Mortars That Use Recycled Aggregates from a Sodium Silicate Process and the Influence of Graphene Oxide as a Nano-Addition. Materials 2023, 16, 7167. https://doi.org/10.3390/ma16227167
Ruiz Martinez JD, Cifuentes H, Rios JD, Ariza P, Leiva C. Development of Mortars That Use Recycled Aggregates from a Sodium Silicate Process and the Influence of Graphene Oxide as a Nano-Addition. Materials. 2023; 16(22):7167. https://doi.org/10.3390/ma16227167
Chicago/Turabian StyleRuiz Martinez, Jaime D., Héctor Cifuentes, José D. Rios, Pilar Ariza, and Carlos Leiva. 2023. "Development of Mortars That Use Recycled Aggregates from a Sodium Silicate Process and the Influence of Graphene Oxide as a Nano-Addition" Materials 16, no. 22: 7167. https://doi.org/10.3390/ma16227167





