Effect of Synthesis Factors on Microstructure and Thermoelectric Properties of FeTe2 Prepared by Solid-State Reaction
Abstract
:1. Introduction
2. Experiment
2.1. Sample Preparation
2.2. Sample Testing and Characterization
3. Results and Discussion
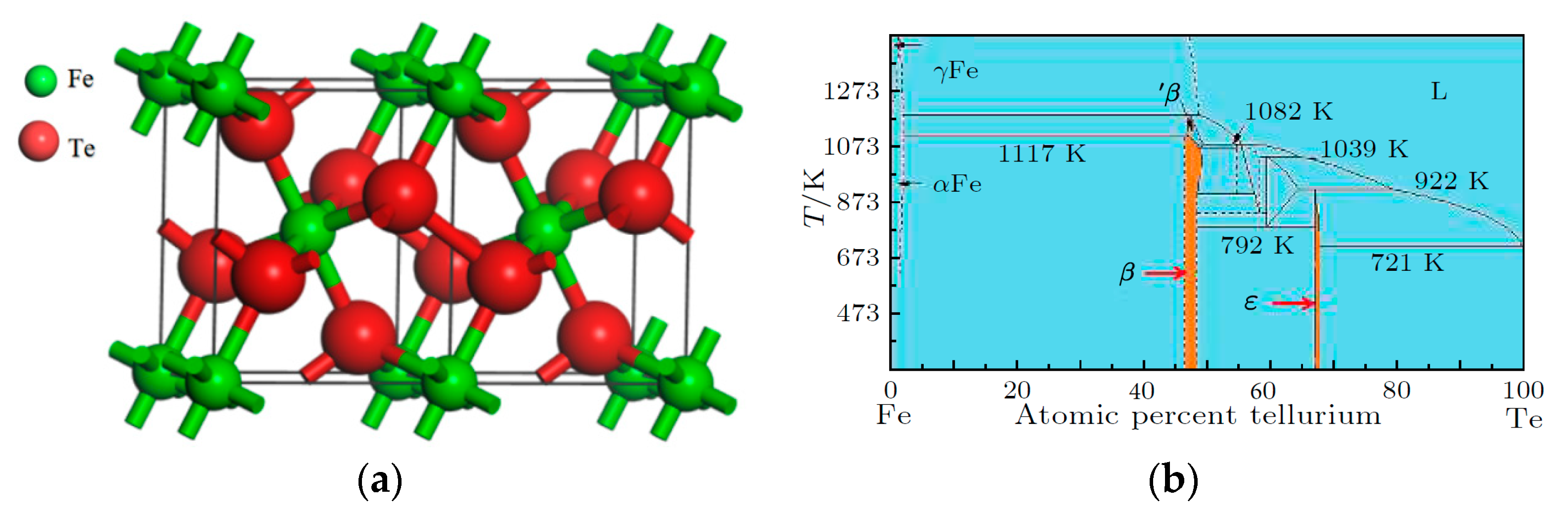
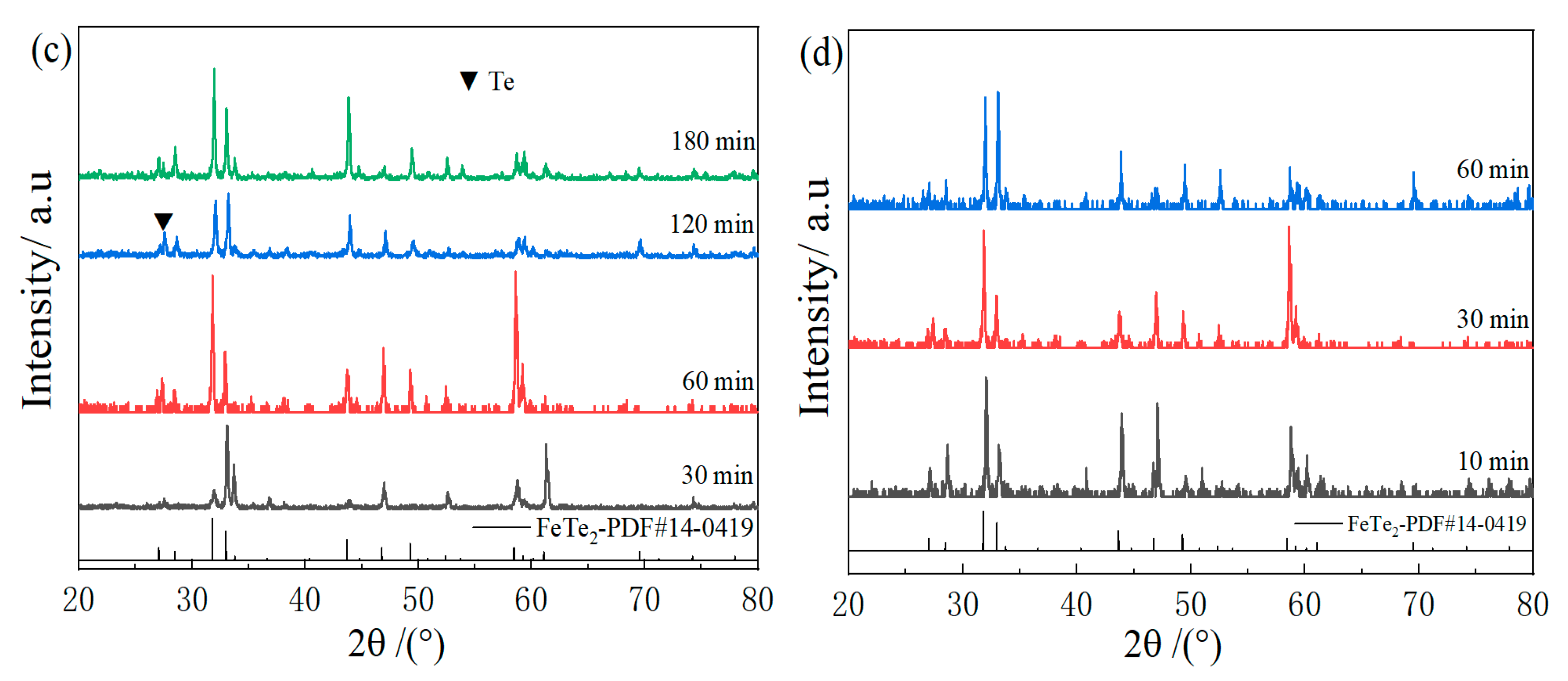
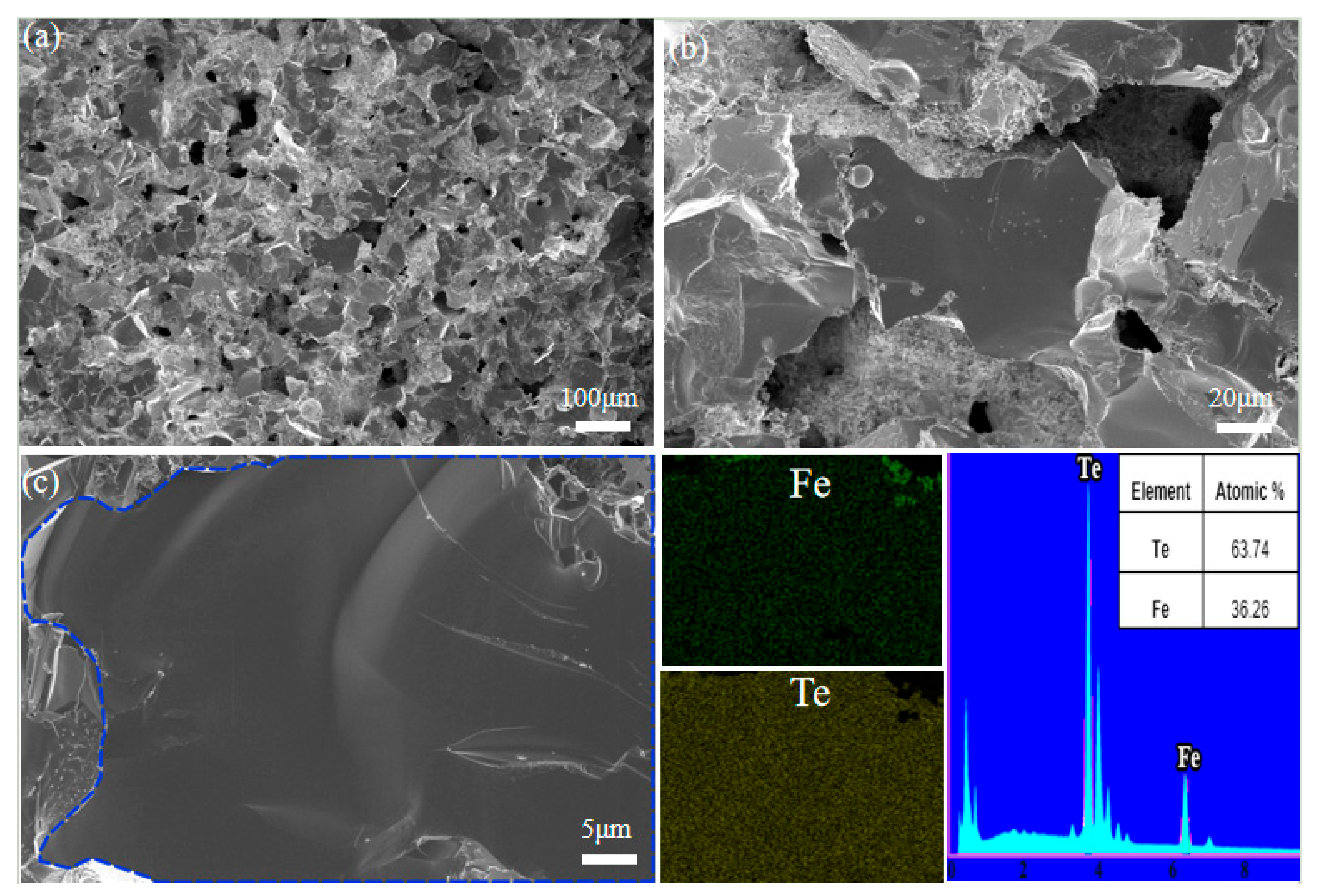
3.1. Phase Composition and Micromorphology Analysis
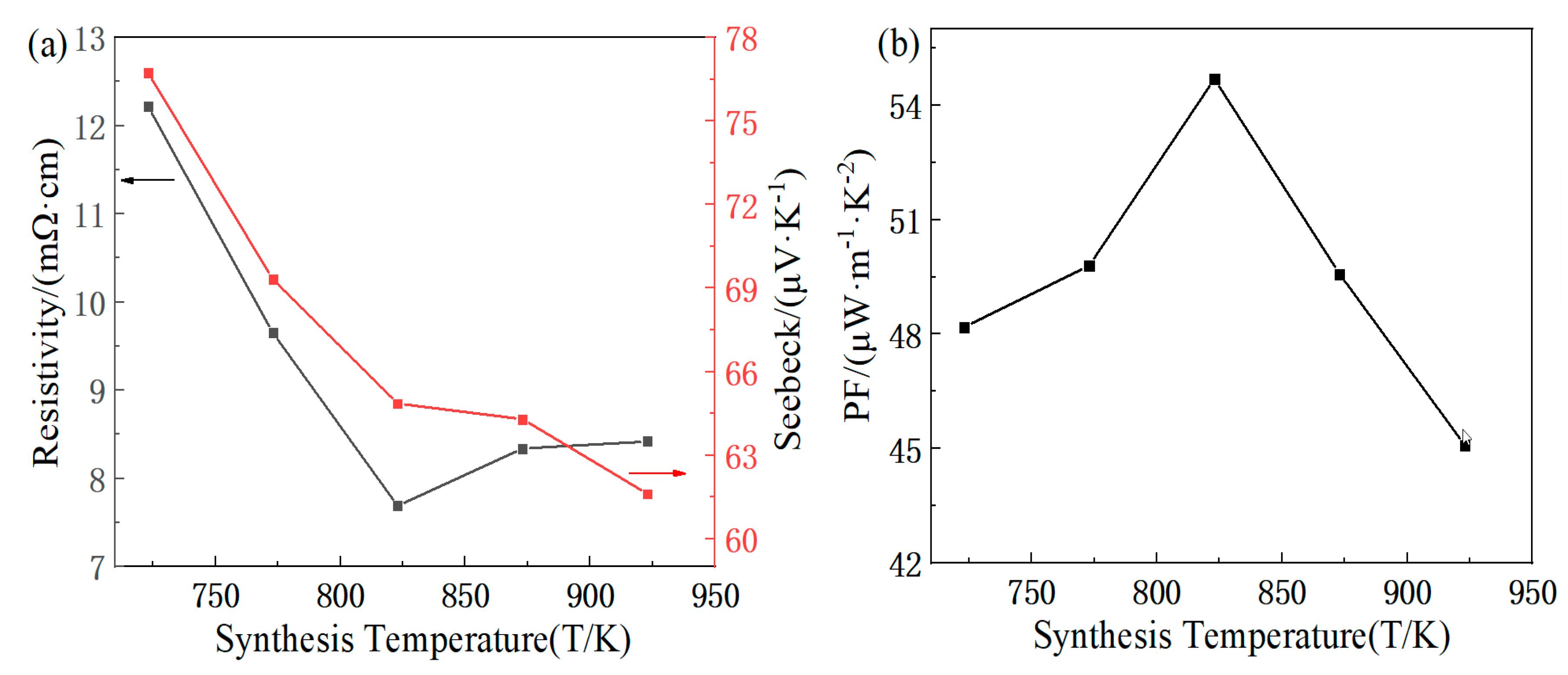
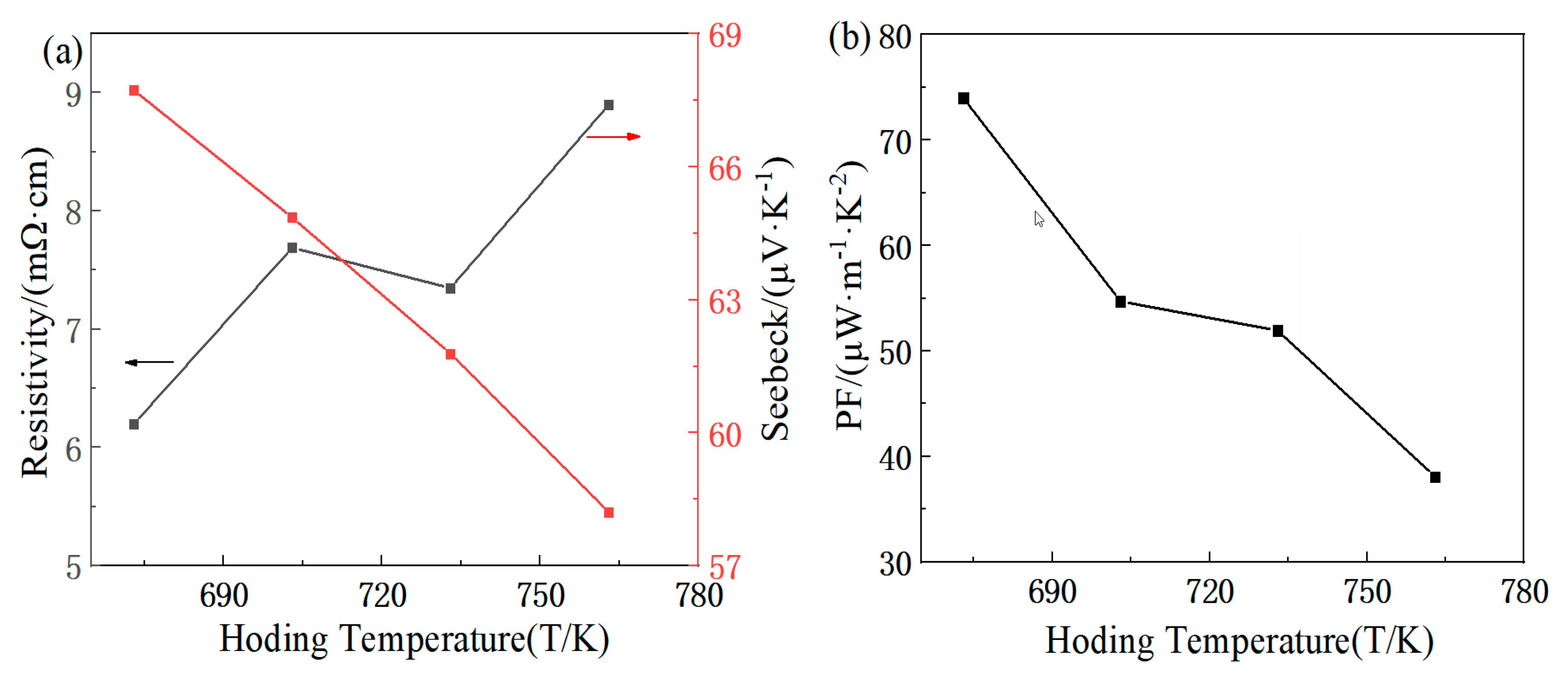
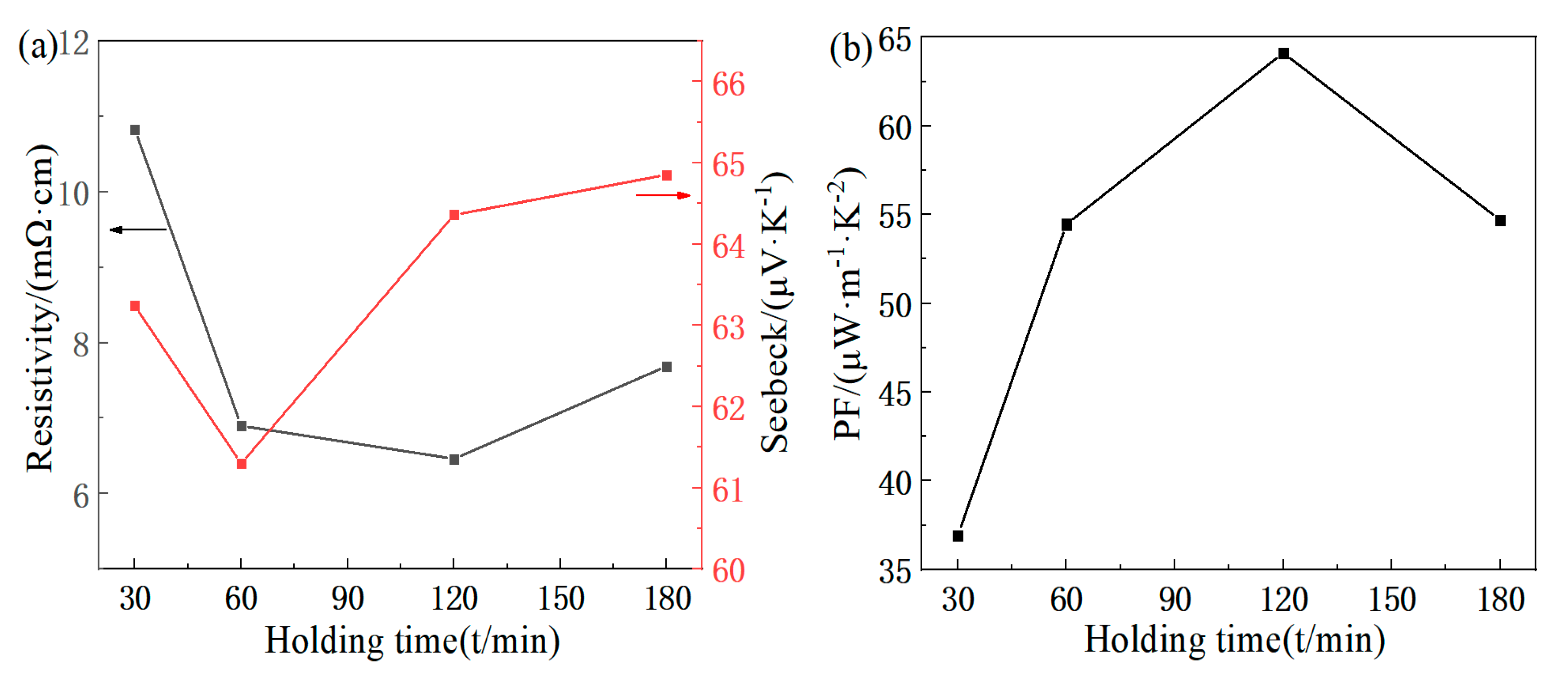
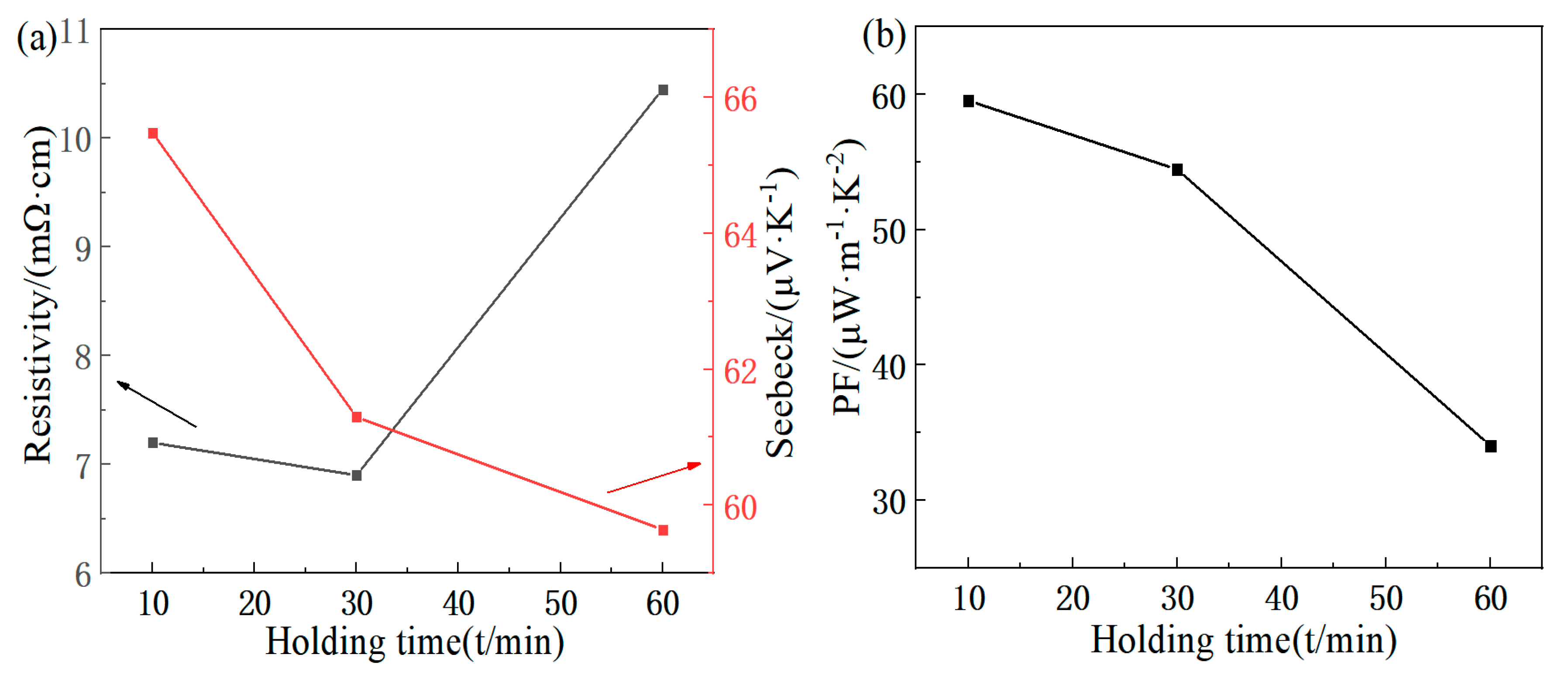
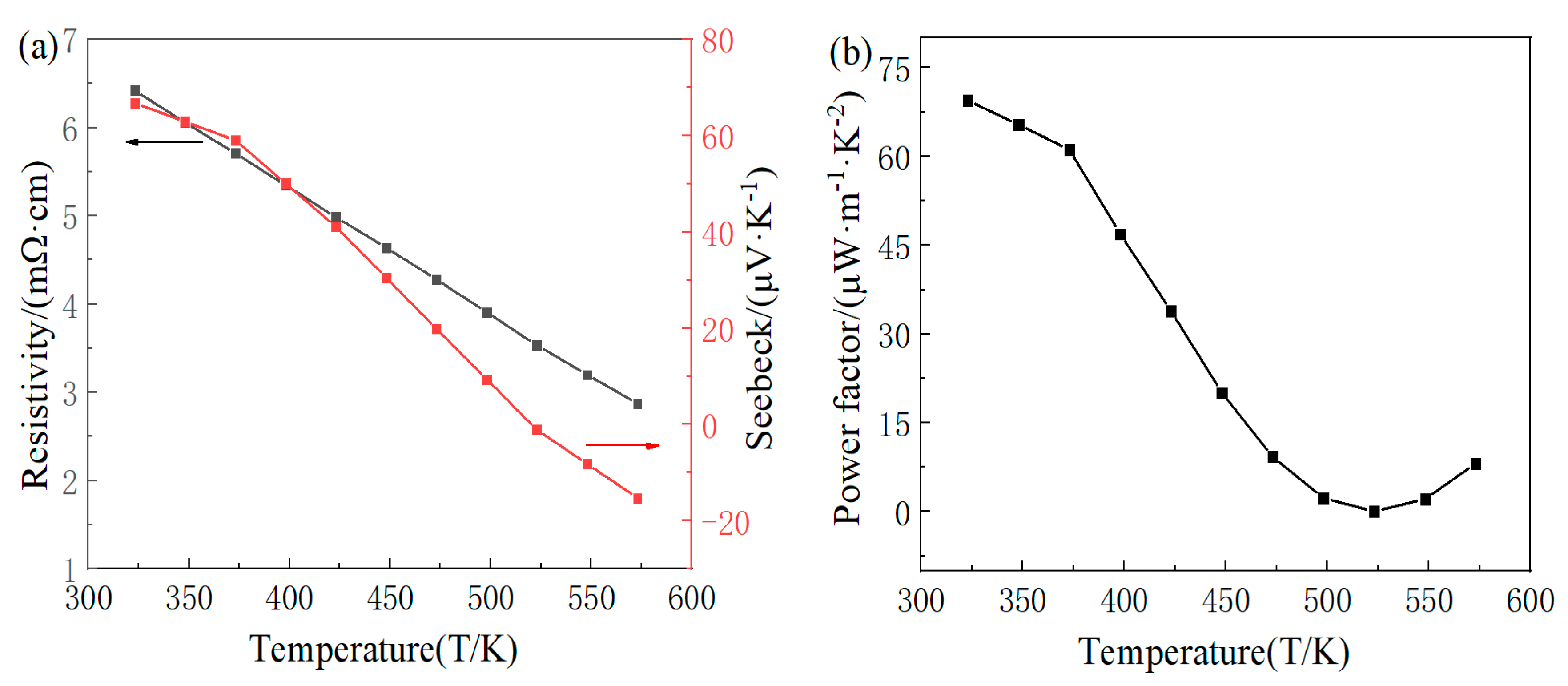
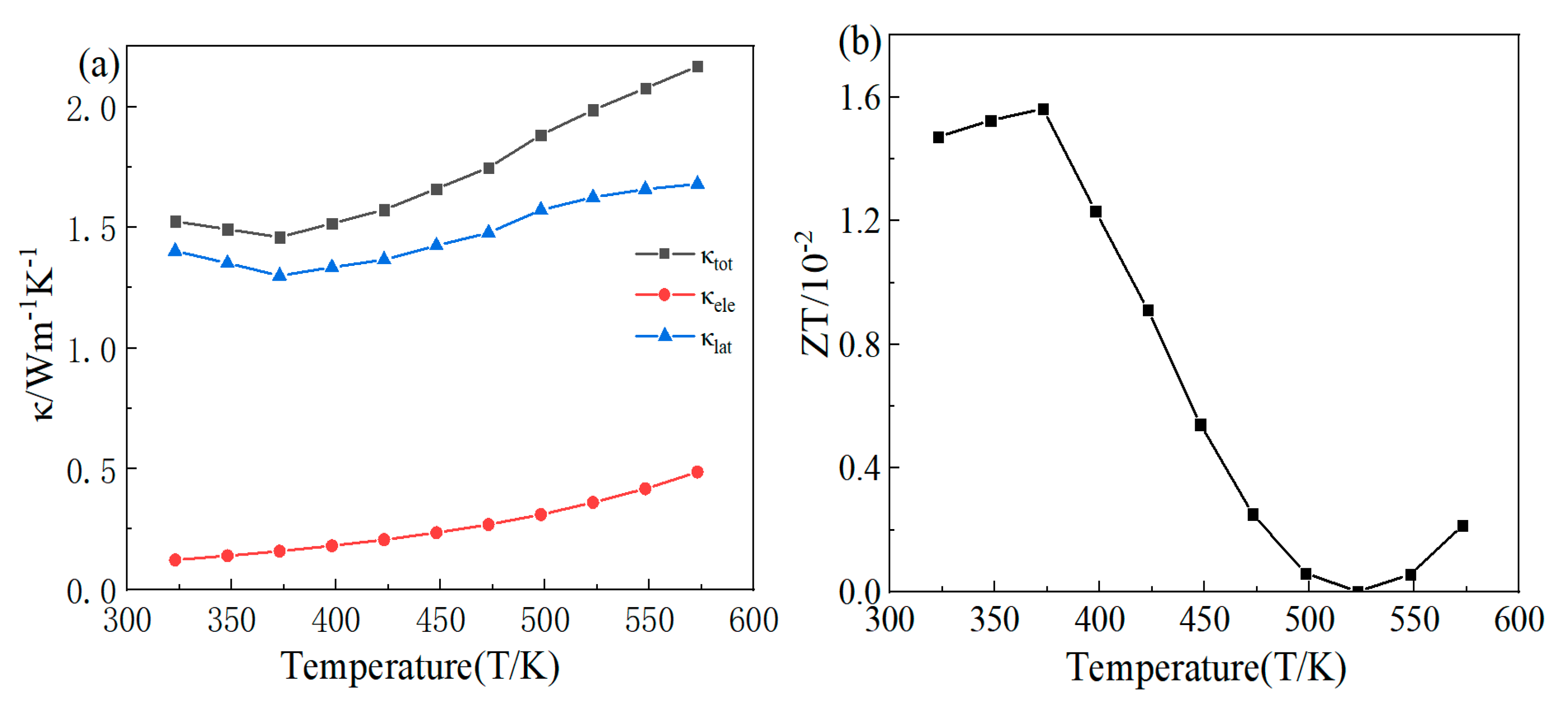
3.2. Analysis of Thermoelectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, G.K.; Wang, S.; Zhou, Z.; Li, X.; Yang, J.; Zhang, W.; Lin, Y.H.; Yang, J.; Nan, C.W. Complex Electronic Structure and Compositing Effect in High Performance Thermoelectric BiCuSeO. Nat. Commun. 2019, 10, 2814. [Google Scholar] [CrossRef]
- Yang, R.; Chen, S.; Fan, W.; Gao, X.; Long, Y.; Wang, W.; Munir, Z.A. Interfacial Properties of Cu/Ni/Mg2Si Joints Prepared in One Step by the Spark Plasma Sintering Method. J. Alloys Compd. 2017, 704, 545–551. [Google Scholar] [CrossRef]
- Zhou, Y. Progress and Trend of PbTe Based Thermoelectric Materials. J. Phys. Conf. Ser. 2022, 2194, 012006. [Google Scholar] [CrossRef]
- Nandihalli, N.; Gregory, D.H.; Mori, T. Energy-Saving Pathways for Thermoelectric Nanomaterial Synthesis: Hydrothermal/Solvothermal, Microwave-Assisted, Solution-Based, and Powder Processing. Adv. Sci. 2022, 9, 2106052. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Ji, Y.; Li, Y.; Qin, B. Effect of Forming Pressure on Thermoelectric Properties of CoSb3-Based Skutterudite Prepared by Solid-State Reaction. Solid State Sci. 2022, 132, 106999. [Google Scholar] [CrossRef]
- Sallehin, N.Z.I.S. A Review on Fabrication Methods for Segmented Thermoelectric Structure. AIP Conf. Proc. 2018, 1972, 030003. [Google Scholar] [CrossRef]
- Wu, T.; Gao, P. Development of Perovskite-Type Materials for Thermoelectric Application. Materials 2018, 11, 999. [Google Scholar] [CrossRef]
- Wei, M.; Ma, H.-L.; Nie, M.-Y.; Li, Y.-Z.; Zheng, Z.-H.; Zhang, X.-H.; Fan, P. Enhanced Thermoelectric Performance of CoSb3 Thin Films by Ag and Ti Co-Doping. Materials 2023, 16, 1271. [Google Scholar] [CrossRef]
- Jin, Y.; Hong, T.; Wang, D.; Xiao, Y.; He, W.; Gao, X.; Qiu, Y.; Zhao, L.-D. Band Structure and Microstructure Modulations Enable High Quality Factor to Elevate Thermoelectric Performance in Ge0.9Sb0.1Te-X%FeTe2. Mater. Today Phys. 2021, 20, 100444. [Google Scholar] [CrossRef]
- Bai, Z.; Ji, Y.; Qin, B. Microstructure and Thermoelectric Properties of Marcasite-Type Compounds XTe2 (X = Fe, Co, Ni) Prepared by Solid-State Reaction. J. Mater. Sci. Mater. Electron. 2023, 34, 461. [Google Scholar] [CrossRef]
- Shin, W.H.; Roh, J.W.; Ryu, B.; Chang, H.J.; Kim, H.S.; Lee, S.; Seo, W.S.; Ahn, K. Enhancing Thermoelectric Performances of Bismuth Antimony Telluride via Synergistic Combination of Multiscale Structuring and Band Alignment by FeTe2 Incorporation. ACS Appl. Mater. Interfaces 2018, 10, 3689–3698. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, H.; Wang, D.; Fu, L.; Zhang, Y.; He, J.; Pennycook, S.J.; Zhao, L.D. Investigations on Electrical and Thermal Transport Properties of Cu2SnSe3 with Unusual Coexisting Nanophases. Mater. Today Phys. 2018, 7, 77–88. [Google Scholar] [CrossRef]
- Soleimani, Z.; Zoras, S.; Ceranic, B.; Shahzad, S.; Cui, Y. A Review on Recent Developments of Thermoelectric Materials for Room-Temperature Applications. Sustain. Energy Technol. Assess. 2020, 37, 100604. [Google Scholar] [CrossRef]
- Zhao, L.-D.; He, J.; Berardan, D.; Lin, Y.; Li, J.-F.; Nan, C.-W.; Dragoe, N. BiCuSeO Oxyselenides: New Promising Thermoelectric Materials. Energy Environ. Sci. 2014, 7, 2900–2924. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Tan, G.; Hao, S.; He, J.; Pei, Y.; Chi, H.; Wang, H.; Gong, S.; Xu, H.; Dravid, V.P.; et al. Ultrahigh Power Factor and Thermoelectric Performance in Hole-Doped Single-Crystal SnSe. Science 2016, 351, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kwak, H.; Kim, H.S.; Bang, J.; Park, H.; Park, O.; Kim, S.I. Evolution of Electrical Transport Properties in FeTe2-CoTe2 Solid Solution System for Optimum Thermoelectric Performance. J. Alloys Compd. 2023, 960, 170850. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Q.; Li, S.; Qin, B.; Su, T. Facile Synthesis and Thermoelectric Performance of BiCu1-XSeO. Mater. Lett. 2021, 301, 130329. [Google Scholar] [CrossRef]
- Goldsmid, H. Bismuth Telluride and Its Alloys as Materials for Thermoelectric Generation. Materials 2014, 7, 2577–2592. [Google Scholar] [CrossRef]
- Tuley, R.; Simpson, K. ZT Optimization: An Application Focus. Materials 2017, 10, 309. [Google Scholar] [CrossRef]
- Fortulan, R.; Aminorroaya Yamini, S. Recent Progress in Multiphase Thermoelectric Materials. Materials 2021, 14, 6059. [Google Scholar] [CrossRef]
- Rahman, J.U.; Nam, W.H.; Jung, Y.; Won, J.H.; Oh, J.; Van Du, N.; Rahman, G.; García-Suárez, V.M.; He, R.; Nielsch, K.; et al. Realizing High Thermoelectric Performance in N-Type Se-Free Bi2Te3 Materials by Spontaneous Incorporation of FeTe2 Nanoinclusions. Energy Environ. Mater. 2023, e12663. [Google Scholar] [CrossRef]
- Viskadourakis, Z.; Drymiskianaki, A.; Papadakis, V.M.; Ioannou, I.; Kyratsi, T.; Kenanakis, G. Thermoelectric Performance of Mechanically Mixed BixSb2-XTe3—ABS Composites. Materials 2021, 14, 1706. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Y.; Wei, B.; Yang, Y.; Yu, D.; Zheng, Y.; He, D.; Zhang, W.; Zou, M.; Lan, J.L.; et al. Compositing Effects for High Thermoelectric Performance of Cu2Se-Based Materials. Nat. Commun. 2023, 14, 2410. [Google Scholar] [CrossRef]
- Liu, W.; Yan, X.; Chen, G.; Ren, Z. Recent advances in thermoelectric nanocomposites. Nano Energy 2012, 1, 42–56. [Google Scholar] [CrossRef]
- Nandihalli, N. Imprints of interfaces in thermoelectric materials. Crit. Rev. Solid State Mater. Sci. 2023, 48, 361–410. [Google Scholar] [CrossRef]
- Kim, S.; Bang, J.; An, J.; Hong, S.; Bang, G.; Shin, W.H.; Kim, T.; Lee, K. Effect of Br Substitution on Thermoelectric Transport Properties in Layered SnSe2. J. Alloys Compd. 2021, 868, 159161. [Google Scholar] [CrossRef]
- Pang, H.; Qiu, Y.; Wang, D.; Qin, Y.; Huang, R.; Yang, Z.; Zhang, X.; Zhao, L.-D. Realizing N-Type SnTe Thermoelectrics with Competitive Performance through Suppressing Sn Vacancies. J. Am. Chem. Soc. 2021, 143, 8538–8542. [Google Scholar] [CrossRef]
- Gudelli, V.K.; Kanchana, V.; Vaitheeswaran, G.; Valsakumar, M.C.; Mahanti, S.D. Thermoelectric Properties of Marcasite and Pyrite FeX2 (X = Se, Te): A First Principle Study. RSC Adv. 2014, 4, 9424. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Sharma, D.K.; Sharma, Y.K.; Chandra, U. High-Pressure Electrical Resistivity Studies on FeSe2 and FeTe2. Proc. AIP Conf. Proc. 2013, 1512, 40–41. [Google Scholar] [CrossRef]
- Capitano, D.; Hu, Z.; Liu, Y.; Tong, X.; Nykypanchuk, D.; Dimarzio, D.; Petrovic, C. Synthesis and Characterization of Ultrathin FeTe2Nanocrystals. ACS Omega 2021, 6, 10537–10546. [Google Scholar] [CrossRef]
- Liu, A.; Chen, X.; Zhang, Z.; Jiang, Y.; Shi, C. Selective Synthesis and Magnetic Properties of FeSe2 and FeTe2 Nanocrystallites Obtained through a Hydrothermal Co-Reduction Route. Solid. State Commun. 2006, 138, 538–541. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, Y.; Zhan, J.; Yu, W.; Yang, L.; Chen, L.; Qian, Y. Synthesis of Nanocrystalline Marcasite Iron Ditelluride FeTe2 in Aqueous Solution. Mater. Sci. Eng. B 2001, 79, 244–246. [Google Scholar] [CrossRef]
- Du, J.; Wang, F.; Jin, L.; Quan, Z.; Bai, Y.; Xu, X. Enhancement of Ferromagnetism in FeTe2 Nanoparticles by Cr Doping. Mater. Lett. 2016, 184, 261–264. [Google Scholar] [CrossRef]
- Chibani, H.; Yahi, H.; Ouettar, C. Tuning the Magnetic Properties of FeTe2 Monolayer Doped by (TM: V, Mn, and Co). J. Magn. Magn. Mater. 2022, 552, 169204. [Google Scholar] [CrossRef]
- Tian, X.H.; Zhang, J.M. The Structural, Elastic, Electronic and Optical Properties of Orthorhombic FeX2 (X=S, Se, Te). Superlattices Microstruct. 2018, 119, 201–211. [Google Scholar] [CrossRef]
- Guo, J.; Liang, S.; Shi, Y.; Li, B.; Hao, C.; Wang, X.; Ma, T. Electrocatalytic Properties of Iron Chalcogenides as Low-Cost Counter Electrode Materials for Dye-Sensitized Solar Cells. RSC Adv. 2015, 5, 72553–72561. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, Y.; Li, Q.; Chu, K. FeTe2 as an Earth-Abundant Metal Telluride Catalyst for Electrocatalytic Nitrogen Fixation. J. Energy Chem. 2021, 56, 259–263. [Google Scholar] [CrossRef]
- Rahman, A.; Zhang, D.; Rehman, M.U.; Zhang, M.; Wang, X.; Dai, R.; Wang, Z.; Tao, X.; Zhang, Z. Multiple Magnetic Phase Transitions, Electrical and Optical Properties of FeTe2 Single Crystals. J. Phys. Condens. Matter 2020, 32, 035808. [Google Scholar] [CrossRef]
- Guo, J.-Y.; Chen, S.-P.; Fan, W.-H.; Wang, Y.-N.; Wu, Y.-C. Improving interface properties of Te based thermoelectric materials and composite electrodes. Acta Phys. 2020, 69, 146801. [Google Scholar] [CrossRef]
- Wang, C.; Chen, F.; Sun, K.; Chen, R.; Li, M.; Zhou, X.; Sun, Y.; Chen, D.; Wang, G. Contributed Review: Instruments for Measuring Seebeck Coefficient of Thin Film Thermoelectric Materials: A Mini-Review. Rev. Sci. Instrum. 2018, 89, 101501. [Google Scholar] [CrossRef]
- Llewellyn, J.P.; Smith, T. The Magnetic Susceptibility of Iron Ditelluride: I. Proc. Phys. Soc. 1959, 74, 65–74. [Google Scholar] [CrossRef]
- Grønvold, F.; Haraldsen, H.; Vihovde, J.; Sörensen, N.A. Phase and Structural Relations in the System Iron Tellurium. Acta Chem. Scand. 1954, 8, 1927–1942. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Qin, B.; Sun, C.; Ji, Y.; Zhao, D. Effect of Synthesis Factors on Microstructure and Thermoelectric Properties of FeTe2 Prepared by Solid-State Reaction. Materials 2023, 16, 7170. https://doi.org/10.3390/ma16227170
Zhang L, Qin B, Sun C, Ji Y, Zhao D. Effect of Synthesis Factors on Microstructure and Thermoelectric Properties of FeTe2 Prepared by Solid-State Reaction. Materials. 2023; 16(22):7170. https://doi.org/10.3390/ma16227170
Chicago/Turabian StyleZhang, Lang, Bingke Qin, Cheng Sun, Yonghua Ji, and Dan Zhao. 2023. "Effect of Synthesis Factors on Microstructure and Thermoelectric Properties of FeTe2 Prepared by Solid-State Reaction" Materials 16, no. 22: 7170. https://doi.org/10.3390/ma16227170
APA StyleZhang, L., Qin, B., Sun, C., Ji, Y., & Zhao, D. (2023). Effect of Synthesis Factors on Microstructure and Thermoelectric Properties of FeTe2 Prepared by Solid-State Reaction. Materials, 16(22), 7170. https://doi.org/10.3390/ma16227170




